Abstract
Objective: This paper describes the GeneTests genetic testing information resource with a focus on the GeneReviews component.
Methods and Findings: The need for authoritative genetic testing information and issues in the development and maintenance of GeneReviews are discussed: Hampered by lack of currency and content deficits, traditional medical information resources such as textbooks and the published literature are generally inadequate sources of genetic testing information. Problems encountered in developing GeneReviews include the evolution of new authorship models and academic and genetics professionals' skepticism about the quality of Web-based publications.
Conclusions: GeneTests is an authoritative, highly used, and well-regarded resource in the international medical community that is intended for health care providers. Future development issues to address include ways to (1) manage the increasing editing and updating load as content grows and (2) address technical and content issues that need to be considered in displaying GeneReviews as a “just in time” resource in the electronic medical record to achieve the project goal of integrating appropriate use of genetic testing into patient care.
Highlights
GeneTests <www.genetests.org>, a genetic testing information resource targeted to health care providers and supported by the National Institutes of Health, strives to incorporate genetic testing into patient care.
GeneTests integrates a directory of international genetics laboratories and genetics clinics, information on resources for health care consumers, and GeneReviews (expert-authored, peer-reviewed disease descriptions).
GeneReviews provides information about a molecular genetic test in the context of the clinical care issues for a patient and the patient's family.
GeneReviews uses an assisted authoring model so that the experts provide their detailed knowledge, and staff structure each entry identically, so that readers know what information they will find and where they will find it.
Currency of each GeneReview is maintained by “quick” revisions as needed when clinically relevant information, such as test availability or treatment protocols, changes significantly and by a formal, comprehensive updating process every two years.
Implications for practice
GeneReviews contrast with stand-alone print articles and peer-reviewed journals in that they are integrated into a set of clinically useful resources and provide continuously updated information on genetic testing.
The GeneReviews context-sensitive illustrated glossary helps make content accessible to a wide range of users, including those with limited understanding of medical genetics terms.
The hybrid text/database model of GeneReviews will allow the same information to be accessible on the Web and at the point of care in the electronic medical record.
INTRODUCTION
GeneTests, an online genetic testing information resource [1], consists of several integrated resources: a genetic testing laboratory directory; a genetics clinics directory; an extensive illustrated glossary of genetic testing terms; and GeneReviews, expert-authored summaries of information about disease diagnosis and management, genetic testing and counseling, and genetic pathogenesis [2]. This paper primarily discusses the development and content of the GeneReviews portion of GeneTests, focusing on the need for the resource, issues in its development, and future directions.
BACKGROUND
The GeneTests resource began production as a directory of genetics laboratories in the 1990s to facilitate location of laboratories offering DNA-based (molecular) genetic testing. Prior to the inception of GeneTests, locating such laboratories was an arduous task that required, in the words of one GeneTests user, “memory, colleagues, literature, and luck.” Funded by a National Library of Medicine contract (NO-1-LM-3506), GeneTests, originally known as Helix, began in 1992 as a “Yellow Pages” for genetics laboratories with listings for about 100 US laboratories offering molecular genetic testing for about 100 diseases. Registered health care providers telephoned in about 10 to 15 daily requests for laboratory information to the project manager who faxed back the search results.
From the first release of Helix/GeneTests, it was clear that providing easy access to molecular genetic testing information through a yellow pages format was only the first step in incorporating appropriate use of molecular genetic testing into patient care. A “users' manual” was needed to help health care providers understand the technical strengths and weaknesses of the molecular genetic testing offered for each disorder, the medical uses of molecular genetic testing in disease diagnosis and patient management, and the genetic counseling implications of the diagnosis of an inherited disorder for both the patient and extended family. An additional consideration for a number of disorders was the ethical implication of predictive testing that identifies the presence of a genetic disorder before it clinically manifests.
To develop this users' manual, grant funding was obtained from the National Institutes of Health (NIH) (1 P41 LMHG06029) in 1997 for Genline: An Electronic Clinical Genetics Knowledge Base. Genline eventually evolved into the GeneReviews portion of the GeneTests resource. GeneReviews is a resource of high-quality content on the use of molecular genetic testing in diagnosis, management, and genetic counseling of inherited disorders. GeneReviews uses a highly structured format with specific content requirements, distributed authorship, and traditional peer review to promote the appropriate use of genetic testing in patient care by providing current, synthesized, easily accessed information free of charge. Table 1 provides descriptions of field content.
Table 1 GeneReviews fields
THE NEED FOR AUTHORITATIVE GENETIC TESTING INFORMATION
GeneReviews fills a gap left by traditional information sources such as textbooks and the published literature that do not adequately address genetic testing.
Inadequacies of traditional medical information sources
The published literature in general does not reflect currently available molecular genetic tests. Textbooks are far too out of date, even when newly published, to accurately convey information on the molecular basis of an inherited disorder, let alone the availability and use of molecular genetic tests.
The peer-reviewed published medical literature is similarly difficult to use when applying molecular genetic testing to patient care. Most published articles about inherited disorders and their related genes focus on the basic science of gene discovery through gene mapping and gene cloning. Aside from identifying a causative gene (which, of course, is a major scientific discovery), the information provided in such publications cannot be used by a clinician in direct care of a patient because most such articles do not report on test methods provided by clinical laboratories. For clinicians to apply gene discoveries to patient care, they must understand the laboratory test methods used to evaluate the gene, the mutation detection rate (ability to identify a mutation in a gene), and false negative rate (inability of the methods used by that laboratory to detect a mutation that is present in a gene).
Reports of data on molecular genetic test sensitivity, specificity, and use in patient care are infrequently published by medical journals and are not available in the volume and timeliness needed for the approximately 1,000 genetic tests that are currently available. Finally, for the busy clinician who has only 10 to 15 minutes per patient visit and is unfamiliar with a certain inherited disorder, the information about a molecular genetic test needs to be accessed quickly and presented in the context of the clinical care issues for the patient and the patient's family.
Further, print publications, and even most electronic publications, cannot meet the information needs of a medical specialty with a rapidly evolving knowledgebase. In current publishing models, both print materials and electronic peer-reviewed articles are static and not continually updated after publication. Print articles and peer-reviewed articles tend to “stand alone” and are not integrated into a set of clinically useful resources (e.g., directory of specialized laboratories and specialized clinical services, consumer health–oriented resources, genomic databases with extensive gene sequence information). It is also unusual for articles to be highly structured or to meet minimum content requirements; rather, the authors provide information that they and their scientific peers deem appropriate for the discovery being reported.
GeneReviews overcomes these deficiencies by focusing on genetic testing information and processes to keep the information on test availability and the understanding of the relationship between genes and their diseases current: continual “quick” revisions as information changes significantly and formal, comprehensive, scheduled updates every two years.
Needs of diverse constituents
Just as print publications suffer problems with currency, they have difficulty to serving the needs of a diverse health care audience. Although the audience for GeneReviews has always been health care professionals, it was decided at the outset of the project to target the level of language to genetics professionals because they were, at that time, the biggest consumers of genetic testing information. With time, GeneReviews became a resource for non-geneticist health care providers as well. Through ongoing interactions with health care providers in primary care and other medical specialties, the GeneReviews editorial staff learned that the specialized vocabulary of medical genetics was off-putting “jargon” to non-geneticist health care providers.
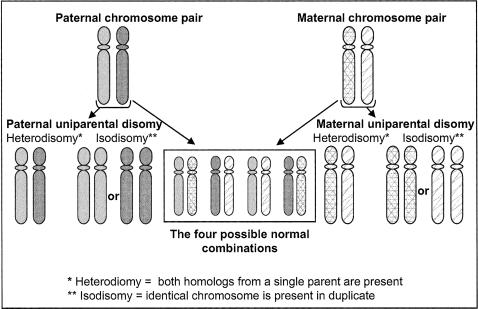
To remedy this problem, GeneReviews staff developed a context-sensitive glossary (funded under the Department of Energy's Ethical, Legal and Social Implications program, DE-FG03–02ER63301/A00) to make the highly specific genetic testing and genetic counseling terms used in each GeneReview entry accessible to this broader audience. Over 225 terms were defined, of which about 100 were illustrated with graphics, “Learn More” (Figure 1) and/or vignettes, “Case Example” or “Disease Example.”
Figure 1.
Glossary illustration of uniparental disomy (UPD)
ISSUES IN THE DEVELOPMENT OF GENEREVIEWS
The development of GeneReviews was complicated by several factors related to health care and academic environments and traditional publishing models. These factors included: concerns about professional turf, the scholarly imprimatur of Web publications, and issues in developing a new publication model that relied on storing text in a database and a collaborative author/ editing model to maximize the efficiency of contributing authors.
Perceived threat to the medical genetics profession
Some medical geneticists expressed concern that accurate, current genetic testing information was the purview of the medical genetics profession and that easy-to-access quality genetic information would obviate the need for medical genetics specialists in the care of persons with inherited disease. However, the converse now seems to be true: easy-to-access information detailing the complexities of genetic test ordering, test result interpretation, and genetic counseling to entire families has defined the role of trained genetics professionals for physicians and other health care providers who tend to be unaware of this specialty.
Academic skepticism
At the inception of GeneReviews in 1995, some academics were concerned that Web-based information was not scholarly, and, therefore, experts could not be enticed to author Web-based materials because work appearing exclusively on the Internet would not enhance their academic credentials. This fear was never realized. GeneReviews was released to the public in October 1998 with 23 expert-authored entries; currently, over 600 experts have authored more than 330 entries. Several authors have commented that the GeneReviews peer-review process is as rigorous as any they have experienced. Recently, an author noted, “This has been a very thorough process. Little did I realize when I took it on, but it gives me great confidence as I use GeneReviews a lot for patients, so thank you for doing it.” Since fall 2005, almost all of the authors scheduled to write new entries were volunteers rather than prospects recruited by the editors.
Term of authorship
When GeneReviews was begun, the prevailing culture for authors was that authoring an “article” was a one-time activity; although authoring a book chapter might involve an occasional solicited update for a future edition. However, writing for the Web required a paradigm shift: If one of the main premises of Web-based publishing is current, high-quality information, then the relationship of authors to published material needed to be redefined.
GeneReviews consequently defined its “term of authorship” in the following manner: following posting of the original entry, each author agrees to a two-year term of authorship, during which time the author agrees to revise the entry as needed when clinically relevant information changes (e.g., test availability changes, treatment protocols change significantly). At the end of two years, the author or authors have the option of terminating their authorship or extending authorship for another two years, during which time they will participate in a formal updating process and provide revisions as needed.
About two-thirds of GeneReviews authors are from the United States, and the remaining authors are international. Authors must have either clinical experience with the disorder or evidence of expertise as reflected in publications. Usually, a non-clinician researcher does not have sufficient clinical expertise to be the sole author. In some instances, members of the scientific (medical) advisory board of an advocacy group serve as coauthors with patient advocates.
Assisted authoring model
At the outset of GeneReviews, the GeneReviews staff thought that highly structured documents presenting predictable types of information in predictable places would be easy to author, easy to read, and ultimately easy to store in a database. The editorial staff, however, quickly learned that although the strength of the distributed author model was the expected invaluable, in-depth content expertise of the authors, a weakness was the diversity in authoring styles and authors' unpredictable use of a seemingly straightforward authoring template.
To assure that the experts could do what they do best while meeting the format and content needs of GeneReviews, the project developed an “assisted authoring model.” In this model, the staff sends the author a document template that contains the disease name, outline of the GeneReview, and completed Genetic Counseling section authored by in-house staff to ensure consistency in language and content. After the authors complete the remainder of the template, the staff edits for format and style, as well as content based on (1) the expertise of four staff members in clinical genetics, laboratory genetics, and genetic counseling; (2) a PubMed search result; and (3) test availability as reflected in the GeneTests Laboratory Directory.
In an iterative process, the staff and authors arrive at a draft suitable for external review. Following external review by two or more content experts, the entry is finalized in another iterative process involving author and staff.
Use of a hybrid text/database model
The project staff concluded that maintenance of data used across the project would be simplified if such data were stored in a database. Thus, data elements used in a GeneReview and elsewhere in the project are stored in the GeneTests database and “pulled” directly into the GeneReview document. Data pulled into each GeneReview include:
title of the GeneReview
Resources
- three tables in the Molecular Genetics section:
- the Molecular Genetics Table that provides the gene symbol, chromosomal locus, and protein name for each relevant gene
- the table providing relevant links to Online Mendelian Inheritance in Man (OMIM) [3]
- the Genomic Databases table with links directly to the relevant entry in basic science genomic databases including “locus specific databases” dedicated to specific genes and “umbrella” databases such as Entrez Gene, Human Gene Mutation Database, GeneCards, Genome DataBase, and GenAtlas.
The hybrid text/database model creates an opportunity for integration of GeneReviews into the electronic medical record (see below).
CURRENT STATE AND USE OF GENEREVIEWS
As of February 2006, GeneReviews included 330 disease entries. About 1 new entry is added each week. The goal of the GeneTests project is to have a GeneReview for each disorder listed in the GeneTests Laboratory Directory.
The entire GeneTests resource, including GeneReviews, is heavily used and highly regarded. In 2002, in response to a request for letters supporting continued NIH funding, the GeneTests project received more than 1,300 signatures on letters attesting to the important role that GeneTests plays in patient care, research, and education. Medical geneticists observed that the 3 essential information resources in their care of patients were PubMed, OMIM, and GeneTests. Educators in family medicine and primary care noted that GeneReviews was the single best educational resource in medical genetics given its high quality and easy accessibility.
Further, many health-focused resources link into GeneReviews entries, in particular, National Library of Medicine information resources such as OMIM, Genetics Home reference entries, and Genes and Disease entries. The complete collection of GeneReviews is also published in the National Center for Biotechnology Information's Bookshelf section, where is it updated weekly.
Use of the resource is also growing rapidly. In 2001, about 2,500 GeneTests records were viewed each day; in 2004, about 25,000 records were viewed each day. Although accurate use data will not be available again until a database redesign is completed, current estimates place database use at about 50,000 records viewed daily.
ISSUES FOR THE FUTURE
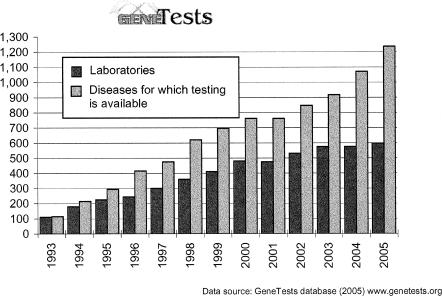
GeneTests is a recognized resource in the international medical community [4–13]. Its growth is steady: the number of new disease listings in the Laboratory Directory is about 100 per year (Figure 2), and about 50 new expert-authored, peer-reviewed GeneReviews are added annually. The 2 most significant issues that GeneTests faces in the near future are the ability to edit increasing numbers of GeneReviews and the need to develop the capability to display GeneReviews sections and subsections in the electronic medical record (EMR) as a “just in time” resource at the point of care.
Figure 2.
Growth of the GeneTest's Laboratory Directory
Adding and maintaining content
Content editing time by the staff for new GeneReviews is between five and thirty hours (average of about ten to fifteen hours). Revisions to accommodate clinically significant changes in test and/or treatment availability take one to two hours of staff content editing. Formal updates every two years take between five and ten hours of staff content editing. These hours do not include author and staff time for administration (i.e., communication with authors, reviewers, and other staff), technical editing, and posting to the Website.
From June 1, 2004, to May 31, 2005, 48 new entries were posted, 65 were revised, and 90 were updated. From June 1, 2005, to May 31, 2006, it is anticipated that 48 new entries will be posted and 140 will be updated. The number of revisions cannot be predicted because they are ongoing as clinically relevant information changes. Given this rate of increase in updates and limited resources to expand the staff, the project will need to identify time-shaving measures for aspects of editing that minimally affect content quality.
Integrating GeneReviews information into electronic medical record systems
To understand the technical and content issues that need to be considered in displaying GeneReviews sections and subsections in the EMR, GeneTests is collaborating with two large health care provider networks with well-developed, in-house support and long experience with the EMR. The premise is that non-geneticist clinicians want to use genetic testing in patient care but understand little about it. Given limited time with patients, they are not interested in viewing or printing long documents describing genetic testing; thus, potential genetic testing questions raised during patient visits (e.g., “How is this condition diagnosed?” “Why should a relative of a person with this disorder be tested?” “How should a person with this condition be followed over time?” “Is prenatal testing available?”) need to be answered by small, stand-alone segments of information.
GeneReviews is highly suited to this use given its dynamic database design [14] and highly structured format. Because it is being redesigned at this time, the GeneTests database can be rebuilt to accomplish the goal of providing self-contained blocks of text that a wide range of EMR systems can use and achieve its goal of integrating appropriate use of genetic testing into patient care.
IMPLICATIONS FOR INFORMATION PROFESSIONALS
GeneTests—comprising GeneReviews, Laboratory Directory, Clinic Directory, and Educational Materials— helps health care providers understand the role of genetic testing in the diagnosis, carrier detection, prenatal diagnosis, and presymptomatic diagnosis of inherited disorders [15]. Information professionals can help those trying to understand these new concepts in patient care by educating clinicians and researchers about the resource and working with users to maximize their ability to extract the information it contains. Similarly, librarians can direct health care consumers, increasingly exposed to often bewildering genetic information, to the GeneTests resource to discuss concepts relevant to their care with their clinicians. By providing authoritative, up-to-date information on genetic test availability and explanations of genetic test use, GeneTests can serve as an important bioinformatics tool for information professionals.
Acknowledgments
This work was accomplished with the dedicated help of the GeneTests staff and editors (in alphabetical order): Cynthia Abair, Patricia K. Baskin, Thomas D. Bird, Suzanne B. Cassidy, Mary Beth Dinulos, Cynthia R. Dolan, Miriam Espeseth, Gerald L. Feldman, Carla J. Gifford, Gina McCullough Grohs, Kathi Marymee, Sergey Mikhaylov, Monica Smersh, Richard J. H. Smith, Roberta Spiro, and Bradley Willson.
Footnotes
* GeneTests is supported by contract no. NO1-LM-4-3503 from the National Institutes of Health.
REFERENCES
- University of Washington. GeneTests. [Web document]. Seattle, WA: The University, 2006. [cited 20 Feb 2006]. <http://www.genetests.org>. [Google Scholar]
- Pagon RA, Tarczy-Hornoch P, Covington ML, Baskin PK, Edwards JE, Espeseth M, Beahler C, Bird TD, Popovich B, Nesbitt C, Dolan C, Marymee K, Hanson NB, Neufeld-Kaiser W, Grohs G McCullough, Kicklighter T, Abair C, Malmin A, Barclay M, and Palepu RD. GeneTests and GeneClinics: genetic testing information for a growing audience. Hum Mutat. 2002 May; 19(5):501–9. [DOI] [PubMed] [Google Scholar]
- Hamosh A, Scott AF, Amberger JS, Bocchini CA, and Mckusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005 Jan 1; 33:D514–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum R, McInnes R, and Willard H. Genetics in medicine. 6th ed. Philadelphia, PA: W. B. Saunders, 2004. [Google Scholar]
- Yoon PW, Chen B, Faucett A, Clyne M, Gwinn M, Lubin IM, Burke W, and Khoury MJ. Public health impact of genetic tests at the end of the 20th century. Genet Med. 2001 Nov–Dec; 3(6):405–10. [DOI] [PubMed] [Google Scholar]
- Press RD. GeneClinics medical genetics knowledge base. Mol Diagn. 1999 Sep; 4(3):256. [PubMed] [Google Scholar]
- Ayme S. Bridging the gap between molecular genetics and metabolic medicine: access to genetic information. Eur J Pediatr. 2000 Dec; 159(suppl. 3):S183–5. [DOI] [PubMed] [Google Scholar]
- Pagon RA. Internet resources in medical genetics. In: Dracopoli N, Morton C, Haines J, Seidman C, Seidman J, Korf B, Smith D, eds. Current protocols in human genetics. New York, NY: J. C. Wiley, 2001:chapter 9.12. [Google Scholar]
- Pagon RA, Pinsky L, and Beahler CC. Online medical genetics resources: a U.S. perspective. BMJ. 2001 Apr 28; 322(7293):1035–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W. Genetic testing. N Engl J Med. 2002 Dec; 347(23):1867–75. [DOI] [PubMed] [Google Scholar]
- Pagon RA, Tarczy-Hornoch P, and Beahler CC. Databases used in genetics clinics (article #574). In: Cooper D, ed. Encyclopedia of the human genome. London, UK: Nature, 2003. [Google Scholar]
- Pagon RA. Uses of databases. In: Korf B, Jorde L, eds. The encyclopedia of genetics, genomics, proteomics, and bioinformatics. London, UK: John Wiley & Sons, 2005:chapter 99. [Google Scholar]
- Pagon RA. Molecular genetic testing for inherited disorders. In: Fuchs J, Podda M, eds. The encyclopedia of medical genomics & proteomics. New York, NY: Marcel Dekker, 2005. [Google Scholar]
- Tarczy-Hornoch P, Shannon P, Baskin P, Espeseth M, and Pagon RA. GeneClinics: a hybrid text/data electronic publishing model using XML applied to clinical genetic testing. J Am Med Inform Assoc. 2000 May–Jun; 7(3):267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagon RA. Genetic diagnosis and counseling. [Web document]. New York, NY: ACP Medicine, WebMD, Jul 2005. [cited 12 Oct 2005]. <http://www.acpmedicine.com>. [Google Scholar]