Abstract
Malignant transformation of melanocytes to melanoma cells closely parallels activation of melanoma inhibitory activity (MIA) expression. We have previously shown that upregulation of MIA occurs on a transcriptional level and involves the highly conserved region (HCR) promoter element. We further observed that the HCR element interacts with the melanoma-associated transcription factor (MATF) and thereby confers strong promoter activation. In this study we identify the peptide sequence of MATF and show that it is identical with the transcription factor HMG1. HMG1 was upregulated in malignant melanoma cells and further activated by hypophosphorylation. Stable antisense-HMG1 expression in melanoma cells led to the reduction of MIA promoter activity and protein expression, indicating that HMG1 is a potent regulator of MIA expression. Interestingly, chromatin immunoprecipitation and electrophoretic mobility shift experiments indicated that HMG1 and the NF-κB family member p65 both interact and bind to the HCR promoter element. In summary, our study proves HMG1 and p65 to be important factors in MIA regulation and melanoma progression.
Previously, we described the isolation of melanoma-inhibitory activity (MIA), an 11-kDa protein secreted from malignant melanoma cells into the tissue culture supernatant (2, 5, 18). Analyzing normal skin and skin-derived melanocytic tumors by semiquantitative reverse transcriptase (RT)-PCR revealed the absence of significant MIA mRNA levels in normal skin and melanocytes, very low levels in benign melanocytic nevi, and high levels in all primary and metastatic malignant melanomas (5, 7, 12, 18). The absence of MIA mRNA expression in benign melanocytes cultured from normal skin biopsies was also reported from a different study (23). We further found that MIA expression in vivo correlates with the progressive malignancy of melanocytic tumors (12, 14) and observed enhanced MIA protein levels in the sera of patients with metastatic melanomas (4, 6, 23).
By analyzing transcriptional mechanisms involved in the regulation of MIA expression, we previously provided an initial characterization of the promoter and showed that it is strongly activated in melanoma cells (5, 14). We identified a 30-bp region in the MIA promoter essentially required for strong and specific activity in human and murine melanoma cells and named it HCR (highly conserved region). This region is subject to binding by the protein MATF (melanoma-associated transcription factor) (12). In further studies, we concentrated on a second cis-regulatory element in the 30-bp region located 5′ of and adjacent to the MATF-binding site, which functions as a silencer element in benign skin melanocytes. The silencer element harbors a T-cell factor-binding site, and we were able to show that the transcriptional repressor CtBP1 is strongly expressed in primary normal human epidermal melanocytes (NHEM) and that it inhibits MIA promoter activity by binding to and inactivating TCF-4 on the MIA promoter. In malignant melanoma cells, CtBP1 expression is lost and the repression of MIA promoter activity is revoked.
In the present study we focused on HCR enhancer activity and identified MATF as the DNA-binding protein HMG1. We further revealed the important role of HMG1/MATF in positively regulating MIA expression by interaction with p65.
MATERIALS AND METHODS
Cell lines and cell culture conditions.
The melanoma cell lines Mel Im, Mel Ei, Mel Wei, Mel Ho, Mel Juso, Mel Ju, SK-Mel-3, SK-Mel-28, and HTZ19d have been described in detail previously (2, 14). The cell lines Mel Ei, Mel Wei, Mel Ho, and Mel Juso were derived from a primary cutaneous melanoma, and Mel Im, Mel Ju, SK-Mel-28, and HTZ19d were derived from metastases of malignant melanomas. For tissue culture, the cells were maintained in Dulbecco's modified Eagle's medium supplemented with penicillin (400 U/ml), streptomycin (50 μg/ml), l-glutamine (300 μg/ml), and 10% fetal calf serum (Sigma, Deisenhofen, Germany) and diluted 1:5 every 3 days.
NHEM derived from normal skin were cultivated in melanocyte medium (MGM-3; Gibco, Eggenstein, Germany) under a humidified atmosphere of 5% CO2 at 37°C. Cells were used in passages 6 to 10 and not later than 3 days after trypsinization. Cells were detached for subcultivation or assay with 0.05% trypsin-0.04% EDTA in phosphate-buffered saline (PBS).
Stable transfection of melanoma cells with antisense HMG1.
A panel of Mel Im cell clones varying with respect to HMG1 expression were established by stable transfection with antisense-HMG1 expression plasmids. Expression was controlled by a cytomegalovirus promoter, and plasmids were cotransfected with pCDNA3 (Invitrogen NV, Leek, Holland), which contains the selectable marker for neomycin resistance. Controls received pCDNA3 alone. Transfections were performed using the Lipofectamine Plus method (Gibco). One day after transfection, cells were placed into selection medium containing 50 μg of G418 (Sigma) per ml. After 25 days of selection, individual G418-resistant colonies were subcloned. The amount of HMG1 expression in these clones was determined by RT-PCR and Western blot analyses.
Transfection experiments.
For transient transfections, 2 × 105 cells per well were seeded into six-well plates and transiently transfected with 0.5 μg of MIA promoter reporter plasmids (1,386 bp of the MIA promoter in pGL3 basic) using the Lipofectamine Plus method (Gibco) according to the manufacturer's instructions. Twenty-four hours after transfection, the cells were lysed and the luciferase activity in the lysate was measured. To normalize transfection efficiency, 0.2 μg of a pRL-TK plasmid (Promega, Mannheim, Germany) was cotransfected and renilla luciferase activity was measured by a luminometric assay (Promega). All transfections experiments were repeated three times.
Western blots.
Cells (3 × 106) were lysed in 200 μl of radioimmunoprecipitation assay (RIPA) buffer (Roche, Mannheim, Germany) and incubated for 15 min at 4°C. Insoluble fragments were removed by centrifugation at 13,000 rpm for 10 min, and the supernatant lysate was immediately shock frozen and stored at −80°C. Seven micrograms of RIPA-cell lysate was loaded per lane, separated on sodium dodecyl sulfate (SDS)-polyacrylamide gradient gels (Invitrogen), and subsequently blotted onto a polyvinylidene difluoride membrane. After being blocked for 1 h with 3% bovine serum albumin-PBS, the membrane was incubated for 16 h with a 1:5,000 dilution of a rabbit polyclonal HMG1 antibody (BD Bioscience, Heidelberg, Germany). Then, the membrane was washed three times in PBS, incubated for 1 h with a 1:3,000 dilution of an alkaline phosphate-coupled secondary anti-mouse immunoglobulin G antibody (AP304A; Chemicon, Hofheim, Germany), and washed again. Finally, immunoreactions were visualized by nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) (Sigma) staining.
Two-dimensional polyacrylamide gel electrophoresis.
First-dimension isoelectric focusing (IEF) was performed using the Protean IEF Cell system (Bio-Rad Laboratories, Munich, Germany). Fifteen-microgram samples of RIPA buffer-Mel Im cell lysate and RIPA buffer-NHEM were diluted in rehydration buffer (8 M urea, 10 mM dithiothreitol [DTT], 1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 0.25% Bio-Lyte). IEF was performed with ReadyStrip IPG strips (width, 7 cm; pH 4 to 7; Bio-Rad) at 250 V for 15 min with a linear increase to 4,000 V within 2.5 h followed by a constant voltage of 4,000 V until 22,000 V · h was reached.
For the second dimension, IPG strips were placed in equilibration buffer (6 M urea, 30% glycerin, and 2% SDS in 0.05 M Tris-HCl) containing 1% DTT or 260 nM iodoacetamide for 10 min each. Each strip was placed on an SDS-4 to 12% polyacrylamide gradient gel (Invitrogen) and run at a constant current of 70 V for 2.5 h. Subsequently, gels were stained with silver or used for Western blotting. Silver staining was performed using a SilverXpress staining kit (Invitrogen) according to the manufacturer's instructions to verify separation and loading.
Immunohistochemistry.
Paraffin-embedded preparations of normal skin and malignant melanomas were screened for HMG1 and tyrosinase protein expression by the avidin-biotin complex method (LSAB2 kit; DAKO, Hamburg, Germany). The tissues were deparaffinized, rehydrated, and subsequently incubated with primary polyclonal HMG1 antibody (dilution, 1:500; BD Bioscience) or monoclonal tyrosinase antibody (dilution, 1:20; Novocastra, Newcastle upon Tyne, United Kingdom) overnight at 4°C. The secondary antibody supplied with the kit was incubated for 30 min at room temperature. Antibody binding was visualized with 3-amino-9-ethylcarbazole solution (LSAB2 kit). Finally, the tissues were counterstained with hemalaun.
RT-PCR.
Expression of HMG1 mRNA was measured by RT-PCR. First-strand cDNA was synthesized using 2 μg of total cellular RNA as the template, 1 μg of random primer (Pharmacia, Freiburg, Germany), 4 μl of 5× First Strand buffer (Gibco), 2 μl of 10 mM DTT, 1 μl (each) of 10 mM concentrations of the deoxynucleoside triphosphates, and 1 μl of Superscript II (Gibco) in a volume of 20 μl. Quantitative real-time PCR was performed on a Lightcycler (Roche). Two microliters of the cDNA template, 2 μl of 25 mM MgCl2, 0.5 μM concentrations of the forward and reverse primers (HMG1 for, GAA GTG CTC AGA GAG GTG G; HMG1 rev, GCA GAA GAG GAA GAA GGC), and 2 μl of SybrGreen LightCycler mix in a total volume of 20 μl were subjected to the following PCR program: 30 s at 95°C (initial denaturation) and then a 20°C/s temperature transition rate up to 95°C for 15 s, 55°C for 3 s, 72°C for 5 s, and an 81°C single-acquisition mode, repeated 40 times (amplification). The PCR was evaluated by melting curve analysis according to the manufacturer's instructions and by checking the PCR products on 1.8% agarose gels.
MIA ELISA.
The amount of secreted MIA protein in cell culture supernatants was determined by using a one-step MIA enzyme-linked immunosorbent assay (ELISA) (Roche) according to the manufacturer's instructions. Briefly, anti-MIA-biotin antibodies were bound to streptavidin-coated wells and incubated with cell culture supernatant. Subsequently, bound MIA was detected by using anti-MIA-peroxidase and ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] solution and an ELISA reader at a wavelength of 405 to 495 nm (6).
Electrophoretic mobility shift assay.
A double-stranded oligonucleotide corresponding to the HMG1-binding site in the MIA promoter (8) (5′-GAG TAG GCA TTT TCT-3′) was phospholabeled and used for gel mobility shift assays. Nuclear extracts of the melanoma cell line Mel Im (8 μg/lane) were incubated with different antibodies (anti-HMG1 [2.5 μg/lane; BD Bioscience] and anti-p65 [4 μg/lane; Santa Cruz, Heidelberg, Germany]), and gel shift assays were performed as described previously (8). Also, nuclear extracts of Mel Im cells transiently transfected with a sense-HMG1 expression plasmid containing an N-terminal flag tag (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys) were used together with an appropriate flag antibody (1 μg/lane; Sigma).
Chromatin immunoprecipitation.
Cells (4 × 106) were collected by trypsinization and fixed in culture medium without fetal calf serum containing 10% fixation solution (0.1 M NaCl, 1 mM EDTA, 0.5 M EGTA, 50 mM Tris-HCl [pH 8], 11% formaldehyde) for 20 min at 4°C. Fixation of transcription factors to the DNA was stopped by the addition of glycine up to a final concentration of 0.125 M. Cells were collected by centrifugation, washed once in PBS, and incubated in Triton lysis buffer (0.25% Triton X-100, 10 mM Tris-HCl [pH 8], 1 mM EDTA, 0.5 mM EGTA, protease inhibitors) for 15 min at room temperature. Chromatin was collected by centrifugation, washed once in NaCl washing buffer (200 mM NaCl, 10 mM Tris-HCl [pH 8], 1 mM EDTA, 0.5 mM EGTA, protease inhibitors), and resuspended in 3 ml of sonication buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 1% deoxycholate, 0.1% SDS, 1 mM EDTA, protease inhibitors). Chromatin was sheared to 200- to 600-bp fragments by sonicating the samples twice for 2 min each time on ice with a Branson B15 sonicator at 50% power. Cell debris was pelleted by centrifugation, and the supernatant containing cross-linked chromatin was stored at −80°C.
For immunoprecipitation, 100 μl of the supernatant was incubated with 4 μg of HMG1 antibody (BD Bioscience), 5 μg of NF-κB p50 antibody (Santa Cruz), and 5 μg each of two different NF-κB p65 antibodies (Santa Cruz and Rockland, Gilbertsville, Pa.) overnight at 4°C. Protein G-Sepharose (Pharmacia) washed in sonication buffer was added to each sample, and incubation took place for 1 h at 4°C on a rotating wheel. After being washed twice in sonication buffer, once in high-salt buffer (10 mM Tris-HCl [pH 7.5], 2 M NaCl, 1% NP-40, 0.5% deoxycholate, 1 mM EDTA), once again in sonication buffer, and finally in Tris-EDTA (TE) buffer, samples were eluted in elution buffer (TE buffer containing 1% SDS and 10 mM DTT). Cross-linking was reversed by incubating samples first in RNase A (50 μg/ml) for 30 min at 37°C and then in proteinase K (500 μg/ml)-0.25% SDS overnight at 37°C followed by incubation at 65°C for 6 h. DNA was purified by phenol-chloroform extraction, precipitated with 20 μg of glycogen as a carrier, and finally dissolved in 20 μl of TE.
PCR amplification was carried out according to the following program: 31 cycles of 1 min at 95°C, 1 min each at 65 and 67°C, and 45 s at 72°C, with a final extension of 10 min at 72°C (MIAfor844, GGA AGC GTC TGG TGA AGA GG; MIArev1256, CCC ACA CCA CCT AGA AGT TGC; negCo for2, GTC ACG CAG GGT GGC ATG C; negCo rev, TGG GCA TAT CAG TGA GCA CAC). As a positive control, sheared chromatin lacking incubation with primary antibody was used in the PCR.
Coimmunoprecipitation.
For coimmunoprecipitation, cell lysates of Mel Im melanoma cells were precleared with 25 μl of protein G-Sepharose (Pharmacia) at 4°C for 6 h. After centrifugation, the supernatant was transferred into a fresh vial and incubated with 5 μg of the appropriate antibody (anti-HMG1 [BD Bioscience], anti-NF-κB p50 [C-19; Santa Cruz]), or one of two different anti-NF-κB p65 antibodies (C-20 [Santa Cruz] or 100-4165 [Rockland]), together with the double-stranded oligonucleotide corresponding to the HMG1-binding site in the MIA promoter while being shaken at 4°C overnight. Then 20 μl of protein G-Sepharose was added for 1 h and the solution was pelleted, washed three times with PBS, resuspended in Laemmli's buffer, heated at 95°C for 10 min, and separated on SDS-15% polyacrylamide gels. HMG1 and p50 were detected by Western blotting.
RESULTS
Previously, we purified MATF, a protein that binds specifically to the HCR element within the MIA promoter (12). MATF was shown to mediate the transactivation of the promoter in melanoma cells and to take part in the melanoma-specific expression of MIA. To further characterize MATF, we performed affinity chromatography using the MATF DNA-binding region within the HCR in the MIA promoter (bp −220 to −192) and nuclear extracts of the melanoma cell line Mel Im. Briefly, to isolate the protein that binds to this specific MIA promoter region, a DNA-binding protein purification kit from Roche was used as suggested by the manufacturer. The multimerized oligonucleotide HCR was generated by self-primed PCR, was coupled to magnetic beads, and served as the binding site. Binding of the nuclear extract of the melanoma cells to the concatemer was performed in a HEPES binding buffer, whereas unspecific binding was repressed by competition with a mutated binding site (for details, please refer to reference 12). A single protein was purified to homogeneity (Fig. 6A in reference12) and cleaved by protease LysC digestion, and peptides were separated by reverse-phase high-performance liquid chromatography. Then the amino acid sequences of two fragments of MATF were determined by peptide microsequencing (TopLab, Martinsried, Germany). Comparison of the two amino acid sequences with those in GenBank by BLAST search revealed that these peptides correspond to the sequence of HMG1 (GenBank accession no. X12597) (Fig. 1).
FIG. 6.
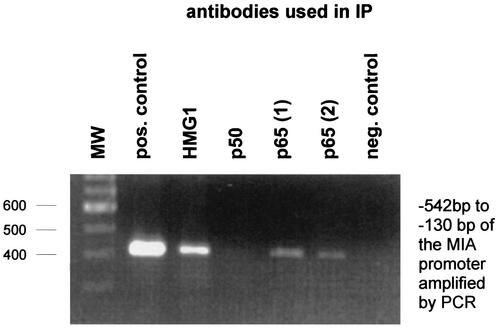
Interaction of HMG1 and p65 of the NF-κB family with the MIA promoter as assayed by chromatin immunoprecipitation (IP). After bound transcription factors were fixed to the chromatin (see Materials and Methods), DNA was sheared by sonication and protein-DNA complexes were used in different immunoprecipitations (with anti-HMG1, anti-p50, and two different anti-p65 antibodies). Subsequently, the DNA was separated from the precipitated protein complexes and used in a specific PCR amplifying the HCR of the MIA promoter (bp −542 to −130). Chromatin immunoprecipitation proved binding of HMG1 and p65 to the region in the MIA promoter in vivo, whereas no binding of p50 was observed. Molecular weight (MW) markers are shown at the left. pos., positive; neg. negative.
FIG. 1.
Peptide sequences of purified MATF. MATF was purified (8) and cleaved by protease LysC digestion. Peptides were purified by reverse-phase HPLC and sequenced, and two peptides were clearly identified (underlined) as HMG1 fragments (GenBank accession no. X12597).
Next, the expression pattern of HMG1 mRNA and protein level in malignant melanoma cell lines were analyzed in comparison to those in NHEM. Western blotting revealed significant upregulation of HMG1 expression in all nine analyzed melanoma cell lines compared to that in melanocytes (Fig. 2A). Quantitative RT-PCR also indicated that mRNA expression was upregulated (data not shown).
FIG. 2.
HMG1 protein expression in malignant melanoma and NHEM. (A) Western blotting revealed strong upregulation of HMG1 expression in nine melanoma cell lines (Mel Im, Mel Ju, Mel Juso, Mel Ho, Mel Wei, Mel Ei, SK-Mel-3, SK-Mel-28, and HTZ19d) compared to that in NHEM. Seven micrograms of protein lysate per lane was applied. Coincubation with a β-actin antibody (Sigma) was used to ensure equal loadings. (B) Analysis of normal skin by immunohistochemistry revealed very weak HMG1 signals in epidermal melanocytes in the basal layer of the skin (panel II, arrows). Tyrosinase staining in serial sections was used to identify the melanocytes (panel I, arrows). All keratinocytes were found to be highly positive for HMG1. In melanoma cells, significant HMG1 expression was found (panels III and IV).
Further, serial tissue sections of normal skin and melanoma biopsies were analyzed by immunohistochemical staining (Fig. 2B). In normal skin, melanocytes were stained for tyrosinase to detect their location in the basal cell layer of the epidermis (panel I). Tyrosinase-positive melanocytes revealed only very faint HMG1 signals, in contrast to the surrounding keratinocytes (panel II). The HMG1 expression found in epidermal keratinocytes was also verified by RT-PCR (data not shown). In malignant melanoma, all tyrosinase-positive cells (panel III) were found to be strongly HMG1 positive (panel IV), indicating that benign and malignant melanocytes in vivo differ in their HMG1 expression patterns.
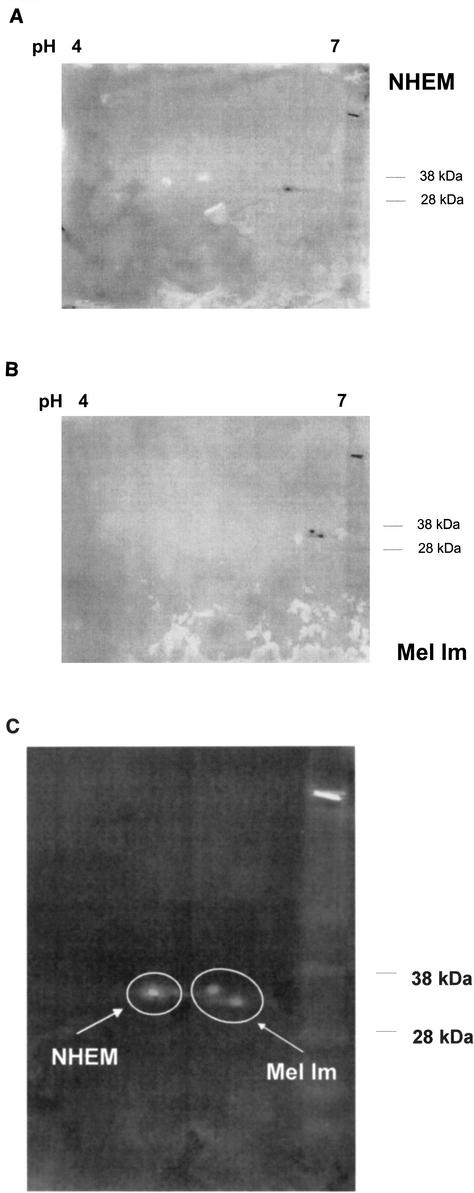
In addition, two-dimensional gel electrophoresis revealed significant differences in the migrating patterns of HMG1 benign melanocytes and malignant melanoma cells (Fig. 3). In melanocytes, HMG1 was detected by Western blotting to have an isoelectric point of 6.4, whereas in the melanoma cell line Mel Im, HMG1 had isoelectric points of 6.55 and 6.65. The posttranslational modification for HMG1 in the melanoma cell line Mel Im was most likely to be hypophosphorylation.
FIG. 3.
Detection of posttranslational modification of HMG1 in malignant melanoma and primary skin melanocytes. By two-dimensional gel electrophoresis and subsequent Western blotting, different electrophoretic mobilities, most likely resulting from a reduction of phosphorylation of HMG1, were detected in melanoma cells (B) and NHEM (A). (C) Overlay to compare the differences in electrophoretic mobilities. As NHEM expressed significantly lower levels of HMG1, a fourfold excess of protein was loaded.
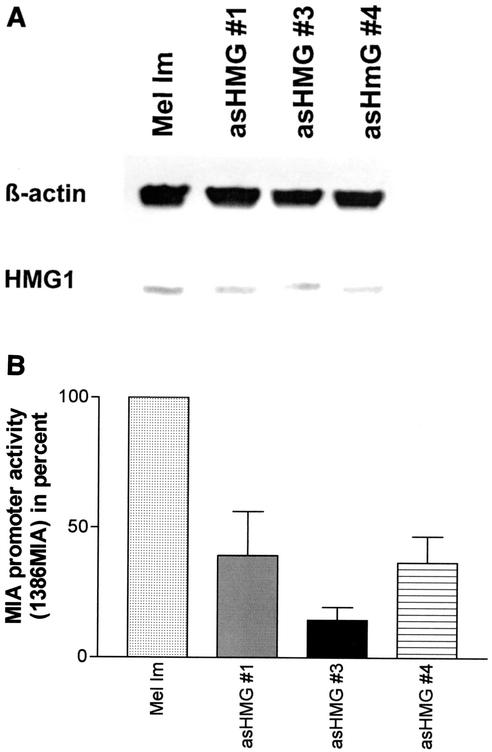
To analyze the functional relevance of HMG1 upregulation in melanoma cells, melanoma cell clones with reduced HMG1 expression were generated. Therefore, an antisense-HMG1 cDNA expression vector was stably transfected into the melanoma cell line Mel Im. HMG1 expression of the cell clones was analyzed by real-time PCR (data not shown) and Western blotting, and three antisense cell clones with significantly reduced HMG1 expression were chosen for further experiments (Fig. 4A).
FIG. 4.
(A) HMG1 protein levels in stably antisense-HMG1 (asHMG #1, 3, and 4)-transfected cell clones compared to those in the parental cell line Mel Im. Expression of HMG1 protein in the parental cell line Mel Im and in stably antisense-HMG1-transfected cell clones was detected by Western blotting with an HMG1 antibody. HMG1 levels in melanoma cells were reduced by stable transfection of antisense-HMG1 expression constructs. Equal loadings were verified by coincubation of the blotting membrane with a β-actin antibody. (B) MIA promoter activity in antisense-HMG1-transfected cell clones. Analysis by luciferase reporter assays of the antisense-HMG1-transfected cell clones revealed a reduced activity of the 1,386-bp fragment of the MIA promoter (1386MIA).
To verify the regulating influence of HMG1 on the activity of the MIA promoter, we transiently transfected the wild-type melanoma cell line Mel Im and the antisense-HMG1 clones with a reporter plasmid carrying 1,386 bp of the MIA promoter in front of a luciferase gene (5). Quantification of luciferase activity revealed significantly reduced MIA promoter activity in the antisense-HMG1 clones (Fig. 4B) compared to that in the melanoma cell line Mel Im (35% ± 9% versus 100%).
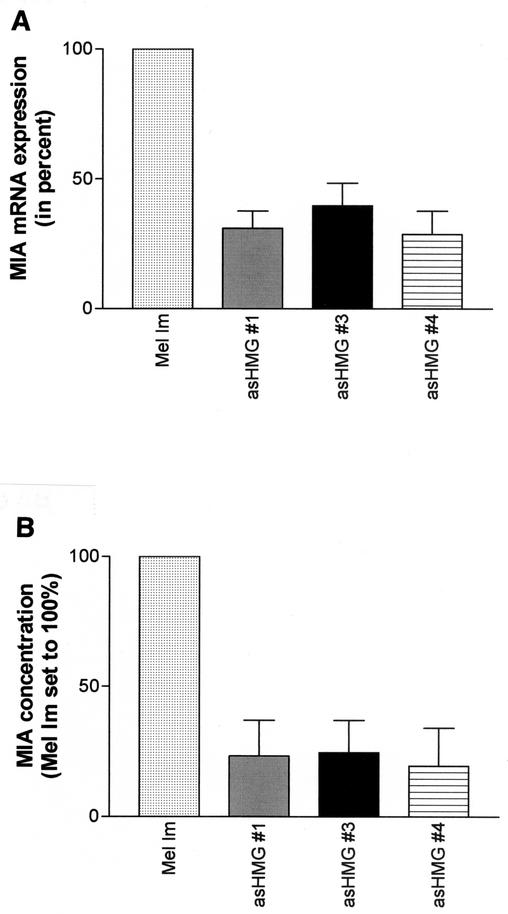
To further validate these data from transient transfections, we also measured endogenous MIA mRNA and protein expression in the antisense-HMG1-transfected melanoma cells. RT-PCR demonstrated a reduction of MIA expression in the antisense-HMG1 cell clones compared to that in the wild-type melanoma cell line Mel Im (33.2% ± 8% versus 100%) (Fig. 5A). Measuring MIA protein expression in the antisense-HMG1 cell clones by a quantitative ELISA (6) showed significant reduction to 22.5% ± 13.5% (Fig. 5B). Consistent with these results, we also observed that overexpression of HMG1 by transient transfection of an expression plasmid led to further induction of MIA expression (data not shown).
FIG. 5.
MIA mRNA and protein expression in antisense-HMG1 (asHMG #1)-transfected cell clones. HMG1 levels in melanoma cells were reduced by stable transfection of antisense-HMG1 expression constructs. Analysis of the cell clones revealed reduced MIA mRNA and protein expression as measured in tissue culture supernatant by RT-PCR (A) and ELISA (B).
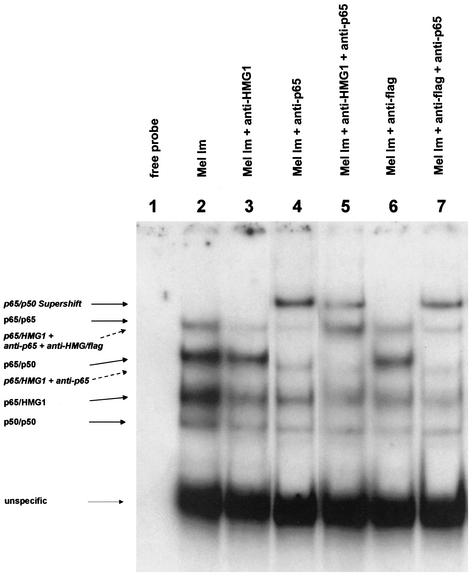
Interestingly, the HMG1-binding site in the MIA promoter is located in close proximity to and partly overlaps an NF-κB binding site. We therefore tested interactions between HMG1 and the NF-κB family proteins p65 and p50 at the HCR cis-regulatory element. Interactions of HMG1 with members of the NF-κB family have been observed previously only in drosophila (8). By chromatin immunoprecipitation, we detected binding of HMG1 and p65 but not p50 to the same region in the MIA promoter in vivo (Fig. 6). We further observed an interaction between HMG1 and p65 in electrophoretic mobility shift assays by supershifting experiments using antibodies against HMG1 and p65 (Fig. 7). As a second approach, nuclear extracts of Mel Im cells transiently transfected with a flag-tagged HMG1 expression plasmid were used for supershifting experiments with a flag antibody. Both the HMG1 antibody and the flag antibody suppress the DNA binding of HMG1, resulting in a decrease of the intensity of one band (bands labeled p65/HMG1 in Fig. 7, lanes 3 and 6). Incubation of the nuclear extracts with an antibody against p65 (lane 4) resulted in supershifting of three bands, two corresponding to the p65-p50 heterodimer or p65-p65 homodimer and the third identical to the band competed by the HMG1 and flag antibodies (labeled p65/HMG1). Coincubation with the antibodies anti-HMG1, anti-flag, and anti-p65 (lanes 5 and 7) resulted in clear supershifted bands (labeled p65/HMG1 + anti-HMG/flag). The bands labeled “unspecific” show equal loadings.
FIG. 7.
Analysis of the direct interaction of HMG1 with NF-κB family members by gel mobility shift assays. Nuclear extracts of the melanoma cell line Mel Im (lane 2), transiently transfected with flag-sense-HMG1 expression plasmids, were incubated with the oligomeric HCR-binding site of the MIA promoter. Lanes 3 and 6 show a decrease in the band intensity of the band shift labeled p65/HMG1 after incubation of the nuclear extracts of Mel Im with anti-HMG1 (lane 3) or anti-flag (lane 6). Band shifting of the bands labeled p65/p65, p65/p50, and p65/HMG1 was observed after incubation of the extracts with anti-p65 (lane 4). In lanes 5 and 7, nuclear extracts were coincubated with two different antibodies: anti-HMG1 and anti-p65 (lane 5) or anti-flag and anti-p65 (lane 7). This resulted in the additional supershifted band of the lane labeled p65/HMG1 + anti-p65 + anti-HMG/flag.
Furthermore, coimmunoprecipitation experiments were performed for additional clarification of direct p65-HMG1 interaction (Fig. 8). These data show that HMG1 directly interacts with p65 but not with p50 (Fig. 8I) in the presence of the HMG1-binding domain on the MIA promoter. As a control, p50 Western blotting of the precipitates was performed (Fig. 8II). p50 was shown to bind to p65, but no precipitation of HMG1 was revealed.
FIG. 8.
Coimmunoprecipitation assays. Coimmunoprecipitation (IP) was utilized to show direct interaction of HMG1 and p65. For coimmunoprecipitation of HMG1 with p65, two different anti-p65 antibodies were used (see Materials and Methods). p50 coimmunoprecipitation is just feasible with p65 from Santa Cruz, as p65 from Rockland binds to the p50-binding site. Western blots were stained with anti-HMG1 (panel I) and anti-p50 (panel II) antibodies, respectively. The data show that HMG1 is able to bind to p65 of the NF-κB family on the MIA promoter.
DISCUSSION
In this study we identified the transcription factor MATF, which binds specifically to the HCR cis-regulatory element of the MIA promoter as the DNA-binding factor HMG1. Further, our data indicate that HMG1 functions as a promoter-specific trans-regulatory protein and that it is involved in the malignant transformation of melanoma cells.
High-mobility-group (HMG) proteins are known to be abundant and ubiquitous nonhistone proteins. They can be classified into three families: HMG1/2/4, HMG14/17, and HMG-I. A new name was recently proposed for the HMG1/2/4 family, specifically, the HMGB family (with HMG1 becoming HMGB1, HMG2 becoming HMGB2, and HMG4 becoming HMGB3). The structures of the three family members are highly conserved. They are composed of three different domains and two DNA-binding domains called HMG boxes (1, 22).
HMG1 binds to the minor groove of double-stranded DNA, causes local distortion of the double helix, and induces a sharp bending (21). These steps allow the formation of larger nucleoprotein complexes where several DNA-binding proteins can contact each other while being bound to their DNA consensus site (22). Therefore, HMG1 has been shown to interact with and enhance the activities of several transcription factors, including the glucocorticoid receptor, homeobox proteins, Pou domain transactivators, TATA-binding protein, and p53 (3, 9, 13, 15, 20, 24). HMG1-deficient mice show a distinct phenotype and die shortly after birth due to hypoglycemia and defects in the activation of glucocorticoid receptor-responsive genes (10). All together, these results suggested that HMG1 does not serve as an unspecific DNA-binding molecule but that it contributes to gene-specific transcriptional regulation.
In the MIA promoter, a sequence-specific DNA motif in the HCR is recognized by HMG1. Both purification by means of DNA affinity chromatography as described previously (12) and chromatin immunoprecipitation as described in this report indicate sequence-specific DNA binding properties. Here, a direct interaction of HMG1 with the MIA promoter HCR was detected in vivo, whereas HMG1 showed no binding to other regions of the MIA promoter. Obviously, a certain sequence specificity of HMG1 and a cis-regulatory function for MIA expression were observed in melanoma cells.
In addition, our data show for the first time strong overexpression of HMG1 in malignant melanoma cell lines at the mRNA and protein levels compared to that in NHEM.
A role of HMG1 in cancer progression has been suggested by several groups. It is known that undifferentiated and rapidly proliferating cells contain larger amounts of HMG proteins than occur in terminally differentiated cells (26). Nestl et al. showed HMG1 to be upregulated in pancreatic carcinoma cells and defined it as a metastasis-specific gene (17). Further, Xiang et al. detected a higher level of expression in gastric and colorectal adenocarcinoma tissues than in noncancerous tissues. They could even reveal a correlation between HMG1 expression and the degree of tumor dedifferentiation, as only 18.2% of the well-differentiated cancers but 60% of moderately and 83.3% of poorly differentiated cancers showed higher expression levels of HMG1 (28). Kawahara et al. reported overexpression of HMG1 in a hepatocellular carcinoma (16).
Further, differences in the isoelectric points of HMG1 in NHEM and melanoma cells were found, suggesting a difference in phosphorylation patterns. Our data suggest that HMG1 in NHEM is likely to be highly phosphorylated. Phosphorylation of HMG1 is known to occur on seryl residues (19, 27). Phosphorylation leads to more negatively charged groups on the protein, causing a DNA binding activity that is reduced by up to 10-fold. Further, the translocation of phosphorylated HMG1 into the nucleus was remarkably delayed (25). Therefore, the hypophosphorylated HMG1 protein present in melanoma cells may also be much more active as a nuclear DNA-binding protein.
Literature data suggested further that DSP1, the drosophila homologue of human HMG1 or -2, binds cooperatively with members of the NF-κB family to specific promoter elements (8, 11). The HMG1 homologue also increased the activity of NF-κB family members on certain promoters (e.g., the drosophila twist promoter). Here we show for the first time HMG1 and p65 interaction in a mammalian system. Our data suggest that HMG1 interacts specifically with p65 in the HCR of the MIA promoter; however, no interaction with p50 was seen. An increase in MIA promoter activity was further shown by cotransfection of HMG1 and p65 (data not shown).
In summary, our data reveal HMG1 and p65 to be important factors in MIA regulation and melanoma progression and further define HMG1 as a gene-specific trans-regulatory protein.
Acknowledgments
We are indebted to Astrid Hamm and Claudia Abschlag for technical assistance.
This work was supported by grants from the DFG and the Deutsche Krebshilfe to A.K.B.
REFERENCES
- 1.Baxevanis, A. D., and D. Landsman. 1995. The HMG-1 box protein family: classification and functional relationships. Nucleic Acids Res. 23:1604-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blesch, A., A. K. Bosserhoff, R. Apfel, C. Behl, B. Hessdoerfer, A. Schmitt, P. Jachimczak, F. Lottspeich, R. Buettner, and U. Bogdahn. 1994. Cloning of a novel malignant melanoma-derived growth-regulatory protein, MIA. Cancer Res. 54:5695-5701. [PubMed] [Google Scholar]
- 3.Boonyaratanakornkit, V., V. Melvin, P. Prendergast, M. Altmann, L. Ronfani, M. E. Bianchi, L. Taraseviciene, S. K. Nordeen, E. A. Allegretto, and D. P. Edwards. 1998. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol. Cell. Biol. 18:4471-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosserhoff, A. K., A. Hauschild, R. Hein, D. Schadendorf, E. Stockflesh, T. Bogenrieder, M. Landthaler, R. Buettner, and W. Stolz. 2000. Elevated MIA serum levels are of relevance for management of metastasized malignant melanomas: results of a German multicenter study. J. Investig. Dermatol. 114:395-396. [DOI] [PubMed] [Google Scholar]
- 5.Bosserhoff, A. K., R. Hein, U. Bogdahn, and R. Buettner. 1996. Structure and promoter analysis of the gene encoding the human melanoma-inhibiting protein MIA. J. Biol. Chem. 271:490-495. [DOI] [PubMed] [Google Scholar]
- 6.Bosserhoff, A. K., M. Kaufmann, B. Kaluza, I. Bartke, H. Zirngibl, R. Hein, W. Stolz, and R. Buettner. 1997. Melanoma-inhibiting activity, a novel serum marker for progression of malignant melanoma. Cancer Res. 57:3149-3153. [PubMed] [Google Scholar]
- 7.Bosserhoff, A. K., M. Moser, R. Hein, M. Landthaler, and R. Buettner. 1999. In situ expression patterns of melanoma-inhibiting activity (MIA) in melanomas and breast cancers. J. Pathol. 187:446-454. [DOI] [PubMed] [Google Scholar]
- 8.Brickman, J. M., M. Adam, and M. Ptashne. 1999. Interactions between an HMG-1 protein and members of the Rel family. Proc. Natl. Acad. Sci. USA 96:10679-10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butteroni, C., M. De Felici, H. R. Scholer, and M. Pesce. 2000. Phage display screening reveals an association between germline-specific transcription factor Oct-4 and multiple cellular proteins. J. Mol. Biol. 304:529-540. [DOI] [PubMed] [Google Scholar]
- 10.Calogero, S., F. Grassi, A. Aguzzi, T. Voigtlander, P. Ferrier, S. Ferrari, and M. E. Bianchi. 1999. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat. Genet. 22:276-280. [DOI] [PubMed] [Google Scholar]
- 11.Decoville, M., M. J. Giraud-Panis, C. Mosrin-Huaman, M. Leng, and D. Locker. 2000. HMG boxes of DSP1 protein interact with the rel homology domain of transcription factors. Nucleic Acids Res. 28:454-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golob, M., R. Buettner, and A. K. Bosserhoff. 2000. Characterization of a transcription factor binding site, specifically activating MIA transcription in melanoma. J. Investig. Dermatol. 115:42-47. [DOI] [PubMed] [Google Scholar]
- 13.Imamura, T., H. Izumi, G. Nagatani, T. Ise, M. Nomoto, Y. Iwamoto, and K. Kohno. 2001. Interaction with p53 enhances binding of cisplatin-modified DNA by high mobility group 1 protein. J. Biol. Chem. 276:7534-7540. [DOI] [PubMed] [Google Scholar]
- 14.Jacob, K., F. Wach, U. Holzapfel, R. Hein, E. Lengyel, R. Buettner, and A. K. Bosserhoff. 1998. In vitro modulation of human melanoma cell invasion and proliferation by all-trans-retinoic acid. Melanoma Res. 8:211-219. [DOI] [PubMed] [Google Scholar]
- 15.Jayaraman, L., N. C. Moorthy, K. G. Murthy, J. L. Manley, M. Bustin, and C. Prives. 1998. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 12:462-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawahara, N., T. Tanaka, A. Yokomizo, H. Nanri, M. Ono, M. Wada, K. Kohno, K. Takenaka, K. Sugimachi, and M. Kuwano. 1996. Enhanced coexpression of thioredoxin and high mobility group protein 1 genes in human hepatocellular carcinoma and the possible association with decreased sensitivity to cisplatin. Cancer Res. 56:5330-5333. [PubMed] [Google Scholar]
- 17.Nestl, A., O. D. Von Stein, K. Zatloukal, W. G. Thies, P. Herrlich, M. Hofmann, and J. P. Sleeman. 2001. Gene expression patterns associated with the metastatic phenotype in rodent and human tumors. Cancer Res. 61:1569-1577. [PubMed] [Google Scholar]
- 18.Perez, R. P., P. Zhang, A. K. Bosserhoff, R. Buettner, and M. Abu-Hadid. 2000. Expression of melanoma inhibitory activity in melanoma and nonmelanoma tissue specimens. Hum. Pathol. 31:1381-1388. [PubMed] [Google Scholar]
- 19.Ramachandran, C., P. Yau, E. M. Bradbury, G. Shyamala, H. Yasuda, and D. A. Walsh. 1984. Phosphorylation of high-mobility-group proteins by the calcium-phospholipid-dependent protein kinase and the cyclic AMP-dependent protein kinase. J. Biol. Chem. 259:13495-13503. [PubMed] [Google Scholar]
- 20.Sutrias-Grau, M., M. E. Bianchi, and J. Bernues. 1999. High mobility group protein 1 interacts specifically with the core domain of human TATA box-binding protein and interferes with transcription factor IIB within the preinitiation complex. J. Biol. Chem. 274:1628-1634. [DOI] [PubMed] [Google Scholar]
- 21.Tang, L., J. Li, D. S. Katz, and J. A. Feng. 2000. Determining the DNA bending angle induced by nonspecific high mobility group-1 (HMG-1) proteins: a novel method. Biochemistry 39:3052-3060. [DOI] [PubMed] [Google Scholar]
- 22.Travers, A. 2000. Recognition of distorted DNA structures by HMG domains. Curr. Opin. Struct. Biol. 10:102-109. [DOI] [PubMed] [Google Scholar]
- 23.van Groningen, J. J., H. P. Bloemers, and G. W. Swart. 1995. Identification of melanoma inhibitory activity and other differentially expressed messenger RNAs in human melanoma cell lines with different metastatic capacity by messenger RNA differential display. Cancer Res. 55:6237-6243. [PubMed] [Google Scholar]
- 24.Verrijdt, G., A. Haelens, E. Schoenmakers, W. Rombauts, and F. Claessens. 2002. Comparative analysis of the influence of the high-mobility group box 1 protein on DNA binding and transcriptional activation by the androgen, glucocorticoid, progesterone and mineralocorticoid receptors. Biochem. J. 361:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisniewski, J. R., E. Schulze, and B. Sapetto. 1994. DNA binding and nuclear translocation of insect high-mobility-group-protein-1 (HMG1) proteins are inhibited by phosphorylation. Eur. J. Biochem. 225:687-693. [DOI] [PubMed] [Google Scholar]
- 26.Wisniewski, J. R., and R. Schwanbeck. 2000. High mobility group I/Y: multifunctional chromosomal proteins causally involved in tumor progression and malignant transformation. Int. J. Mol. Med. 6:409-419. [DOI] [PubMed] [Google Scholar]
- 27.Wisniewski, J. R., Z. Szewczuk, I. Petry, R. Schwanbeck, and U. Renner. 1999. Constitutive phosphorylation of the acidic tails of the high mobility group 1 proteins by casein kinase II alters their conformation, stability, and DNA binding specificity. J. Biol. Chem. 274:20116-20122. [PubMed] [Google Scholar]
- 28.Xiang, Y. Y., D. Y. Wang, M. Tanaka, M. Suzuki, E. Kiyokawa, H. Igarashi, Y. Naito, Q. Shen, and H. Sugimura. 1997. Expression of high-mobility group-1 mRNA in human gastrointestinal adenocarcinoma and corresponding noncancerous mucosa. Int. J. Cancer 74:1-6. [DOI] [PubMed] [Google Scholar]