Abstract
The stabilities of colistin and colistin methanesulfonate (CMS) in different aqueous media were studied by specific high-performance liquid chromatography (HPLC) methods. Colistin was stable in water at 4 and 37°C for up to 60 days and 120 h, respectively. However, degradation was observed when colistin was stored in isotonic phosphate buffer (0.067 M, pH 7.4) and human plasma at 37°C. The stability of CMS from three different sources in water was explored by strong-anion-exchange (SAX) HPLC for CMS and by measuring the concentrations of colistin formed from the hydrolysis of CMS. The peaks of CMS in SAX HPLC disappeared almost completely after 12 h at 37°C, but appeared to remain intact for up to 2 days at 4°C. Over the same period, there was no formation of colistin at 4°C. In water, phosphate buffer, and plasma, there was rapid formation of colistin within 24 to 48 h at 37°C from the three sources of CMS. The hydrolysis products were assumed to be a complex mixture of many different sulfomethyl derivatives, including colistin. The stability of a fourth source of CMS in Mueller-Hinton broth examined during 30 min at 37°C revealed no formation of colistin. Along with previous microbiological studies, this suggested that different sulfomethyl CMSs possess intrinsic antibacterial activity. These results will be helpful for understanding the pharmacokinetics and pharmacodynamics of colistin and CMS in humans and animals.
Colistin, also known as polymyxin E, is a cationic lipopeptide antibiotic isolated in 1950 from Bacillus polymyxa subsp. colistinus (5). It is most notable for its activity toward the multidrug-resistant organism Pseudomonas aeruginosa and low rates of resistance (10). For patients with cystic fibrosis, infections of the respiratory tract by the ubiquitous organism P. aeruginosa present a significant problem, because many strains of this organism are resistant to the commonly prescribed aminoglycosides and other antipseudomonal antibiotics.
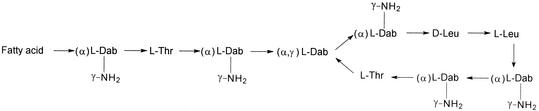
Colistin comprises a cyclic heptapeptide and a tripeptide side chain acylated at the N terminus by a fatty acid. At least 30 different components have been detected in colistin (11). The two main components are colistin A (polymyxin E1) and colistin B (polymyxin E2), which differ only in the fatty acid side chain (Fig. 1). Two different forms of colistin are available for clinical use: colistin sulfate for topical use and sodium colistin methanesulfonate (CMS) for parenteral and aerosol therapy. CMS is produced by a sulfomethylation reaction in which the primary amine groups on α,γ-diaminobutyric acid (Fig. 1) are reacted with formaldehyde followed by sodium bisulfite. Although the in vitro antibacterial potency is decreased by sulfomethylation, undesirable side effects, such as painful irritation at subcutaneous or intramuscular injection sites, are avoided, and toxicity is decreased significantly (2, 3, 13).
FIG. 1.
Chemical structures of colistin A and B. “Fatty acid” represents 6-methyloctanoic acid for colistin A and 6-methylheptanoic acid for colistin B. Dab, α,γ-Diaminobutyric acid. α and γ indicate the respective -NH2 involved in the peptide linkage.
Since there are five primary amine groups in colistin, all of them have the potential to be sulfomethylated. Although Barnett et al. indicated that all five groups were fully substituted, based on the results of elemental analysis, no detailed results were provided (2). Two papers have investigated the chemical homogeneity of CMS by using electrophoresis and bioassay (2, 3). Since the results were based on antibacterial activity, no direct proof was provided for the presence of colistin. CMS is readily hydrolyzed in aqueous solution (2). Assuming that hydrolysis of the five sulfomethyl groups was random, there could be up to 32 (25) products, including colistin, arising from the hydrolysis of CMS in aqueous solution. Since colistin and CMS have different antibacterial activities, pharmacokinetics, and pharmacodynamics (1), an understanding of the stabilities of colistin and CMS in plasma and aqueous solutions, such as water and buffer, is very important for interpreting results from pharmacokinetic and pharmacodynamic studies.
We have developed a high-performance liquid chromatography (HPLC) method for the assay of colistin in human plasma (7), which, in contrast to the bioassay (6), has superior sensitivity and specificity for quantitating colistin in biological fluids. The present paper describes an adaptation of our previous method (7) for the analysis of colistin in aqueous solution, together with a description of an HPLC method using a strong-anion-exchange (SAX) column for the analysis of CMS in water. These methods have been used to investigate the stability of colistin and CMS in aqueous media. Because CMS from different sources may exhibit distinct toxicities (2), CMS from four suppliers was used for the stability study. The stabilities of colistin sulfate (from one source) and sodium CMS (from three sources) were studied with three media: water at 4 and 37°C, isotonic phosphate buffer (pH 7.4, 0.067 M) at 37°C, and human plasma at 37°C. The stability of CMS from a fourth source, used in a previous microbiological study (9), was examined with Mueller-Hinton broth at 37°C.
MATERIALS AND METHODS
Chemicals and materials.
9-Fluorenylmethyl chloroformate (FMOC-Cl) was obtained from Sigma (St. Louis, Mo.). Colistin sulfate (lot 49H0635) was purchased from Sigma (produced in Denmark). Sodium CMS was purchased from three different sources—from Sigma (lot 40K0601; produced in Denmark), as Coly-Mycin M from Dumex (batch no. A1680442; Copenhagen, Denmark), and as Coly-Mycin M from Parke-Davis (batch no. 01389PZ; Caringbah, Australia). Colomycin was obtained as a gift from Pharmax (batch no. 11060; Kent, United Kingdom). All samples of CMS were presumed pure and to exist as the penta(methanesulfonic acid) derivatives. Netilmicin sulfate was obtained from Schering-Plough (Madison, N.J.). Trichloroacetic acid, boric acid, sodium hydrogen carbonate, sodium sulfate, and sodium hydroxide of analytical grade, as well as acetonitrile, methanol, tetrahydrofuran, and acetone (all of HPLC grade) were obtained from BDH Laboratory Supplies (Poole, England). Mueller-Hinton broth was from Oxoid (Hampshire, England). Water was purified with a Milli-Q system (Millipore Corp., Bedford, Mass.). Solid-phase extraction (SPE) cartridges (C18, 100 mg) were supplied by Waters (Milford, Mass.).
HPLC apparatus.
The HPLC system comprised a LC-10AS pump, a SIL-10ADvp autoinjector (Shimadzu, Kyoto, Japan) and either an SPD-6A UV spectrophotometric detector (Shimadzu) or 470 scanning fluorescence detector (Waters) connected to a multi-instrument data acquisition and processing system (Maxima 820; Waters). All analyses were carried out at ambient temperature.
Stability in water.
Solutions of colistin sulfate and solutions of sodium CMS (from Sigma, Pharmax, and Parke-Davis) were prepared with Milli-Q water at a concentration of 0.1 mg/ml, and 10-ml aliquots of each were transferred into 20-ml plastic bottles (Super Polyethylene Vial; Packard, Böblingen, Germany) kept at 4 ± 0.2 and 37 ± 0.1°C. Milli-Q water was used as the control. Samples (0.5 ml) were taken at 0, 2, 4, 7, 14, 21, 28, and 60 days from the solutions kept at 4°C and at 0, 2, 4, 6, 12, 24, 48, 72, 96, and 120 h from the solutions kept at 37°C and stored immediately at −20°C pending analysis. Samples from the solutions of CMS were examined by HPLC method I (described below). Levels of colistin in the colistin sulfate solutions and formed by hydrolysis in the CMS solutions were determined by HPLC method II (described below).
Stability in isotonic phosphate buffer (0.067 M, pH 7.4) at 37°C.
Solutions of colistin sulfate and sodium CMS (Sigma, Pharmax, and Parke-Davis) were prepared with isotonic phosphate buffer (0.067 M, pH 7.4) at a concentration of 0.1 mg/ml, and 10-ml aliquots of each were transferred into 20-ml plastic bottles (Packard) and kept at 37 ± 0.1°C. Isotonic phosphate buffer (0.067 M, pH 7.4) was used as the control. Samples (0.5 ml) were taken from the solutions at 0, 2, 4, 6, 12, 24, 48, 72, 96, and 120 h and stored immediately at −20°C pending analysis. Levels of colistin in the solutions of colistin sulfate and formed by hydrolysis in the CMS solutions were determined by HPLC method II.
Stability in human plasma.
Blank human plasma (10 ml; donation no. O5881373; Australian Red Cross, Adelaide, Australia) was adjusted to pH 7.4 with phosphoric acid (85% [wt/wt]) and spiked with colistin sulfate or sodium CMS (Sigma, Pharmax, and Parke-Davis) at a concentration of 20 μg/ml. The spiked and control (drug-free) plasma samples were sterilized by being passed through a 0.2-μm-pore-diameter Minisart filter (Sartorius, Germany) into sterile 50-ml centrifuge tubes (Greiner Labortechik, Kremsmuenster, Austria) and kept at 37 ± 0.1°C. At 0, 2, 4, 6, 12, 24, 48, 72, 96, and 120 h, samples (0.5 ml) were removed aseptically and immediately stored at −20°C pending analysis. The levels of colistin in the colistin sulfate solutions and formed by hydrolysis in the solutions of CMS were determined by HPLC method II.
Stability of CMS in Mueller-Hinton broth.
Mueller-Hinton broth (10 ml; Oxoid, Hampshire, England) was spiked with sodium CMS (Dumex) at a concentration of 1,024 μg/ml and kept at 37 ± 0.1°C. Samples (0.5 ml) were taken at 0, 5, 10, 15, and 30 min and stored at −20°C until assay. The level of colistin formed from the hydrolysis of CMS was determined by HPLC method II.
HPLC method I.
Components in aliquots (50 μl) of the solutions of CMS prepared in water were separated on a steel SAX column (250 by 53 by 4.6 mm inside diameter) packed with 5-μm-diameter Spherisorb (Alltech, Deerfield, Ill.), through which a mobile phase consisting of acetonitrile:1.2 mM sodium sulfate (70:30 [vol/vol]) was pumped at 1 ml/min. The eluent was monitored for its UV absorbance at 210 nm.
HPLC method II.
Concentrations of colistin were determined according to the method of Li et al. (7). Aliquots of the aqueous solutions (50 μl), pretreated plasma (250 μl), or Mueller-Hinton broth (100 μl) were passed through SPE cartridges that had been conditioned with 1 ml of methanol and equilibrated with 1 ml of carbonate buffer (pH 10). FMOC-Cl (30 μl, 100 mM) was added to the cartridge and allowed to react for 10 min. The FMOC derivatives of colistin were eluted from the cartridges in 900 μl of acetone and mixed with 600 μl of 0.2 M boric acid. The final mixture was chromatographed on a steel column (250 by 4.6 mm inside diameter) packed with 5-μm-diameter Ultrasphere C18 (Beckman, Berkeley, Calif.) through which a mobile phase of acetonitrile-tetrahydrofuran-water (87:4:13 [vol/vol]) was pumped at 1 ml/min. Detection was by fluorescence (λex, 260 nm; λem, 315 nm).
Data analysis.
The percentages of colistin formed by the hydrolysis of CMS were calculated on a molar basis. Colistin is a multicomponent antibiotic, and colistin A and B always comprised more than 80% of the total (4). Colistin A and B were therefore set as the markers for quantitation of colistin. In the present study, the molecular weight of colistin was set at 1,163, the average of the individual values for colistin A (Mw, 1,170) and B (Mw, 1,156), and for colistin sulfate was 1,408. The molecular weight of CMS was set at 1,743, which represents the average of the molecular weights of penta(methanesulfonic acid) CMS A (Mw, 1,750) and B (Mw, 1,736). Although the ratios of colistin A and B may vary in different sources of colistin and CMS, the usage of average molecular weights would have little impact given the molecular weight difference of only 14.
RESULTS
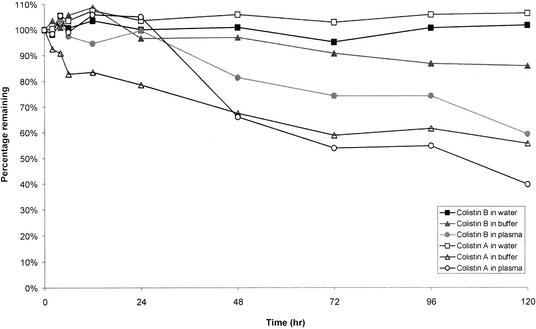
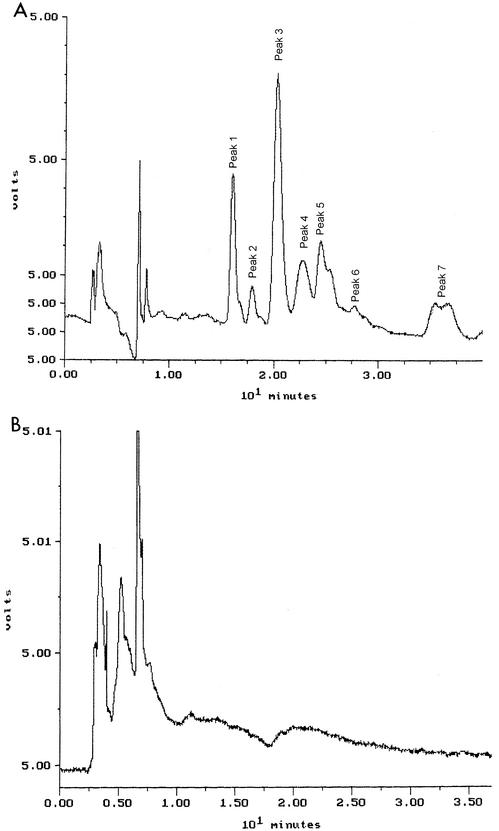
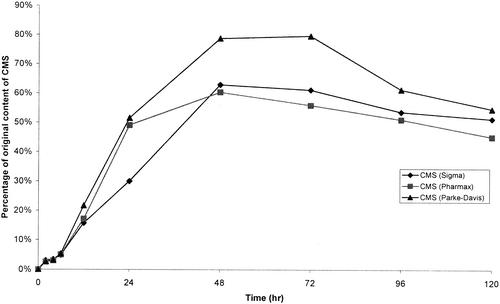
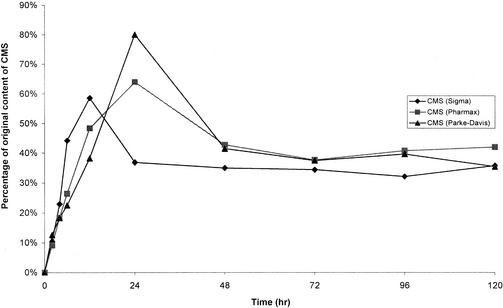
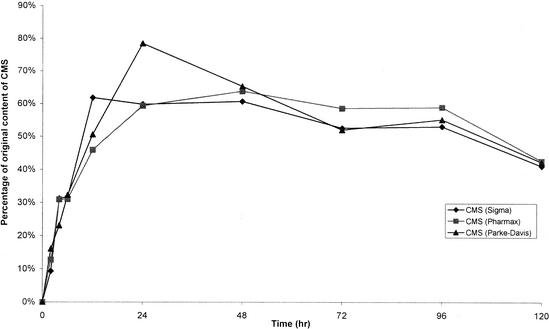
Colistin was very stable in water at 4°C. After 60 days, the percentages of colistin A and B remaining were 97.4 and 105.3%, respectively. There were no decreases in the concentrations of colistin A and B stored in water at 37°C for up to 120 h (Fig. 2). However, they were not stable in isotonic phosphate buffer (pH 7.4) and plasma at 37°C (Fig. 2). Figure 3A shows a typical chromatogram of a fresh solution containing 0.1 mg of CMS per ml (Sigma). The chromatogram contained two dominant peaks (peaks 1 and 3) and several minor ones (peaks 2, 4, 5, 6, and 7). Figure 3B shows a typical chromatogram of CMS in water stored for 12 h at 37°C. CMS hydrolyzed in water, isotonic phosphate buffer (pH 7.4), and plasma at 37°C, and the time courses of formation of colistin in the three sources of CMS are shown in Fig. 4, 5, and 6, respectively.
FIG. 2.
Percentage of initial concentrations of colistin A and B remaining after storage at 37°C in water, isotonic phosphate buffer (0.067 M, pH 7.4) and human plasma (pH adjusted to 7.4 with phosphoric acid).
FIG. 3.
(A) Typical chromatogram obtained for a fresh solution of CMS in water examined by SAX HPLC. For experimental details, see the text. (B) Typical chromatogram of CMS in water stored for 12 h at 37°C.
FIG. 4.
Levels of colistin in samples of CMS in water kept at 37°C, expressed as a percentage of the original CMS on a molar basis.
DISCUSSION
Stability of colistin in water, buffer, and human plasma.
Colistin was stable in water at 4 and 37°C. However, there were decreases in the concentrations of colistin in isotonic phosphate buffer (pH 7.4) and plasma at 37°C (Fig. 2). The pH values in phosphate buffer and plasma were approximately 1.5 units above the values measured in water (between 5.63 and 5.98), which may account for the greater instability in these two media compared with in water. Furthermore, in phosphate buffer and plasma at 37°C, colistin A appeared to be less stable than colistin B. The reasons remain unknown, but the different fatty acid chains—the only difference in their chemical structures—may be a contributing factor.
Stability of CMS in water, buffer, human plasma, and Mueller-Hinton broth.
Barnett et al. (2) and Beveridge et al. (3) observed marked increases in microbiological activity when aqueous solutions of CMS were incubated at 37°C and concluded that CMS was converted to colistin. Although electrophoresis and detection with bioautograms were used to explore the conversion in their studies, no direct evidence was provided for the formation of colistin from CMS. In the present study, a reported HPLC method (7) was used to explore the conversion of CMS to colistin. Barnett et al. (2) pointed out that CMS made in the United States had higher toxicity in mice (50% lethal dose [LD50] = 220 mg/kg of body weight) than Colomycin produced in the United Kingdom (LD50 = 650 mg/kg). Given the known greater toxicity of colistin compared with CMS (13), the two samples might have different percentages of colistin or other hydrolysis products arising from manufacture or from hydrolysis during storage. Therefore, the stabilities of three different sources of CMS from Sigma, Parke-Davis, and Pharmax were examined.
First, the stability of CMS in water at 37°C was explored by quantitating colistin formed from the hydrolysis of CMS (Fig. 4). There was no colistin measurable in the fresh solutions of CMS prepared from the three sources. The limit of quantification was 0.4 μg/ml, which is equivalent to levels of colistin in all samples of <0.4%. The time to reach the maximum concentration of colistin differed slightly among the samples from three sources (Fig. 4). After 48 h, the highest levels of conversion to colistin were 62.8% (Sigma) and 60.3% (Pharmax), while a maximal conversion of 79.6% was observed at 72 h for CMS from Parke-Davis. No significant change in pH was observed, with pHs ranging between 6.35 and 6.90. Although colistin is stable in water at 37°C (Fig. 2), concentrations of colistin formed from hydrolysis of CMS decreased after reaching a maximum. The data in Fig. 2 demonstrated that colistin is more stable at pH values ranging from 5.63 to 5.98 than at pH 7.4. Therefore, the higher pH values in the solutions of CMS in water, compared with colistin in water, may account for the decrease in concentrations of colistin.
To the best of our knowledge, no HPLC method for separating and detecting the components of CMS has been reported previously. In the present study, a method introducing the use of a SAX HPLC column was developed for CMS in water (HPLC method I). An eluent with a high organic content (acetonitrile-1.2 mM sodium sulfate at 70:30 [vol/vol]) was used in order to achieve good retention of CMS. Several attempts using electrospray ionization mass spectrometry failed to identify peaks 1 and 3 in Fig. 3A. Nevertheless, all seven peaks have been proven to be related to colistin A and/or B by adapting a specific HPLC method developed in our laboratory for the assay of CMS in plasma (8). Milli-Q water was used as the control, and no peaks appeared between the retention times of peaks 1 and 7. When colistin was chromatographed, no peaks were apparent.
When chromatographing the CMS samples incubated in water at 37°C by using HPLC method I, the chromatograms showed progressive decreases in the areas of all seven peaks after 2 h; these peaks had disappeared after 12 h of storage (Fig. 3B). It is proposed that the disappearance of the peaks is a consequence of hydrolysis of the methanesulfonate functional groups. Colistin was not detectable under the chromatographic conditions of method I. However, from measuring the appearance of colistin in the CMS solutions by HPLC method II, only about 20% of the CMS had been hydrolyzed completely to colistin by 12 h. This suggested that a large fraction of the CMS lost from samples, as observed in the chromatograms with HPLC method I (Fig. 3), was in the form of partial methanesulfonate derivatives of colistin, which could account for the peaks appearing within the retention time of 10 min in Fig. 3B.
After 2 days at 4°C, no colistin was detected from the hydrolysis of CMS, and the appearance of the chromatograms for HPLC method I was quantitatively identical to that of the fresh solutions. After 60 days, approximately 30% of the CMS was converted to colistin. CMS from all three sources displayed similar rates of hydrolysis at 4°C. Overall, the results suggest that solutions of CMS should be prepared freshly for pharmacological studies to minimize the errors in interpretation caused by hydrolysis. Hence, improper storage of CMS solutions before administration, generating the much more toxic agent colistin, may have been one of the reasons for the differences in toxicity of CMS from different sources reported by Barnett et al. (2).
The stability of CMS was also explored with isotonic phosphate buffer (0.067 M, pH 7.4) (Fig. 5) and human plasma at 37°C (Fig. 6). HPLC method I could not be used to examine the hydrolysis of CMS in buffer and plasma—probably because of the impact of electrolytes in both systems. The formation of colistin from CMS was complicated by the observed instability of colistin in both media (Fig. 2). During the first 24 h, the rate of formation of colistin from CMS was more rapid in buffer (Fig. 5) and human plasma (Fig. 6) than in water (Fig. 4). The pH of buffer and plasma was between 0.5 and 1 units above the values measured in the solutions of CMS in water, which may explain the greater rate of formation of colistin. The rapid formation of colistin in buffer, with an estimated 20% conversion from CMS after 4 h, corresponds well with the rapid increase in the antibacterial activity of solutions of CMS, when tested against Escherichia coli after 4 h of storage at 37°C in 0.1 M phosphate buffer (pH 7.0) (3). Other reports have shown that the antibacterial activities of colistin against P. aeruginosa (9), E. coli, Enterobacter aerogenes, and Klebsiella pneumoniae were 3- to 10-fold the activity of CMS (13). Therefore, even a 20% conversion to colistin within 4 h would increase the antibacterial activity considerably. The concentrations of colistin increased to a maximum between 12 and 24 h for all three samples of CMS stored in buffer before decreasing to constant values beyond 48 h of storage (Fig. 5). It is difficult to explain the observations beyond 48 h. In contrast, the concentrations of colistin decreased slowly after reaching a maximum in human plasma (Fig. 6). This is not unexpected given the steady decrease in concentrations of colistin when the substance was stored in plasma at 37°C (Fig. 2). There was no substantial difference among the three sources of CMS in the time course of formation of colistin, although CMS from Parke-Davis always had a higher maximum conversion than CMS from Sigma and Pharmax (Fig. 4 to 6).
FIG. 5.
Levels of colistin in samples of CMS in isotonic phosphate buffer (0.067 M, pH 7.4) kept at 37°C, expressed as a percentage of the original CMS on a molar basis.
FIG. 6.
Levels of colistin in samples of CMS in human plasma kept at 37°C, expressed as a percentage of the original CMS on a molar basis.
Reed et al. recently reported on the pharmacokinetics of colistin following intravenous administration of CMS to patients with cystic fibrosis (12). Prior to HPLC, samples of plasma were treated at 54°C for 2 h in the presence of 3 M perchloric acid and 0.1 M hydrochloric acid. According to our data on the stability of CMS presented above, a 2-h heat pretreatment at 54°C may accelerate the hydrolysis of CMS to colistin. This was supported by data showing considerable formation of colistin when CMS was incubated in 2 M hydrochloric acid for 2 h (3). Therefore, it is likely that the concentrations of colistin measured by Reed et al. (12) represented the sum of colistin originally in plasma when the samples were collected plus colistin formed from the hydrolysis of CMS during sample pretreatment. A previous report from our laboratory (8) has shown that CMS is not converted to colistin during preparation of plasma samples for derivatization of colistin. When assaying for CMS (8) and colistin (7) in plasma collected following the administration of a dose of CMS, it is recommended that samples be frozen rapidly and stored at −20°C pending analysis.
No colistin was detected when CMS (Dumex) at a concentration of 1,024 μg/ml was stored for 30 min in Mueller-Hinton broth at 37°C. With a limit of quantification in broth of 0.2 μg/ml, this is equivalent to a <0.2% conversion to colistin. The initial concentration of CMS (Dumex) was equivalent to 64 times the MIC of CMS when the same batch was tested against P. aeruginosa ATCC 27853 in a previous in vitro pharmacodynamic study of colistin and CMS (9). According to that study, when the killing effect of CMS on the same strain was examined, the log10 CFU/ml decreased from 6.08 to 2.78 in the first 15 min (9). Our present observation of insignificant conversion of CMS to colistin in Mueller-Hinton broth in the first 30 min demonstrates that the sulfomethyl derivatives possess intrinsic antibacterial activity. However, the comparative antibacterial activities of the numerous partially derivatized (or hydrolyzed) forms of CMS remain unknown. Therefore, it is anticipated that the pharmacodynamics of CMS in vitro and in vivo will be extremely complex.
In conclusion, the stabilities of colistin and CMS in aqueous solutions were studied by specific HPLC methods. Colistin was very stable in water, but less stable in isotonic phosphate buffer and human plasma at 37°C. The degradation of CMS was shown by examining the disappearance of CMS by SAX HPLC and the appearance of formed colistin by reverse-phase HPLC. This is the first report using a specific HPLC method to measure the concentrations of colistin formed from the hydrolysis of CMS in different aqueous solutions. These results will be helpful for understanding the pharmacokinetics and pharmacodynamics of colistin and CMS in humans and animals. They also show that when concentrations of colistin are being measured in biological fluids following the administration of sodium CMS, the hydrolysis of CMS to colistin during sample storage and preparation for analysis should be minimized to avoid falsely elevated values.
Acknowledgments
We thank David Shacklefold for helpful advice.
REFERENCES
- 1.Al-Khayyat, A. A., and A. L. Aronson. 1973. Pharmacologic and toxicologic studies with the polymyxins. II. Comparative pharmacologic studies of the sulfate and methanesulfonate salts of polymyxin B and colistin in dogs. Chemotherapy 19:82-97. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, M., S. R. Bushby, and S. Wilkinson. 1964. Sodium sulphomethyl derivatives of polymyxins. Br. J. Pharmacol. 23:552-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beveridge, E. G., and A. J. Martin. 1967. Sodium sulphomethyl derivatives of polymyxins. Br. J. Pharmacol. Chemother. 29:125-135. [DOI] [PMC free article] [PubMed]
- 4.Decolin, D., P. Leroy, A. Nicolas, and P. Archimbault. 1997. Hyphenated liquid chromatographic method for the determination of colistin residues in bovine tissues. J. Chromatogr. Sci. 35:557-564. [DOI] [PubMed] [Google Scholar]
- 5.Koyama, Y., A. Kurosasa, A. Tsuchiya, and K. Takakuta. 1950. A new antibiotic “colistin” produced by spore-forming soil bacteria. J. Antibiot. 3:457-458. [Google Scholar]
- 6.Leroy, P., D. Decolin, S. Nicolas, P. Archimbault, and A. Nicolas. 1989. Residue determination of two co-administered antibacterial agents—cephalexin and colistin—in calf tissues using high-performance liquid chromatography and microbiological methods. J. Pharm. Biomed. Anal. 7:1837-1846. [DOI] [PubMed] [Google Scholar]
- 7.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, K. Coulthard, and D. W. Johnson. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 761:167-175. [DOI] [PubMed] [Google Scholar]
- 8.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, K. Coulthard, and J. Valentine. 2002. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob. Agents Chemother. 46:3304-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, J., J. Turnidge, R. Milne, R. L. Nation, and K. Coulthard. 2001. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Littlewood, J. M., C. Koch, P. A. Lambert, N. Hoiby, J. S. Elborn, S. P. Conway, R. Dinwiddie, and F. Duncan-Skingle. 2000. A ten year review of Colomycin. Respir. Med. 94:632-640. [DOI] [PubMed] [Google Scholar]
- 11.Orwa, J. A., A. Van Gerven, E. Roets, and J. Hoogmartens. 2000. Development and validation of a liquid chromatography method for analysis of colistin sulphate. Chromatographia 51:433-436. [Google Scholar]
- 12.Reed, M. D., R. C. Stern, M. A. O'Riordan, and J. L. Blumer. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645-654. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz, B. S., M. R. Warren, F. A. Barkley, and L. Landis. 1960. Microbiological and pharmacological studies of colistin sulphate and sodium colistin methanesulfonate. Antibiot. Annu. 1959-1960:41-60. [PubMed] [Google Scholar]