Abstract
Annexins are highly conserved proteins that are characterized by their ability to interact with phospholipids in a calcium-dependent manner. Although diverse functions have been ascribed to annexins based on in vitro analyses, their in vivo functions still remain unclear. The intensively studied annexin A5 has been identified by its effects on blood coagulation, and subsequently, its function as a calcium-specific ion channel was described. In vitro experiments and expression studies suggested a potential role of annexin A5 during calcification processes in vivo, especially in endochondral ossification. To gain insights into the relevance of annexin A5 in this process, we generated an annexin A5-deficient mouse mutant. Mice lacking annexin A5 are viable, are fertile, and reveal no significant alterations in the biochemical parameters characteristic for metabolic or functional defects. Neither the development of skeletal elements nor the in vitro calcification properties of isolated chondrocytes is significantly impaired by the absence of annexin A5. Therefore, annexin A5 is dispensable for the formation and maintenance of skeletal elements in the mouse and may possibly be pointing to a compensatory effect of other members from the annexin family due to their high functional and structural similarity.
Annexins represent a large family of proteins defined by their ability to bind to phospholipids in a calcium-dependent manner (21, 27). Members of this multigene family, classified to 27 subfamilies, are found in most eukaryotic organisms (25, 26). All annexins are composed of a highly conserved protein core domain consisting of four or eight homologous repeats, each harboring a Ca2+-binding site, whereas variable domains at the N termini define the individual characters of each annexin. Structural analysis of annexin A5 and other members of the family revealed that the four repeats formed by five α-helices are grouped symmetrically around a central pore (10, 20). Although protein and gene structures as well as the biochemical features of annexins were analyzed extensively, the in vivo functions of the individual members of the annexin family remain unclear (23). In vitro experiments have linked the functions of annexins to signal transduction events, apoptosis (32), blood coagulation processes (45), and vesicle trafficking, as well as calcium signaling and transport (4), but the demonstration of these functions in vivo is still pending. The coexpression of different annexins with potentially overlapping functions in most tissues complicates the in vivo analysis of individual annexins.
Annexin A5 was originally purified from chondrocytes as a potential receptor for collagen type II (24, 41) and independently isolated from placenta as a protein with anticoagulant activity (5). Later, its capacity to form voltage-dependent calcium channels in phospholipid bilayers was recognized and investigated in great detail (9, 11, 19). It became evident that annexin A5 is located inside of cells but that it can also be secreted and is thereby detected in blood plasma. Additionally, annexin A5 is able to bind to the outer surfaces of cells, mediated by its high affinity to phosphatidylserine (2, 29). Surface presentation of this phospholipid represents a characteristic event during the early stages of apoptosis, and therefore, annexin A5 binding has become an important tool for detecting apoptotic changes (16).
An essential role of annexin A5 during the mineralization of tissues has been assumed, especially during the process of endochondral ossification (40). By interacting with the extracellular matrix components collagen II and X, annexin A5 can mediate the influx of Ca2+ into the matrix vesicles secreted from hypertrophic cartilage at the growth plate and thereby initiate the calcification of cartilage (12, 13). Furthermore, this observation is supported by in vivo expression studies showing that the annexin A5 gene (Anxa5) is specifically expressed in skeletal elements during embryogenesis (1). In the early stages of development (9.5- to 12.5-days-postcoitum embryos), Anxa5 expression was detected in cells associated with the developing vasculature. In later stages (>13.5 days postcoitum), a strong expression was observed in cartilage anlagen, such as the vertebral bodies, neural arches, and ribs, as well as the digits of the limb buds. In adult mice, Anxa5 gene expression is maintained in cartilaginous tissues as well as in bone. Therefore, these data suggest a potential role of annexin A5 during skeletal development.
Here, we analyze the consequences of annexin A5 deficiency, generated by a gene-targeting approach, for the development of skeletal elements in the mouse. Annexin A5-deficient mice are viable and fertile and show no obviously altered phenotype. The lack of significant changes in the development of cartilage and bone indicates that either only marginal contributions of annexin A5 to the ossification processes were made or that this could be due to the functional compensation by other members of the annexin family.
MATERIALS AND METHODS
Generation of targeting construct.
The murine annexin A5 gene (Anxa5) was isolated from a 129/SvJ genomic library in the lambda fix II vector (Stratagene, La Jolla, Calif.) by standard methods. Mapping of a 25-kb region corresponded to published data (34). Various subclones (pBluescriptII) representing regions from intron 2 to intron 6 were generated and used for the construction of the targeting vector. A 5-kbp fragment containing exon 3 was amplified by PCR with primers Ex3fus (5′-CGGGGTACCTTCATGGCCTTCCGAAGG-3′) and Revlong (5′-GGAAACAGCTATGACCATG-3′). The resulting product was inserted via KpnI-EcoRV sites into the vector pUC19 and combined with the flanking 5′ upstream EcoRV-SacI fragment of 5.5 kbp. A LacZ cassette from the vector pSV-β-galactosidase (Promega) was inserted in frame with exon 3 by using KpnI-SalI sites. A 1.5-kbp ApaI-XbaI fragment including exon 5 was cloned into pNeoflox8 containing a neomycin cassette flanked by loxP sites (kindly supplied by W. Müller, Cologne, Germany), and the exon3-LacZ cassette was inserted after NotI digestion into pNeoflox8, resulting in the targeting vector pAVLacZ3.5 (Fig. 1).
FIG. 1.
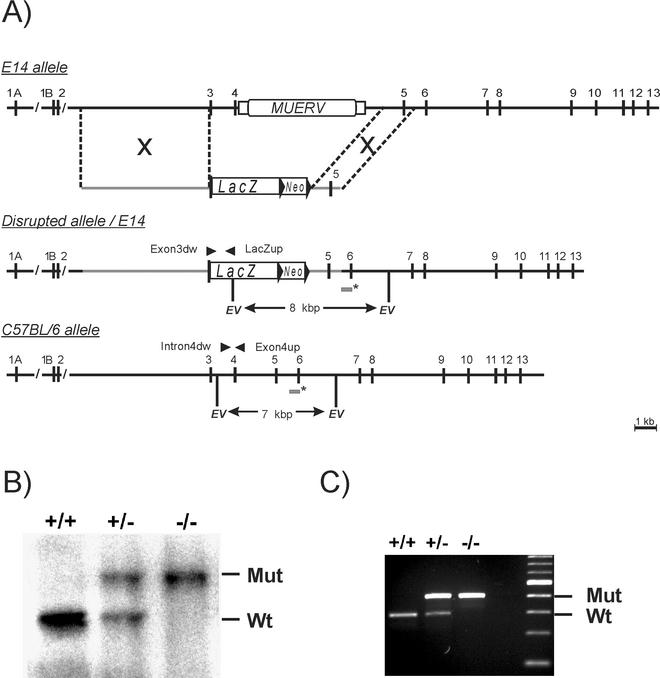
(A) Generation of annexin A5-deficient mice. The structures of the wild-type allele of the Anxa5 gene in the 129/SvJ (E14) and C57BL/6 mouse strains, the targeting vector, and the disrupted allele are shown with numbered exons (vertical bars) and introns. The presence of MuERV is shown. LacZ and neomycin (Neo) cassettes are marked in the targeting construct, and regions of homology are indicated (grey lines). The sizes of the EcoRV fragments, detected by a probe specific for exon 6 (asterisks), are 7 and 12.5 kbp for the wild-type alleles of C57BL/6 and 129/SvJ (E14) mice as well as 8 kbp for the disrupted allele, respectively. (B) Southern blot analysis of offspring from heterozygous intercrosses digested with EcoRV and hybridized with the probe exon 6. Mut, mutant; Wt, wild type. (C) PCR analysis of isolated DNA results in fragments of 301 bp for the wild type and of 449 bp for the disrupted allele.
Generation of an annexin A5-deficient mouse strain.
The targeting construct pAVLacZ3.5 was linearized by ApaI digestion and transfected into E14.1 embryonic stem (ES) cells by electroporation (28), and resistant cells were selected in the presence of G418 (0.2 mg/ml) as described previously (43). A total of 384 clones were picked and grown. Isolated DNA was screened by Southern blotting and hybridization for correct homologous recombination. An 815-bp fragment including exon 6 was amplified by PCR with primers Intron5dw (5′-TCTCAAGCAGTATTAGCAGCAAAGATC-3′) and Exon6up (5′-TGGCACTGAGTTCTTCAGGTGTCCTTGA-3′) and used as a probe for Southern blot analysis. Correct clones were hybridized also with a probe corresponding to sequences from intron 2. Additionally, a neomycin-specific probe was used to test for unique integration of the targeting construct. Three ES cell clones containing the correctly targeted Anxa5 allele were selected (clones A9, E11, and B11), expanded, injected into C57BL/6 blastocysts, and used for the generation of chimera. Chimeric offspring were screened for germ line transmission by coat color and PCR as well as Southern blot analysis. For one clone (A9), correct transmission of the mutated allele could be found. Mice heterozygous for the mutated allele were mated to establish a breeding colony. Genotyping of mice was performed by PCR detecting the wild type with primers Exon4dw (5′-GAAGCAATGCTCAGCGCCAGGA-3′) and Intron4up (5′-CTGTACTCTATCACTATCACTGACTGTTTAATC-3′) and the mutated allele with primers Exon3dw (5′-CGAGAGGCACTGTGACTGACTTCCCTGGAT-3′) and LacZup (5′-GCCAGTTTGAGGGGACGACGACAG-3′).
Immunohistochemistry and detection of β-galactosidase expression.
Embryos, organs, and limbs of newborn mice were isolated and snap frozen in Tissue-Tek (Sakura, Tokyo, Japan). Immunostainings of microtome sections (5 to 10 μm thick) were performed with primary antibodies specific for annexin A5 (rabbit polyclonal antibody; Hyphen BioMed) and Cy5-labeled secondary antibodies (Dianova) as described earlier (1, 33). Sections were stained for β-galactosidase activity, and calcium deposits were visualized by staining with 0.5% (wt/vol) alizarin red as described previously (8).
Immunoblotting.
Tissue samples (150 mg) were homogenized on ice in 2 ml of lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 0.05% Triton X-100, 0.5% sodium deoxycholate, 2.5 mM EGTA, 2 mM phenylmethylsulfonyl fluoride). After centrifugation, the supernatants were collected and protein concentrations were determined (Pierce, Rockford, Ill.). Equal amounts of protein (20 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% acrylamide) and transferred onto reinforced nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Primary antibodies specific for annexin A2 (goat polyclonal antibody, 1:20; Santa Cruz), annexin A5 (rabbit polyclonal antibody, 1:100; Hyphen BioMed), annexin A6 (goat polyclonal antibody, 1:100; Santa Cruz), and annexin A7 (mouse monoclonal hybridoma supernatant; kindly provided by A. A. Noegel, Cologne, Germany) were used for immunoblotting and detected by species-specific secondary antibodies labeled with horseradish peroxidase (Bio-Rad). Peroxidase activity was visualized by chemoluminescence according to standard procedures.
Analysis of biochemical parameters in blood plasma.
Sex- and age-matched mice (six animals per genotype, 5 month of age) were anesthetized with ether, and blood samples were taken by puncturing the retro-orbital sinus. The samples were quickly frozen on dry ice and analyzed for biomedical parameters as described previously (31).
Staining of the skeleton in newborn mice.
Skeletal preparation and staining of newborns with 0.5% alizarin red and 0.015% alcian blue were performed as described previously (8).
Chondrocyte culture.
Chondrocytes were isolated from the epiphyseal cartilage of the forelimbs and hindquarters of newborn mice by trypsin digestion (1%) for 30 min at 37°C followed by collagenase digestion (1 mg/ml; Roche, Basel, Switzerland) overnight in Dulbecco's modified Eagle's medium-F12 (DMEM-F12; Gibco) containing 10% fetal calf serum (Gibco). Chondrocytes were washed three times in DMEM-F12, and 2 × 105 cells were plated onto 12-well plates (Becton Dickinson) in DMEM-F12 containing 10% (vol/vol) fetal calf serum, 50 U of penicillin/ml, 0.1 mg of streptomycin/ml, and 1 mM pyruvate. When chondrocytes reached confluence (day 7), 10 mM glycerophosphate, 10 mM CaCl2, and 50 μg of ascorbate/ml were added. The cultures were incubated at 37°C in 10% CO2, and the medium was changed daily. Eight days after adding supplements, the cells were fixed for 5 min in 100% methanol and washed three times with phosphate-buffered saline. To localize calcium deposits, cells were stained with 0.5% alizarin red for 10 min at room temperature, washed three times with phosphate-buffered saline, and cleared with 2% potassium hydroxide. Stainings were analyzed by light microscopy and quantified by histogram software (Adobe Photoshop).
RESULTS
Generation of Anxa5-null mutant mice.
The annexin A5 gene (Anxa5) of the 129/SvJ mouse strain contains 13 exons, with two alternatively spliced first exons, 1A and 1B, and a murine endogenous retrovirus (MuERV) integrated into intron 4 of the gene, as shown recently (34). We confirmed this organization in the 129/SvJ strain, but in contrast, Southern blotting and PCR analysis using DNA from the C57BL/6 mouse strain revealed the absence of this retroviral element within the Anxa5 gene of this strain (Fig. 1). This difference had to be taken into account for the generation of a targeting construct, based on DNA isolated from 129/SvJ mice, as well as for the analysis of animals with a mixed genetic background (129/SvJ × C57BL/6). In order to inactivate the Anxa5 gene, we generated a targeting construct interrupting exon 3 and deleting exon 4 of the Anxa5 gene. A LacZ cassette was fused in frame to the second ATG initiation codon of exon 3, together with a neomycin selection cassette and flanking genomic sequences as indicated in Fig. 1. Exon 3 represents the first common exon found in all known splice products. The mutated allele therefore lacked exon 4, together with the MuERV-related element, and no functional mRNA should be produced (Fig. 1). ES cells were transfected and tested for homologous recombination by Southern blotting with an exon 6-specific probe (data not shown). Three out of the 384 clones (A9, B11, and E11) with correct integration of the targeting vector were used for blastocyst injection. Single and correct integration was further proven by the use of additional probes (neomycin and intron 2) as well as PCR. Chimeric males were crossed with C57BL/6 females to establish strains with a mixed genetic background heterozygous for the mutated allele. The strain derived from the ES cell clone A9 showed the correct integration after germ line transmission, as tested by Southern blotting (Fig. 1B) and PCR analysis (Fig. 1C), and was used for further analysis.
PCR genotyping of 147 offspring from heterozygous intercrosses demonstrated Mendelian inheritance of the mutated allele (+/+, 24%; +/−, 48%; −/−, 27%). Therefore, the deficiency of the Anxa5 gene causes no significant effect on embryonic development. Heterozygous as well as homozygous Anxa5-deficient mice were vital and fertile and showed no obvious phenotypic or behavioral abnormalities.
The ablation of Anxa5 mRNA expression was confirmed by reverse transcription-PCR analysis. No correct full-length Anxa5 mRNA could be detected in homozygous mice (data not shown). Yet small amounts of a truncated mRNA were detected by using reverse transcription-PCR with specific primers from exons 3 and 6 (data not shown), which represented an aberrantly spliced variant. However, no corresponding protein could be detected by immunoblotting or immunohistochemistry in mice with mutated Anxa5 alleles (Fig. 2 and 4). Additionally, recombinant expression of this aberrant mutant protein is instable and lost the characteristic biochemical property of annexin A5 to bind to phosphatidylserine (data not shown).
FIG. 2.
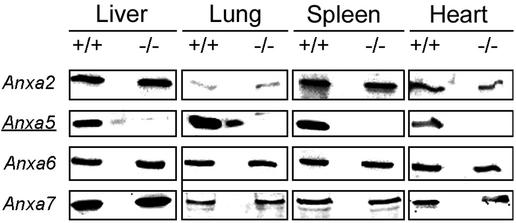
Expression of annexin A2, A5, A6, and A7 in organs from wild-type (+/+) and annexin A5-deficient (−/−) mice. Protein samples of tissue lysates (20 μg of total protein per lane) from the liver, lung, spleen, and heart were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the presence of annexins A2, A5, A6, and A7 was detected by immunoblotting with specific antibodies. To avoid contamination with proteins from neighboring lanes, an empty lane separated the wild-type and annexin A5-deficient lanes.
FIG. 4.
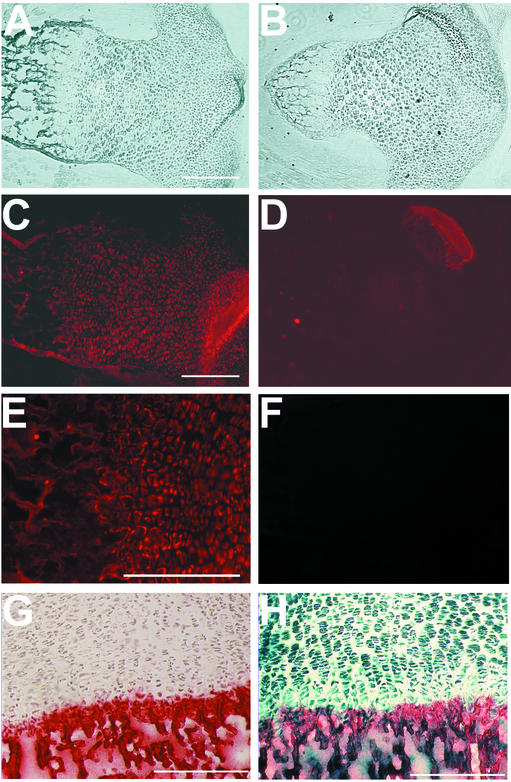
Expression of annexin A5 in the tibia of wild-type (+/+) (A, C, E, and G) and annexin A5-deficient (−/−) (B, D, and H) mice. (A and B) Phase contrast image of sections from the tibia. (C and D) Detection of annexin A5 protein by immunohistochemistry. (E) Higher magnification of panel C. (F) Immunostaining with the secondary antibody as negative control. (G and H) Parallel staining for β-galactosidase activity by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) substrate (blue) and for calcium deposits by alizarin red. Bars, 250 μm.
Potential effects on the expression of other members of the annexin family were tested by immunoblot analysis of organ lysates from adult annexin A5-deficient mice (Fig. 2). Annexin A5 was absent in the liver, lung, spleen, and heart, whereas annexins A2, A6, and A7 were expressed in annexin A5-deficient mice in amounts comparable to those in wild-type controls. Thus, no altered regulation of distinct members of the annexin family on the level of protein expression was detectable. The absence of annexin A5 in null mutant mice was further confirmed by the immunohistochemistry of newborns with annexin A5-specific antibodies (Fig. 4).
Clinical-chemical parameters are normal in annexin A5-deficient mice.
In order to test for biochemical defects, we examined a number of clinically relevant parameters in the blood plasma of age-matched (5 months) females from wild-type and homozygous mutants (Table 1).
TABLE 1.
Determination of biochemical parametersa in the blood plasma of wild-type (+/+) and Anxa5-deficient (−/−) age-matched female mice (5 months)
| Genotype | Calcium (mmol/liter) | Alkaline phophatase (U/liter) | Glucose (g/liter) | Cholesterin (g/liter) | Triglyceride (g/liter) | Uric acid (mg/liter) | Total protein (g/liter) |
|---|---|---|---|---|---|---|---|
| +/+ | 2.6 ± 0.2 | 141 ± 26 | 2.51 ± 0.41 | 0.77 ± 0.12 | 0.72 ± 0.18 | 3 ± 1 | 54 ± 4 |
| −/− | 2.5 ± 0.2 | 185 ± 35 | 2.70 ± 0.3 | 0.88 ± 0.22 | 0.91 ± 0.3 | 4 ± 3 | 55 ± 3 |
Concentrations, mean values, and standard deviations (six animals each) are given for each parameter.
The levels of total protein, glucose, cholesterol, triglycerides, uric acid, calcium, and alkaline phosphatase were determined (31), but for none of the parameters was a significant deviation from those of wild-type mice detectable. This indicates that annexin A5-deficient mice do not develop any severe defects during the first 5 months of life in association with glucose and lipoprotein metabolism, hepatic functions, or parameters linked to the ossification processes detectable in the blood plasma.
Normal development of skeletal elements in annexin A5-deficient mice.
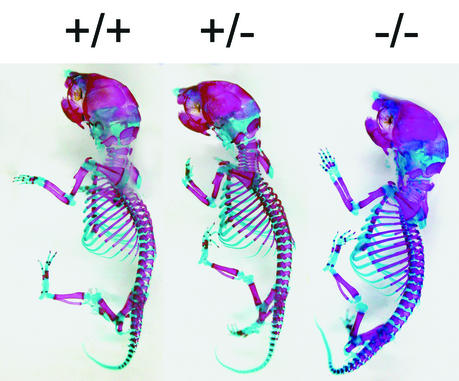
Annexin A5-deficient animals are indistinguishable in size and behavior from wild-type mice up to an age of 1 year. Adult mice lacking annexin A5 displayed no obvious abnormalities in the developing skeleton, and no skeletal elements were missing or deformed. Further, X-ray analysis of the skeleton from 6-month-old animals revealed no apparent differences in size or in the density of the bone (data not shown). To exclude a possible delay in skeletal development, we focused our interest on the early development of skeletal structures in newborn animals by staining cartilaginous and bony structures with alcian blue and alizarin red (Fig. 3). The size of the skeletal elements and the relative ratio of cartilage to bone in annexin A5-deficient mice were indistinguishable from those of wild-type animals. Histological analysis of the tibia from newborn animals (Fig. 4D) displayed no overt changes in the organization of the growth plate in the absence of annexin A5. Significant differences in the level of the calcification of cartilage between wild-type and annexin A5-deficient tibia were not detected (Fig. 4G and H). Although annexin A5 was found highly expressed in cartilage and bone, as shown by expression of the Anxa5-LacZ fusion protein (Fig. 4H) and the immunohistochemical distribution of annexin A5 (Fig. 4C and E), the deficiency causes no obvious consequences during development and in adulthood.
FIG. 3.
Cartilage and bone develop normally in newborns lacking annexin A5. The skeletons from newborn littermates of different genotypes (+/+, +/−, and −/−) were stained with alcian blue and alizarin red, detecting cartilage and bony structures, respectively.
In vitro calcification by chondrocytes.
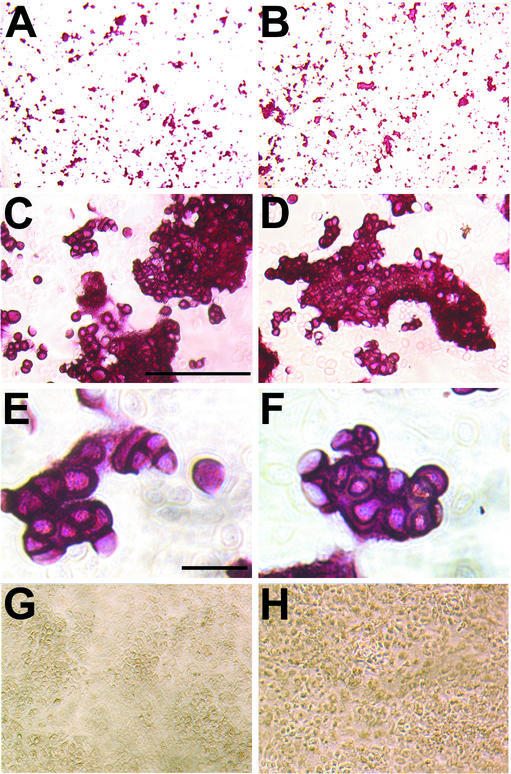
Based on previous evidence for a role of annexin A5 in the calcification of cartilage (15), we tested the ability of isolated chondrocytes to form mineral deposits. Chondrocytes of newborns were isolated and cultured in the absence or presence of β-glycerophosphate, CaCl2, and ascorbate, essential components to induce calcification in vitro (38). No detectable calcification could be observed in the absence of supplementations (Fig. 5G and H), while cell clusters with dense matrix deposits appeared already at day 6 in both wild-type and annexin A5-deficient cells in the presence of supplementation. At day 8, calcium mineral deposits in chondrocyte cultures were stained with alizarin red (Fig. 5). Histogram analyses of images taken from chondrocyte cultures (Fig. 5A and B) showed that the proportions of stained and unstained areas as well as the overall intensity of staining were not significantly different between wild-type or annexin A5-deficient chondrocytes. Additionally, the morphology of calcified cell clusters from annexin A5-deficient chondrocytes did not differ from that of wild-type clusters when analyzed at a higher magnification. Therefore, in vitro calcification of chondrocytes was obviously not siginficantly impaired by the ablation of annexin A5 expression.
FIG. 5.
In vitro calcification of isolated chondrocytes from wild-type (A, C, E, and G) and annexin A5-deficient (B, D, F, and H) mice. (A and B) Chondrocytes were induced for 8 days in medium supplemented with 10 mM glycerophosphate, 10 mM CaCl2, and 50 μg of ascorbate/ml; stained for mineral deposits with 0.5% alizarin red; and cleared with 2% potassium hydroxide. (C and D) Higher magnifications of panels A and B, respectively, are shown. Bar, 250 μm. (E and F) Single cell clusters at higher magnifications of panels C and D, respectively, are shown. Bar, 50 μm. (G and H) Chondrocytes cultured in medium without induction are shown.
DISCUSSION
Annexin A5 represents one of the most intensely studied members of the annexin family by in vitro methods. Nevertheless, the in vivo functions of annexin A5 still remain unclear, similar to those of most other members of the family (23). Biochemical data obtained from in vitro cell culture experiments as well as in vivo expression studies suggested a potential function for annexin A5 in the development and growth of the skeleton (1, 15). It has been proposed that annexin A5 represents an important calcium channel in the matrix vesicles involved in the endochondral ossification of the growth plate (14, 15, 44). Accordingly, it has been assumed that annexin A5 mediates the Ca2+ uptake of matrix vesicles in hypertrophic chondrocytes stimulated by interaction with the extracellular matrix components collagen II and collagen X, leading to the calcification of cartilage within the growth plate.
Surprisingly, the data presented here clearly indicate that the presence of annexin A5 is not essential for cartilage development, endochondral ossification, or the formation of bone. Neither the formation nor the structure and function of the developing skeleton is affected in annexin A5-deficient newborns and in adult animals, as indicated by unchanged levels of calcium and alkaline phosphatase in the blood plasma, by X-ray studies of the skeleton, and by histological analysis of the growth plate. The obtained result was not expected due to the fact that the protein is detected at high levels in the bone and cartilage during embryonic development as well as during adulthood (1, 15, 35). A potential explanation relies on the redundant functions of annexins, which could functionally compensate for the annexin A5 deficiency. Indeed, two other annexins with selective calcium channel activity, annexins A6 and A2, are also found in matrix vesicles within the growth plate (3). It is conceivable that these annexins may be sufficient for the calcification process. This suggestion is further supported by the finding that the in vitro calcification of isolated chondrocytes is comparable between wild-type and annexin A5-deficient animals. However, there is no indication for a significant compensatory upregulation of annexin A2, A6, or A7 in different organs in mice lacking annexin A5. The normal level of annexin A6 especially was unexpected, as annexin A6 colocalizes with annexin A5 in some other tissues also, like the bile duct of the liver (39) or the heart (22). A comparable result was described previously for annexin A6-deficient mice, which also did not show any upregulation of other members of the annexin family, including annexin A5 (7).
Recently, an annexin A5-deficient mutant was established in the DT40 cell line and in these cells, a reduced susceptibility to various apoptotic stimuli was observed (6, 17). Although these data point to a role of annexin A5 in calcium-dependent apoptosis, the lack of annexin A5 may be largely tolerable in vivo. Yet we cannot exclude at the moment whether, under various stress conditions, specific phenotypes will become apparent.
It has been proposed that annexin A5 plays a role in pregnancy loss associated with antiphospholipid syndrome in humans (30). Annexin A5 should form an antithrombotic shield on the syncytium of the placental villi, and a disruption of this shield by autoimmune antibodies could induce placental thrombosis and intrauterine growth retardation. This is further supported by the finding that the injection of annexin A5-specific antibodies into pregnant mice leads to placental infarction and fetal losses (42). Therefore, we expected an impact of annexin A5 deficiency on the developing fetuses and/or litter size or deviations from Mendelian inheritance. No significant differences were seen upon heterozygous crossings. To exclude the possibility of a functional substitution of annexin A5 from maternal tissues, we also tested intercrosses of homozygous mutants (data not shown). Again, we could not detect a significant reduction in the litter size, but extended analyses of embryonic stages and litter sizes may be essential for a final conclusion. This finding may also be due to the fact that the placental structures in the mouse and human are organized differently; hence, the results of annexin A5-deficient mice most likely do not fully reflect the situation in humans (36). Additionally, there are controversial data about the role of annexin A5 in antiphospholipid syndrome. Some studies failed to detect anti-annexin A5 antibodies or did not find any correlation between annexin A5 displacement by antiphospholipid antibodies and increased thrombogenicity of the cell surface (18, 37).
In this paper, we describe the generation of an annexin A5-deficient mouse model by gene targeting and the analysis of its in vivo consequences. Our data imply that the ablation of annexin A5 has no significant pathological effect on vitality or fertility or on the formation of cartilage and bone, especially during endochondral ossification. Hence, the major questions for future studies will focus on the detailed analysis of subtle pathological consequences and on the analysis of potentially compensatory effects by other annexins.
Acknowledgments
The project was supported by Deutsche Forschungsgemeinschaft (Po 340/4).
Vector pNeoflox-8 was kindly provided by W. Müller, Cologne, Germany. Analysis of blood samples was kindly performed by M. Mohr and E. Wolf, Munich, Germany. We thank A. A. Noegel for providing us with the annexin A7 antibody.
REFERENCES
- 1.Brachvogel, B., H. Welzel, H. Moch, K. von der Mark, C. Hofmann, and E. Pöschl. 2001. Sequential expression of annexin A5 in the vasculature and skeletal elements during mouse development. Mech. Dev. 109:389-393. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty, M. J., S. West, R. L. Heimark, K. Fujikawa, and J. F. Tait. 1990. Placental anticoagulant protein-I: measurement in extracellular fluids and cells of the hemostatic system. J. Lab. Clin. Med. 115:174-181. [PubMed] [Google Scholar]
- 3.Genge, B. R., L. N. Wu, and R. E. Wuthier. 1989. Identification of phospholipid-dependent calcium-binding proteins as constituents of matrix vesicles. J. Biol. Chem. 264:10917-10921. [PubMed] [Google Scholar]
- 4.Gerke, V., and S. E. Moss. 1997. Annexins and membrane dynamics. Biochim. Biophys. Acta 1357:129-154. [DOI] [PubMed] [Google Scholar]
- 5.Grundmann, U., K. J. Abel, H. Bohn, H. Lobermann, F. Lottspeich, and H. Kupper. 1988. Characterization of cDNA encoding human placental anticoagulant protein (PP4): homology with the lipocortin family. Proc. Natl. Acad. Sci. USA 85:3708-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkins, T. E., D. Das, B. Young, and S. E. Moss. 2002. DT40 cells lacking the Ca2+-binding protein annexin 5 are resistant to Ca2+-dependent apoptosis. Proc. Natl. Acad. Sci. USA 99:8054-8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins, T. E., J. Roes, D. Rees, J. Monkhouse, and S. E. Moss. 1999. Immunological development and cardiovascular function are normal in annexin VI null mutant mice. Mol. Cell. Biol. 19:8028-8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan, B., R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 9.Huber, R., R. Berendes, A. Burger, M. Schneider, A. Karshikov, H. Luecke, J. Romisch, and E. Paques. 1992. Crystal and molecular structure of human annexin V after refinement. Implications for structure, membrane binding and ion channel formation of the annexin family of proteins. J. Mol. Biol. 223:683-704. [DOI] [PubMed] [Google Scholar]
- 10.Huber, R., J. Romisch, and E. P. Paques. 1990. The crystal and molecular structure of human annexin V, an anticoagulant protein that binds to calcium and membranes. EMBO J. 9:3867-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber, R., M. Schneider, I. Mayr, J. Romisch, and E. P. Paques. 1990. The calcium binding sites in human annexin V by crystal structure analysis at 2.0 Å resolution. Implications for membrane binding and calcium channel activity. FEBS Lett. 275:15-21. [DOI] [PubMed] [Google Scholar]
- 12.Kirsch, T., G. Harrison, E. E. Golub, and H. D. Nah. 2000. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J. Biol. Chem. 275:35577-35583. [DOI] [PubMed] [Google Scholar]
- 13.Kirsch, T., Y. Ishikawa, F. Mwale, and R. E. Wuthier. 1994. Roles of the nucleational core complex and collagens (types II and X) in calcification of growth plate cartilage matrix vesicles. J. Biol. Chem. 269:20103-20109. [Erratum, 269:25234.] [PubMed]
- 14.Kirsch, T., H. D. Nah, D. R. Demuth, G. Harrison, E. E. Golub, S. L. Adams, and M. Pacifici. 1997. Annexin V-mediated calcium flux across membranes is dependent on the lipid composition: implications for cartilage mineralization. Biochemistry 36:3359-3367. [DOI] [PubMed] [Google Scholar]
- 15.Kirsch, T., and R. E. Wuthier. 1994. Stimulation of calcification of growth plate cartilage matrix vesicles by binding to type II and X collagens. J. Biol. Chem. 269:11462-11469. [PubMed] [Google Scholar]
- 16.Koopman, G., C. P. Reutelingsperger, G. A. Kuijten, R. M. Keehnen, S. T. Pals, and M. H. van Oers. 1994. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415-1420. [PubMed] [Google Scholar]
- 17.Kubista, H., T. E. Hawkins, D. R. Patel, H. T. Haigler, and S. E. Moss. 1999. Annexin 5 mediates a peroxide-induced Ca(2+) influx in B cells. Curr. Biol. 9:1403-1406. [DOI] [PubMed] [Google Scholar]
- 18.Lakasing, L., J. S. Campa, R. Poston, M. A. Khamashta, and L. Poston. 1999. Normal expression of tissue factor, thrombomodulin, and annexin V in placentas from women with antiphospholipid syndrome. Am. J. Obstet. Gynecol. 181:180-189. [DOI] [PubMed] [Google Scholar]
- 19.Liemann, S., J. Benz, A. Burger, D. Voges, A. Hofmann, R. Huber, and P. Gottig. 1996. Structural and functional characterisation of the voltage sensor in the ion channel human annexin V. J. Mol. Biol. 258:555-561. [DOI] [PubMed] [Google Scholar]
- 20.Liemann, S., and R. Huber. 1997. Three-dimensional structure of annexins. Cell. Mol. Life Sci. 53:516-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liemann, S., and A. Lewit-Bentley. 1995. Annexins: a novel family of calcium- and membrane-binding proteins in search of a function. Structure 3:233-237. [DOI] [PubMed] [Google Scholar]
- 22.Matteo, R. G., and C. S. Moravec. 2000. Immunolocalization of annexins IV, V and VI in the failing and non-failing human heart. Cardiovasc. Res. 45:961-970. [DOI] [PubMed] [Google Scholar]
- 23.Mollenhauer, J. 1997. Annexins: what are they good for? Cell. Mol. Life Sci. 53:506-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollenhauer, J., and K. von der Mark. 1983. Isolation and characterization of a collagen-binding glycoprotein from chondrocyte membranes. EMBO J. 2:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan, R. O., and M. P. Fernandez. 1997. Annexin gene structures and molecular evolutionary genetics. Cell. Mol. Life Sci. 53:508-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan, R. O., and M. Pilar Fernandez. 1997. Distinct annexin subfamilies in plants and protists diverged prior to animal annexins and from a common ancestor. J. Mol. Evol. 44:178-188. [DOI] [PubMed] [Google Scholar]
- 27.Moss, S., H. C. Edwards, and M. J. Crumpton. 1991. Diversity in the annexin family, p. 535-566. In C. W. Heizmann (ed.), Novel calcium-binding proteins. Springer-Verlag KG, Berlin, Germany.
- 28.Neubauer, H., A. Cumano, M. Muller, H. Wu, U. Huffstadt, and K. Pfeffer. 1998. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93:397-409. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffle, M., F. Ruggiero, H. Hofmann, M. P. Fernandez, O. Selmin, Y. Yamada, R. Garrone, and K. von der Mark. 1988. Biosynthesis, secretion and extracellular localization of anchorin CII, a collagen-binding protein of the calpactin family. EMBO J. 7:2335-2342. [Erratum, 9:1336, 1990.] [DOI] [PMC free article] [PubMed]
- 30.Rand, J. H. 2000. Antiphospholipid antibody-mediated disruption of the annexin-V antithrombotic shield: a thrombogenic mechanism for the antiphospholipid syndrome. J. Autoimmun. 15:107-111. [DOI] [PubMed] [Google Scholar]
- 31.Rathkolb, B., T. Decker, E. Fuchs, D. Soewarto, C. Fella, S. Heffner, W. Pargent, R. Wanke, R. Balling, M. Hrabe de Angelis, H. J. Kolb, and E. Wolf. 2000. The clinical-chemical screen in the Munich ENU Mouse Mutagenesis Project: screening for clinically relevant phenotypes. Mamm. Genome 11:543-546. [DOI] [PubMed] [Google Scholar]
- 32.Reutelingsperger, C. P., and W. L. van Heerde. 1997. Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell. Mol. Life Sci. 53:527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ringelmann, B., C. Röder, R. Hallmann, M. A. Maley, M. J. Davies, M. D. Grounds, and L. M. Sorokin. 1999. Expression of laminin α1, α2, α4, and α5 chains, fibronectin, and tenascin-C in skeletal muscle of dystrophic 129ReJ Dy/Dy mice. Exp. Cell. Res. 246:165-182. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Garcia, M. I., R. O. Morgan, M. R. Fernandez, P. Bances, and M. P. Fernandez. 1999. Mouse annexin V genomic organization includes an endogenous retrovirus. Biochem. J. 337:125-131. [PMC free article] [PubMed] [Google Scholar]
- 35.Rojas, E., N. Arispe, H. T. Haigler, A. L. Burns, and H. B. Pollard. 1992. Identification of annexins as calcium channels in biological membranes. Bone Miner. 17:214-218. [DOI] [PubMed] [Google Scholar]
- 36.Rossant, J., and J. C. Cross. 2001. Placental development: lessons from mouse mutants. Nat. Rev. Genet. 2:538-548. [DOI] [PubMed] [Google Scholar]
- 37.Siaka, C., M. Lambert, C. Caron, J. Amiral, E. Hachulla, P. Y. Hatron, and J. Goudemand. 1999. Low prevalence of anti-annexin V antibodies in antiphospholipid syndrome with fetal loss. Rev. Med. Interne. 20:762-765. (In French.) [DOI] [PubMed]
- 38.Thomas, J. T., R. P. Boot-Handford, and M. E. Grant. 1990. Modulation of type X collagen gene expression by calcium beta-glycerophosphate and levamisole: implications for a possible role for type X collagen in endochondral bone formation. J. Cell Sci. 95:639-648. [DOI] [PubMed] [Google Scholar]
- 39.Thorin, B., G. Gache, T. Dubois, R. Grataroli, N. Domingo, F. Russo-Marie, and H. Lafont. 1995. Annexin VI is secreted in human bile. Biochem. Biophys. Res. Commun. 209:1039-1045. [DOI] [PubMed] [Google Scholar]
- 40.von der Mark, K., and J. Mollenhauer. 1997. Annexin V interactions with collagen. Cell. Mol. Life Sci. 53:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von der Mark, K., J. Mollenhauer, P. K. Muller, and M. Pfaffle. 1985. Anchorin CII, a type II collagen-binding glycoprotein from chondrocyte membranes. Ann. N. Y. Acad. Sci. 460:214-223. [DOI] [PubMed] [Google Scholar]
- 42.Wang, X., B. Campos, M. A. Kaetzel, and J. R. Dedman. 1999. Annexin V is critical in the maintenance of murine placental integrity. Am. J. Obstet. Gynecol. 180:1008-1016. [DOI] [PubMed] [Google Scholar]
- 43.Wurst, W., and A. L. Joyner. 1993. Production of targeted embryonic stem cell clones. IRL Press, Oxford University Press, Oxford, United Kingdom.
- 44.Wuthier, R. E., L. N. Wu, G. R. Sauer, B. R. Genge, T. Yoshimori, and Y. Ishikawa. 1992. Mechanism of matrix vesicle calcification: characterization of ion channels and the nucleational core of growth plate vesicles. Bone Miner. 17:290-295. [DOI] [PubMed] [Google Scholar]
- 45.Yoshizaki, H., S. Tanabe, K. Arai, A. Murakami, Y. Wada, M. Ohkuchi, Y. Hashimoto, and M. Maki. 1992. Effects of calphobindin II (annexin VI) on procoagulant and anticoagulant activities of cultured endothelial cells. Chem. Pharm. Bull. (Tokyo) 40:1860-1863. [DOI] [PubMed] [Google Scholar]