Abstract
The general transcription factor TFIIE plays essential roles in both transcription initiation and the transition from initiation to elongation. Previously, we systematically deleted the structural motifs and characteristic sequences of the small subunit of human TFIIE (hTFIIEβ) to map its functional regions. Here we introduced point mutations into two regions located near the carboxy terminus of hTFIIEβ and identified the functionally essential amino acid residues that bind to RNA polymerase II (Pol II), the general transcription factors, and single-stranded DNA. Although most residues identified were essential for transcription initiation, use of an in vitro transcription assay with a linearized template revealed that several residues in the carboxy-terminal helix-loop region are crucially involved in the transition stage. Mutations in these residues also affected the ability of hTFIIEβ to stimulate TFIIH-mediated phosphorylation of the carboxy-terminal heptapeptide repeats of the largest subunit of Pol II. Furthermore, these mutations conspicuously augmented the binding of hTFIIEβ to the p44 subunit of TFIIH. The antibody study indicated that they thus altered the conformation of one side of TFIIH, consisting of p44, XPD, and Cdk-activating kinase subunits, that is essential for the transition stage. This is an important clue for elucidating the molecular mechanisms involved in the transition stage.
In eukaryotes, the expression of protein-coding genes is strictly regulated at the level of transcription by RNA polymerase II (Pol II). Once signals from outside the nucleus are received and the condensed form of the inactive chromatin is activated and remodeled by chromatin-modulating factors, five general transcription factors (TFIIB, TFIID, TFIIE, TFIIF, and TFIIH) together with Pol II form the preinitiation complex (PIC) on the core promoter. Formation of this complex is assisted by various transcriptional activators, cofactors, and mediators (for reviews, see references 19, 25, and 43). Analyses of the PIC assembly pathway using isolated general transcription factors have revealed that the factors can assemble stepwise in vitro. This process commences with the binding of TFIID to the TATA box on the core promoter and ends with TFIIE and TFIIH joining the PIC (reviewed in references 10, 26, 34, and 42). It is widely accepted that TFIIE and TFIIH stabilize and activate the PIC by binding to all the other general transcription factors as well as to Pol II and at the same time open up the double-stranded DNA (dsDNA) at the region from −9 to +2, adjacent to the transcription initiation site (+1), in a manner that is dependent on dATP hydrolysis (14, 56). This process is known as promoter melting. These various functions of TFIIE and TFIIH have been revealed recently by three types of studies. First, photo-cross-linking studies demonstrated that TFIIEβ binds directly to the core promoter region between positions −14 and −2, which is where the promoter melts upon transcription initiation (5, 41). Second, two-dimensional crystallography of yeast Saccharomyces cerevisiae TFIIE (yTFIIE) with Pol II demonstrated that yTFIIE binds to the active center of Pol II, which is located near the transcription initiation site on the promoter (20). Third, short mismatched heteroduplex DNA around the transcription initiation site in topologically relaxed linear templates was shown to eliminate the requirement for TFIIE, TFIIH, and ATP (6, 13, 38, 52). After promoter melting, initiation occurs through the action of nucleoside triphosphates. TFIIE and TFIIH both appear to play important roles in the subsequent transition from initiation to elongation, a process known as promoter clearance (or promoter escape) (6, 9, 17, 56). However, unlike the well-understood role of these general transcription factors in transcription initiation, their role in the transition stage is not exactly clear.
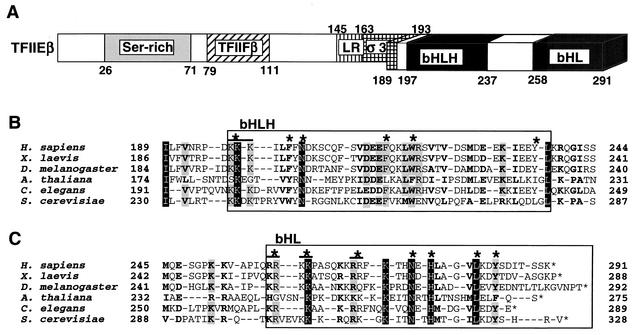
Human TFIIE (hTFIIE) consists of α and β subunits and forms an α2β2 heterotetramer (26, 31). The hTFIIEα subunit possesses 439 amino acid residues, and the region essential for basal transcription is located within the amino (N)-terminal half of the molecule, in which all of the structural motifs are located (28, 30). This N-terminal half shows good homology with the recently identified archaebacterial TFIIEα homologs, which do not possess a region corresponding to the carboxy (C)-terminal half of hTFIIEα (1, 11). The acidic region near the C terminus is the only region in the C-terminal half of the protein that binds directly to TFIIH and that has a stimulatory effect on basal transcription. The hTFIIEβ subunit is smaller (291 amino acid residues) (48). We recently mapped three functional regions on this subunit, namely, a central core (residues 66 to 146), a basic helix-loop-helix (bHLH) (residues 197 to 237), and a C-terminal basic helix-loop (bHL) (residues 258 to 291) by using deletion mutation studies (32, 33, 48) (Fig. 1A). The central core region was recently found to bind to dsDNA, and its three-dimensional structure was a winged-helix motif or, in other words, a forkhead motif (33). It was also found that TFIIEβ binds to single-stranded DNA (ssDNA) through the basic region of bHL. Two other general transcription factors, TFIIB and TFIIFβ (RAP30), also bind to this region (32).
FIG. 1.
Structural features of hTFIIEβ. (A) Schematic diagram of the structural motifs and characteristic sequences of hTFIIEβ. Included residues are shown. Ser-rich, serine-rich sequence; TFIIFβ, region similar to the dsDNA-binding region of TFIIFβ (RAP30); LR, leucine repeat motif; σ3, region similar to the bacterial σ factor subdomain 3; bHLH, bHLH motif; bHL, a bHLH motif-like sequence that lacks the second helix. The C-terminal region (residues 189 to 291) containing bHLH and bHL has been drawn stereographically. (B) Sequence alignment of the bHLH region of hTFIIEβ with that of TFIIβ from the following species (sources of sequence data are in parentheses): Homo sapiens (48), Xenopus laevis (27), Drosophila melanogaster (54), Arabidopsis thaliana (GenBank accession no. CAB79101), C. elegans (56), and S. cerevisiae (7). Sequences corresponding to the bHLH motif are boxed. Asterisks above sequences, mutated residues. A double mutant is indicated by overlining and an asterisk. (C) Sequence alignment of the bHL region of hTFIIEβ with those of other species. Sequences corresponding to the bHL region are boxed. Mutated residues and double mutants are indicated as in panel B. Completely identical residues are shaded in black, and residues identical in five species are shaded in gray. Conserved similar residues are in boldface. Identical and similar amino acids were identified as described previously (27, 48).
Human TFIIH consists of nine subunits and has three ATP-dependent catalytic activities, namely, a kinase activity that phosphorylates the C-terminal domain (CTD) of the largest subunit of Pol II, a DNA-dependent ATPase activity, and a DNA helicase activity (reviewed in reference 50). Recent three-dimensional structural analyses of human and yeast Saccharomyces cerevisiae TFIIH by electron microscopy have revealed that the six core subunits (XPB, XPD, p62, p52, p44, and p34) form a ring-like structure through which dsDNA may pass (2, 45). The other three subunits are Cdk-activating kinase (CAK) subunits (Cdk7, cyclin H, and MAT1) that form a spherical structure sitting on the ring through their binding to XPD (4, 45). The CTD of Pol II contains multiple repeats of the heptapeptide sequence YSPTSPS (52 in humans and 27 in S. cerevisiae) (57), and the CAK subcomplex, in the context of the holo-TFIIH complex, appears to act as the CTD kinase that phosphorylates these repeats, especially the serine residues at positions 2 and 5 (Ser-2 and Ser-5) (22, 55, 56).
A PIC constituted with purified general transcription factors preferentially recruits the unphosphorylated form of Pol II (Pol IIa) (21). CTD phosphorylation occurs after promoter melting, primarily between transcription initiation and the transition from initiation to elongation, and converts Pol IIa into the highly phosphorylated processive form (Pol IIo) (29, 56). During transcription initiation and the transition from initiation to elongation, TFIIE regulates the three enzymatic activities of TFIIH. In particular, experiments with a TFIIE homolog from the nematode Caenorhabditis elegans (ceTFIIE) showed that the TFIIE-mediated stimulation of Ser-5 phosphorylation by the CTD kinase activity of TFIIH is essential for the transition from initiation to elongation (56). It has recently also been demonstrated that this event is important for recruiting the mRNA processing enzymes to the nascent transcript, presumably reflecting the fact that mRNA processing (splicing, capping, and polyadenylation) occurs during and/or after transcription (reviewed in references 19, 24, 35, and 40).
Based on these observations, we further investigated the role that TFIIE plays in the mechanisms of the two sequential transcription steps, initiation and the transition from initiation to elongation. For this purpose, we introduced a series of point mutations into the C-terminal bHLH and bHL regions of hTFIIEβ (32) and expressed them in bacteria. We used in vitro binding assays to test the ability of these mutants to bind to Pol II, the general transcription factors, and ssDNA. We also examined the effect of these mutations on the phosphorylation of the CTD of the largest subunit of Pol II and on transcription with supercoiled or linearized templates. We found that most of the mutations affected the transcription initiation stage. However, we were also able to identify, for the first time, residues in TFIIE that are essential for its role in the transition stage. The functional mechanisms involved in the transition stage have been started to be extensively explored (8, 16, 36, 37), and thus our findings will greatly aid in the elucidation of these mechanisms.
MATERIALS AND METHODS
Construction of hTFIIEβ point mutants.
The plasmid p2EB contains the complete open reading frame of hTFIIEβ cDNA cloned into the pBluescript II SK(−) phagemid (Stratagene) (48). For subcloning the full-length hTFIIEβ cDNA into a bacterial expression vector, the oligonucleotide hEB1T (5′-AGGAGAGTGAGATCATATGGACCCAAGCCTGTTGAGAG-3′) was designed to create an NdeI site (underlined) at the first methionine codon of hTFIIEβ cDNA and to disrupt a BamHI site by changing the third nucleotide of the second aspartate codon (T to C; boldface and underlined). The oligonucleotide hEB1B (5′-AACTATTCGCCTGGATCCCTATTTGCTGAAGTAATGTC-3′) was designed to create a BamHI site right after the stop codon (underlined). PCR was performed with both oligonucleotides and the plasmid p2EB as a template. After three cycles of PCR with an annealing temperature of 37°C, another 30 cycles of PCR with an annealing temperature of 55°C were performed. The PCR products (approximately 900 bp) were purified, blunt ended with the Klenow fragment of Escherichia coli DNA polymerase I, phosphorylated with T4 polynucleotide kinase, and subcloned into the SmaI site of pBluescript SK(−) (Stratagene). The nucleotide sequences of the cloned PCR products were confirmed by using an ABI PRISM 310 genetic analyzer (Applied Biosystems). The NdeI-BamHI fragment of hTFIIEβ cDNA was then subcloned into the pET28a vector (Novagen) to construct a plasmid expressing hTFIIEβ with an N-terminal six-histidine (His) tag (6His-hTFIIEβ). The six-His-tagged coding region of TFIIEβ was digested with XbaI (which cuts upstream of the 5′ portion of the coding sequence for the six-His tag) and BamHI (which cuts at the 3′ end) and transferred into pET21b (Novagen) to utilize the ampicillin resistance conferred by pET21b for screening by site-directed mutagenesis. By using the site-directed mutagenesis kit Mutan-K (TaKaRa) with the 6His-hTFIIEβ pET21b expression plasmid as a template, various oligonucleotide-mediated point mutants were created (18). A restriction site was placed in each oligonucleotide to select for properly mutated plasmids as described elsewhere (28, 32), and the mutants were then checked by sequencing with an ABI PRISM 310 genetic analyzer (Applied Biosystems). (Due to the large number of oligonucleotides used, we have refrained from describing their exact sequences, but this information will be provided on request.)
Expression and purification of recombinant proteins.
Recombinant point mutant hTFIIEβ proteins were expressed in E. coli BL21(DE3)pLysS by induction with isopropyl-β-d-thiogalactopyranoside (IPTG) (47). For general purification, soluble bacterial lysates were used. For miniscale preparations, lysates (1 ml) representing 50 to 100 ml of culture were mixed directly with 400 μl of buffer B (20 mM Tris-HCl [pH 7.9 at 4°C], 0.5 mM EDTA, 10% [vol/vol] glycerol, 1 mM phenylmethylsulfonyl fluoride [PMSF], 2 μg of antipain/ml, 2 μg of aprotinin/ml, 1 μg of leupeptin/ml, 0.8 μg of pepstatin/ml, 10 mM 2-mercaptoethanol) containing 500 mM NaCl (BB500) and 100 μl of Ni-nitrilotriacetic acid-agarose resin (Qiagen) and incubated for 4 h at 4°C. The resin samples were washed twice with 1 ml of BB500, twice with 1 ml of buffer D (20 mM Tris-HCl [pH 7.9 at 4°C], 20% [vol/vol] glycerol, 1 mM PMSF, 10 mM 2-mercaptoethanol) containing 500 mM KCl (BD500), and twice with 500 μl of BD500 containing 20 mM imidazole-HCl (pH 7.9). Bound proteins were eluted twice with 300 μl of BD500 containing 100 mM imidazole-HCl (pH 7.9). Typical preparations were >80% pure, judging by Coomassie blue staining of a sodium dodecyl sulfate (SDS)-polyacrylamide gel. Large-scale purification of recombinant six-His-tagged human TATA-binding protein, TFIIB, TFIIF, and TFIIE has been described in detail elsewhere (23, 28, 32, 51).
To construct the glutathione S-transferase (GST)-tagged SII (TFIIS) expression vector, the NcoI-EcoRI fragment of the mouse SII cDNA clone (pSII-3) (12) was subcloned into pGEX-2TL(+), which was digested with the same restriction enzyme. Proteins made by fusing GST to general transcription factors and SII were expressed in E. coli BL21(DE3)pLysS by IPTG induction. Cells were harvested from 50 to 100 ml of culture, resuspended in 1 ml of BB500, and sonicated. Soluble lysates were separated from insoluble debris by ultracentrifugation at 20,000 × g in a 50.2 Ti rotor (Coulter-Beckman) and stored at −80°C until use.
Preparation of Pol II affinity column and Pol II binding assay.
Pol II was purified to near homogeneity from HeLa nuclear pellets by three ion-exchange columns, namely, DEAE-cellulose (DE52; Whatman), DEAE-Sephadex A-25 (Amersham Pharmacia Biotech), and phosphocellulose (P11; Whatman), followed by one high-performance liquid chromatography DEAE 5PW (Tosoh) column as described before (49). DE52-purified anti-CTD antibody 8WG16 (100 μg) and 200 μl (packed volume) of protein G-Sepharose 4 Fast Flow (Amersham Pharmacia Biotech) were incubated in 400 μl of buffer C (20 mM Tris-HCl [pH 7.9 at 4°C], 0.5 mM EDTA, 20% [vol/vol] glycerol, 0.5 mM PMSF, 10 mM 2-mercaptoethanol, 0.002% [vol/vol] Nonidet P-40) containing 100 mM KCl (BC100) and 200 μg of bovine serum albumin (BSA)/ml for 2 h at 4°C with rotation. The protein G-Sepharose beads were then precipitated and washed twice with 500 μl of buffer C containing 1 M KCl (BC1000) and twice with 500 μl of BC500. Purified Pol II (50 μg) equilibrated with BC500 was then incubated with the prepared anti-Pol II antibody-protein G beads in a 500-μl reaction volume for 4 h at 4°C with rotation. The Pol II-bound resin was washed twice with 500 μl of BC500 and twice with 500 μl of BC100 and resuspended with 1.8 ml of BC100. For Pol II binding assays with hTFIIEβ point mutants, 40 μl of the Pol II-bound resin suspension was incubated with 300 ng of each hTFIIEβ mutant overnight at 4°C with rotation. The resin was then washed twice with 500 μl of BC200 and once with 500 μl of BC100, and bound hTFIIEβ mutants were detected by Western blotting with anti-hTFIIEβ rabbit polyclonal antisera.
GST pull-down assay.
GST fusion proteins were used for protein interaction assays (32). Each protein to be tested (200 ng) was incubated for 4 h at 4°C with rotation with lysates containing 400 ng of GST fusion proteins together with 5 μl (packed volume) of glutathione-Sepharose (Amersham Pharmacia Biotech) in a 500-μl reaction volume with BC100 containing 200 μg of BSA/ml. The glutathione-Sepharose resin was washed twice with 500 μl of buffer C containing 200 mM KCl (BC200) and once with 500 μl of BC100 and then boiled in SDS sample buffer. The proteins released from the resin were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting with anti-hTFIIEβ rabbit antiserum (1:3,000 dilution) after transferring them to an Immobilon-P polyvinylidene difluoride membrane (Millipore) as described previously (28). Signals were detected with an enhanced chemiluminescence detection system (ECL kit; Amersham Pharmacia Biotech) and RX-U film (Fuji Film) after incubation of the immunoblots with horseradish peroxidase-linked secondary antibodies against rabbit or rat immunoglobulin G.
ssDNA binding assay.
His-tagged TFIIEβ point mutants (400 ng) were incubated with 5 μl (packed volume) of ssDNA-agarose (Invitrogen) in a 500-μl reaction volume of BC100 containing 200 μg of BSA/ml for 4 h at 4°C with rotation. The ssDNA-agarose resin was washed twice with 500 μl of buffer C containing 250 mM KCl (BC250) and once with 500 μl of BC100, boiled in SDS sample buffer, and analyzed by SDS-PAGE (12% acrylamide). Bound mutants were detected by Western blotting with anti-hTFIIEβ rabbit polyclonal antisera as described above.
In vitro transcription assay.
TFIIH was purified either from HeLa nuclear extracts or from cytoplasmic S100 fractions as previously described (29). Other recombinant general transcription factors as well as native Pol II were purified as described above. In vitro transcription was carried out as described previously (28). The plasmid pML(C2AT)Δ-50, which contains the adenovirus type 2 major late (AdML) promoter and which gives a 390-nucleotide (nt) transcript, was used as either a supercoiled or a linearized template for basal-transcription assays (56). To prepare the linearized template, the plasmid pML(C2AT)Δ-50 was digested with SmaI. Autoradiography was performed at −80°C with Fuji RX-U X-ray film. The incorporation of [α-32P]CTP into the transcripts was quantified with a Fuji BAS2500 Bio-Imaging analyzer.
Kinase assay.
In vitro kinase assays, carried out as described elsewhere (29), used the general transcription factors together with Pol II and a DNA fragment containing AdML promoter sequences from −39 to +29. Phosphorylation reactions were carried out at 30°C for 1 h and stopped by adding 75 μl of phosphorylation stop solution (10 mM EDTA, 0.1% NP-40, 0.05% SDS). Phosphorylated proteins were precipitated with trichloroacetic acid, analyzed by SDS-PAGE (5.5% acrylamide), and detected by autoradiography performed at −80°C with RX-U X-ray film (Fuji Film). The extent of 32P phosphorylation of the CTD of the largest subunit of Pol II was quantified with a Fuji BAS2500 Bio-Imaging analyzer.
RESULTS
Identification of residues in the bHLH and bHL regions of hTFIIEβ involved in binding to RNA polymerase II, general transcription factors, and ssDNA. Three regions on hTFIIEβ have been found to play essential roles in TFIIE functions, namely, the central core (residues 66 to 146), bHLH (residues 197 to 237), and bHL (residues 258 to 291) regions (32, 33). Molecular analysis of the central core region has been extensive and shows that it has dsDNA-binding activity and a winged-helix structure (33). The C-terminal regions bHLH and bHL have been much less well characterized, although it is known that these regions bind several transcription factors as well as ssDNA (32). In this study, we characterized these multivalent regions by introducing point mutations, primarily at the residues shown to be evolutionarily conserved among various species (Fig. 1), by site-directed mutagenesis using mutated oligonucleotides (17). Each mutant was expressed in bacteria, and the soluble fractions of the sonicated cell lysates were purified with Ni-nitrilotriacetic acid-agarose (Qiagen). The purity of these preparations is shown by SDS-PAGE (Fig. 2). All mutants were soluble after expression and could therefore be easily purified.
FIG. 2.
Purified hTFIIEβ proteins with point mutations in the bHLH and bHL regions. (A) SDS-PAGE of the purified hTFIIEβ proteins with point mutations in the bHLH region. Histidine-tagged point mutants were expressed in E. coli BL21(DE3)pLysS and purified, and 400 ng of each was subjected to SDS-PAGE (12% acrylamide) and stained with Coomassie blue. Lane 1, wild-type hTFIIEβ; lanes 2 to 13, mutant proteins. The sizes of the molecular weight markers are indicated on the right. Mutated residues are indicated at the top of each lane. (B) SDS-PAGE of the purified hTFIIEβ proteins with point mutations in the bHL region. Expression, purification, and SDS-PAGE were performed as described for panel A. Lane 1, wild-type hTFIIEβ; lanes 2 to 20, mutant proteins.
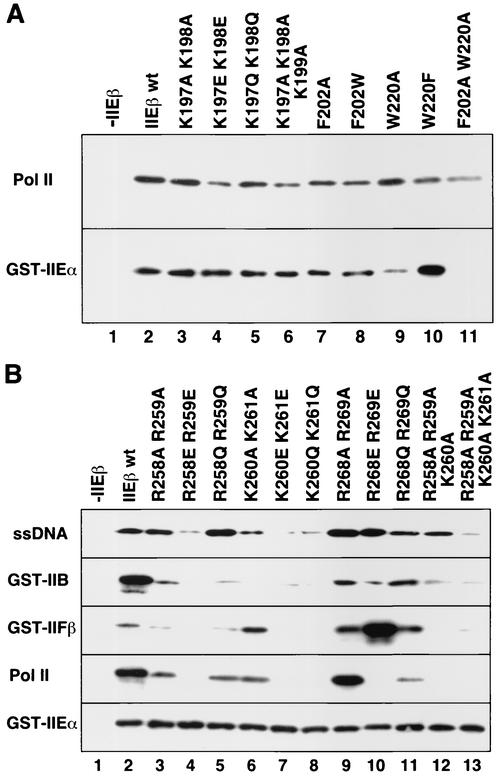
The ability of the bHLH and bHL mutants to bind to ssDNA, Pol II, and GST-tagged general transcription factors was examined by in vitro binding studies (Fig. 3). Mutations in the basic residues of both regions reduced binding to Pol II. In particular, Pol II binding of the bHL mutants was severely reduced when double mutations that changed the charge from positive to negative were introduced at the basic residues, that is, R258E R259E, K260E K261E, and R268E R269E (Fig. 3B, Pol II, lanes 4, 7, and 10, respectively). Pol II binding of the bHL mutants was also reduced when there were three or four consecutive replacements of the basic residues with alanine, that is, R258A R259A K260A and R258A R259A K260A K261A (Fig. 3B, Pol II, lanes 12 and 13, respectively). In contrast, point mutations in the basic residues of bHLH only weakly affected Pol II binding (Fig. 3A, Pol II, lanes 4 and 6, versus B, Pol II, lanes 4, 7, 10, 12, and 13). Furthermore, a weak reduction in Pol II binding was observed when the bHLH aromatic residues Phe-202 and Trp-220 were replaced (F202W, W220F, and F202A W220A; Fig. 3A, Pol II, lanes 8, 10, and 11, respectively). Thus, it appears that, while hTFIIEβ binds to Pol II via both bHLH and bHL regions, particularly strong binding occurs with the N-terminal basic residues of bHL, namely, Arg-258, Arg-259, Lys-260, and Lys-261.
FIG. 3.
Ability of the hTFIIEβ mutants to bind to ssDNA, general transcription factors, and Pol II. (A) Binding of hTFIIEβ proteins with point mutations in the bHLH region. Mutant hTFIIEβ proteins were mixed with Pol II (top) or GST-hTFIIEα (GST-IIEα; bottom) bound to protein G-Sepharose beads (Pol II) or glutathione-Sepharose resin (GST-IIEα). The mixtures were rotated for 4 h at 4°C, washed, and subjected to SDS-PAGE. Bound mutants were detected by Western blotting with anti-hTFIIEβ antisera. Lane 1, no hTFIIEβ was added (−IIEβ); lane 2, wild-type hTFIIEβ (IIEβ wt); lanes 3 to 11, point mutant proteins. Mutated residues are indicated at the top of each lane. (B) Binding of hTFIIEβ proteins with point mutations in the bHL region. Binding assays were performed as in panel A. ssDNA, GST-TFIIB (GST-IIB), GST-TFIIFβ (GST-IIFβ), Pol II, and GST-hTFIIEα (GST-IIEα) bound to agarose (ssDNA), glutathione-Sepharose (general transcription factors), or protein G-Sepharose beads (Pol II). Lane 1, no hTFIIEβ (−IIEβ); lane 2, wild-type hTFIIEβ; lanes 3 to 13, point mutant proteins. Mutated residues are indicated at the top of each lane.
The binding of hTFIIEβ to GST-hTFIIEα was predominantly affected by mutations involving the aromatic residue Trp-220 in the bHLH region (W220A and F202A W220A; Fig. 3A, GST-IIEα, lanes 9 and 11, respectively). Only a faint reduction in binding could be observed with the F202W mutant (Fig. 3A, GST-IIEα, lane 8), indicating the prominence of Trp-220 in this function. In contrast, mutations of the other aromatic residues in bHLH (F216Y, F216A, F216W, Y236F, and Y236A) did not affect hTFIIEβ binding to either Pol II or hTFIIEα (data not shown). In addition, none of the bHL mutations affected the binding of hTFIIEβ to hTFIIEα (Fig. 3B, GST-IIEα).
We next investigated the effect of the bHL point mutations on the binding of hTFIIEβ to TFIIB, TFIIFβ, and ssDNA. As the bHL mutations do not affect the binding of hTFIIEβ to hTFIIEα, GST-hTFIIEα was used as a positive control for the binding of hTFIIEβ to the other factors and ssDNA (Fig. 3B, GST-IIEα). The same bHL mutations that affected hTFIIEβ binding to Pol II also affected binding to GST-TFIIB (Fig. 3B, compare GST-IIB and Pol II). Similarly, mutations at the N-terminal basic residues of bHL (Arg-258, Arg-259, Lys-260, and Lys-261) also reduced the binding of hTFIIEβ to ssDNA, especially when double mutations changing the charge from basic to acidic were introduced (R258E R259E and K260E K261E; Fig. 3B, lanes 4 and 7, ssDNA versus Pol II). However, unlike what was found for Pol II binding, only a weak effect of the R268E R269E double mutation was observed (Fig. 3B, lane 10, ssDNA versus Pol II). Thus, hTFIIEβ appears to bind to ssDNA with its N-terminal half of the bHL basic region, namely, at Arg-258, Arg-259, Lys-260, and Lys-261. Furthermore, the ssDNA binding involves the Lys-260 and Lys-261 residues more strongly than the preceding Arg-258 and Arg-259 residues (Fig. 3B, ssDNA, lanes 6 to 8 versus lanes 3 to 5).
hTFIIEβ binding to GST-TFIIFβ was affected by the same bHL point mutations that greatly reduced its binding to ssDNA (R258E R259E and K260E K261E; Fig. 3B, ssDNA and GST-IIFβ, lanes 4 and 7, respectively). However, this pattern was opposite to the ssDNA binding pattern in that TFIIFβ binding involves the Arg-258 and Arg-259 residues more strongly than Lys-260 and Lys-261 (Fig. 3B, GST-IIFβ, lanes 3 to 5 versus lanes 6 to 8). In contrast, mutations at the C-terminal basic residues Arg-268 and Arg-269, especially the double mutation R268E R269E (Fig. 3B, ssDNA and GST-IIFβ, lane 10) actually increased hTFIIEβ binding to GST-TFIIFβ.
Our earlier studies examining the effects of deletions in hTFIIEβ on ssDNA, GST-TFIIB, and GST-TFIIFβ binding revealed that deletion of bHL abolished the ability of hTFIIEβ to bind to all of these substrates (32). Intriguingly, however, our point-mutational analyses in this study revealed that different regions in the bHL domain are involved in each of these functions. In other words, hTFIIEβ binds to ssDNA and TFIIFβ via the N-terminal half of the basic region of bHL, while binding to TFIIB and Pol II is mediated by the entire basic region of bHL. Our studies also showed that hTFIIEβ binding to hTFIIEα appears to involve only the aromatic residue Trp-220 in bHLH. Note that since other point mutations of the C-terminal bHL residues, Asn-274, His-276, Leu-281, and Tyr-284 (Fig. 2B, lanes 10 to 20) did not affect binding to Pol II, hTFIIEα, ssDNA, TFIIB, and TFIIFβ, the associated binding results are not presented in Fig. 3 (data not shown).
Aromatic residues in bHLH are important for transcription with a supercoiled template.
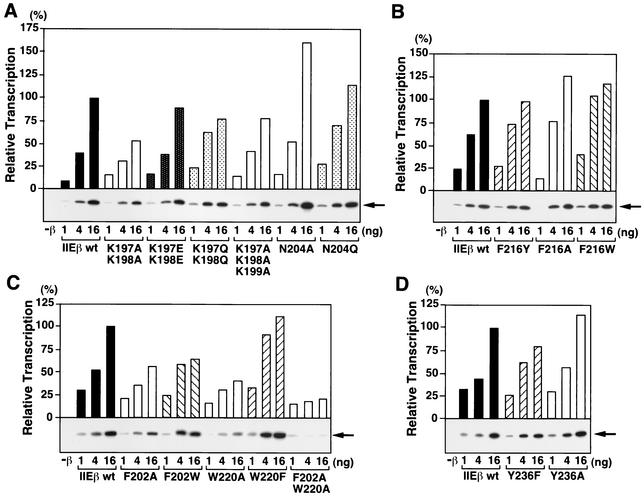
We assessed the effect of the bHLH point mutations on basal transcription with a supercoiled template containing the AdML promoter (Fig. 4). The mutations at the two aromatic residues Phe-202 and Trp-220 that affected hTFIIEβ binding to both Pol II and hTFIIEα (F202A, W202A, and F202A W202A; Fig. 3A, lanes 7, 8, and 11, respectively) also reduced the transcription activity of hTFIIEβ to approximately 20 to 60% of the wild-type activity (Fig. 4C). In clear contrast to this, mutation of the other two aromatic residues in bHLH (Phe-216 and Tyr-236) had almost no effect on transcription (Fig. 4B and D). Similarly, mutations at the basic residues in bHLH had little effect on transcription (Fig. 4A).
FIG. 4.
Effect of bHLH point mutations on the basal transcription activity of hTFIIEβ with a supercoiled template. In vitro transcription assays with a supercoiled template were carried out with increasing amounts (1, 4, and 16 ng) of wild-type hTFIIEβ (IIEβ wt) or hTFIIEβ proteins with point mutations in the bHLH region. After the transcription reaction, radiolabeled transcripts were subjected to urea-PAGE and detected by autoradiography (bottom of each graph). Each transcript was quantified by a Fuji BAS2500 Bio-Imaging analyzer. Relative transcription activities (bars) of the mutant hTFIIEβ proteins were calculated by defining the transcription activity of 16 ng of wild-type hTFIIEβ as 100%. Mutated residues are indicated at the bottom of each panel. As a control, transcription was carried out without the hTFIIEβ protein (−β). Arrows (right side of each panel), positions of the 390-nt transcripts.
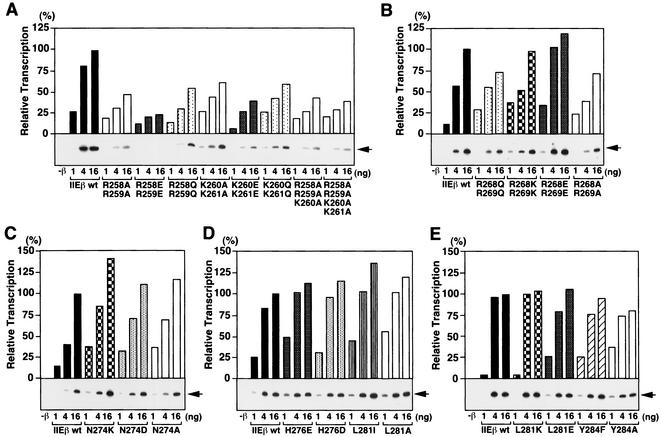
Basic residues in bHL are important for transcription with a supercoiled template.
The effect of the bHL point mutations on basal transcription with a supercoiled template was also tested (Fig. 5). The point mutations at basic residues Arg-258, Arg-259, Lys-260, and Lys-261 reduced transcription (Fig. 5A). In contrast, point mutations at the other basic residues in this basic region, namely, Arg-268 and Arg-269, did not affect transcription activity as much, although the double point mutations R268Q R269Q and R268A R269A slightly reduced the activity to around 70% of the wild type (Fig. 5B). Mutations at the C-terminal residues (Asn-274, His-276, Leu-281, and Tyr-284) did not affect transcription (Fig. 5C to E). Thus, the same bHL point mutations that affect hTFIIEβ binding to ssDNA, TFIIB, TFIIFβ, and Pol II also affect transcription with a supercoiled template.
FIG. 5.
Effect of bHL point mutations on the basal transcription activity of hTFIIEβ with a supercoiled template. In vitro transcription assays were performed as described for Fig. 4 with wild-type hTFIIEβ (IIEβ wt) or hTFIIEβ proteins with mutations in the bHL region. Arrows are as defined for Fig. 4.
Aromatic residues in bHLH are important for TFIIH-mediated CTD phosphorylation.
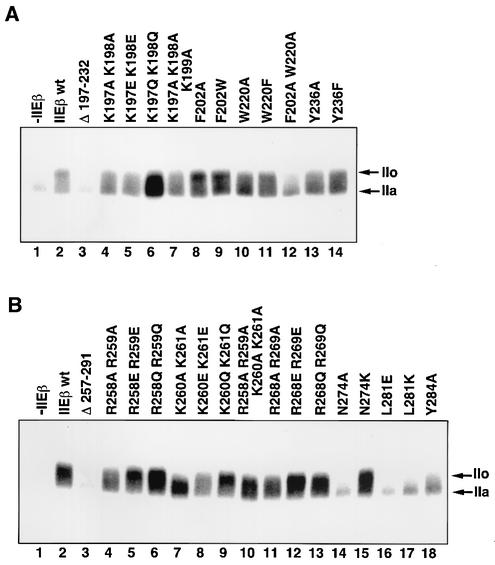
It has been demonstrated that phosphorylation of the CTD of the largest subunit of Pol II correlates with transcription activity and is essential for at least the transition stage (28, 32, 56). Thus, we examined the effect of the bHLH point mutations on CTD phosphorylation. Complete deletion of the bHLH region (residues 197 to 232) did not stimulate CTD phosphorylation (Fig. 6A, lane 3), but all bHLH point mutations stimulated it to some extent compared to the result in the absence of hTFIIEβ (Fig. 6A, lanes 4 to 14 versus lane 1). The F202A W220A double mutant showed a particularly severe defect although F202A and W220A single mutants showed weak defects (Fig. 6A, lane 12 versus lanes 8 and 10). In contrast, mutations of the aromatic residue Tyr-236 produced almost complete CTD phosphorylation to the hyperphosphorylated IIo form (Fig. 6A, lanes 13 and 14). Interestingly, mutations at the basic residues of bHLH also reduced CTD phosphorylation (Fig. 6A, lanes 4 to 7). These observations correlate well in general with the transcription results shown in Fig. 4.
FIG. 6.
Effects of bHLH and bHL point mutations on TFIIH-mediated CTD phosphorylation. (A) bHLH point mutations. Kinase assays were performed under the conditions of active initiation complex formation. Lane 1, no hTFIIEβ; lane 2, wild-type (wt) hTFIIEβ; lane 3, hTFIIEβ bHLH deletion mutant; lanes 4 to 14, hTFIIEβ with point mutations in the bHLH region. The mutated residues are indicated at the top of each lane. The phosphorylation of the largest subunit of Pol II was analyzed on a 5.5% acrylamide-SDS gel and detected by autoradiography. Arrows, phosphorylated form of the largest subunit of Pol II (IIo) and the unphosphorylated form (IIa). (B) bHL point mutations. Kinase assays were performed as described for panel A. Lane 1, no hTFIIEβ; lane 2, wild-type hTFIIEβ; lane 3, hTFIIEβ bHL deletion mutant; lanes 4 to 18, hTFIIEβ with point mutations in the bHL region. Arrows, IIo and IIa.
C-terminal conserved residues in addition to basic residues in bHL are important for TFIIH-mediated CTD phosphorylation.
Mutations in the basic bHL residues reduced CTD phosphorylation, especially the three mutations K260A K261A, K260E K261E, and R258A R259A K260A K261A (Fig. 6B, lanes 7, 8, and 10, respectively). These mutations coincide to some extent with those that affect transcription with a supercoiled template and the binding of hTFIIEβ to the other general transcription factors and ssDNA. However, there were some notable differences. For example, R258E R259E stimulated CTD phosphorylation perfectly well (Fig. 6B, lane 5) but reduced the transcription activity of hTFIIEβ to 20% of the wild-type activity (Fig. 5A). It also reduced hTFIIEβ binding to TFIIB, TFIIFβ, Pol II, and ssDNA (Fig. 3B, lane 4). Also surprising were the point mutations at Asn-274, Leu-281, and Tyr-284, which are conserved among all species from budding yeast to humans and which are located in the C-terminal helix-and-loop structure of bHL. The mutations N274A, L281E, L281K, and Y284A were almost completely unable to stimulate CTD phosphorylation (Fig. 6B, lanes 14 and 16 to 18, respectively). When we checked the effect of mutating another conserved residue adjacent to these residues, His-276, we found that the H276K and H276E mutants were also almost completely unable to stimulate CTD phosphorylation (data not shown). Despite this, these mutations did not affect transcription with a supercoiled template or binding to TFIIB, hTFIIEα, TFIIFβ, Pol II, and ssDNA (Fig. 5D and E and data not shown).
C-terminal conserved residues in bHL are also essential for the transcriptional transition stage.
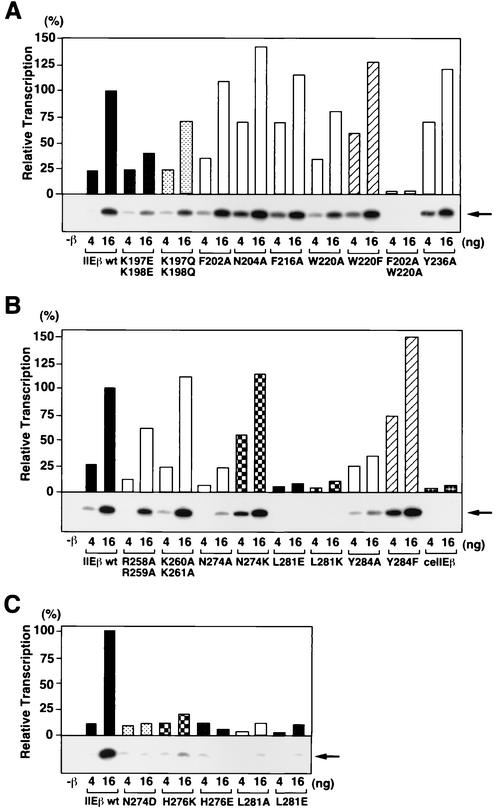
Since we observed that mutations in the bHL helix and loop affected CTD phosphorylation (Fig. 6B) but not other activities of hTFIIEβ, we asked whether these mutations affected transcription with a linearized template (Fig. 7). Our earlier studies had shown that, while transcription with a supercoiled template reflects the transcription initiation activity of the factors constituting the transcription system, transcription with a linearized template reflects the ability of these factors to support the transition from transcription initiation to elongation (56). We found that the bHL helix and loop mutants (N274A, L281E, L281K, and Y284A mutants) and their related mutants (N274D, L281A, and L281E mutants) as well as the mutants with substitutions at the conserved His-276 residue (H276K and H276E) all showed severe defects in supporting this transcription (5 to 35% of the wild-type activity) (Fig. 7B and C), despite the fact that these mutants all supported transcription with a supercoiled template with more than 80% of the wild-type activity (Fig. 5C to E). As expected, no defects were observed when these mutants were also tested in the abortive initiation assay (data not shown). Thus, these residues in the C-terminal helix and loop regions of bHL (Asn-274, His-276, Leu-281, and Tyr-284) might also be involved in the transition stage.
FIG. 7.
Effects of bHLH and bHL point mutations on basal transcription activity with a linearized template. (A) bHLH point mutations. In vitro transcription assays were performed as described for Fig. 4 except with a linearized template. Increasing amounts (4 and 16 ng) of wild-type hTFIIEβ (IIEβ wt) and hTFIIEβ proteins with mutations in the bHLH region were tested. After the transcription reaction, the radiolabeled transcripts were subjected to urea-PAGE and detected by autoradiography (below graph). The transcripts were quantified with a Fuji BAS2500 Bio-Imaging analyzer. Relative transcription activities (bars) of the mutant hTFIIEβ proteins were calculated by defining the transcription activity of 16 ng of wild-type hTFIIEβ as 100%. Mutated residues are indicated at the bottom. As a control, transcription was carried out without the hTFIIEβ protein (−β). Arrow (right), 390-nt transcripts. (B and C) bHL point mutations. Transcription was performed as described for panel A. Mutated residues are indicated at the bottom. As a control, transcription was carried out without the hTFIIEβ protein (−β). Arrows (right), 390-nt transcripts. ceTFIIEβ was also used as a negative control in panel B.
When we examined the effect of the point mutations in bHLH on transcription with a linearized template, we found that the F202A W220A mutant showed very low activity (2% of the wild type) (Fig. 7A). This mutant also showed low activity in transcription with a supercoiled template, but it was not as low as that with the linearized template (20% of the wild type) (Fig. 4C). This suggests that these residues may also be involved in both transcription initiation and the transition to elongation. In contrast, all the mutations at the basic residues in both bHLH and bHL regions that reduced transcription with a supercoiled template (around 20 to 60% of the wild type) (Fig. 4A and 5A and B) resulted in equivalent activities in transcription with a linearized template (Fig. 7A and B and data not shown). As an additional decrease in transcription with a linearized template was not observed, this suggests that these residues are involved mainly in transcription initiation.
Point mutations at C-terminal bHL residues involved in the transition stage augment the interaction between hTFIIEβ and the p44 subunit of TFIIH.
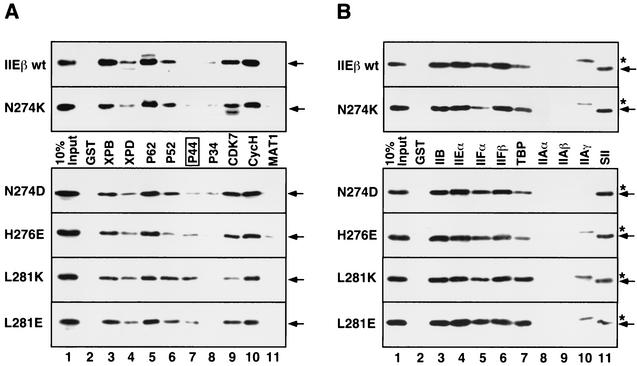
We next investigated why the mutations in the C-terminal bHL region affect the transition stage by first examining the effect of these mutations on hTFIIEβ binding to each subunit of all the general transcription factors, intact Pol II, and ssDNA (Fig. 8 and data not shown). The mutants defective in the transition stage had mutations that affected only the binding of hTFIIEβ to the TFIIH subunits (Fig. 8A). We then performed GST pull-down assays with the nine GST-tagged TFIIH subunits and used an anti-hTFIIEβ polyclonal antibody in Western blotting to detect which of the mutants bound to the TFIIH subunits. The top two sections of Fig. 8 show the binding of hTFIIEβ proteins (wild type and N274K mutant) that are capable of supporting transcription; the lower four sections of Fig. 8 shows the binding of the mutants that are severely defective in transcription with a linearized template but that are almost normal in transcription with a supercoiled template. All the hTFIIEβ proteins bound well to the TFIIH subunits except that the mutants defective in transcription with a linearized template bound three- to fivefold better to the p44 subunit than wild-type hTFIIEβ (Fig. 8A, lane 7). In contrast, none of the mutants differed in their abilities to bind to the GST-tagged subunits of other general transcription factors, to intact Pol II, or to ssDNA (Fig. 8B and data not shown). Since the general transcription elongation factor SII (TFIIS) was found to bind to wild-type hTFIIEβ via the C-terminal bHL region (Y. Ohkuma, unpublished data), we tested the effects of the mutations in the bHL C-terminal helix and loop on TFIIS binding (Fig. 8B). None of the mutations affected SII binding to hTFIIEβ (Fig. 8B, lane 11). Thus, it appears that p44 function may be affected by the mutations in the bHL C-terminal helix and loop. This is quite intriguing because p44 has recently been reported to play a role in promoter escape, which occurs at the transition stage (53). Furthermore, p44 acts together with XPD to tightly associate with CAK (3, 4, 46).
FIG. 8.
Effect of point mutations at the C-terminal residues of bHL on binding to the subunits of general transcription factors. (A) GST pull-down assays were performed with GST-tagged TFIIH subunits and hTFIIEβ proteins with point mutations in C-terminal bHL residues. Bound mutants were detected by Western blotting with anti-hTFIIEβ antisera. The upper two sections reveal the binding of the hTFIIEβ proteins that support transcription with a linearized template, namely, the wild-type hTFIIEβ (IIEβ wt) and the N274K mutant. The lower four sections show the binding of the four mutants (identified at the left) unable to support transcription with a linearized template. Arrows (right), hTFIIEβ proteins. The transcriptionally negative mutants showed augmented binding to p44 (lane 7, box). Lane 1, 10% input of each mutant; lane 2, GST alone instead of GST-tagged TFIIH subunits. (B) GST pull-down assays were performed with GST-tagged subunits of all the general transcription factors except for TFIIH and the TATA-binding protein (TBP)-associated factor subunits of TFIID. The assay was performed as described for Fig. 8A. GST-SII was also used as a positive control for this assay. Arrows (right), hTFIIEβ proteins; asterisks, contaminating proteins in the TFIIAγ fraction that are cross-reactive with the anti-hTFIIEβ antisera.
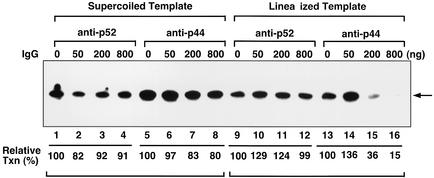
Transcription with a linearized template is specifically inhibited by an antibody against p44.
Since the point mutations at the C terminus of bHL that affect transcription with a linearized template also enhance the ability of hTFIIEβ to bind to p44, we assessed the effects of the antibodies against p44 and another TFIIH subunit, p52, on wild-type hTFIIEβ transcription with supercoiled and linearized templates (Fig. 9). We tested the antibodies against p44 (N-17) and p52 (C-19) (Santa Cruz Biotechnology). The antibody against p44 inhibited transcription with a linearized template (15% of wild-type activity) but not as much when the supercoiled template was used (80% of the wild-type activity) (Fig. 9, lanes 13 to 16 versus lanes 5 to 8). In contrast, the antibody against p52 did not affect transcription with either DNA template (Fig. 9, lanes 1 to 4, 9 to 12, and 17 to 20, and data not shown). Thus, p44 appears to regulate TFIIH function at the transition stage. It seems that the point mutations at the C terminus of bHL alter the conformation of TFIIEβ and that this enhances its binding to p44, which then inhibits TFIIH function at the transition stage. Given that these mutations also fail to support TFIIH-mediated CTD phosphorylation of the largest subunit of Pol II (Fig. 6B), it may be that the altered conformation resulting from these mutations also affects the steric positioning of XPD and CAK in the TFIIH complex.
FIG. 9.
Effects of antibodies on transcription with supercoiled and linearized templates. Increasing amounts of the anti-p52 antibody against the C terminus of p52 (C-19) or the anti-p44 antibody against the N terminus of p44 (N-17) (0, 50, 200, and 800 ng) were incubated with 20 ng of TFIIH for 4 h at 4°C. In vitro transcription assays with wild-type hTFIIEβ were then performed as described in the legends of Fig. 4, 5, and 7. Transcripts incorporating [α-32P]CTP were subjected to urea-PAGE and detected by autoradiography. The radioactivity of each transcript was quantified by a Fuji BAS2500 Bio-Imaging analyzer. Relative transcription activities were calculated by defining the activity in the absence of each antibody as 100% (lanes 1, 5, 9, and 14). Arrow, 390-nt transcripts. IgG, immunoglobulin G.
DISCUSSION
To determine when and how hTFIIE is involved in transcription initiation and the transition to elongation, in the paper presented here we determined which of the residues in the two C-terminal regions of hTFIIEβ, bHLH and bHL, are involved in binding to several factors as well as to ssDNA. We also assessed their effects on in vitro transcription with supercoiled and linearized templates. These observations are summarized in Fig. 10. To identify residues involved in transcription initiation events, we studied the binding of the hTFIIEβ point mutants to Pol II, ssDNA, TFIIB, hTFIIEα, and TFIIFβ and the ability of these mutants to support transcription with a supercoiled template. To identify residues participating in the transition stage, we examined the abilities of the point mutants to support the phosphorylation of the CTD of the largest subunit of Pol II and transcription with a linearized template. Two aromatic residues (Phe-202 and Trp-220) appeared to be strongly involved in binding to hTFIIEα and affected both transcription initiation and the transition to elongation (Fig. 3A and 4). In contrast, several N-terminal basic residues in bHL (Arg-258, Arg-259, Lys-260, and Lys-261) were much more strongly involved in binding to Pol II, ssDNA, TFIIB, and TFIIFβ, and mutations in these residues severely affected transcription initiation (Fig. 3B and 5). However, mutations of the C-terminal residues in bHL (Asn-274, His-276, Leu-281, and Tyr-284) markedly affected CTD phosphorylation and transcription with a linearized template although they did not affect transcription with a supercoiled template as much (Fig. 5 to 7), indicating their involvement in the transition stage. Mutations in the C-terminal bHL residues also resulted in an abnormally augmented binding of hTFIIEβ to the p44 subunit of TFIIH (Fig. 8). This result was supported by the antibody experiment to determine the effects on transcription with both linear and supercoiled templates. The antibody against p44 inhibited transcription with a linearized template but not that with a supercoiled template (Fig. 9). These observations strongly suggest that p44 is involved in the transition stage, which confirms observations made previously (46, 53).
FIG. 10.
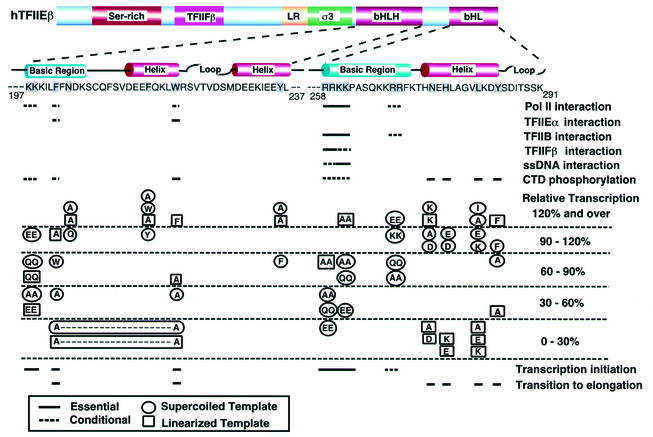
Summary of the effects of point mutations in bHLH and bHL on hTFIIEβ functions. The results of all our functional studies are summarized here. Mutated residues are shadowed. Solid lines, functionally essential residues; dashed lines, conditional residues. In the column of relative transcription activities, the circled mutated residues represent transcription with a supercoiled template, while the boxed mutated residues represent transcription with a linearized template.
The bHLH and bHL regions of hTFIIEβ may play distinct roles before and at transcription initiation.
As summarized in Fig. 10, we found that the binding of hTFIIEα to hTFIIEβ is mediated only by bHLH (Fig. 3). This binding involves the same two aromatic residues that may possibly be involved in both transcription initiation and the transition from initiation to elongation (Phe-202 and Trp-220). Pol II binds to both the bHLH and bHL regions, but the N-terminal basic residues in bHL (Arg-258, Arg-259, Lys-260, and Lys-261) are mainly involved in this binding. In contrast, TFIIB, TFIIFβ, and ssDNA bind only to bHL. The bHL residues involved in all binding events are all basic, but each substrate shows a slightly different binding specificity. That is, TFIIB binds primarily via the four basic residues (Arg-258, Arg-259, Lys-260, and Lys-261) but two additional basic residues (Arg-268 and Arg-269) are also involved, albeit less significantly. This pattern is similar to the Pol II binding pattern but differs slightly with regard to the R258Q R259Q and K260A K261A mutants. In contrast, TFIIFβ binds strongly via Arg-258 and Arg-259 and weakly via Lys-260 and Lys-261. ssDNA shows a pattern opposite to this in that it binds strongly via Lys-260 and Lys-261 and weakly via Arg-258 and Arg-259. That so many different components can apparently bind to the same region of bHL can be explained by the fact that TFIIE is an α2β2 heterotetramer complex and thus two hTFIIEβ subunits are available for simultaneous binding. Furthermore, TFIIE plays a role in several distinct stages of transcription, and thus rapid sequential binding of these various elements on bHL may occur.
The DNA-protein photo-cross-linking studies (5, 15, 41) have shown that the core promoter region wraps around Pol II and that this event is assisted by hTFIIEβ, TFIIFα (RAP74), and TFIIFβ (RAP30). Two hTFIIEβ subunits bind around the TATA box and the promoter melting (−10 to −2) and downstream promoter (+10 to +20) regions. These observations together with our functional analyses suggest that one TFIIEβ subunit orients its N terminus toward the upstream promoter region while the C-terminal basic region of its bHL region binds to TFIIB and TFIIFβ between the TATA box and the −10 region. Meanwhile, this TFIIEβ subunit uses its bHLH region to bind to hTFIIEα. The second hTFIIEβ orients its N terminus toward the downstream promoter region, and its bHLH and bHL regions both bind to Pol II near the transcription initiation site (+1). Since we now know that hTFIIEβ possesses a dsDNA-binding region in its central core domain (amino acid residues 66 to 146), which is located in the N-terminal part of the protein (33), we can also imagine that hTFIIEβ binds to the above-described three regions of the core promoter via its dsDNA-binding region. This conformation would change upon promoter melting and transcription initiation, resulting in the release of TFIIB and TFIIFβ by the hTFIIEβ subunit facing the downstream promoter region. This subunit then binds to the ssDNA region melted around −9 to +2. This would stabilize this region and allow Pol II to initiate efficient transcription. We believe that in contrast to that of bHL, the bHLH association with hTFIIEα and Pol II does not alter after transcription is initiated. Thus, we postulate that the dynamic changes at the bHL region are responsible for the change in the TFIIE function before and at transcription initiation. We are currently testing these hypotheses.
C-terminal bHL residues are involved in the transition from transcription initiation to elongation by binding to p44.
It is believed that transcription with a supercoiled template reflects transcription initiation activity while transcription with a linearized template reflects the activity to transit from transcription initiation to elongation (6, 9, 39, 53, 56). In our previous paper, we studied the activity of TFIIE in three in vitro transcription assays, namely, an assay of transcription with a supercoiled template, an abortive initiation assay examining the addition of one nucleotide at the initiation site, and an assay of transcription with a linearized template (56). We observed that the activities of TFIIE in transcription initiation and transcription with a supercoiled template correlated well but that additional TFIIE and TFIIH functions are needed to complete transcription with a linearized template. This was shown by the fact that, although the ceTFIIEβ subunit could partially support transcription with a supercoiled template when it replaced its human counterpart (30 to 50% of the wild-type hTFIIEβ activity), it could not complete transcription with a linearized template (less than 5% of wild-type activity). These observations support the notion that, once transcription is initiated at the initiation site of supercoiled templates, no additional requirements are needed to complete transcription, probably because the torsional stress from negative supercoiling is converted into the energy needed to open up the dsDNA, thereby allowing Pol II transcription to proceed (9, 39). In contrast, extension of initiated transcripts through the transcription transition stage (promoter escape) might require TFIIH helicase activity when linearized templates are used (3, 6, 17, 53). Thus, our observations as well as the data presented by Tremeau-Bravard and colleagues showing p44 involvement in the transcription transition stage (promoter escape) (53) clearly indicate that transcription with a supercoiled template reflects transcription initiation activity while transcription with a linearized template reflects the ability to transit from transcription initiation to elongation. On the basis of this, we found that several C-terminal residues in bHL (Asn-274, His-276, Leu-281, and Tyr-284) appear to participate in the transition stage since point mutations in them solely affect transcription with a linearized template (Fig. 5 and 7B and C). Mutations in these residues also affect the ability of hTFIIEβ to stimulate CTD phosphorylation (Fig. 6B and 7B and C). Furthermore, these mutations enhance the ability of hTFIIEβ to bind to p44 (Fig. 8A), which is known to be involved in the transcription transition stage. If this is the case, this would be the first time amino acid residues involved in the transition stage have been identified.
With regard to bHLH, mutations in the aromatic residues Phe-202 and Trp-220 affected transcription with a supercoiled template but reduced transcription with a linearized template even more, indicating that these residues may function in both transcription initiation and the transition stage. In contrast, the basic residues in both bHLH and bHL regions affected transcription with supercoiled and linear templates to equivalent extents, suggesting that they may function primarily in transcription initiation.
It has recently been reported that the p44 and XPD subunits of TFIIH play important roles in the transition stage by associating with each other and, for XPD, associating with the CAK subunits (3, 4, 46, 53). We found that mutating C-terminal bHL enhanced hTFIIEβ binding to p44 (Fig. 8A). Furthermore, an antibody against the N terminus of p44 inhibited transcription with a linearized template (Fig. 9, lanes 13 to 16). It is known that the N-terminal half of p44 binds directly to XPD and stimulates XPD helicase activity and is therefore essential for the transition stage (promoter escape) (4, 46). It appears that the core six subunits (XPB, XPD, p62, p52, p44, and p34) of TFIIH form a ring-like structure while the three CAK subunits (Cdk7, cyclin H, and MAT1) form a spherical structure. The spherical structure is located on the ring because of the association of CAK with XPD. It is likely that CAK also associates to some extent with p44 since TFIIH mutants from xeroderma pigmentosum patients failed to bind to both p44 and CAK (2, 3, 4, 45) and since the proper functioning of TFIIH at the transition stage requires that p44 and XPD adopt a particular conformation. This is supported by the observation that mutations in p44 or XPD abolish binding to each other and, for XPD mutants, to CAK (3, 4, 46). Recent interaction studies have shown that XPD interacts with CAK in the TFIIH complex via the MAT1 subunit (44). Thus, the TFIIH ring may have two sides with distinct functions. One side of the ring consists of XPD and p44 and is involved in the transition while the other side, containing XPB, plays a role in transcription initiation (44). It is possible that hTFIIEβ binds to the p44/XPD side of TFIIH and thereby induces the proper conformation of TFIIH, which is essential for the transition stage.
We did not observe any effect on Pol II binding of point mutations in the C-terminal conserved residues of bHL. Nevertheless, it is still possible that these mutations affect the interaction with the various individual subunits of Pol II. Therefore, we are currently studying which subunits of Pol II directly bind to TFIIEβ.
The transition stage has been far less extensively analyzed than transcription initiation. One reason for this is that the transition studies have tended to focus closely on TFIIH, Pol II, and DNA. However, as we show here, the mechanical steps involved in the transition stage will not be elucidated unless the roles of TFIIE are taken into account since TFIIE is also actively involved in this stage (6, 56, 58). Our observations here will thus be useful in helping us understand the molecular mechanisms involved in the transition from transcription initiation to elongation.
Acknowledgments
We thank Shunji Natori for providing the mouse SII cDNA clone pSII-3, Thomas Oelgeschläger, Sohail Malik, and Tetsuro Kokubo for critically reading the manuscript, Yoshinori Watanabe and Mitsuo Iwasaki for technical assistance, and Yoshifumi Nishimura, Masahiko Okuda, Katsuhiko Kamada, and our present and former laboratory members for helpful discussions.
This work was supported in part by grants from the Ministry of Education, Science and Culture of Japan (F.H. and Y.O.), the Japan Society for the Promotion of Science (Y.O.), the Biodesign Research Program of the Institute of Physical and Chemical Research (RIKEN) (F.H.), and the Core Research for Evolutional Science and Technology (F.H. and Y.O.).
REFERENCES
- 1.Bell, S. D., A. B. Brinkman, J. van der Oost, and S. P. Jackson. 2001. The archaeal TFIIEα homologue facilitates transcription initiation by enhancing TATA-box recognition. EMBO Rep. 2:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, W.-H., and R. D. Kornberg. 2000. Electron crystal structure of the transcription factor and DNA repair complex, core TFIIH. Cell 102:609-613. [DOI] [PubMed] [Google Scholar]
- 3.Coin, F., E. Bergmann, A. Tremeau-Bravard, and J.-M. Egly. 1999. Mutations in XPB and XPD helicases found in xeroderma pigmentosum patients impair the transcription function of TFIIH. EMBO J. 18:1357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coin, F., J.-C. Marinoni, C. Rodolfo, S. Fribourg, A. M. Pedrini, and J.-M. Egly. 1998. Mutations in the XPD helicase gene result in XP and TTD phenotypes, preventing interaction between XPD and the p44 subunit of TFIIH. Nat. Genet. 20:184-188. [DOI] [PubMed] [Google Scholar]
- 5.Douziech, M., F. Coin, J. M. Chipoulet, Y. Arai, Y. Ohkuma, J. M. Egly, and B. Coulombe. 2000. Mechanism of promoter melting by the xeroderma pigmentosum complementation group B helicase of transcription factor IIH revealed by protein-DNA photo-cross-linking. Mol. Cell. Biol. 20:8168-8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvir, A., R. C. Conaway, and J. W. Conaway. 1997. A role for TFIIH in controlling the activity of early RNA polymerase II elongation complexes. Proc. Natl. Acad. Sci. USA 94:9006-9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feaver, W. J., N. L. Henry, D. A. Bushnell, M. H. Sayre, J. H. Brickner, O. Gileadi, and R. D. Kornberg. 1994. Yeast TFIIE. Cloning, expression, and homology to vertebrate proteins. J. Biol. Chem. 269:27549-27553. [PubMed] [Google Scholar]
- 8.Fiedler, U., and H. T. M. Timmers. 2001. Analysis of the open region of RNA polymerase II transcription complex in the early phase of elongation. Nucleic Acids Res. 29:2706-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodrich, J. A., and R. Tjian. 1994. Transcription factors IIE and IIH and ATP hydrolysis direct promoter clearance by RNA polymerase II. Cell 77:145-156. [DOI] [PubMed] [Google Scholar]
- 10.Hampsey, M. 1998. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol. Mol. Biol. Rev. 62:465-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanzelka, B. L., T. J. Darcy, and J. N. Reeve. 2001. TFE, an archaeal transcription factor in Methanobacterium thermoautotrophicum related to eucaryal transcription factor TFIIEα. J. Bacteriol. 183:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirashima, S., H. Hirai, Y. Nakanishi, and S. Natori. 1988. Molecular cloning and characterization of cDNA for eukaryotic transcription factor S-II. J. Biol. Chem. 263:3858-3863. [PubMed] [Google Scholar]
- 13.Holstege, F. C. P., P. C. van der Vliet, and H. T. M. Timmers. 1996. Opening of an RNA polymerase II promoter occurs in two distinct steps and requires the basal transcription factors IIE and IIH. EMBO J. 15:1666-1677. [PMC free article] [PubMed] [Google Scholar]
- 14.Holstege, F. C. P., U. Fiedler, and H. T. M. Timmers. 1997. Three transitions in the RNA polymerase II transcription complex during initiation. EMBO J. 16:7468-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, T.-K., R. H. Ebright, and D. Reinberg. 2000. Mechanism of ATP-dependent promoter melting by transcription factor IIH. Science 288:1418-1421. [DOI] [PubMed] [Google Scholar]
- 16.Kugel, J. F., and J. A. Goodrich. 2002. Translocation after synthesis of a four-nucleotide RNA commits RNA polymerase II to promoter escape. Mol. Cell. Biol. 22:762-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar, K. P., S. Akoulitchev, and D. Reinberg. 1998. Promoter-proximal stalling results from the inability to recruit transcription factor IIH to the transcription complex and is a regulated event. Proc. Natl. Acad. Sci. USA 95:9767-9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunkel, T. A., J. D. Roberts, and R. A. Zakour. 1987. Rapid and efficient site-specific mutagenesis without phenotypic selections. Methods Enzymol. 154:367-382. [DOI] [PubMed] [Google Scholar]
- 19.Lee, T. I., and R. A. Young. 2000. Transcription of eukaryotic protein-coding genes. Annu. Rev. Genet. 34:77-137. [DOI] [PubMed] [Google Scholar]
- 20.Leuther, K. K., D. A. Bushnell, and R. D. Kornberg. 1996. Two-dimensional crystallography of TFIIB- and IIE-RNA polymerase II complexes: implications for start site selection and initiation complex formation. Cell 85:773-779. [DOI] [PubMed] [Google Scholar]
- 21.Lu, H., O. Flores, R. Weinmann, and D. Reinberg. 1991. The nonphosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc. Natl. Acad. Sci. USA 88:10004-10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, H., L. Zawel, L. Fisher, J.-M. Egly, and D. Reinberg. 1992. Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase II. Nature 358:641-645. [DOI] [PubMed] [Google Scholar]
- 23.Malik, S., K. Hisatake, H. Sumimoto, M. Horikoshi, and R. G. Roeder. 1991. Sequence of general transcription factor TFIIB and relationships to other initiation factors. Proc. Natl. Acad. Sci. USA 88:9553-9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maniatis, T., and R. Reed. 2002. An extensive network of coupling among gene expression machines. Nature 416:499-506. [DOI] [PubMed] [Google Scholar]
- 25.Näär, A. M., B. D. Lemon, and R. Tjian. 2001. Transcriptional coactivator complexes. Annu. Rev. Biochem. 70:475-501. [DOI] [PubMed] [Google Scholar]
- 26.Ohkuma, Y. 1997. Multiple functions of general transcription factors TFIIE and TFIIH in transcription: possible points of regulation by trans-acting factors. J. Biochem. (Tokyo) 122:481-489. [DOI] [PubMed] [Google Scholar]
- 27.Ohkuma, Y., S. Hashimoto, R. G. Roeder, and M. Horikoshi. 1992. Structural conservation of putative functional motifs between Xenopus and human TFIIE-β. Nucleic Acids Res. 20:4363.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohkuma, Y., S. Hashimoto, C. K. Wang, M. Horikoshi, and R. G. Roeder. 1995. Analysis of the role of TFIIE in basal transcription and TFIIH-mediated carboxy-terminal domain phosphorylation through structure-function studies of TFIIE-α. Mol. Cell. Biol. 15:4856-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohkuma, Y., and R. G. Roeder. 1994. Regulation of TFIIH ATPase and kinase activities by TFIIE during active initiation complex formation. Nature 368:160-163. [DOI] [PubMed] [Google Scholar]
- 30.Ohkuma, Y., H. Sumimoto, A. Hoffmann, S. Shimasaki, M. Horikoshi, and R. G. Roeder. 1991. Structural motifs and potential σ homologies in the large subunit of human general transcription factor TFIIE. Nature 354:398-401. [DOI] [PubMed] [Google Scholar]
- 31.Ohkuma, Y., H. Sumimoto, M. Horikoshi, and R. G. Roeder. 1990. Factors involved in specific transcription by mammalian RNA polymerase II: purification and characterization of general transcription factor TFIIE. Proc. Natl. Acad. Sci. USA 87:9163-9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto, T., S. Yamamoto, Y. Watanabe, T. Ohta, F. Hanaoka, R. G. Roeder, and Y. Ohkuma. 1998. Analysis of the role of TFIIE in transcriptional regulation through structure-function studies of the TFIIEβ subunit. J. Biol. Chem. 273:19866-19876. [DOI] [PubMed] [Google Scholar]
- 33.Okuda, M., Y. Watanabe, H. Okamura, F. Hanaoka, Y. Ohkuma, and Y. Nishimura. 2000. Structure of the central core domain of TFIIEβ with a novel double-stranded DNA-binding surface. EMBO J. 19:1346-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orphanides, G., T. Lagrange, and D. Reinberg. 1996. The general transcription factors of RNA polymerase II. Genes Dev. 10:2657-2683. [DOI] [PubMed] [Google Scholar]
- 35.Orphanides, G., and D. Reinberg. 2002. A unified theory of gene expression. Cell 108:439-451. [DOI] [PubMed] [Google Scholar]
- 36.Pal, M., and D. S. Luse. 2002. Strong natural pausing by RNA polymerase II within 10 bases of transcription start may result in repeated slippage and reextension of the nascent RNA. Mol. Cell. Biol. 22:30-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal, M., D. McKean, and D. S. Luse. 2001. Promoter clearance by RNA polymerase II is an extended, multistep process strongly affected by sequence. Mol. Cell. Biol. 21:5815-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan, G., and J. Greenblatt. 1994. Initiation of transcription by RNA polymerase II is limited by melting of the promoter DNA in the region immediately upstream of the initiation site. J. Biol. Chem. 269:30101-30104. [PubMed] [Google Scholar]
- 39.Parvin, J. D., and P. A. Sharp. 1993. DNA topology and a minimal set of basal factors for transcription by RNA polymerase II. Cell 73:533-540. [DOI] [PubMed] [Google Scholar]
- 40.Proudfoot, N. J., A. Furger, and M. J. Dye. 2002. Integrating mRNA processing with transcription. Cell 108:501-512. [DOI] [PubMed] [Google Scholar]
- 41.Robert, F., D. Forget, J. Li, J. Greenblatt, and B. Coulombe. 1996. Localization of subunits of transcription factors IIE and IIF immediately upstream of the transcriptional initiation site of the adenovirus major late promoter. J. Biol. Chem. 271:8517-8520. [DOI] [PubMed] [Google Scholar]
- 42.Roeder, R. G. 1996. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci. 21:327-335. [PubMed] [Google Scholar]
- 43.Roeder, R. G. 1998. Role of general and gene-specific cofactors in the regulation of eukaryotic transcription. Cold Spring Harbor Symp. Quant. Biol. 63:201-218. [DOI] [PubMed] [Google Scholar]
- 44.Sandrock, B., and J.-M. Egly. 2001. A yeast four-hybrid system identifies Cdk-activating kinase as a regulator of the XPD helicase, a subunit of transcription factor IIH. J. Biol. Chem. 276:35328-35333. [DOI] [PubMed] [Google Scholar]
- 45.Schultz, P., S. Fribourg, A. Poterszman, V. Mallouh, D. Moras, and J.-M. Egly. 2000. Molecular structure of human TFIIH. Cell 102:599-607. [DOI] [PubMed] [Google Scholar]
- 46.Seroz, T., C. Perez, E. Bergmann, J. Bradsher, and J.-M. Egly. 2000. p44/SSL1, the regulatory subunit of the XPD/RAD3 helicase, plays a crucial role in the transcriptional activity of TFIIH. J. Biol. Chem. 275:33260-33266. [DOI] [PubMed] [Google Scholar]
- 47.Studier, W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 48.Sumimoto, H., Y. Ohkuma, E. Sinn, H. Kato, S. Shimasaki, M. Horikoshi, and R. G. Roeder. 1991. Conserved sequence motifs in the small subunit of human general transcription factor TFIIE. Nature 354:401-404. [DOI] [PubMed] [Google Scholar]
- 49.Sumimoto, H., Y. Ohkuma, T. Yamamoto, M. Horikoshi, and R. G. Roeder. 1990. Factors involved in specific transcription by mammalian RNA polymerase II: identification of general transcription factor TFIIG. Proc. Natl. Acad. Sci. USA 87:9158-9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svejstrup, J. Q., P. Vichi, and J.-M. Egly. 1996. The multiple roles of transcription/repair factor TFIIH. Trends Biochem. Sci. 21:346-350. [PubMed] [Google Scholar]
- 51.Takada, R., Y. Nakatani, A. Hoffmann, T. Kokubo, S. Hasegawa, R. G. Roeder, and M. Horikoshi. 1992. Identification of human TFIID components and direct interaction between a 250-kDa polypeptide and the TATA box-binding protein (TFIIDτ). Proc. Natl. Acad. Sci. USA 89:11809-11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tantin, D., and M. Carey. 1994. A heteroduplex template circumvents the energetic requirement for ATP during activated transcription by RNA polymerase II. J. Biol. Chem. 269:17397-17400. [PubMed] [Google Scholar]
- 53.Tremeau-Bravard, A., C. Perez, and J.-M. Egly. 2001. A role of the C-terminal part of p44 in the promoter escape activity of transcription factor IIH. J. Biol. Chem. 276:27693-27697. [DOI] [PubMed] [Google Scholar]
- 54.Wang, X., S. K. Hansen, R. Ratts, S. Zhou, A. J. Snook, and W. Zehring. 1997. Drosophila TFIIE: purification, cloning, and functional reconstitution. Proc. Natl. Acad. Sci. USA 94:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe, Y., H. Fujimoto, T. Watanabe, T. Maekawa, C. Masutani, F. Hanaoka, and Y. Ohkuma. 2000. Modulation of TFIIH-associated kinase activity by complex formation and its relationship with CTD phosphorylation of RNA polymerase II. Genes Cells 5:407-423. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto, S., Y. Watanabe, P. J. van der Spek, T. Watanabe, H. Fujimoto, F. Hanaoka, and Y. Ohkuma. 2001. Studies of nematode TFIIE function reveal a link between Ser-5 phosphorylation of RNA polymerase II and the transition from transcription initiation to elongation. Mol. Cell. Biol. 21:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young, R. A. 1991. RNA polymerase II. Annu. Rev. Biochem. 60:689-715. [DOI] [PubMed] [Google Scholar]
- 58.Zawel, L., K. P. Kumar, and D. Reinberg. 1995. Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev. 9:1479-1490. [DOI] [PubMed] [Google Scholar]