Abstract
Human cells are more resistant to both immortalization and malignant transformation than rodent cells. Recent studies have established the basic genetic requirements for the transformation of human cells, but much of this work relied on the expression of transforming proteins derived from DNA tumor viruses. We constructed an isogenic panel of human fibroblast cell lines using a combination of gene targeting and ectopic expression of dominantly acting mutants of cellular genes. Abolition of p21Cip1/Waf1 and p16Ink4a functions prevented oncogenically activated Ras from inducing growth arrest and was sufficient for limited anchorage-independent growth but not tumorigenesis. Deletion of the tumor suppressor p53 combined with abolition of p16Ink4a function failed to mimic the introduction of simian virus 40 large T antigen, indicating that large T antigen may target additional cellular functions. Ha-Ras and Myc cooperated only to a limited extent, but in the absence of Ras, Myc cooperated strongly with the simian virus 40 small t antigen to elicit aggressive anchorage-independent growth. The experiments reported here further define specific components of human transformation pathways.
A state of irreversible growth arrest, commonly referred to as replicative senescence, has been documented in many normal human cells after a period of rapid proliferation in cell culture (20, 68). Since the proliferative period seems to be limited by the number of elapsed cell divisions, rather than chronological time, and indefinite proliferation (referred to as immortalization) depends on the accumulation of genetic lesions, it has been proposed that the senescence response may have evolved as a defense against the development of malignancy (8, 11). Indeed, most tumor cells bear mutations in the p53 and/or Rb pathways, both of which have been implicated in the establishment of replicative senescence (3, 60).
A number of significant differences have been documented between human and rodent cells in the regulation of the senescence response. Many rodent cell types either express telomerase or can spontaneously activate telomerase after a relatively limited culture period (46). Rodent cells are also more susceptible to malignant transformation. For example, normal mouse embryo fibroblasts are easily transformed by the combined expression of an activated oncogene, such as Ha-RasG12V (referred to hereafter simply as Ras), and an immortalizing function, such as Myc (29), adenovirus E1a (53), simian virus 40 (SV40) large T antigen (LT) (38), or human papillomavirus E6 or E7 (32, 45). These viral proteins all have the ability to interfere with the normal functions of the cellular p53 and/or retinoblastoma (Rb) proteins. The importance of the p53 and Rb pathways in preventing tumor formation was further confirmed by mouse knockout studies, which showed that mouse embryo fibroblasts derived from p53−/− (22), p19 Arf−/− (24), or Rb/p107/p130−/− (55) animals could be transformed by activated Ras alone.
In contrast, both the senescence and transformation mechanisms are more stringently regulated in human cells (11, 56). The great majority of normal human cells do not express human telomerase (hTERT) activity (27), and immortalization is an extremely rare event. Likewise, Myc and Ras fail to transform primary human cells on their own (10, 11, 17, 81). More recent work has shown that Ras actually elicits a senescence-like arrest in both primary human and rodent cells (58). This somewhat unexpected finding can be viewed as yet another defense mechanism against inappropriate oncogenic signaling present in normal cells. In rodent cells, Ras-induced arrest can be eliminated by lesions in either the p53 or Rb pathways (58); however, in human cells, both pathways must be compromised (18, 44, 58, 75). Furthermore, bypassing Ras-induced arrest is not sufficient for full oncogenic transformation of human cells (18, 39, 44). Transformation of human foreskin fibroblasts, mammary epithelial cells, or keratinocytes has been shown to require the additional expression of SV40 small antigen (ST) (12, 18), which interferes with the function of protein phosphatase 2A (PP2A) (43, 80).
We have previously used gene targeting to knock out the p21 (6) and p53 (7) genes in normal, nonimmortalized human fibroblasts and used the resulting cell lines to study both replicative and induced senescence states. We presented data indicating that p53, p21, and Rb act sequentially and constitute the major pathway for establishing growth arrest in response to telomere attrition (75). p21 appears to be the major effector downstream of p53 responsible for both the establishment of replicative senescence (6) and p14ARF-induced premature senescence (75). In the studies reported here, we have expanded this genetic system by constructing additional isogenic cell lines to investigate the roles that p53, p21, and p16 play in premature senescence and transformation induced by oncogenic Ras. These experiments were stimulated by our desire to deduce clear-cut genetic functions for the steps required to convert a normal human cell into a malignant one. Most of the prior work in this area was predicated on the expression of viral transforming proteins, which are known to target multiple cellular proteins. We found that abolition of p21 and p16 functions was sufficient to bypass Ras-induced growth arrest and elicit limited anchorage-independent growth in response to Ras transformation. Loss of p53 gave the same results as loss of p21, confirming that p21 is a major p53 effector. Somewhat surprisingly, loss of p53 and p16 function did not mimic the introduction of LT in transformation elicited by the combination of Ras and ST, indicating that LT may target additional cellular function(s). In the presence of LT and ST, Myc cooperated to a limited extent with Ras to enhance anchorage-independent growth but did not further enhance in vivo tumorigenicity. Finally, in the absence of Ras, Myc cooperated with ST to elicit aggressive anchorage-independent growth, but the resulting cells did not form tumors in nude mice.
MATERIALS AND METHODS
Cell lines and culture conditions.
LF1 is a normal human fibroblast cell strain derived from embryonic lung tissue (6). The p21−/− and p53−/− derivatives of LF1 have been described previously, as have their hTERT-immortalized derivatives (6, 7, 75). hTERT was introduced in all cases using retrovirus vector infection followed by drug selection. When originally derived, LF1/TERT cells were grown for more than 100 doublings past their calculated senescence point and were shown to express telomerase activity at the beginning and end of the passaging regimen. p21−/− and p53−/− cells were immortalized close to the end of their natural proliferative life span; introduction of empty vector did not yield any colonies, and all derived clones expressed telomerase activity. All three cell lines have been subjected to multiple rounds of drug selections, and in no case was loss of immortalized phenotype seen during genetic selection or subsequent passaging. All LF1 derivatives were cultured in Ham's F-10 medium supplemented with 15% fetal bovine serum, glutamine, penicillin, and streptomycin. Cultures were incubated at 37°C in an atmosphere of 93% N2, 5% CO2, and 2% O2 (75, 76). The amphotropic Phoenix packaging cell line (70) was cultured at 37°C in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum in an atmosphere of 5% CO2 in air.
Retrovirus vectors.
Retrovirus vectors of the pBabe series (40) and the pWZL-blasticidin (WZL-blast) retrovirus vector were obtained from J. Morgenstern (Millenium Pharmaceuticals). The pBabe-mEYFP vector was constructed by substituting the drug resistance gene in pBabe-puro with the membrane-bound enhanced yellow fluorescence protein (mEYFP) cDNA (Clontech). Ha-RasG12V cDNA was obtained from S. Lowe (Cold Spring Harbor) and subcloned into pBabe-mEYFP. hTERT, LT, and ST cDNAs (18, 37) were obtained from R. Weinberg (Whitehead Institute for Biomedical Research). The hTERT cDNA was subcloned into pBabe-puro, and SV40 ST cDNA was subcloned into pBabe-bleo. The cDNA encoding a Cdk4R24C-cyclin D1 fusion protein (49) (DK) was obtained from R. N. Rao (Eli Lilly & Co.) and subcloned into the pWZL-blast, pBabe-puro, and pBabe-hygro vectors. Mouse c-Myc was tagged at the N terminus with a hemagglutinin (HA) tag and subcloned into the pWZL-blast and pBabe-bleo vectors. The amphotropic Phoenix packaging cell line (70) was obtained from G. Nolan (Stanford) and used according to the provided protocols (http://www.stanford.edu/group/nolan). The following drug concentrations were used for selection: hygromycin, 100 μg/ml; G418, 500 μg/ml; puromycin, 1 μg/ml; blasticidin, 3 μg/ml; and bleomycin, 500 μg/ml.
Immunoblotting.
Immunoblotting analysis was performed as described previously (74, 75). Anti-p21 antibody C-19 (sc-397), anti-p53 antibody FL-393 (sc-6243), anti-Cdk4 antibody C-22 (sc-260), anti-LT+ST antibody Pab 108 (sc-148) were from Santa Cruz. Anti-c-Myc antibody 06-340 was from Upstate Biotechnology. Anti-HA tag antibody (MMS-101P) was from Covance. Anti-Ras antibody Ab-3 (OP40) and anti-Mdm-2 antibody Ab-1 (OP46) were from Calbiochem.
Soft-agar growth and mouse tumorigenicity assays.
Soft-agar growth assays were performed as described previously (2). The infection efficiencies were determined prior to plating in soft agar by photographing random fields 48 h after infection under fluorescence and phase-contrast illumination and calculating the frequency of green cells as a percentage of total cells. At the time of plating in soft agar, cultures were trypsinized and counted, and 104 or 105 total cells were mixed with 1.5 ml of 0.4% Noble agar-DMEM (top layer) and then poured on top of 5 ml of solidified 0.8% Noble agar-DMEM (bottom layer) in 6-cm-diameter dishes. Cells were fed weekly by overlaying with 1.5 ml of fresh top layer solution. After 3 weeks, colonies were counted, and pictures were taken. Tumorigenicity assays were performed as described previously (17, 18) with minor modifications. A total of 3 × 106 cells were resuspended in 50 μl of phosphate-buffered saline, mixed with 50 μl of Matrigel solution, and immediately injected subcutaneously into nude mice. Each cell line was injected into four animals. Each animal was injected with Ras-infected cells in the right flank and with control cells infected with empty vector in the left flank. Female mice of the strain BALB/cAnNCrl-nuBR were obtained from Charles River at 8 weeks of age and injected within 1 week. Animals were not irradiated or otherwise treated.
Flow cytometry.
Exponentially growing cells were trypsinized, fixed in ethanol (70% final concentration), and stored at 4°C as described previously (35). Immediately before use, cells were stained with propidium iodide and analyzed in a Becton-Dickinson FACSCalibur instrument.
RESULTS
Construction of cell lines.
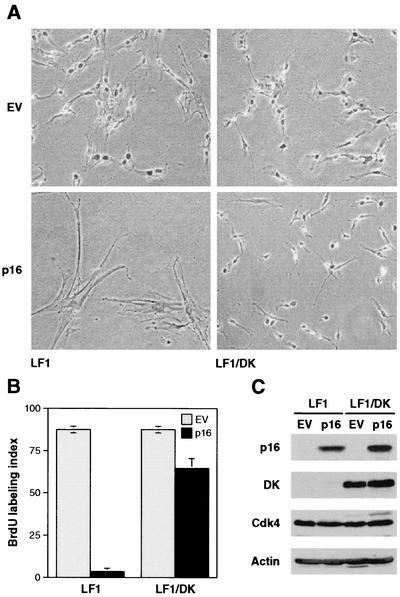
The starting objective of this study was to derive an isogenic set of cell lines in which to test the functional requirements for the p53 and Rb pathways (Fig. 1). The parental cell line was the LF1 lung fibroblast (6). Gene targeting was used to eliminate the function of the p21 and p53 genes (6, 7). To phenocopy loss of function of p16, we expressed the R24C mutant of Cdk4 which does not bind p16 and is thus insensitive to its inhibitory effects (78). This mutant has been frequently used to enforce p16-insensitive Cdk4 activity (18, 41, 48, 50, 58, 65) and is most effective when coexpressed with cyclin D. Since the scope of our genetic manipulations was limited by the relatively small number of dominant selectable markers, we chose to express a fusion protein between Cdk4R24C and cyclin D1 (DK) (28, 49). This strategy allows the stoichiometric expression of Cdk4R24C and cyclin D1 from a single retrovirus vector. Expression of DK in LF1 cells elicited a limited extension of life span (approximately five population doublings [data not shown]) at the end of which the cultures entered into a typical senescence (M1)-like state, very similar to the findings of Morris and coworkers (41) who used Cdk4R24C. Furthermore, LF1/DK cells were immune to the inhibitory effects of p16 expression (Fig. 2). These findings indicate that the expressed DK protein has biological activity, and that, as expected, it renders cells resistant to the inhibitory effects of elevated p16 expression.
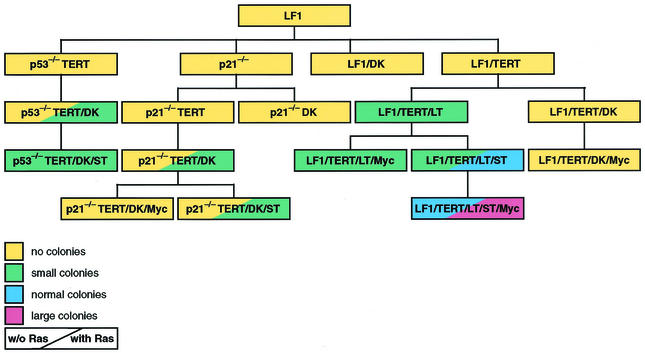
FIG. 1.
Schematic representation of cell line pedigrees and anchorage-independent growth phenotypes. Primary data for soft-agar colony formation are shown in Fig. 5 and Table 2. Details of cell line construction are shown in Table 1. Colony sizes were defined as follows (see also Fig. 5). Small colonies were multicellular aggregates estimated to contain 30 to 100 cells. These colonies were visible only microscopically and were estimated to be 0.1 mm or less in diameter. Normal colonies were macroscopically visible colonies estimated to be between 0.2 and 1.0 mm in diameter. Large colonies were macroscopically visible colonies estimated to be >1.0 mm in diameter.
FIG. 2.
DK-expressing cells are resistant to p16-induced premature senescence. (A) Photomicrographs of LF1 and LF1/DK cells infected with p16-expressing retrovirus. Cells were infected with pBabe-puro/Ras and pBabe-puro empty vector (EV) viruses. Pictures were taken 5 days after the start of puromycin selection. (B) Bromodeoxyuridine (BrdU) incorporation assays. Virus-infected and puromycin-selected cultures were labeled for 48 h with BrdU. Following immunohistochemical staining, total and BrdU-positive nuclei were counted in random fields. (C) Expression of p16 and DK proteins. Virus-infected and puromycin-selected cultures were harvested, and immunoblots were probed with the indicated antibodies. Cdk4 and DK proteins were both visualized by using an anti-Cdk4 antibody.
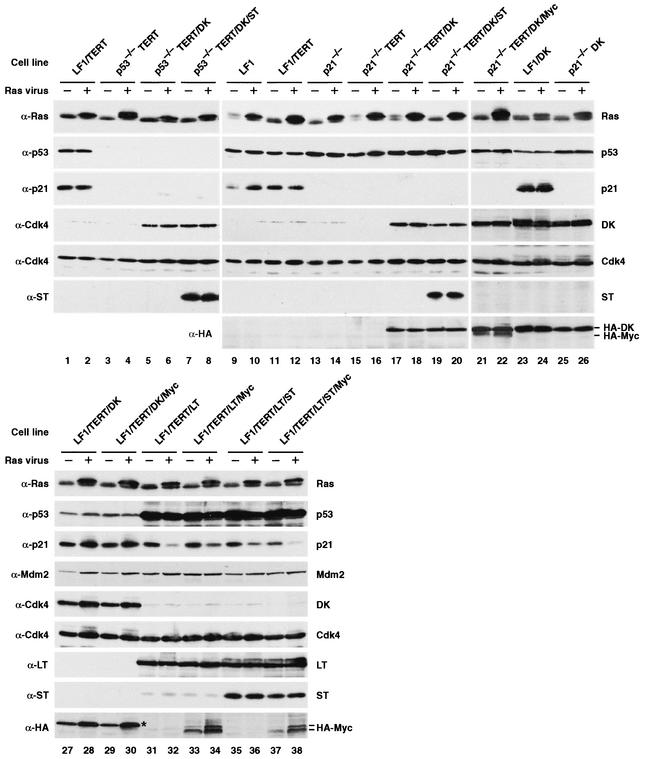
The pedigrees for all cell lines are shown in Fig. 1, and the retrovirus vectors used in their construction are summarized in Table 1. In all cases of immortalization with hTERT, cell lines were tested for telomerase activity using the telomere repeat amplification protocol assay (25, 74) and subsequently passaged extensively to verify the immortalized phenotype. Expression of the relevant proteins was demonstrated in all cell lines by immunoblotting (Fig. 3). Proliferation was measured during exponential growth phase using standard growth curves; the doubling times are shown in Table 1. The expression of DK in all cases accelerated proliferation. The expression of LT, ST, and Myc, in various combinations, also enhanced proliferation. The fastest growing cell line, LF1/TERT/LT/ST/Myc (doubling time of 20 h) was accelerated more than twofold relative to the LF1/TERT cell line (doubling time of 43 h) from which it was derived.
TABLE 1.
Construction and proliferation rates of cell lines
| Cell line | Construction of cell line | Doubling time (h)a |
|---|---|---|
| LF1 | No virus | 34b |
| LF1/TERT | LF1 plus pBabe-puro/hTERT (clonal) | 43 |
| LF1/TERT/DK | LF1/TERT plus pWZL-blast/DK (clonal) | 43 |
| LF1/TERT/DK/Myc | LF1/TERT/DK plus pBabe-bleo/Myc (pooled) | 43 |
| LF1/TERT/LT | LF1/TERT plus Neo-LT (pooled) | 43 |
| LF1/TERT/LT/Myc | LF1/TERT/LT plus pWZL-blast/Myc (pooled) | 25 |
| LF1/TERT/LT/ST | LF1/TERT/LT plus pBabe-hygro/ST (pooled) | 27 |
| LF1/TERT/LT/ST/Myc | LF1/TERT/LT/ST plus pWZL-blast/Myc (pooled) | 20 |
| p21−/− | No virus | 48c |
| p21−/−TERT | p21−/− plus pBabe-puro/hTERT (pooled) | 66 |
| p21−/−TERT/DK | p21−/−TERT plus pWZL-blast/DK (clonal) | 60 |
| p21−/−TERT/DK/ST | p21−/−TERT/DK plus pBabe-bleo/ST (pooled) | 60 |
| p21−/−TERT/DK/Myc | p21−/−TERT/DK plus pBabe-bleo/Myc (pooled) | 30 |
| p53−/−TERT | p53−/− plus pBabe-puro/hTERT (clonal) | 74 |
| p53−/−TERT/DK | p53−/−TERT plus pBabe-hygro/DK (pooled) | 43 |
| p53−/−TERT/DK/ST | p53−/−TERT/DK plus pBabe-bleo/ST (pooled) | 43 |
The variance associated with the doubling times was within 5 to 10% of the mean doubling time given for each cell line.
Growth of nonimmortalized LF1 cells was determined at mid passage.
Growth of nonimmortalized p21−/− cells was determined at late passage.
FIG. 3.
Immunoblot analysis of Ras-infected pools of cells. Exponentially growing cultures were infected with pBabe-mEYFP/Ras or empty pBabe-mEYFP vectors. Cultures were not subjected to selection with any drugs and were kept in the exponential growth phase by subculturing as needed. Six days after infection, four 10-cm-diameter dishes were harvested, pooled, and processed for immunoblotting. The cell line is indicated above each pair of lanes, and the virus used to infect the cells is indicated above each lane as follows: −, empty vector; +, Ras vector. The antibodies used (anti-Ras [α-Ras], etc.) are indicated to the left of the gels. The proteins detected are indicated to the right of the gels. DK, which has a HA tag, can be detected with both anti-Cdk4 and anti-HA antibodies. HA-tagged Myc (HA-Myc) was detected with anti-HA antibody and migrates as a doublet (lanes 33, 34, 37, and 38). The upper band of this doublet comigrates with the DK protein, which is also detected by the HA antibody (lanes 21 and 22). Note the relatively low expression of Myc in the LF1/TERT/DK/Myc cell line (lanes 29 and 30) (the position of the DK protein is marked by an asterisk), whereas the Myc protein is easily seen in the p21−/−TERT/DK/Myc cell line (lanes 21 and 22).
Ras does not induce growth arrest in p21−/−TERT/DK cells.
Overexpression of Ras in normal human fibroblasts results in a senescence-like state (58), and immortalization with hTERT does not abolish this response (74). Ras failed to induce premature senescence in cells in which both the p53 and Rb pathways are inactivated by E1a (58), E6 plus E7 (39), or LT (18). Ras was, however, able to induce premature senescence in human fibroblasts with disrupted p21 or p53 genes (75), as well as in human fibroblasts coexpressing the R24C mutant of Cdk4 and cyclin D1 (58). Furthermore, LT mutants unable to interact with either Rb or p53 were unable to protect human fibroblasts from Ras-induced arrest (18). In agreement, in our hands, LF1 fibroblasts expressing only the DK fusion protein displayed a clear premature senescence response after infection with a Ras-expressing retrovirus vector (data not shown).
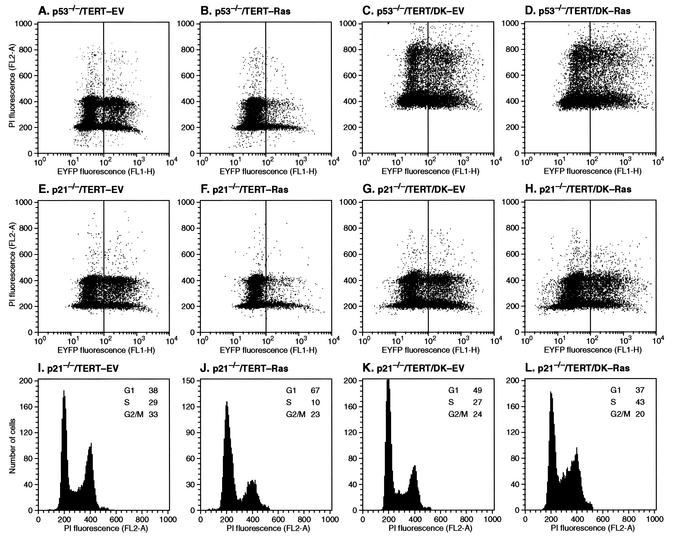
To investigate the effects of abolition of both p21 and p16 function, p21−/−TERT/DK cells were infected with the pBabe-mEYFP/Ras retrovirus vector (or empty vector control). Seven days after infection, the cultures were harvested, fixed, stained with propidium iodide, and analyzed by two-parameter flow cytometry for mEYFP expression and cell cycle distribution (Fig. 4). As expected, in p21−/−TERT cells not expressing DK, Ras reduced the fraction of mEYFP-positive cells (Fig. 4E and F), indicating that the cells were at a proliferative disadvantage. Furthermore, the mEYFP-positive cells displayed a cell cycle distribution indicative of growth arrest, namely, an increase in G0/G1 fractions and a decrease in S and G2 fractions (Fig. 4I and J). The approximately threefold decrease in S-phase content is especially noteworthy. In contrast, infection of p21−/−TERT/DK cells with the pBabe-mEYFP/Ras retrovirus vector did not elicit any signs of cell cycle arrest (Fig. 4G, H, K, and L); in fact, S-phase content was significantly increased in response to Ras (Fig. 4K and L). p53−/− cells behaved in a fashion similar to that of p21−/− cells: Ras elicited cell cycle arrest in the absence of DK (Fig. 4A and B) but not in the presence of DK (Fig. 4C and D). Unfortunately, the p53−/−TERT/DK cell line became tetraploid in the course of these experiments, which complicated the subsequent cell cycle analysis. Taken together, our results are in agreement with most previous studies indicating that interference with both p53 and pRb pathways is necessary to avoid Ras-induced growth arrest. Furthermore, we have pinpointed p21 as the critical downstream effector of p53 and have established the minimal sufficient intervention as the joint abolition of p21 and p16 function.
FIG. 4.
Ras expression does not inhibit the proliferation of p53−/−TERT/DK and p21−/−TERT/DK cells. The cell lines were infected and propagated as described in the legend to Fig. 3. Seven days after infection, cells were harvested, fixed with 70% ethanol, stained with propidium iodide (PI), and analyzed by two-parameter flow cytometry. (A to H) Dot plots of PI and mEYFP fluorescence (aggregates were gated out on the forward scatter/side scatter dot plot). (I to L) PI fluorescence histograms of green (high mEYFP fluorescence) cells from panels E to H. EV, empty virus (pBabe-mEYFP).
Losses of p21 and p16 function are the minimum requirements for anchorage-independent growth in response to Ras.
Having established the requirements to bypass the proliferative inhibition by Ras, we proceeded to investigate the minimum requirements to transform human fibroblasts to anchorage-independent growth. Since many of the cell lines are multiply marked with drug resistance markers and to achieve maximum consistency among the different cell lines, Ras was introduced using the pBabe-mEYFP retrovirus vector (empty pBabe-mEYFP vector was used as the control in all cases). Two days after infection, the cultures were observed under a fluorescence microscope to assess the efficiency of infection. Seven days after infection, the cultures were harvested by trypsinization and plated in soft agar. Aliquots were also processed for immunoblotting to ascertain the expression of the Ras protein, as well as all the other relevant proteins in each cell line (Fig. 3). Soft-agar plates were photographed at 3 weeks, and the incubation was continued, with regular feeding, for up to 5 weeks. Representative photomicrographs of the soft-agar plates are shown in Fig. 5, quantitative data are presented in Table 2, and the data are summarized in Fig. 1. Finally, all Ras-infected pools were injected into nude mice to determine in vivo tumorigenicity (Table 3).
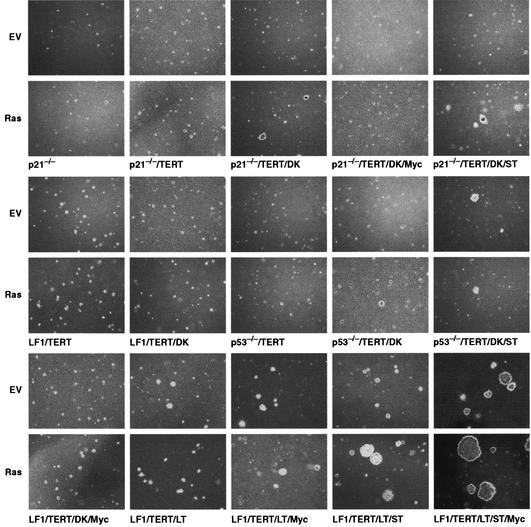
FIG. 5.
Soft-agar colony assays. The indicated cell lines were infected with pBabe-mEYFP/Ras virus (Ras) or empty vector virus (EV) (pBabe-mEYFP) and propagated as described in the legend to Fig. 3. Six days after infection, cells were harvested by trypsinization, and 104 or 105 cells were plated in soft agar as indicated in Materials and Methods. Photomicrographs were taken under phase-contrast illumination 21 days after plating. All photomicrographs are shown on the same scale to illustrate relative colony sizes. It should be noted that p21−/−TERT/DK/MYC cells appeared to disintegrate when placed in soft agar, rather than simply failed to proliferate.
TABLE 2.
Soft-agar plating efficiencies
| Cell line | Plating efficiencya
|
|||
|---|---|---|---|---|
| Empty EYFP vector
|
EYFP-Ras vector
|
|||
| Total coloniesb | Macro coloniesc | Total coloniesb | Macro coloniesc | |
| LF1/TERT | 0 | 0 | 0 | 0 |
| LF1/TERT/DK | 0 | 0 | 0 | 0 |
| LF1/TERT/DK/Myc | 0 | 0 | 0 | 0 |
| LF1/TERT/LT | 54 | 0 | 53 | 0 |
| LF1/TERT/LT/Myc | 53 | 0 | 57 | 0 |
| LF1/TERT/LT/ST | 60 | 0 | 97 | 56 |
| LF1/TERT/LT/ST/Myc | 70 | 62 | 100 | 70 |
| p21−/−TERT | 0 | 0 | 0 | 0 |
| p21−/−TERT/DK | 0 | 0 | 18 | 0 |
| p21−/−TERT/DK/ST | 0 | 0 | 20 | 0 |
| p21−/−TERT/DK/Myc | 0 | 0 | 0 | 0 |
| p53−/−TERT | 0 | 0 | 0 | 0 |
| p53−/−TERT/DK | 0 | 0 | 21 | 0 |
| p53−/−TERT/DK/ST | 12 | 0 | 24 | 0 |
All plating efficiencies have been adjusted for the infection efficiency of each culture with the EYFP and EYFP-Ras vectors. Infection efficiencies were in the range of 20 to 40%. Plating efficiencies are expressed as relative percentages (see footnotes b and c). Absolute plating efficiencies (total colonies per cells plated, adjusted for infection efficiency) were also determined and were in the range of 1 to 5%. For example, the absolute plating efficiencies of LF1/TERT/LT/ST cells infected with EYFP-Ras, LF1/TERT/LT/ST/Myc cells infected with EYFP, and LF1/TERT/LT/ST/Myc cells infected with EYFP-Ras3 were 5.4, 5.9, and 5.7%, respectively.
Plating efficiencies are expressed relative to the most efficient example, LF1/TERT/LT/ST/Myc cells infected with EYFP-Ras virus, which has been set at 100%. Total colonies are defined as all multicellular aggregates estimated to contain 30 to 100 cells and all macroscopic colonies. This category thus includes the small, normal, and large colony morphologies defined in the legend to Fig. 1 and depicted in Fig. 5.
Plating efficiencies are expressed as a percentage of the total colonies for any given cell-virus combination. Macroscopic (Macro) colonies are defined as all colonies of >0.2 mm in diameter. This category thus includes the normal and large colony morphologies defined in the legend to Fig. 1 and depicted in Fig. 5. Note that macroscopic colonies were observed for only the LF1/TERT/LT/ST cells infected with EYFP-Ras virus, LF1/TERT/LT/ST/Myc cells infected with empty EYFP virus, and LF1/TERT/LT/ST/Myc cells infected with EYFP-Ras virus.
TABLE 3.
Tumor formationa
| Cell line | Animal | Time at sacrifice (wk)/tumor diameter (cm) |
|---|---|---|
| LF1/TERT/LT/ST | 1 | 11/1.7 |
| 2 | 17/1.5 | |
| 3 | TFb | |
| 4 | TF | |
| LF1/TERT/LT/ST/Myc | 1 | 11/2.0, 7/2.0 |
| 2 | TF, 11/0.7 | |
| 3 | TF, TF | |
| 4 | TF | |
| BJELR | 1 | 11/2.13 |
| 2 | 11/1.70 | |
| 3 | 11/1.02 | |
| 4 | TF |
Each cell line was injected into four animals, and each animal was injected with Ras-infected cells in the right flank and with control cells infected with empty virus in the left flank. The tumor data presented in the table are for Ras-infected cells; cells infected with empty vector did not form tumors in any of the animals. The following cell lines were also injected but did not display any tumor formation for up to 6 months: p21−/−TERT, p21−/−/TERT/DK, p21−/−TERT/DK/Myc, p21−/−TERT/DK/ST, p53−/−TERT/DK, p53−/−TERT/DK/ST, LF1/TERT/DK, LF1/TERT/LT, LF1/TERT/DK/Myc, and LF1/TERT/LT/Myc. BJELR cells also exhibited tumors (two of three injected animals) in the NIH-bg-nu-xidBR mouse strain (Charles River) (data not shown). As controls, BJELT cells (18) were injected into the left flanks of animals receiving BJELR cells. BJELT cells did not form tumors in any of the animals. Two experiments were done with mice injected with the LF1/TERT/LT/ST/Myc cell line (three mice in the second experiment).
TF, tumor-free.
Cell lines LF1, LF1/DK, LF1/TERT, LF1/TERT/DK, p21−/−, p21−/−TERT, and p53−/−TERT did not form colonies in soft agar either with or without Ras infection, even after 5 weeks of incubation, consistent with the observation that the growth of all these cell lines is inhibited by Ras. p21−/−TERT/DK cells infected with Ras formed small but clearly delineated colonies after 3 weeks of incubation (control vector-infected cells did not form colonies up to 5 weeks). The colonies were densely packed with cells, and after picking expansion with glass capillaries and expanded, the cells could be replated in soft agar with similar plating efficiencies. p21−/−TERT/DK cells infected with Ras did not form tumors in nude mice up to 6 months after injection. Furthermore, a cell line established from a soft-agar colony that replated well in soft agar did not form tumors in vivo. Nonimmortalized p21−/−DK cells did not produce colonies in soft agar after infection with Ras; this failure is explained by the fact that due to the multiple genetic interventions, these cultures were near the end of their proliferative life span. p53−/−TERT/DK cells were not transformed by Ras to any greater extent than p21−/−TERT/DK cells, either in the soft-agar or nude mouse assays. Thus, by both assays, the loss of p21 or p53 produces very similar end points. We conclude that a minimum of two clearly delineated genetic alterations are required by Ras to elicit anchorage-independent growth: loss of p21 and p16 functions.
Loss of p53 and p16 function is not equivalent to expression of LT.
Since it has recently been demonstrated that ST is required in addition to LT for full transformation of human fibroblasts by Ras (18, 81), we introduced ST into p53−/−TERT/DK and p21−/−TERT/DK cells and repeated the Ras transformation assays. Surprisingly, although both the soft-agar plating efficiency and colony size were somewhat improved, transformation by Ras clearly did not reach the level of robustness elicited by the combination of LT and ST. Furthermore, expression of Ras in p53−/−TERT/DK/ST and p21−/−TERT/DK/ST cells did not result in tumor formation in nude mice, up to 6 months after injection into the animals (Table 3). Expression of ST allowed p53−/−TERT/DK but not p21−/−TERT/DK cells to form small colonies in soft agar even without Ras, but the introduction of Ras did not further enhance growth in soft agar.
A number of factors may have contributed to the observed small colony size in soft agar and lack of tumor formation in vivo by p53−/−TERT/DK/ST/Ras cells. First, the expression of Ras by the pBabe-mEYFP vector could be insufficient, despite the fact that very good expression (Fig. 3) was documented by immunoblotting the pools of cells at the time of plating or injection. Second, the LF1 strain of human lung fibroblasts could be more resistant to transformation than the BJ strain used in prior experiments (17, 18). Third, the conditions of soft-agar plating and especially the mouse tumorigenicity assays could be sufficiently different from those used previously. To address these issues, we performed a number of control experiments. First, TERT, LT, and ST retroviruses were introduced into LF1 fibroblasts to derive a series of cell lines equivalent to those previously constructed in the BJ fibroblast background (17, 18). The appropriate expression of LT and ST proteins was verified by immunoblotting (Fig. 3). Second, BJ fibroblasts expressing TERT, LT, ST, and Ras (BJELR) were used as controls in side-by-side soft-agar and nude mouse assays. LF1/TERT/LT/ST cells were transformed efficiently by the pBabe-mEYFP/Ras vector to anchorage-independent growth (Fig. 5), and the size range of the soft-agar colonies was comparable to that shown by BJELR cells (data not shown). Thus, it appears that the pBabe-mEYFP/Ras vector is competent to transform LF1/TERT fibroblasts if coexpressed with LT and ST. Furthermore, both LF1/TERT/LT/ST/Ras and BJELR cells produced in vivo tumors in our hands (Table 3). Therefore, the most reasonable explanation for the failure of Ras to fully transform p53−/−TERT/DK cells is that the expression of LT elicits (or abolishes) a cellular response that goes beyond the loss of p53 and p16 function.
Myc cooperates with ST to promote strong anchorage-independent growth but not in vivo transformation.
Since c-Myc has been well documented to collaborate with Ras in the transformation of rodent cells (29), it was of interest to introduce ectopic c-Myc expression into our isogenic panel of cell lines. In most cases, c-Myc was a strong growth-promoting agent, shortening exponential-phase doubling times by as much as twofold (Table 1). The one exception was the LF1/TERT/DK/Myc cell line; however, these cells expressed only low levels of Myc (Fig. 3, lanes 29 and 30), possibly due to the induction of apoptosis by high ectopic Myc expression. In contrast, p21−/−TERT/DK/Myc cells displayed easily demonstrable ectopic Myc expression (Fig. 3, lanes 21 and 22), significantly accelerated proliferation, and a distinct small and compact cell shape. However, when deprived of anchorage, p21−/−TERT/DK/Myc cells both in the absence and presence of Ras underwent apoptosis. As expected, expression of LT protected cells from Myc-induced apoptosis, and ectopic Myc expression significantly augmented the proliferation of LF1/TERT/LT cells, both in the presence (Table 1) and absence (Fig. 5) of anchorage. Surprisingly, Myc did not cooperate with Ras under these conditions, and LF1/TERT/LT/Myc cells formed only small colonies in soft agar both in the absence and presence of Ras (Fig. 5).
Perhaps the most surprising result was the promotion of strong anchorage-independent growth by the introduction of Myc into the LF1/TERT/LT/ST cell line (Fig. 5). This effect occurred in the absence of Ras and was thus the result of cooperation between Myc and ST. Exponential-phase proliferation rates were the highest of all cell lines in the panel (20 h [Table 1]), an increase of more than twofold relative to the parental LF1/TERT cell line (43 h). Both the plating efficiency and colony size in soft agar were significantly enhanced and equivalent to that elicited by Ras in LF1/TERT/LT/ST cells. However, in stark contrast to LF1/TERT/LT/ST/Ras cells, which were highly tumorigenic in vivo, LF1/TERT/LT/ST/Myc cells showed only negative results by the nude mouse assay (Table 3). Introduction of Ras into LF1/TERT/LT/ST/Myc cells further enhanced colony size in soft agar but did not increase the in vivo tumorigenicity beyond that seen with LF1/TERT/LT/ST/Ras cells.
DISCUSSION
It has been known for quite some time that human cells are much more resistant to both immortalization and malignant transformation than their rodent counterparts (56). However, other than sweeping generalizations that it takes more genetic lesions to fully transform a human cell, the mechanistic underpinnings of this observation have remained elusive. Only recently has complete transformation of normal human cells been achieved with defined genetic interventions (17, 18, 34, 51, 81), namely, the expression of hTERT, SV40 LT, SV40 ST, and Ras. However, since some of these interventions entailed the expression of DNA tumor virus oncoproteins, the corresponding list of equivalent cellular functions is still not completely understood. This is because the viral oncoproteins target multiple cellular effectors, some of which remain poorly defined or even unknown. We demonstrate here that a homozygous knockout of p21 combined with the expression of a p16-insensitive Cdk4-cyclin D1 fusion protein suffices to overcome the arrest induced by Ras. This finding is consistent with previous studies utilizing viral oncoproteins that demonstrated a need to interfere with both the p53 and Rb pathways in human cells (14, 18, 39, 58, 84) and furthermore establish the loss of p16 and p21 functions as the minimum necessary requirements.
A recent report (4) presented evidence that human fibroblasts deficient solely in p16 function are resistant to Ras-induced senescence. The cells in that study were derived from a patient with a homozygous 19-bp deletion in the second exon of the CDKN2A locus that affects the coding region of both the p16Ink4a and p19ARF proteins. Extensive evidence was presented by the researchers that p16 was completely inactive and that while Arf was expressed as a partially frameshifted protein, it retained full activity. Although this study at face value contradicts our finding of a requirement for the combined loss of p16 and p21, there are several intriguing parallels. Most importantly, in both cases, the introduction of Ras failed to arrest the cells, induced anchorage-independent growth with a small colony morphology in soft agar, and failed to produce tumors in nude mice. Furthermore, Brookes et al. (4) found no evidence for either activation of p53 or induction of p21 in response to Ras. This interesting finding, which is at odds with our results as well as those of several other groups (18, 30, 31, 58, 75, 84), nevertheless explains why the requirement to abolish p21 function was apparently absent in their experiments. The reasons why Ras can induce p21 in some experiments but not in other experiments are not clear and will require further study. Besides the usual differences in methodologies used by the different laboratories, the possibility that the frameshifted Arf protein expressed from the mutant CDKN2A locus possesses some degree of abnormal biological activity needs to be further evaluated. For example, Ras can affect p53 activity directly through Mdm2 (52), and the frameshifted Arf protein, through its ability to bind to Mdm2, may interact with this pathway.
Several lines of evidence indicate that the small soft-agar colonies produced by p53−/−TERT/DK/Ras and p21−/−TERT/DK/Ras cells represent a biologically significant phenotype. First, colonies were observed in repeated trials and did not emerge from cells infected with empty vector or from cells in which either p16 or p21 functions were singly abolished. Second, when colonies were recovered from the soft agar and expanded into cell lines, they retained ectopic Ras expression and replated in soft agar with similar plating efficiencies and colony morphology. Cells that did not express ectopic Ras could be recovered from the soft-agar plates but did not replate. These results indicate that the soft-agar colony formation requires Ras and is unlikely to depend on the acquirement of secondary mutations. The reasons for the slow growth in soft agar have not been explored in detail. One clear contributing factor is the slow growth of the parental p53−/−TERT/DK and p21−/−TERT/DK cells, which was not significantly accelerated by Ras. The fact that low oxygen conditions have been reported to promote soft-agar colony formation may explain, in part, why anchorage-independent growth of TERT-, LT-, and Ras-expressing cells was not previously documented. Thus, in addition to the study of Brookes et al. (4) discussed above, the data presented here are the first delineation of minimum genetic requirements in terms of defined cellular functions for anchorage-independent growth. We propose that anchorage-independent growth of a human fibroblast requires a minimum of three genetic events: activation of Ras and elimination of p16 and p21 functions. Whether activation of telomerase is required only for extension of life span or also has a component in the anchorage-independent phenotype itself (69, 81) is not addressed by our experiments, because the p21−/−DK cell line was close to the end of its replicative life span at the time of Ras transformation. It also should be kept in mind that our results with fibroblasts may not apply to other cell types, for example epithelial cells, which may have very different requirements for immortalization and transformation.
Surprisingly, we observed that Ras-expressing p53−/−TERT/DK/ST and p21−/−TERT/DK/ST cells formed only small colonies in soft agar. Since we have previously shown that coexpression of a dominantly acting mutant of p53 (p53DD), a Cdk4 mutant (Cdk4R24C), and cyclin D1 functionally replace LT in the transformation of human fibroblasts (18), these observations suggest that the genetic abolition of p53 and the expression of the p53DD mutant protein are not functionally equivalent. We make this suggestion because the coexpression of Cdk4R24C and cyclin D1 should be equivalent to the expression of the DK fusion protein (49). However, the p53DD mutant has known gain-of-function properties, indicating that some of these other functions may participate in cell transformation (73). For example, the p53DD protein not only binds wild-type p53, thus preventing the activation of p53-dependent transcriptional targets, but also interacts with p53 cellular partners such as Mdm2 and p300/CBP (59). The interaction of p53DD with wild-type p53 also serves to stabilize p53 against turnover and acts as a substrate for many kinases that act on p53 (21). In addition, since p53 forms complexes with the related proteins, p63 and p73, the p53DD mutant may perturb the functions of these proteins in addition to its effects on p53 (36, 72).
Moreover, LT has biological activities in addition to those that target cellular p53 and Rb proteins, and although the ability of LT to cooperate in the transformation of human cells does not appear to require its J domain (18), other effectors may also participate in transformation (66, 67, 71, 82, 83). For example, LT forms a trimeric complex with p53 and Mdm2 (1), and Mdm2 can promote growth arrest by p53-independent pathways (5, 9). Since Ras induces Mdm2 transcription through AP-1 and Ets sites in the Mdm2 promoter (52), the interaction of p53DD with Mdm2 may antagonize Ras-induced, Mdm2-dependent, p53-independent growth inhibition. In addition, LT, the adenoviral E1A, and p53DD proteins each interact with p53 and p300/CBP (1), and the interaction of E1A and p300/CBP was recently shown to play an important role in human fibroblasts transformed by E1A, Mdm2, and Ras (57). Since the genetic abolition of p53 severs this interaction, these observations suggest several plausible mechanisms that could explain why the p53−/−TERT/DK/ST cells described in this report fail to form large, anchorage-independent colonies. Moreover, since tumor-associated p53 mutants also exhibit gain-of-function properties (10), further work will be necessary to delineate the p53-related activities that conspire to transform human cells.
The ability of ST to promote tumor growth in conjunction with LT and Ras depends on its ability to bind PP2A (18, 43, 80). PP2A is a heterotrimeric serine/threonine phosphatase with numerous cellular targets and biological functions (23). Although PP2A is essential for viability (13, 15, 33), in a general sense its activity has antiproliferative effects (23). Its targets include (among many others) the mitogen-activated protein kinase pathway (63, 64), G1 cyclin-dependent kinases (79), p70 S6 protein kinase (77), mitochondrial Bcl2 (54), and Mdm2 (42). ST binds and interferes with PP2A function (43, 80), which results in the upregulation of mitogen-activated protein kinase pathway activity and Ras signaling (64). The specific targets of PP2A that are relevant to the ability of ST to promote tumor growth have not been identified. However, it is unlikely that this function will be limited to a simple augmentation of Ras signaling, especially in the case of ST and Myc cooperation. This is evident from the observation that on the LF1/TERT/LT background, Myc and Ras cooperated only weakly, whereas Myc and ST showed strong cooperation. It should be noted that in the present study, the cooperative effects between Myc and ST were observed in the context of LT-expressing cells. Although it is reasonable that the function of LT in these experiments was to antagonize the pro-apoptotic effects of Myc and/or to alleviate the growth arrest caused by Ras, we were unable to test these possibilities more directly because of a lack of suitable drug-resistant retrovirus vectors for further genetic manipulations.
The major focus of this study was to continue to resolve a minimum human fibroblast transformation pathway into single and well-defined cellular functions. Previous work has defined four distinct categories of functional requirements: acquisition of indefinite life span, expression of growth- and transformation-promoting functions, elimination of growth-inhibitory effectors, and escape from apoptotic surveillance mechanisms (16, 19). The cellular p53 and Rb pathways are almost universally compromised in human cancers (62) and have also been strongly implicated in bypassing replicative senescence (3, 60). The extent to which mutations in these pathways are necessary for immortalization in addition to the expression of telomerase has been controversial (26, 47, 50, 61). The very significant need to compromise the p53 and Rb pathways is in large part due to their function in surveillance mechanisms. We have shown here that loss of p21 and p16 function is sufficient to escape the surveillance of inappropriate oncogenic signaling, and thus allow Ras to elicit limited anchorage-independent growth. p53 mutations are much more frequent in human cancers than p21 mutations; at least one reasonable explanation is that loss of p53 provides additional escape from many apoptotic surveillance mechanisms, which allows the expression of growth-promoting functions such as Myc. We have also shown that full transformation and tumorigenesis require ST and possibly another yet undefined function that can be contributed by LT. The precise definition of this activity and the targets of PP2A that are the likely downstream effectors of ST are currently under investigation.
Acknowledgments
We gratefully acknowledge J. Morgenstern for the pWZL-blast retrovirus vector; S. Lowe for Ha-RasG12V cDNA; R. Weinberg for hTERT, LT, and ST cDNAs; R. N. Rao for the Cdk4R24C-cyclin D1 fusion protein; and G. Nolan for the amphotropic Phoenix cell line.
This work was supported in part by NIH grants R01AG16694 and R01GM41690 to J.M.S. and by NIH grant K01CA94223, a Kimmel Scholar Award, U.S. DOD grant DAMD17-01-1-0049, and a Doris Duke Clinical Scientist Development Award to W.C.H.
REFERENCES
- 1.Ali, S. H., and J. A. DeCaprio. 2001. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 11:15-23. [DOI] [PubMed] [Google Scholar]
- 2.Bazarov, A. V., S. Adachi, S. F. Li, M. K. Mateyak, S. Wei, and J. M. Sedivy. 2001. A modest reduction in c-myc expression has minimal effects on cell growth and apoptosis but dramatically reduces susceptibility to Ras and Raf transformation. Cancer Res. 61:1178-1186. [PubMed] [Google Scholar]
- 3.Bringold, F., and M. Serrano. 2000. Tumor suppressors and oncogenes in cellular senescence. Exp. Gerontol. 35:317-329. [DOI] [PubMed] [Google Scholar]
- 4.Brookes, S., J. Rowe, M. Ruas, S. Llanos, P. A. Clark, M. Lomax, M. C. James, R. Vatcheva, S. Bates, K. H. Vousden, D. Parry, N. Gruis, N. Smit, W. Bergman, and G. Peters. 2002. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 21:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, D. R., C. A. Thomas, and S. P. Deb. 1998. The human oncoprotein MDM2 arrests the cell cycle: elimination of its cell-cycle-inhibitory function induces tumorigenesis. EMBO J. 17:2513-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, J. P., W. Wei, and J. M. Sedivy. 1997. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 277:831-834. [DOI] [PubMed] [Google Scholar]
- 7.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 8.Campisi, J. 2000. Cancer, aging and cellular senescence. In Vivo 14:183-188. [PubMed] [Google Scholar]
- 9.Daujat, S., H. Neel, and J. Piette. 2001. MDM2: life without p53. Trends Genet. 17:459-464. [DOI] [PubMed] [Google Scholar]
- 10.Dittmer, D., S. Pati, G. Zambetti, S. Chu, A. K. Teresky, M. Moore, C. Finlay, and A. J. Levine. 1993. Gain of function mutations in p53. Nat. Genet. 4:42-46. [DOI] [PubMed] [Google Scholar]
- 11.Drayton, S., and G. Peters. 2002. Immortalisation and transformation revisited. Curr. Opin. Genet. Dev. 12:98-104. [DOI] [PubMed] [Google Scholar]
- 12.Elenbaas, B., L. Spirio, F. Koerner, M. D. Fleming, D. B. Zimonjic, J. L. Donaher, N. C. Popescu, W. C. Hahn, and R. A. Weinberg. 2001. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 15:50-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans, D. R., T. Myles, J. Hofsteenge, and B. A. Hemmings. 1999. Functional expression of human PP2Ac in yeast permits the identification of novel C-terminal and dominant-defective mutant forms. J. Biol. Chem. 274:24038-24046. [DOI] [PubMed] [Google Scholar]
- 14.Fanton, C. P., M. McMahon, and R. O. Pieper. 2001. Dual growth arrest pathways in astrocytes and astrocytic tumors in response to Raf-1 activation. J. Biol. Chem. 276:18871-18877. [DOI] [PubMed] [Google Scholar]
- 15.Gotz, J., A. Probst, E. Ehler, B. Hemmings, and W. Kues. 1998. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Cα. Proc. Natl. Acad. Sci. USA 95:12370-12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, D. R., and G. I. Evan. 2002. A matter of life and death. Cancer Cell 1:19-30. [DOI] [PubMed] [Google Scholar]
- 17.Hahn, W. C., C. M. Counter, A. S. Lundberg, R. L. Beijersbergen, M. W. Brooks, and R. A. Weinberg. 1999. Creation of human tumour cells with defined genetic elements. Nature 400:464-468. [DOI] [PubMed] [Google Scholar]
- 18.Hahn, W. C., S. K. Dessain, M. W. Brooks, J. E. King, B. Elenbaas, D. M. Sabatini, J. A. DeCaprio, and R. A. Weinberg. 2002. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 22:2111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 20.Hayflick, L., and P. S. Moorhead. 1961. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25:585-621. [DOI] [PubMed] [Google Scholar]
- 21.Hickman, E. S., M. C. Moroni, and K. Helin. 2002. The role of p53 and pRB in apoptosis and cancer. Curr. Opin. Genet. Dev. 12:60-66. [DOI] [PubMed] [Google Scholar]
- 22.Jacks, T. 1996. Lessons from the p53 mutant mouse. J. Cancer Res. Clin. Oncol. 122:319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353:417-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamijo, T., E. van de Kamp, M. J. Chong, F. Zindy, J. A. Diehl, C. J. Sherr, and P. J. McKinnon. 1999. Loss of the ARF tumor suppressor reverses premature replicative arrest but not radiation hypersensitivity arising from disabled atm function. Cancer Res. 59:2464-2469. [PubMed] [Google Scholar]
- 25.Kim, N. W., M. Platyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. C. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 26.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 27.Kolquist, K. A., L. W. Ellisen, C. M. Counter, M. Meyerson, L. K. Tan, R. A. Weinberg, D. A. Haber, and W. L. Gerald. 1998. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat. Genet. 19:182-186. [DOI] [PubMed] [Google Scholar]
- 28.Konstantinidis, A. K., R. Radhakrishnan, F. Gu, and W. K. Yeh. 1998. Purification, characterization, and kinetic mechanism of cyclin D1/Cdk4, a major target for cell cycle regulation. J. Biol. Chem. 273:26506-26515. [DOI] [PubMed] [Google Scholar]
- 29.Land, H., L. F. Parada, and R. A. Weinberg. 1983. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 304:596-602. [DOI] [PubMed] [Google Scholar]
- 30.Lee, A. C., B. E. Fenster, H. Ito, K. Takeda, N. S. Bae, T. Hirai, Z. X. Yu, V. J. Ferrans, B. H. Howard, and T. Finkel. 1999. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274:7936-7940. [DOI] [PubMed] [Google Scholar]
- 31.Lin, A. W., M. Barradas, J. C. Stone, L. V. Aelst, M. Serrano, and S. W. Lowe. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, Z., J. Ghai, R. S. Ostrow, R. C. McGlennen, and A. J. Faras. 1994. The E6 gene of human papillomavirus type 16 is sufficient for transformation of baby rat kidney cells in cotransfection with activated Ha-ras. Virology 201:388-396. [DOI] [PubMed] [Google Scholar]
- 33.Lizotte, D. L., D. D. McManus, H. R. Cohen, and A. DeLong. 1999. Functional expression of human and Arabidopsis protein phosphatase 2A in Saccharomyces cerevisiae and isolation of dominant-defective mutants. Gene 234:35-44. [DOI] [PubMed] [Google Scholar]
- 34.MacKenzie, K. L., S. Franco, A. J. Naiyer, C. May, M. Sadelain, S. Rafii, and M. A. S. Moore. 2002. Multiple stages of malignant transformation of human endothelial cells modelled by co-expression of telomerase reverse transcriptase, SV40 T antigen and oncogenic N-ras. Oncogene 21:4200-4211. [DOI] [PubMed] [Google Scholar]
- 35.Mateyak, M. K., A. J. Obaya, S. Adachi, and J. M. Sedivy. 1997. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 8:1039-1048. [PubMed] [Google Scholar]
- 36.Melino, G., V. De Laurenzi, and K. H. Vousden. 2002. p73: friend or foe in tumorigenesis. Nat. Rev. Cancer 2:605-615. [DOI] [PubMed] [Google Scholar]
- 37.Meyerson, M., C. M. Counter, E. N. Eaton, L. W. Ellisen, P. Steiner, S. D. Caddle, L. Ziaugra, R. L. Beijersbergen, M. J. Davidoff, Q. Liu, S. Bacchetti, D. A. Haber, and R. A. Weinberg. 1997. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90:785-795. [DOI] [PubMed] [Google Scholar]
- 38.Michalovitz, D., L. Fischer-Fantuzzi, C. Vesco, J. M. Pipas, and M. Oren. 1987. Activated Ha-ras can cooperate with defective simian virus 40 in the transformation of nonestablished rat embryo fibroblasts. J. Virol. 61:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales, C. P., S. E. Holt, M. Ouellette, K. J. Kaur, Y. Yan, K. S. Wilson, M. A. White, W. E. Wright, and J. W. Shay. 1999. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 21:115-118. [DOI] [PubMed] [Google Scholar]
- 40.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris, M., P. Hepburn, and D. Wynford-Thomas. 2002. Sequential extension of proliferative lifespan in human fibroblasts induced by over-expression of CDK4 or 6 and loss of p53 function. Oncogene 21:4277-4288. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto, K., H. Li, M. R. Jensen, T. Zhang, Y. Taya, S. S. Thorgeirsson, and C. Prives. 2002. Cyclin G recruits PP2A to dephosphorylate Mdm2. Mol. Cell 9:761-771. [DOI] [PubMed] [Google Scholar]
- 43.Pallas, D. C., L. K. Shahrik, B. L. Martin, S. Jaspers, T. B. Miller, D. L. Brautigan, and T. M. Roberts. 1990. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell 60:167-176. [DOI] [PubMed] [Google Scholar]
- 44.Peeper, D. S., J. H. Dannenberg, S. Douma, H. te Riele, and R. Bernards. 2001. Escape from premature senescence is not sufficient for oncogenic transformation by Ras. Nat. Cell Biol. 3:198-203. [DOI] [PubMed] [Google Scholar]
- 45.Phelps, W. C., C. L. Yee, K. Munger, and P. M. Howley. 1988. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell 53:539-547. [DOI] [PubMed] [Google Scholar]
- 46.Prowse, K. R., and C. W. Greider. 1995. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl. Acad. Sci. USA 92:4818-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramirez, R. D., C. P. Morales, B. S. Herbert, J. M. Rohde, C. Passons, J. W. Shay, and W. E. Wright. 2001. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rane, S. G., S. C. Cosenza, R. V. Mettus, and E. P. Reddy. 2002. Germ line transmission of the Cdk4R24C mutation facilitates tumorigenesis and escape from cellular senescence. Mol. Cell. Biol. 22:644-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao, R. N., N. B. Stamm, K. Otto, S. Kovacevic, S. A. Watkins, P. Rutherford, S. Lemke, K. Cocke, R. P. Beckmann, K. Houck, D. Johnson, and B. J. Skidmore. 1999. Conditional transformation of rat embryo fibroblast cells by a cyclin D1-cdk4 fusion gene. Oncogene 18:6343-6356. [DOI] [PubMed] [Google Scholar]
- 50.Rheinwald, J. G., W. C. Hahn, M. R. Ramsey, J. Y. Wu, Z. Guo, H. Tsao, M. De Luca, C. Catricala, and K. M. O'Toole. 2002. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell. Biol. 22:5157-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rich, J. N., C. Guo, R. E. McLendon, D. D. Bigner, X. F. Wang, and C. M. Counter. 2001. A genetically tractable model of human glioma formation. Cancer Res. 61:3556-3560. [PubMed] [Google Scholar]
- 52.Ries, S., C. Biederer, D. Woods, O. Shifman, S. Shirasawa, T. Sasazuki, M. McMahon, M. Oren, and F. McCormick. 2000. Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell 103:321-330. [DOI] [PubMed] [Google Scholar]
- 53.Ruley, H. E. 1983. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature 304:602-606. [DOI] [PubMed] [Google Scholar]
- 54.Ruvolo, P. P., X. Deng, T. Ito, B. K. Carr, and W. S. May. 1999. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J. Biol. Chem. 274:20296-20300. [DOI] [PubMed] [Google Scholar]
- 55.Sage, J., G. J. Mulligan, L. D. Attardi, A. Miller, S. Chen, B. Williams, E. Theodorou, and T. Jacks. 2000. Targeted disruption of the three Rb-related genes leads to loss of G1 control and immortalization. Genes Dev. 14:3037-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sager, R. 1984. Resistance of human cells to oncogenic transformation. Cancer Cells 2:487-493. [Google Scholar]
- 57.Seger, Y. R., M. Garcia-Cao, S. Piccini, C. L. Cunsolo, C. Doglioni, M. A. Blasco, G. J. Hannon, and R. Maestro. 2002. Transformation of normal human cells in the absence of telomerase activation. Cancer Cell 2:401-413. [DOI] [PubMed] [Google Scholar]
- 58.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 59.Shaulian, E., A. Zauberman, D. Ginsberg, and M. Oren. 1992. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol. Cell. Biol. 12:5581-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherr, C. J. 2000. Cancer cell cycles revisited. Cancer Res. 60:3689-3695. [PubMed] [Google Scholar]
- 61.Sherr, C. J., and R. A. DePinho. 2000. Cellular senescence: mitotic clock or culture shock? Cell 102:407-410. [DOI] [PubMed] [Google Scholar]
- 62.Sherr, C. J., and F. McCormick. 2002. The RB and p53 pathways in cancer. Cancer Cell 2:103-112. [DOI] [PubMed] [Google Scholar]
- 63.Silverstein, A. M., C. A. Barrow, A. J. Davis, and M. C. Mumby. 2002. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc. Natl. Acad. Sci. USA 99:4221-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sontag, E., S. Fedorov, C. Kamibayashi, D. Robbins, M. Cobb, and M. C. Mumby. 1993. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell 75:887-897. [DOI] [PubMed] [Google Scholar]
- 65.Sotillo, R., P. Dubus, J. Martin, E. de la Cueva, S. Ortega, M. Malumbra, and M. Barbacid. 2001. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. 20:6637-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Srinivasan, A., A. J. McClellan, J. Vartikar, I. Marks, P. Cantalupo, Y. Li, P. Whyte, K. Rundell, J. L. Brodsky, and J. M. Pipas. 1997. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol. Cell. Biol. 17:4761-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srinivasan, A., K. W. Peden, and J. M. Pipas. 1989. The large tumor antigen of simian virus 40 encodes at least two distinct transforming functions. J. Virol. 63:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stannulis-Praeger, B. 1987. Cellular senescence revisited: a review. Mech. Ageing Dev. 38:1-48. [DOI] [PubMed] [Google Scholar]
- 69.Stewart, S. A., W. C. Hahn, B. F. O'Connor, E. N. Banner, A. S. Lundberg, P. Modha, H. Mizuno, M. W. Brooks, M. Fleming, D. B. Zimonjic, N. C. Popescu, and R. A. Weinberg. 2002. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc. Natl. Acad. Sci. USA 99:12606-12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swift, S., J. Lorence, P. Achacoso, and G. P. Nolan. 1999. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. In R. Coico (ed.), Current protocols in immunology, vol. 2. John Wiley & Sons, New York, N.Y. [DOI] [PubMed]
- 71.Thompson, D. L., D. Kalderon, A. E. Smith, and M. J. Tevethia. 1990. Dissociation of Rb-binding and anchorage-independent growth from immortalization and tumorigenicity using SV40 mutants producing N-terminally truncated large T antigens. Virology 178:15-34. [DOI] [PubMed] [Google Scholar]
- 72.Urist, M., and C. Prives. 2002. p53 leans on its siblings. Cancer Cell 1:311-313. [DOI] [PubMed] [Google Scholar]
- 73.Vousden, K. H. 2002. Activation of the p53 tumor suppressor protein. Biochim. Biophys. Acta 1602:47-59. [DOI] [PubMed] [Google Scholar]
- 74.Wei, S., and J. M. Sedivy. 1999. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 59:1539-1543. [PubMed] [Google Scholar]
- 75.Wei, W., R. M. Hemmer, and J. M. Sedivy. 2001. Role of p14ARF in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 21:6748-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei, W., and J. M. Sedivy. 1999. Differentiation between senescence (M1) and crisis (M2) in human fibroblast cultures. Exp. Cell Res. 253:519-522. [DOI] [PubMed] [Google Scholar]
- 77.Westphal, R. S., R. L. Coffee, Jr., A. Marotta, S. L. Pelech, and B. E. Wadzinski. 1999. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J. Biol. Chem. 274:687-692. [DOI] [PubMed] [Google Scholar]
- 78.Wolfel, T., M. Hauer, J. Schneider, M. Serrano, E. Wolfel, E. Klehmann-Hieb, E. De Plaen, T. Hankeln, K. H. Meyer zum Buschenfelde, and D. Beach. 1995. A p16INK4a-insensitive CDK4 mutant targeted by cytotoxic T lymphocytes in a human melanoma. Science 269:1281-1284. [DOI] [PubMed] [Google Scholar]
- 79.Yan, Y., and M. C. Mumby. 1999. Distinct roles for PP1 and PP2A in phosphorylation of the retinoblastoma protein. J. Biol. Chem. 274:31917-31924. [DOI] [PubMed] [Google Scholar]
- 80.Yang, S. I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 11:1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu, J., A. Boyapati, and K. Rundell. 2001. Critical role for SV40 small-t antigen in human cell transformation. Virology 290:192-198. [DOI] [PubMed] [Google Scholar]
- 82.Zalvide, J., and J. A. DeCaprio. 1995. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol. Cell. Biol. 15:5800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu, J., P. W. Rice, L. Gorsch, M. Abate, and C. N. Cole. 1992. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J. Virol. 66:2780-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu, J., D. Woods, M. MacMahon, and J. M. Bishop. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12:2997-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]