Abstract
NF-κB plays crucial roles in the nervous system, including potential roles in long-term responses to synaptic plasticity, pro- or antiapoptotic effects during developmental cell death, and neurodegenerative disorders. We report here the characterization of signaling pathways leading to the constitutive activation of NF-κB in primary cultures of neonatal cerebellar granule neurons, consecutive to calcium entry into the cytosol. We found that opening of calcium channels at the plasma membrane and at intracellular stores is indispensable for the basal NF-κB activity. We demonstrated further that three cellular sensors of the cytosolic Ca2+ levels, calmodulin, protein kinases C (PKCs), and the p21ras/phosphatidylinositol 3-kinase (PI3K)/Akt pathway are simultaneously involved in the steps linking the Ca2+ second messenger to NF-κB activity. Calmodulin triggers the activity of calcineurin, a phosphatase which plays a role in the basal NF-κB activity, while stimulation of both the calmodulin kinase II and Akt kinase pathways results in the up-regulation of the transcriptional potential of the p65 subunit of NF-κB. Finally, using pharmacological and molecular approaches, we analyze interactions between these three pathways at different levels and demonstrate a connection between PKCs and PI3K. All three components converge towards NF-κB, at the level of both nuclear translocation and transcriptional activity. These results stand in contrast to the situation in nonneuronal cells, which either do not respond to Ca2+ or do not simultaneously activate all three cascades. By using a global approach in studying signaling pathways in neurons, these results provide further evidence to validate the concept of networks of transducing cascades, specific to cells and to physiological situations.
NF-κB is a transcription factor which has recently been demonstrated to be involved in both survival and apoptosis of neurons. NF-κB is a homo- or heterodimer of proteins belonging to the NF-κB/Rel family, which contains five subunits identified in mammalian cells: RelA or p65, RelB, c-rel, p50, and p52. The dimer p50/p65 is the most prominent and is considered to be the prototype of the NF-κB factors (45, 88). In contrast to p50, p52, and p65, which are ubiquitous, RelB and c-rel are restricted mainly to the lymphoid tissues (29). In unstimulated cells, NF-κB proteins remain in the cytoplasm bound to inhibitory IκB molecules, which mask their nuclear localization signal. Three of these inhibitory molecules have been described: IκBα, IκBβ, and IκBɛ (93). Following cellular stimulation, IκB proteins become phosphorylated by the IκB kinase (IKK) complex, ubiquitinated, and finally degraded by the proteasome complex. The recent identification of a high-molecular-weight complex containing two kinases (IκB kinase alpha [IKKα] and IKKβ) and at least one regulatory subunit (NEMO/IKKγ/IKKAP), has led to the hypothesis that this complex may constitute an integrator of all signals, contributing to the exquisite regulation of NF-κB activity (37). NF-κB is then released and translocated to the nucleus, where it activates its target genes by binding to specific sites in their regulatory regions. One of these genes encodes IκBα. Neosynthesized IκBα molecules are able to retrieve NF-κB from the nucleus, leading to a negative feedback, which contributes to the transient nature of the activation (1). Although the mechanisms leading to the degradation of the IκB proteins are relatively well understood, the precise steps of signal transduction which result in the activation of the high-molecular-weight kinase complex remain to be elucidated. In addition to the control of NF-κB activity exerted at the nuclear translocation level, it has further been shown that a second level of regulation may be found at the level of the transactivating capacity of the p65 subunit, which can be phosphorylated in its transactivating domain. Stimuli such as tumor necrosis factor alpha (TNF-α) or direct IKK activation result in the phosphorylation of serines 529 and 536, respectively, thereby potentiating the transactivating efficiency of this subunit and resulting in a second level of regulation independent from the nuclear translocation process (76, 89).
In the nervous system, NF-κB is modulated under physiological and pathological conditions, including developmental cell death and acute or chronic neurodegenerative disorders (3, 56). NF-κB has been associated with synaptic plasticity since it is present in synaptic terminals and can be activated locally in such synapses (42, 58). Moreover, physiological signals such as glutamate receptor binding and membrane depolarization induce NF-κB activation in hippocampal pyramidal neurons and cerebellar granule neurons in cell culture (30, 32, 43). NF-κB activity is also greatly increased in brain cells following excitotoxic and apoptotic insults. Thus, several studies have documented increased levels of NF-κB activation in brain tissues in rodent models of stroke, cardiac arrest, transient global or focal ischemia (13, 15, 77, 99), traumatic shock (96), or seizure (31, 55, 69, 75). In addition, activation of NF-κB before experimental insults such as exposure to glutamate, glucose deprivation, β-amyloid peptide, or oxidative molecules has been shown to protect neurons against apoptosis (2, 14, 41). In contrast, treatment of neurons with κB decoy DNA which selectively blocks NF-κB activity abolished the protective effect of small doses of TNF-α (57). In vivo, administration of κB decoy DNA to mice via intraventricular infusion before administration of the glutamate agonist kainate resulted in a significant increase of neuronal death in the CA1 and CA3 regions of the hippocampus, and mice lacking p50 exhibited increased damage to hippocampal pyramidal neurons (98). Finally, studies of postmortem brain tissues from patients with neurodegenerative diseases such as Alzheimer's or Parkinson's diseases revealed increased NF-κB activity closely associated with the neurodegenerative process (36, 41). These results strongly suggest that NF-κB regulates apoptosis in response to stress in the nervous system, in addition to regulating apoptosis in a large variety of cells and tissues.
Because NF-κB plays such crucial functions in the nervous system (51, 56, 62), it was important to identify the signaling pathways leading to its activation in neurons. As stimulation of glutamate receptors results in membrane depolarization which opens Ca2+ channels, leading to a rise in the intracellular Ca2+ concentration, we hypothesized that Ca2+ could play an important role in NF-κB activity in neurons. Induction of NF-κB by various stimuli has already been shown to require Ca2+ for proper signal transduction (27, 33, 34, 40, 64). In addition, the Ca2+ signal may also synergize with other signaling pathways, as is the case for protein kinase C (PKC) activation in T lymphocytes (26, 82, 86). However, the exact process involved in transducing the Ca2+ signal remains to be elucidated.
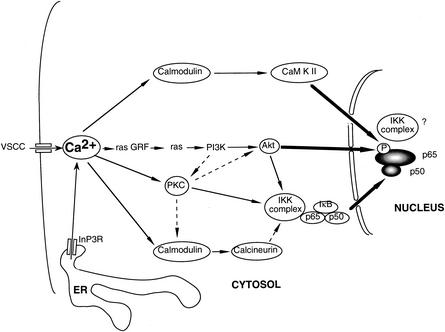
To address this question, we have analyzed the transduction pathways which activate NF-κB downstream of the Ca2+ signal in primary cultures of neonatal cerebellar granule neurons. We chose to use these cells because they may reflect in vitro the physiological state of development, where cells require external signals such as the establishment of functional synaptic connections and stimulation by neurotrophic factors for survival in vivo. We demonstrate here that the rise of intracellular Ca2+, through opening of L-voltage-sensitive Ca2+ channels (L-VSCCs) at the plasma membrane and indirect opening of In3P receptors associated with the intracellular stores of Ca2+, is responsible for the basal NF-κB activity in these cells. We demonstrated further that subsequent steps in signal transduction involve the major cellular sensors of Ca2+ levels, calmodulin, and PKCs, as well as the ras/phosphatidylinositol 3-kinase (PI3K)/Akt kinase pathway. Moreover, we found a complex functional interplay between these three pathways, involving connections between PI3K and PKC pathways on one hand and convergence towards NF-κB at the level of both nuclear translocation and transcriptional activity on the other. Altogether, these results suggest that instead of being controlled by linear and independent signaling pathways, NF-κB activity is regulated by a network of transduction cascades controlled by the level of intracellular Ca2+ in neurons. Our results provide a new framework for our understanding of how cross talk integrates a variety of signals in a physiological context for neurons.
MATERIALS AND METHODS
Plasmid constructs.
The (Igκ)3conaluc plasmid has been described elsewhere (78). Briefly, it was produced using three copies of the NF-κB/Rel-binding site of the immunoglobulin κ light chain inserted immediately upstream of the conalbumin (cona) minimal promoter. This enhancer-promoter element was cloned into SalI-HindIII-digested pUC-luc. As negative control for this construct, conaluc carries the conalbumin promoter alone, cloned into the SalI-HindIII site of pUC-luc. pUC-luc is derived from pUC18 and contains the luciferase gene cloned into the HindIII-BamHI fragment. The Gal4-p65, Gal4-p65 S529A, Gal4-p65 S536A, and Gal4-p65 S529A/S536A constructs were obtained from L. Madrid (University of Virginia, Charlottesville). The sequence coding for the DNA-binding element from the yeast Saccharomyces cerevisiae Gal4 coding sequence (amino acids 1 to 146) is fused to the C-proximal part of the human p65 (amino acids 520 to 550). The TP1-Gal4-luc plasmid was constructed by inserting 10 Gal4-binding sequences into the TP1-luc plasmid, obtaining the TP1-Gal4 promoter in front of the luciferase reporter gene (B. Kempes and H. Gruffat, Institut für Klinische Molekularbiologie und Tumorgenetik, Munich, Germany). The IκBα plasmid and its dominant-interfering mutant IκBα DN (where the two serines 32 and 36 are mutated into alanines) were described elsewhere (92). The plasmid pRasDN (rasDN) expressing the p21ras dominant-negative mutant was obtained from M. Karin, University of California—San Diego. The dominant-negative mutants for the p85 regulatory subunit of the PI3K (pGEX-Δp85) deleted from its p110 binding site (amino acids 479 to 513) was kindly given by Masto Kasuga, Kobe University, Kobe, Japan; we subcloned this cDNA into pCDNA3 in the BamHI-EcoRI site, to obtain p85DN. Constitutively active (AktCA) and dominant-negative (AktDN) forms of Akt were generous gifts from Thomas F. Franke, Columbia University, New York, N.Y. (respectively, myrAkt-HA with a myristoylation tag added, and HA-Akt [K179 M]). The plasmids expressing the calcineurin A (pCNA) and B (pCNB) subunits were gifts from G. R. Crabtree, Stanford University, Stanford, Calif., and Michel Kobr, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, respectively. Primer A1 (5′-TTGCTCGGATCCTAGTTTCTGATGACTTCTTCCGG-3′), for the 5′ extremity of the coding sequence, and primer A2 (5′-TTTTTTAAGCTTGCGCCGGTGCGGTCGGGG-3′), which creates a new stop codon to replace amino acid 398, were used in PCR amplification form the pCNA plasmid to obtain the constitutively active mutant CNAΔCaM from which the calmodulin-binding domain has been deleted. To generate the CNADN dominant-negative mutant, three additional oligonucleotides were used: A2 for the stop codon and C1 (5′-TTAGGGGACTATGTTAATAGAGGGTACTTCAGT-3′) and C2 (5′-ACTGAAGTACCCTCTATTAACATAGTCCCCTAA-3′) to generate the mutation replacing Asp at amino acid 130 with Asn. In the first step, two fragments were amplified by PCR using the pCNA plasmid as the template, with A1 and C2 for the first fragment and C1 and A2 for the second. In the second step, the two fragments were used as the template for PCR amplification, with A1 and A2 to reconstitute the whole molecule. The sequence of the resulting DNA was determined, and the constructs were ligated into an expression vector pcDNA3 (Invitrogen, Carlsbad, Calif.) and tested for functional activity in Jurkat T lymphocytes by transient transfection with an NF-AT-responsive reporter plasmid. The plasmid EF1lacZ was obtained by inserting the promoter of the human elongation factor 1 (EF1) gene, cut by HindIII-XbaI and blunt ended for XbaI, into the plasmid pSKTnlslacZ cut by HindIII-EcoRV. This plasmid contains the lacZ gene downstream of this insertion point. The NF-AT reporter plasmid was obtained from O. Acuto (Pasteur Institute, Paris, France). Dominant-negative constructs for Ca2+/calmodulin-dependent protein kinase II (CaMKII) and CaMKIV were kindly given by T. Grundström (Umeå University, Umeå, Sweden) and J.-P. Loeffler (Université Louis Pasteur, Strasbourg, France), respectively. CREB-reporter vectors were from Stratagene (La Jolla, Calif.).
Cell culture.
Granule cells were dissociated from the cerebella of 7-day-old Swiss mice as described previously (10); cultured in Dulbecco's modified Eagle medium (DMEM) with HEPES modification (Sigma, St. Quentin-Fallavier, France) containing 1.8 mM CaCl2 supplemented with heat-inactivated 5% fetal calf serum (FCS), 5% horse serum, and gentamicin (100 μg/ml); and plated on poly-l-lysine-treated plates at a density of 3 × 105 to 4 × 105 cells per cm2. After 24 h, 10 μM cytosine arabinofuranoside was added to the culture to inhibit the growth of mitotically active cells. HeLa cells were cultured in DMEM supplemented with 10% FCS, penicillin (100 U/ml), and streptomycin (100 mg/liter). Jurkat cells were cultured in RPMI medium supplemented with 10% FCS, penicillin (100 U/ml), and streptomycin (100 μg/ml).
Cellular transfection and luciferase reporter assays.
HeLa cells were transfected by the Ca2+ phosphate coprecipitation method. Twenty micrograms of total DNA was used for a 10-cm-diameter dish. Jurkat cells were transfected using 2 μg of total DNA for 5 × 106 cells using DEAE-dextran as previously described (91). Neurons were transfected after 2 days in culture in 24-well plates using TFX-50 (Promega, Charbonnières, France) liposomes according to the manufacturer's specification. Briefly, the ratio of DNA to TFX-50 was 1 μg:4.5 μl in 200 μl of serum-free DMEM for 3 h. Each well received 1 μg of total DNA, which was subdivided into the following: 0.75 μg of (Igκ)3conaluc or 0.08 μg of Gal4-p65 plus 0.7 μg of TP1-Gal4-luc, to which was added 0.25 μg of EF1lacZ vector. When other constructs were cotransfected, 0.4 μg of (Igκ)3conaluc or 0.04 μg of Gal4-p65 plus 0.35 μg of TP1-Gal4-luc were used, completed by 0.6 or 0.35 μg of the additional plasmid, respectively, and by 0.25 μg of EF1lacZ. Each transfection was performed in triplicate. Cells were visually examined before and at the end of the drug treatment to check for necrosis or apoptosis. For each 24-well plate, two controls treated with solvent only were done. Forty-eight hours after transfection, or 24 h after transfection when using dominant-interfering constructs (in order to avoid potential pitfalls associated with cell death), neurons were treated with the appropriate drug for 6 h before lysis and measurement of the luciferase and β-galactosidase activities (β-gal Genetic Reporter System II; Clontech, Palo Alto, Calif.) in an Autolumat LB953 luminometer (Berthold Analytical Instruments, Bad Wilbad, Germany). The values expressed in relative light units (RLU) ranged for the control from 3,000 to 1,0000 RLU for luciferase and 1,000 to 3,000 RLU for β-galactosidase. Results were normalized with an internal β-galactosidase-expressing plasmid (EF1lacZ) and expressed as a multiple of the control value corresponding to neurons treated with the solvent only. Results are expressed as means ± standard errors (SE) and were obtained from at least three separate experiments. Data were analyzed by a two-tailed Student's t test.
Pharmacology and drug treatment.
Neurons were pretreated with inhibitors for 6 h; this time period was chosen because it corresponds to two half-life periods for luciferase and also minimizes apoptosis due to various treatments. In a separate experiment, neuronal survival was estimated after treatment with various drugs by measuring the amount of colored formazan by the reduction of 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma) (11). All the working concentrations used for the different drugs did not significantly alter the MTT value compared to that observed in untreated neurons (data not shown). Pharmacological agents used were nimodipin, nifedipin, ω-conotoxin GVIA, ω-conotoxin MVIIC, TMB8, BAPTA-AM [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetra(acetyoxymethyl) ester], heparin, ryanodine, caffeine, thapsigargin, cyclosporine, FK506, bis-indoyl-maleimide (BIM), LY294002, wortmannin, W7, and calmidazolium (all from Sigma) and Gö6976, H89, KN62, and KN93 (from Calbiochem, Meudon, France). Locke's solution (pH 7.4) was made freshly and contained NaCl (154 mM), KCl (5.6 mM), NaHCO3 (3.6 mM), CaCl2 (2.3 mM), glucose (5.6 mM), and HEPES (5 mM).
Immunoblot analysis.
Cells were washed twice in phosphate-buffered saline and lysed in 250 μl of 1× TNE (50 mM Tris [pH 8.0], 1% NP-40, and 2 mM EDTA), supplemented with a 10-μg/ml concentration of each of the protease inhibitors leupeptin, aprotinin, N-tosyl-l-phenylalanine chloromethyl ketone, N-p-tosyl-l-lysine chloromethyl ketone, and phenylmethylsulfonyl fluoride as well as the phosphatase inhibitors sodium fluoride (50 mM), sodium orthovanadate (1 mM), and β-glycerophosphate (25 mM). Aliquots (200 μg) of cellular extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoblots were performed according to a previously described protocol (90). For anti-phosphorylated threonine 308 (T308) and anti-Akt immunoblots (Upstate Biotechnology Inc., Lake Placid, N.Y.), we used antiserum at a 1/1,000 dilution (for enhanced chemiluminescence). For anti-phosphorylated p38 and whole p38 kinase, antisera were used at a 1/1,000 dilution (Cell Signaling Technology, Beverly, Mass.). Proteins transferred to Immobilon membranes (Millipore, Molsheim, France) were revealed with the AmershamPharmaciaBiotech (Orsay, France) ECL system for direct immunoblotting of total cell extracts. Immunoreactive products were detected by autoradiography.
Nuclear extracts and electrophoretic mobility shift assay (EMSA).
After stimulation, cells were lysed in 200 μl of cold EMSA buffer (50 mM Tris-HCl [pH 7.9], 10 mM KCl, 1 mM EDTA, 0.2% NP-40, glycerol 10%, antiprotease cocktail [Boehringer-Mannheim, Mannheim, Germany]), incubated for 4 min at 4°C, and centrifuged for 3 min at 2,800 × g at 4°C (Eppendorf). The pellet was resuspended in 20 μl of cold nuclear extraction buffer (400 mM NaCl, 20% glycerol, 20 mM HEPES, 10 mM KCl, 1 mM EDTA, antiprotease cocktail), shaken at 4°C for 30 min, and centrifuged for 10 min at 15,000 × g at 4°C. Supernatants corresponding to the nuclear extract were quickly frozen at −80°C. Gel retardation assays for detection of NF-κB were performed with the oligonucleotides derived from the H-2Kb promoter as previously described (78). The binding reactions were performed in a total volume of 20 μl for 30 min at room temperature by adding 20 μg of nuclear extract, 10 μl of 2× binding buffer (8% Ficoll, 40 mM HEPES [pH 7.5], 140 mM KCl, 4 mM dithiothreitol, bovine serum albumin [200 μg/ml], 0.002% NP-40), 1 μg of poly(dI:dC), and 1 μl of 32P-labeled κB probe. For supershifts, 0.5 μl of serum 1226 (anti-p65) or 1157 (anti-p50) was added (Nancy Rice). The reaction mixtures were separated on a 5% polyacrylamide gel in 0.5× Tris-borate-EDTA (TBE) buffer at 200 V for 1.5 h. Gels were dried and exposed for 24 to 48 h.
Kinase assays.
Cellular extracts were obtained from cells treated for 10 min with various inducers or pretreated for 30 min with various inhibitors. Cells were lysed in a solution containing 10 mM HEPES (pH 7.6), 10 mM KCl, 2 mM MgCl2, 0.1 mM EDTA supplemented with 1 mM sodium orthovanadate, 1 mM sodium fluoride, 10 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, protease inhibitors (Boehringer Mannheim cocktail), and 1% NP-40. CaMKII and PKC assay kit were used (Upstate Biotechnology Inc.). CaMKII activity was assayed utilizing a peptide substrate (KKALRRQETVDAL) with relative selectivity for CaMKII. The reaction mixture containing 10 μl of substrate, 10 μl of inhibitors of PKA and PKC, and 10 μl of MgCl2-[γ-32P]ATP mixture was incubated at 30°C for 10 min. PKC activity was assayed utilizing a peptide substrate (QKRPS8QRSKYL) with relative selectivity for PKCs. The reaction mixture containing 10 μl of substrate, 10 μl of other Ser/Thr kinases such as PKA and CaMKII, 10 μl of phosphatidylserine (0.5 mg/ml) and diacylglycerol (0.05 mg/ml), and 10 μl of MgCl2-[γ-32P]ATP mixture was incubated at 30°C for 10 min. The phosphorylated substrate was separated from the residual [γ-32P]ATP using p81 phosphocellulose paper. The papers were washed twice in 0.75% H3PO4 and then in acetone for 2 min, and the bound radioactivity was quantified with a scintillation counter. Blanks to correct for nonspecific binding of [γ-32P]ATP and its breakdown products to the phosphocellulose paper and controls for phosphorylation of endogenous proteins in the sample were performed, and CaMKII or PKC activity was expressed as counts per minute per milligram of protein.
RESULTS
NF-κB is required for cerebellar granule cell survival.
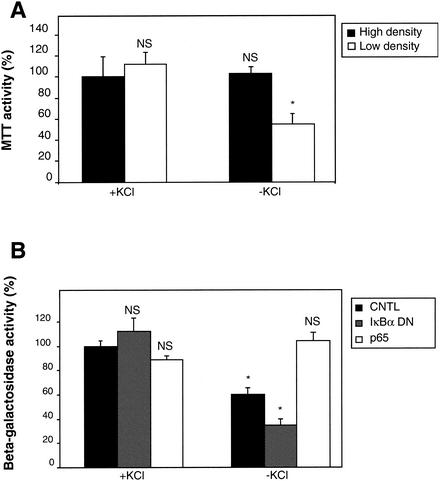
Cerebellar granule neurons are dependent upon membrane depolarization and subsequent extracellular calcium entry, elicited by KCl in the culture medium, for their survival. This activity mimics natural activities resulting from synaptic connections that start to be established after birth in the cerebellum. In order to understand the role played by NF-κB in this phenomenon, we designed an experiment to determine if NF-κB activity is required for neuronal survival. First, we analyzed cell culture conditions under which deprivation of KCl was detrimental (Fig. 1A). We measured cell survival by monitoring mitochondrial activity with tetrazolium salts (MTT). We found that at 4 days of culture in vitro (DIV 4), a moderate cell loss of 40% was observed only if neurons were cultured at low density (150,000 cells/cm2), in contrast with high-density cultures (400,000 cells/cm2). To analyze the role of NF-κB in cerebellar granule neurons subjected to KCl deprivation, we cotransfected a reporter construct containing a housekeeping promoter for the EF1 controlling the expression of the lacZ gene (EF1-lacZ). This vector monitoring cell survival was cotransfected with either an empty vector (pcDNA3), a dominant negative construct for IκBα blocking NF-κB activity (IκBα DN), or a plasmid coding for p65, to stimulate this activity (Fig. 2B and 2C for controls). Investigating the role of NF-κB at DIV 4 and at low density, we found that the expression of the NF-κB-blocking construct (IκBα DN) reduced cell survival by 50% (from 60 to 30%) (Fig. 1B). In contrast, expression of p65 rescued the cell by restoring a full 100% activity. As controls, expression of these constructs did not modify EF1lacZ activity when KCl was present. From these results we concluded that NF-κB is able to protect cerebellar granule neurons from cell death due to KCl deprivation at early stages of neuronal maturation. This result confirms similar data from other studies showing that NF-κB is required for cerebellar granule neurons survival (46, 67, 95).
FIG. 1.
NF-κB protects cerebellar granule cells from cell death following KCl deprivation. (A) To determine experimental conditions of stress, neurons were plated onto 24-wells dishes at high (dense; 400,000 cells/cm2) or at low (diluted; 150,000 cells/cm2) density. At DIV 4, cells were washed once and replaced in medium containing either 25 mM KCl (+KCl) or 5 mM KCl (−KCl). Neuronal survival was assessed 24 h later, using tetrazolium salts (MTT) (see Materials and Methods for more details). (B) Neurons were plated at low density as determined in panel A and transfected after 2 days in culture with 1 μg of either an empty vector (pcDNA3 [CNTL]); a dominant-negative mutant for IκBα, blocking NF-κB activity (IκBα DN); or a vector expressing the p65 subunit from NF-κB. In order to monitor precisely the survival rate of the transfected neurons, in each case 0.25 μg of a plasmid containing the reporter lacZ gene under the control of the EF1 promoter was cotransfected. Forty-eight hours later, neurons were washed and the medium was replaced with either high- or low-KCl containing medium. Twenty-four hours later, the β-galactosidase activity was measured. Symbols and abbreviations: error bars, SE; *, significant; NS, not significant.
FIG. 2.
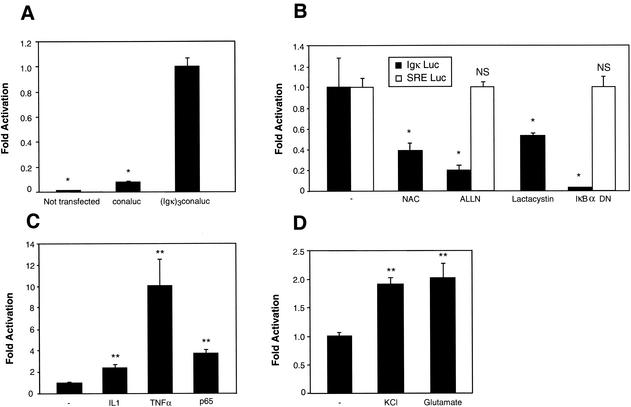
Basal and inducible NF-κB activity in neurons. Cerebellar granule neurons were transfected with 0.75 μg of the NF-κB-responsive (Igκ)3conaluc plasmid, together with 0.25 μg of EF1lacZ normalization vector, unless specified otherwise. Cells were harvested 48 h later, and the luciferase as well as the β-galactosidase activities were measured. (A, B, and C) Neurons were transfected 2 days after seeding. Data are the means + SE (error bars) of measures done in triplicate, expressed as fractions of the normalized level of activation obtained with control neurons treated with 25 mM of KCl, and are representative of three independent experiments. Symbols: * and **, P < 0.01 and P < 0.05, respectively, for significant difference compared to the untreated control, determined by Student's test; -, control neurons treated with solvent only. (A) A basal level of NF-κB activity is observed in untreated primary cultures of cerebellar granule neurons. Neurons were transfected with either the conaluc control plasmid or with the (Igκ)3conaluc vector as indicated. As a control, the background activity of untransfected neurons was also measured (Not transfected). (B) Inhibitors of NF-κB antagonize the transactivation of (Igκ)3conaluc construct in neurons. Forty-eight hours after transfection with (Igκ)3conaluc and EF1lacZ plasmids, neurons were exposed for 6 h to antioxidant N-acetyl-cysteine (NAC) (1 mM), inhibitors of proteasome (ALLN [100 μM] and lactacystin [10 μM]) or cotransfected with a dominant-negative mutant of IκBα (IκBα DN). In parallel, an unrelated pathway involving the SRE was tested as control. SRE Luc (0.75 μg) and EF1lacZ (0.25 μg) vectors were transfected, and neurons were treated in a manner similar to that indicated above. (C) NF-κB activity is further inducible over its basal level by cytokines in neurons. Cells with the reporter plasmids described in panel B were subjected to a 6-h induction by IL-1β at 10 ng/ml (IL-1) or by TNF-α (100 U/ml) before harvesting. A 0.1-μg aliquot of pcDNA3 or of a murine p65 cDNA cloned into pcDNA3 was transfected in parallel, together with 0.75 μg of (Igκ)3conaluc and 0.25 μg of EF1lacZ, as controls. (D) NF-κB can be induced by neuronal signals. Cerebellar granule neurons were left to mature for 6 days in culture after plating and before transfection. At 8 days in culture, neurons were subjected to a 5-min pulse with 100 mM KCl or 100 μM glutamate in Locke's solution as indicated. Cells were then washed carefully twice with Locke's solution and replaced in their initial medium. The luciferase and β-galactosidase activities were recorded 6 h later.
NF-κB exhibits a basal level of activity in cerebellar granule cells.
Having shown that NF-κB plays a role in the survival of cerebellar granule neurons, we then decided to analyze the pathways leading to NF-κB activation in these cells. We first checked whether a basal NF-κB activity could be detected in neurons placed in medium containing a high KCl concentration (25 mM), thus allowing their survival. For this purpose, we first measured NF-κB activity by transient transfection of a κB-dependent luciferase construct, (Igκ)3conaluc, and a control plasmid (conaluc). We found that in unstimulated neurons kept for 4 days in culture, a basal NF-κB activity was clearly visible (10-fold the activity of the control plasmid [Fig. 2A]).
To confirm that the observed luciferase activity was effectively due to NF-κB, we used inhibitors acting at different steps of the NF-κB pathway. An antioxidant agent, N-acetyl-cysteine, and two inhibitors of the proteasome pathway, N-acetyl-leucine-leucine-norleucinal (ALLN) and lactacystin, were tested during 6 h on neurons. In parallel, IκBα DN, a dominant-negative mutant of the IκBα molecule in which serines 32 and 36 have been mutated into alanines, was cotransfected with the reporter plasmid, and the activities were analyzed 24 h later. Although there was some variation between inhibitors, all these molecules reduced the luciferase activity by 45 to 90% (Fig. 2B). The IκBα DN construct was the more potent inhibitor, therefore confirming that the luciferase activity reflects the endogenous NF-κB activity. We concluded from these experiments that a basal level of NF-κB activity can be observed in cerebellar granule cells.
As shown in Fig. 1A, using high cellular densities and medium complemented with serum did not lead to significant neuronal apoptosis, 24 h after switching from a high to a low KCl concentration. We therefore used these conditions in all the subsequent experiments, in order to avoid potential apoptosis after blocking NF-κB activity. To clearly demonstrate that under our culture conditions, neuronal survival is not affected by various inhibitors of the NF-κB signaling pathway, we used a construct in which the luciferase reporter gene expression is driven by a serum-responsive element (SRE) in transfection assays performed in parallel. In Fig. 2B we show that the control level produced by the SRE Luc vector is unaffected by ALLN, lactacystin, or the expression of the dominant-negative construct IκBα DN. However, this activity is effectively dependent upon the level of serum present in the culture medium, since reducing the level of FCS or horse serum from 10 to 0.1% for 6 h inhibits it totally, while survival monitored by the β-galactosidase activity produced by the EF1-LacZ reporter construct was left unchanged (not shown). These results therefore demonstrate that when using culture conditions such as high cellular densities, high serum, and 4 days in culture, the addition of various agents that inhibit NF-κB activity does not affect neuronal survival unspecifically.
To assess whether NF-κB could be further induced in these neurons, we used two cytokines, TNF-α and interleukin 1β (IL-1β), which are mediators of inflammation and potent inducers of NF-κB in a large panel of cells (59). Both TNF-α and IL-1β stimulated NF-κB, 10- and 3-fold, respectively, and cotransfection with a vector expressing the p65 subunit produced a 4-fold induction (Fig. 2C). The neurotransmitter glutamate and the depolarizing molecule KCl were assayed as specific signals for mature neurons after 8 days in culture. When administered for 5 min at 100 μM and 100 mM for glutamate and KCl, respectively, both elicited a twofold stimulation of the luciferase activity 6 h after stimulation (Fig. 2D), confirming previous results obtained by different groups (30, 32, 43). These results show that the basal NF-κB activity in neurons can be further stimulated, either by cytokines or by depolarizing agents.
Basal NF-κB activity is dependent upon calcium influx.
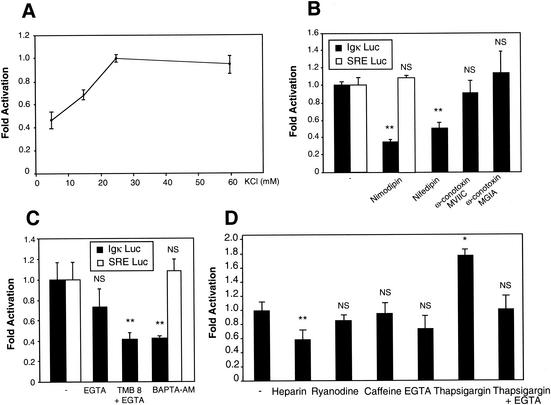
In the following experiments, we decided to focus exclusively on the basal NF-κB activity in cerebellar granule neurons, hypothesizing that it is due to a depolarizing stimulus. Given the requirement of cerebellar granule neurons for elevated cytosolic Ca2+, elicited by the addition of KCl in the medium, we therefore hypothesized that Ca2+ could play a crucial role in the activation of NF-κB in cerebellar granule neurons. To test this possibility, we first measured the NF-κB activity using the (Igκ)3conaluc plasmid in neurons exposed to culture medium containing various KCl concentrations. In response to membrane depolarization, NF-κB activity increased proportionally to the concentration of KCl (5 to 25 mM) and then reached a plateau (Fig. 3A). It appears therefore that the usual concentration of KCl (25 mM) used to maintain cerebellar granule neurons in culture is also optimum with regard to NF-κB activity. Different types of voltage-sensitive Ca2+ channels have been described. To identify the ones involved in this activity, we used nimodipin (10 μM) and nifedipin (10 μM) as pharmacological antagonists of fast L-channels and ω-conotoxin MVIIC (10 μM) and ω-conotoxin GVIA (10 μM) as inhibitors of, respectively, Q and N slow voltage-dependent Ca2+ channels. The results show that only the inhibition of the L-channel decreased NF-κB activity (Fig. 3B). Since the entry of Ca2+ through L-channels results in the elevation of intracellular Ca2+ concentration, we then investigated whether this increase was necessary for the NF-κB activity observed in neurons depolarized with 25 mM of extracellular KCl. Cerebellar granule cells were treated for 6 h with chelators of intracellular Ca2+ in the presence of 25 mM KCl, after transfection with the (Igκ)3conaluc plasmid. Two chelating molecules, TMB8 (200 nM) plus EGTA (1 mM) and BAPTA-AM (50 μM), were able to reduce the level of luciferase activity to about 40% of its basal value (Fig. 3C). These results demonstrate the role of Ca2+ channels and elevation of intracellular Ca2+ in regulating the constitutive NF-κB activity in neurons. As further controls, the activities displayed by the control vector SRE Luc were not affected by nimodipin (Fig. 3B) and BAPTA-AM (Fig. 3C), demonstrating the absence of unspecific effects.
FIG. 3.
The rise of intracellular Ca2+ following membrane depolarization is essential for NF-κB basal activity. Primary cerebellar granule neurons were transfected with 0.75 μg of (Igκ)3conaluc plasmid, together with 0.25 μg of EF1lacZ normalization vector. To control the specificity of action of some drugs used, 0.75 μg of SRE Luc plasmid, monitoring a pathway unrelated to NF-κB, together with 0.25 μg of EF1lacZ normalization vector was also transfected in parallel. Neuronal extracts were assayed for luciferase and β-galactosidase activity 48 h later. Data are the means ± SE (error bars) of measures done in triplicate, expressed as fractions of the normalized level of activation obtained with control neurons treated with 25 mM of KCl (-) and are representative of three independent experiments. Symbols and abbreviations: * and **, P < 0.01 and P < 0.05, respectively, for significant difference compared to inhibitor-free control, determined by Student's test; NS, no statistical difference with the control. (A) Dose response of NF-κB activity to the depolarizing agent KCl. Neurons were exposed 6 h to medium containing various concentrations of KCl before measuring their luciferase and β-galactosidase activities. (B) Ca2+ entry through L-VSCCs is required for the NF-κB activity. Transfected neurons were exposed 6 h to inhibitors of L-VSCCs (nimodipin and nifedipin [10 μM]), N-channels (ω-conotoxin GVIA [10 μM]), or Q-channels (ω-conotoxin MVIIC [10 μM]). The luciferase and β-galactosidase activities were then measured. (C) Intracellular Ca2+ chelators inhibit NF-κB activity. Neurons transfected with (Igκ)3conaluc and EF1lacZ were exposed 6 h to EGTA (1 mM), TMB8 (200 nM) plus EGTA, or BAPTA-AM (50 μM) as indicated before recording the luciferase and β-galactosidase activities. (D) Involvement of the intracellular Ca2+ stores in NF-κB activity. Neurons were subjected to treatment with various inhibitors for 6 h, 48 h after transfection with the same plasmid as indicated above. Heparin (50 μg/ml) and ryanodine (2 mM) inhibit InP3 and ryanodine ligand-gated ER Ca2+ channels, respectively. Caffeine (1 mM) triggers the release of Ca2+ by the ER. Thapsigargin is an inhibitor of the ER Ca2+-ATPase which is responsible for the reuptake of Ca2+ into the intracellular stores.
One of the major events following the rise of intracellular Ca2+ concentration due to extracellular Ca2+ entry is the release of Ca2+ contained in the intracellular stores, mainly in the endoplasmic reticulum (ER), modulating the pulse of cytosolic Ca2+ concentration (87). To check the possibility that calcium release from intracellular stores could regulate NF-κB activity, we used inhibitors of specific Ca2+ channels. Two types of channels have been identified, one responding to In3P, which can be inhibited by heparin (50 μg/ml), and the ryanodine receptor, named after its specific inhibitor ryanodine (2 mM). Our results show that heparin can significantly reduce the NF-κB basal activity, but not ryanodine (Fig. 3D). Caffeine (1 mM), a potent stimulant of Ca2+ release from the intracellular stores associated with ryanodine receptors, did not alter the NF-κB activity. In contrast, when thapsigargin was used on neurons, this inhibitor of the Ca2+ pump stimulated the basal activity 1.8-fold. This result suggests that the pumping of the Ca2+ to the intracellular stores contributes to limit the cytoplasmic concentration of free Ca2+ and that inhibition of the uptake increases this concentration, leading to an elevated NF-κB activity. As a control, adding EGTA to the cells together with thapsigargin resulted in the inhibition of the stimulating effect of the latter (Fig. 3D).
Together, these data demonstrate that membrane depolarization, leading to Ca2+ influx into the cytoplasm is one of the mechanisms by which NF-κB, is constitutively activated in neonatal cerebellar granule neurons.
Calmodulin is involved in Ca2+-mediated NF-κB activity.
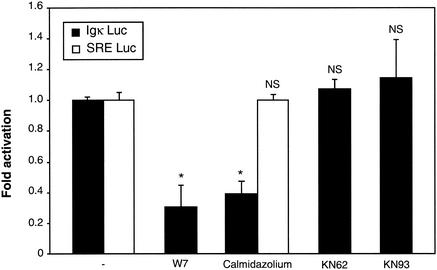
Since calmodulin, a small calcium-binding protein plays a pivotal role in the Ca2+ signaling pathway and is able to trigger the activity of many kinases and phosphatases following binding of Ca2+, we explored the possibility that this molecule could be involved in NF-κB basal activity. Thus, we examined whether antagonists of calmodulin were able to block NF-κB activation, using the luciferase reporter gene assay in granule cerebellar cells. Both W7 (50 μM) and calmidazolium (5 mM), two specific inhibitors of calmodulin, led to a significant decrease in NF-κB basal activity (Fig. 4). In contrast, no such effect was observed on the SRE Luc control vector, invalidating the possibility of a necrotic or apoptotic effect.
FIG. 4.
Involvement of calmodulin in NF-κB-mediated transcriptional activity. Neurons transfected with the (Igκ)3conaluc plasmid, together with the EF1lacZ normalization vector, were treated with inhibitors of calmodulin, W7 (5 μM) and calmidazolium (5 mM), for 6 h. Inhibitors of calmodulin kinases, KN62 and KN93 (each at 1 mM), were used in parallel, before harvesting the cells and measuring their luciferase and β-galactosidase activities. To check that neurons were not affected in an unspecific manner by these treatments, the SRE Luc plasmid and the EF1lacZ vector were transfected, and neurons were treated with calmidazolium. Data are the means + SE (error bars) of measures done in triplicate, expressed as fractions of the normalized level of activation obtained with control neurons treated with 25 mM of KCl (-), and are representative of three independent experiments. Symbols and abbreviations: *, P < 0.01 (significant difference as determined by Student's test, compared to the inhibitor-free control); NS, no statistical difference with the control.
We next asked whether calmodulin kinases, which are activated subsequently to the binding of Ca2+ to calmodulin, were part of the NF-κB activation pathway. KN62 (1 mM) and KN93 (1 mM), two potent and specific inhibitors of calmodulin-kinases, were unable to modify the level of NF-κB activity.
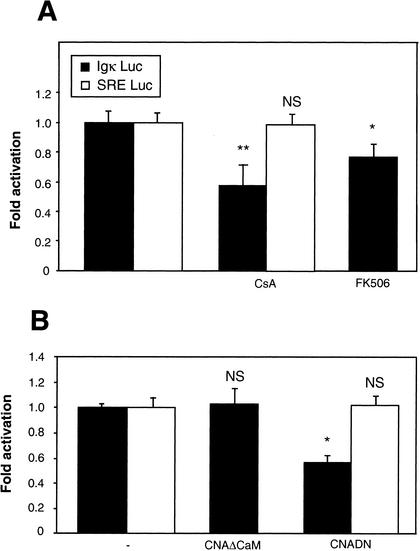
We finally determined whether calcineurin could play a role in the calcium-calmodulin pathway to regulate NF-κB; this serine-threonine phosphatase has already been shown to be involved in the synergistic induction of NF-κB by Ca2+ and phorbol esters in T lymphocytes (26, 82, 86). Calcineurin is activated by the binding of Ca2+ to calmodulin, which dissociates the two proteins and allows the catalytic site of calcineurin to become accessible. Neurons were exposed for 6 h to cyclosporine (1 mM) or to FK506 (1 mM), two inhibitors of calcineurin acting on different associated immunophilins (cyclophilin and FKBP), after transfection with the reporter plasmids. Cyclosporine was more potent in inhibiting NF-κB, reducing its activity by 40%, while FK506 reached only 20% (Fig. 5A). To confirm this result by a different approach, we cotransfected the NF-κB reporter plasmid with an excess amount (2:1) of expression vectors coding for calcineurin mutants. In the control experiment, an empty vector (pCDNA3) was used as a carrier (see Materials and Methods for details). A constitutively active form of calcineurin, CNAΔCaM, from which the calmodulin-binding domain has been deleted, did not modify the NF-κB level under the normal conditions of culture. In contrast, a dominant-negative mutant, in which the Asp of the catalytic site was mutated to an Asn (CNADN) reduced the luciferase activity to 60%, which is comparable to the activity obtained in the presence of cyclosporine (Fig. 5B). Control experiments conducted with the SRE Luc vector showed that pharmacological agents as well as dominant-interfering constructs did not affect unspecifically the expression potential of treated cerebellar granule neurons. These data therefore demonstrate that, as already described for the activation of T lymphocytes by calcium, the calmodulin-calcineurin pathway plays a role in the constitutive activation of NF-κB in neuronal cells.
FIG. 5.
Requirement of calcineurin in NF-κB basal activity. (A) Pharmacological approach. Primary cerebellar granule neurons were transfected with 0.75 μg of (Igκ)3conaluc plasmid, together with 0.25 μg of the EF1lacZ normalization vector. After 48 h, cells were treated for 6 h with cyclosporine (CsA) (2 μg/ml) and FK506 (1 mM), two inhibitors of calcineurin, before harvesting and recording the luciferase and β-galactosidase activities. Control experiments with SRE Luc constructs were done in parallel. Results are expressed as fractions of the normalized level of activation obtained with control neurons treated with 25 mM of KCl and are representative of three independent experiments. Data are the means + SE (error bars) of measures done in triplicate, expressed as fractions of the normalized level of activation obtained with control neurons treated with 25 mM of KCl (-) and are representative of three independent experiments. (B) Molecular approach. In addition to plasmids described above, neurons were transfected with expression vectors encoding either a constitutively activated form of calcineurin (CNAΔCaM) (0.6 μg), or the dominant-negative construct of the same molecule (CNADN) (0.6 μg), with 0.4 μg of the (Igκ)3conaluc plasmid (see Materials and Methods for more details). In the control experiment, the expression vector was replaced by an empty vector, pcDNA3. Luciferase and β-galactosidase levels were measured 24 h posttransfection in order to avoid potential artifacts associated with cell death. In parallel, an unrelated pathway involving the SRE was tested as control: SRE Luc (0.75 μg) and EF1lacZ (0.25 μg) vectors were transfected and neurons treated in a similar manner as indicated above. Symbols and abbreviations: *, P < 0.01 (significant difference determined by Student's test, compared to the inhibitor-free control); NS, no statistical difference with the control.
PKCs are involved in the activation of NF-κB in neurons.
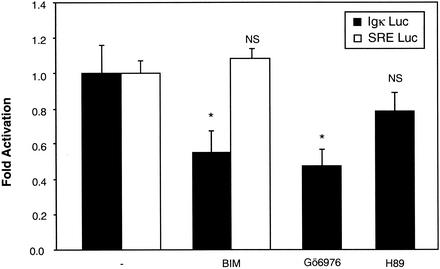
We next addressed the question as to whether Ca2+-responsive PKCs (PKCα, PKCβ, and PKCγ) (38) could be involved in the NF-κB activation pathway in neurons. Using the same transfection assay, neurons were exposed for 6 h to BIM (1 μM), a potent and specific inhibitor of all PKCs, and to Gö6976, a specific inhibitor of the calcium-responsive PKCs, namely, PKCα and PKCβI (3 μM) (53). Both drugs reduced the NF-κB activity to approximately 60% of its basal level, indicating that PKCs are responsible in part for NF-κB activity (Fig. 6). In contrast, the SRE-driven control luciferase gene remained unaffected by treatment with BIM. As a second control, H89 (5 μM), an inhibitor of PKA, did not alter significantly this NF-κB basal activity.
FIG. 6.
NF-κB basal activity involves calcium-dependent members of the PKC family. Neurons transfected with the two reporter plasmids as described in Fig. 3 were subjected to treatment with inhibitors of PKCs. BIM (1 μM) is a potent and selective inhibitor of PKCs, while Gö6976 (3 μM) selectively inhibits Ca2+-dependent PKCα and PKCβI isozymes. As a control, H89 (5 μM), an inhibitor of PKA, was used. As a second control, SRE Luc (0.75 μg) and EF1lacZ (0.25 μg) were transfected, and neurons were subjected to the same treatment as for those transfected with (Igκ)3conaluc plasmids. Data are the means + SE (error bars) of measures done in triplicate, expressed as fractions of the normalized level of activation obtained with control neurons treated with 25 mM of KCl (-) and are representative of three independent experiments. Symbols and abbreviations: * and **, P < 0.01 and P < 0.05, respectively, for significant difference determined by Student's test, compared to the inhibitor-free control; NS, no statistical difference with the control.
The pathway ras/PI3K/Akt is another component of the NF-κB pathway in neurons.
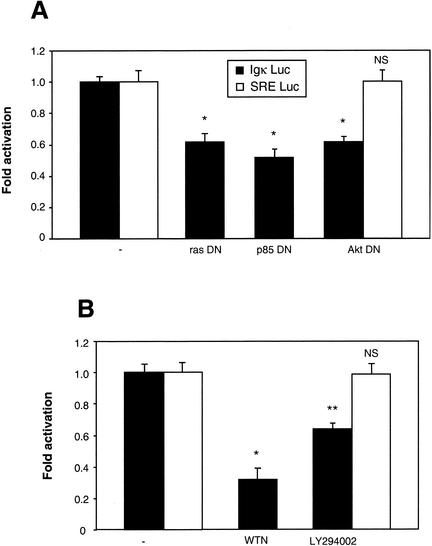
Recent papers have shown that the small GTP-binding protein p21ras (ras) can be activated by Ca2+ signaling, by several potential mechanisms (23). Ras activates the PI3K, which in turn activates the Akt/PKB kinase (48). This last kinase has been suggested to stimulate NF-κB by activating the IKK complex, although this is still a debated issue (5, 19, 44, 63, 74). On the other hand, Akt activation has been suggested to lead to the phosphorylation of the p65 subunit of NF-κB and thereby enhance its transactivation potential (52, 80, 89). To investigate the possibility that this pathway is involved in the constitutive activation of NF-κB observed upon neuron depolarization, we measured NF-κB activity in cotransfection experiments with dominant-negative constructs for ras, PI3K regulatory subunit B, and Akt. Twenty-four hours after transfection the luciferase activity was analyzed, which revealed that all dominant-negative constructs led to a significant inhibition of NF-κB, reducing its activity to around 60% of its normal value (Fig. 7A). To confirm this result, neurons were subjected to 6 h of treatment with wortmannin or LY294002, two inhibitors of PI3K. Both products, and more potently wortmannin, were efficient in reducing the level of luciferase activity (Fig. 7B). In addition, to carefully check that inhibiting the Akt pathways either by a dominant-interfering construct (Akt DN) (Fig. 7A) or by blocking PI3K (LY294002, Fig. 7B) does not provoke neuronal apoptosis under our cell culture conditions, we performed an additional control; we did not detect any unspecific inhibition of luciferase activity controlled by the SRE sequence. These results emphasize the importance of the ras/PI3K/Akt pathway in the basal NF-κB activity observed in primary neurons.
FIG. 7.
The ras/PI3K/Akt pathway is necessary for the basal NF-κB activation in neurons. (A) Inhibitory effect of dominant-negative constructs for ras, PI3K, and Akt. Neurons were cotransfected with (Igκ)3conaluc and EF1lacZ plasmids, together with expression vectors coding for dominant-negative constructs of c-ras (rasDN), the regulating subunit of PI3K (p85DN), or the dominant-interfering vector HA-Akt (K179 M) of the Akt kinase (AktDN) at a ratio of 2:1 with the reporter plasmids. In the control experiment (-), the expression vector was replaced by an empty vector, pcDNA3. Luciferase and β-galactosidase levels were measured 24 h posttransfection in order to avoid potential artifacts associated with cell death. (B) Effects of pharmacological inhibitors of PI3K on the level of NF-κB activity. Neurons were transfected as described in the legend to Fig. 3 and treated with wortmannin (WTN) (2 μM) or LY294002 (25 μM), two specific inhibitors of the PI3K, before recording their luciferase and β-galactosidase activities. Control experiments designed to monitor unspecific inhibitions were performed in parallel using SRE Luc (0.75 μg) and EF1lacZ (0.25 μg) plasmids. Data are the means + SE (error bars) of measures done in triplicate, expressed as fractions of the normalized level of activation obtained with neurons treated with 25 mM of KCl, and are representative of three independent experiments. Symbols and abbreviations: * and **, P < 0.01 and P < 0.05, respectively, for significant difference determined by Student's test, compared to the inhibitor-free control; NS, no statistical difference with the control.
Calcium influxes regulate NF-κB activity at the nuclear translocation level.
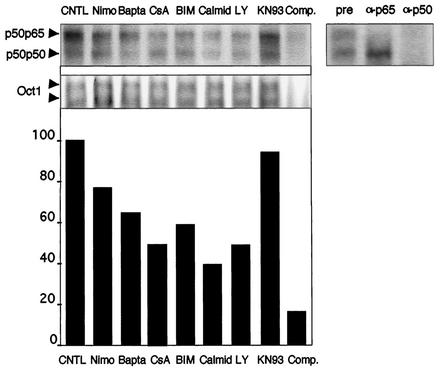
Having shown the involvement of three major pathways mediating signals triggered by intracellular Ca2+ entry, such as PKC, calmodulin-calcineurin, and ras/PI3K/Akt, in the regulation of the basal NF-κB activity, we next decided to determine whether this activity is regulated at the nuclear translocation or at the transcriptional level. We used EMSA to determine the level of nuclear NF-κB, in cells subjected to various inhibitors of the transduction pathways evidenced above (Fig. 8). Two bands were detected, identified as the p50/p65 dimer for the upper one, and as the p50/p50 molecule for the lower one (supershift experiments in the upper right panel). A control experiment was performed with a probe binding the transcription factor Oct-1 (middle panel). This control experiment also demonstrates that the treatments with the various inhibitors were not detrimental to neurons. The intensities of the bands were quantified for both types of dimers, added, and normalized to that of Oct-1 and are presented in the lower panel. We found that inhibitors of the Ca2+ channels (nimodipin), as well as intracellular Ca2+ chelators (BAPTA) led to a significant reduction (30%) of the nuclear level of NF-κB. Similarly, inhibition of calmodulin (with calmidazolium), calcineurin (with cyclosporine), PKC (with BIM), and PI3K (with LY294002) led to an important reduction (50 to 60%) of the nuclear levels of NF-κB. Altogether, these results strongly suggest that both calmodulin and calcineurin, PKC, and PI3K pathways exert a potent regulating effect at the level of nuclear translocation of NF-κB.
FIG. 8.
Calcium and calcium-activated signaling pathways regulate NF-κB activity at the nuclear translocation level in the neurons. Primary cerebellar granule neurons were treated with inhibitors of fast L-Ca2+ channels (nimodipin [Nimo]), chelators of intracellular Ca2+ (BAPTA-AM [Bapta]), inhibitors of calcineurin (cyclosporine [CsA]), PKC (BIM), calmodulin (calmidazolium [Calmid]), PI3K (LY294002 [LY]), and CaMKs (KN93) for 2 h. Nuclear extracts were subjected to a gel shift analysis using a probe for NF-κB (left upper panel), and compared to extracts from control neurons (CNTL). A 100-fold molar excess of the cold probe added to a control extract almost completely abolished the binding of the two bands detected (Comp.). Supershift experiments with antibodies directed against the p65 (α-p65) and the p50 (α-p50) subunits (right upper panel) demonstrate the nature of the two retarded complexes marked by arrows (pre, preimmune serum). As a control for gel loading and for drug specificity, extracts were also analyzed with a probe binding the Oct-1 transcription factor (middle panel). A quantification of NF-κB binding was performed by summing the intensities for the two bands in each lane and normalization to Oct-1(lower panel).
Calcium entry is involved in the activation of NF-κB at the transcriptional level in neurons.
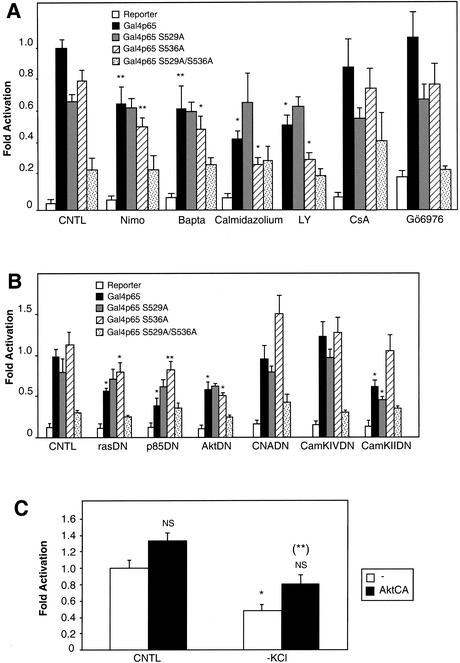
To further analyze whether a control of the basal NF-κB activity may also be exerted over the transcriptional level, we performed cotransfection experiments using Gal4p65, a fusion protein between the transactivating domain of p65 (amino acids 520 to 550) and the DNA-binding domain of the yeast Gal4 transactivator (amino acids 1 to 146), together with a reporter plasmid containing the luciferase gene under the control of a Gal4-responsive element (TP1-Gal4-luc). In the transactivating domain, two residues, serines 529 and 536, have been shown to mediate the action of TNF-α and IKK, respectively (76, 89). We therefore used constructs with mutations for each of these serines (Gal4p65 S529A and Gal4p65 S536A) or both of them (Gal4p65 S529A/S536A) to determine whether the pathways analyzed above were involved in the transactivating activity of the p65 subunit. The results presented in Fig. 9 reveal that blocking Ca2+ entry or intracellular Ca2+ availability resulted in an inhibition of the transactivating level of Gal4p65 and Gal4p65 S536A by about 35%, but not of Gal4p65 S529A, suggesting that serine 529 is the only target of this transactivating regulation (Fig. 9A). Similar results were obtained using calmidazolium, an inhibitor of calmodulin, and LY294002, a blocking agent for PI3K. In contrast, calcineurin (inhibited by cyclosporine) and PKC (inhibited by Gö6976) did not play a role in this regulation. We confirmed this pharmacological approach, using dominant-negative mutants for the ras/PI3K/Akt pathway in cotransfection experiments, with the results shown in Fig. 9B. As mentioned above, Akt is able to elicit phosphorylation of the p65 subunit of NF-κB on its transactivating domain, thereby increasing its transactivating efficiency (52, 80, 89). All mutants of the kinases involved in this pathway reduced the efficiency of the transactivating domain of p65 to 40 to 60% of its full level, indicating that the ras/PI3K/Akt pathway plays a role in the transactivating activity. In contrast, a dominant-negative form of calcineurin (CNADN) had no effect. It is noteworthy that the activity of the Gal4p65 S529A mutant was not affected by these treatments (excepted with a dominant-negative construct coding for the IKKβ [data not shown] and, surprisingly, with a dominant-negative construct for CaMKII), as well as that of the double mutant Gal4p65 S529A/S536A, which was totally unaffected, thus invalidating the possibility of unspecific mechanisms like necrosis or apoptosis to account for the observed inhibitions of NF-κB activity. In addition, when used alone, the reporter plasmid elicited only a residual, but constant activity in neurons.
FIG. 9.
Calcium entry and the Akt pathway stimulate the transactivating potential of p65 in primary neurons. Neurons were transfected with either pcDNA3, Gal4p65, Gal4p65 S529A, Gal4p65 S536A, or Gal4p65 S529A/S536A vectors, together with a plasmid encoding a Gal4-responsive luciferase construct (TP1-Gal4-luc) and the EF1lacZ normalizing vector. Data are the means + SE (error bars) of measures done in triplicate, expressed as fractions of the normalized level of activation obtained with neurons treated with 25 mM of KCl, and are representative of three independent experiments. Symbols and abbreviations: * and **, P < 0.01 and P < 0.05, respectively, for significant difference determined by Student's test, compared to the inhibitor-free control; NS, no statistical difference with the control. (A) Pharmacological blockers of PI3K inhibit the activity of the transactivating domain of p65 in a serine 529-dependent manner. Nimodipin (Nimo) (10 μM) is a Ca2+ channel blocker, BAPTA-AM (Bapta) (50 μM) is an intracellular Ca2+ chelator, calmidazolium (5 μM) inhibits specifically calmodulin, and LY294002 (LY) (25 μM) is a potent inhibitor of PI3K. Cyclosporine (CsA) (2 μg/ml) inhibits the activity of calcineurin, and Gö6976 (3 μM) is an inhibitor of PKCs. These inhibitors were added to neurons for 6 h. (B) Dominant-negative constructs inhibit the transcriptional potential of the p65 transactivating domain. The plasmids described above were cotransfected with dominant-interfering mutants for p21ras (rasDN), PI3K regulating subunit (p85DN) or Akt (AktDN) at a ratio of 1:1 with the reporter plasmids and at a ratio of 10:1 with the empty vector, pcDNA3, or each of the Gal4p65, Gal4p65 S529A, Gal4p65 S536A, Gal4p65 S529A/S536A expression vectors (see Materials and Methods). Dominant-interfering mutants for calcineurin (CNADN) and calmodulin kinases CaMKIV and CaMKII were also tested. Luciferase and β-galactosidase levels were measured 24 h posttransfection in order to avoid potential artifacts associated with cell death. Results are expressed as activation with respect to the level obtained with control neurons transfected with the (Igκ)3conaluc together with an empty expression vector (-), and normalized with the β-galactosidase activity for each well. (C) An active form of Akt kinase rescues NF-κβ activity following KCl deprivation. Primary cerebellar granule neurons weretransfected at 3 DIV with the vectors Gal4p65, TP1-Gal4-luc, and EF1lacZ. Twenty-four hours later, the cultured medium was either replaced with culture medium supplemented with 25 mM KCl (CNTL) or 5 mM KCl (−KCl). Six hours later, luciferase and β-galactosidase activities were recorded, and the luciferase activity normalized. Symbols and abbreviations: NS and *, no statistical difference and a significant difference (P < 0.01) with the control (CNTL, open bar: 25 mM KCl and transfection of an empty vector); (**), significant difference (P < 0.05) with mock-transfected neurons placed in medium supplemented with 5 mM KCl (−KCl, open bar).
To further confirm that Akt kinase is effectively able to stimulate the transactivation potential of NF-κB in primary neurons, we cotransfected vectors expressing the constitutively activated form of Akt (AktCA) into cerebellar granule neurons, together with the Gal4p65 and TP1-Gal4-luc expression vectors. While expression of the activated form did not unequivocally induce a modification of the constitutive level of NF-κB-driven luciferase activity, deprivation of KCl, which diminishes this activity by 40%, was partially compensated for by the activated form of Akt kinase (Fig. 9C). These results therefore confirm and extend to primary neurons previous results showing that Akt kinase stimulates the activity of the transactivation domain of p65.
Cross talks between pathways in neurons.
The results presented above demonstrate that signal transduction in neurons may use at least three different pathways which contribute to the NF-κB transcriptional activity: calmodulin and calcineurin, the PKC pathway, and the ras/PI3K/Akt cascade. In an attempt to evaluate the interrelations between these three different pathways, we designed experiments in which simultaneous inhibition of molecules involved in two different pathways were used. In these assays, we hypothesized that if the two specific inhibitors used simultaneously have a stronger effect than each of the inhibitors alone, the two pathways are likely to be independent, and each would contribute to the resulting NF-κB-dependent activity. On the contrary, if the effect of the two inhibitors used simultaneously is equivalent to the effect of one inhibitor, then it is probable that the two pathways are not independent. These assays were all performed using the (Igκ)3conaluc reporter vector, which monitors the global NF-κB activity, thus recording the resulting action from both nuclear translocation and transcriptional activities.
Results listed in Table 1 show that, when recording NF-κB activity, simultaneous inhibition of both Akt and calmodulin (82% inhibition) is more efficient than inhibition of calmodulin alone (69%) or Akt alone (37%), indicating therefore that the two pathways are not directly linked (Table 1, calmodulin plus Akt). We then determined the relationship between PKCs and calmodulin pathways and found an additive effect of the inhibition of both pathways (68%) (Table 1, calmodulin plus PKC), indicating that the two pathways may be independent with regard to NF-κB. In contrast, we found that inhibition of PKCs and Akt (Table 1, PKC plus Akt) was not additive, suggesting that PKC and Akt do not use independent pathways for the stimulation of NF-κB. To determine whether PKCs and calcineurin have an additive or synergistic effect on NF-κB activity, by analogy with results found in T cells, we treated neurons with inhibitors of calcineurin and PKC. We found that the use of inhibitors of both pathways simultaneously resulted in a stronger inhibition (80%), which was statistically different from that observed with each individual inhibitor (61% for CNA and 42% for PKC), suggesting a convergence of the calcineurin- and PKC-driven NF-κB activation pathways (Table 1, PKC plus calcineurin).
TABLE 1.
Interactions between main Ca2+-to-NF-κB signaling pathwaysa
| Pathways | % Inhibition of NF-κB activity with inhibition of:
|
Pb | Commentc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaM | PKC | Akt | CNA | CaM + PKC | CaM + Akt | PKC + Akt | CNA + PKC | |||
| CaM + Akt | 69 | 37 | 82 | <0.05 | + | |||||
| CaM + PKC | 56 | 23 | 68 | <0.05 | + | |||||
| PKC + Akt | 26 | 33 | 37 | NS | = | |||||
| PKC + CNA | 42 | 61 | 80 | <0.05 | + | |||||
Main interactions between signaling pathways for NF-κB. All experiments where conducted as in Fig. 2. Calmodulin (CaM) was inhibited using W7; PKC and Akt pathways were blocked using BIM and wortmannin, respectively; and calcineurin (CNA) was inhibited by cyclosporine.
A P of <0.05 indicates significant differences determined by Student's test for double inhibition compared to each single inhibition. NS, not significantly different.
Symbols: =, the value resulting from inhibition of both pathways is equal to the lowest value resulting from inhibition of a single pathway; +, the percentage of inhibition obtained by inhibiting both pathways is lower than that resulting from inhibition of a single pathway.
Calmodulin, PKC, and Akt activities are dependent upon Ca2+ influx in cerebellar granule neurons.
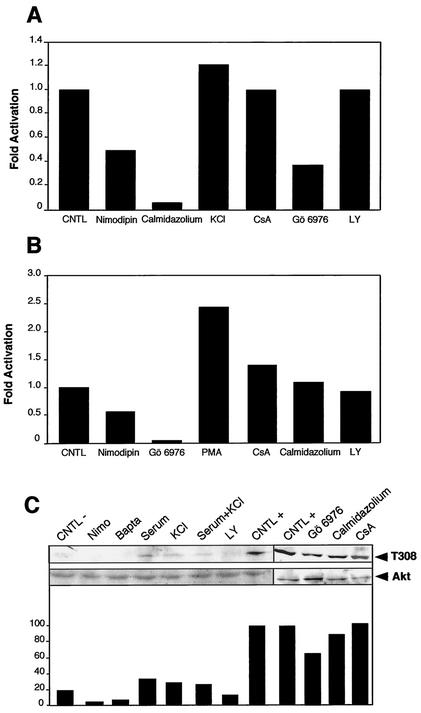
To confirm the involvement of calmodulin, PKC, and Akt, their activities were monitored. Calmodulin activity was monitored indirectly by analyzing a kinase activity which requires calmodulin to function (CaMKII), thereby reflecting calmodulin's levels of activation. Neuronal extracts were analyzed for CaMKII activity using myelin basic protein as a substrate, [γ-32P]ATP, and inhibitors of PKA and PKC (Fig. 10A). In neurons treated with nimodipin, an antagonist of Ca2+ channels, the activity was reduced by 50%, while increasing the extracellular concentration of KCl to 60 mM stimulated the activity by 25%. This result confirms the role of Ca2+ entry in the basal activity of calmodulin in these neurons. As a control, calmidazolium, a potent blocker of calmodulin, totally abolished the activity, demonstrating the specificity of the reaction. Control experiments with drugs blocking unrelated pathways, like calcineurin (cyclosporine) or PI3K (LY294002) had no effects. Unexpectedly, Gö6976, which specifically inhibits some Ca2+-activated PKCs, reduced the calmodulin activity by 60%, suggesting a possible link between PKC and calmodulin in these cells. Neuronal extracts were analyzed in a similar manner for PKC activity using a specific peptide as substrate, [γ-32P]ATP, and inhibitors of PKA and calmodulin. When neurons were treated with nimodipin, PKC activity was reduced by a factor of two, confirming the role of Ca2+ in its basal activity (Fig. 10B). Phorbol myristate acetate (PMA) stimulated this activity 2.5-fold, while a specific inhibitor of Ca2+-activated PKC (Gö6976) reduced the activity to a basal level, confirming the specificity of the reaction. Further controls with blocking agents against calcineurin (cyclosporine) and calmodulin (calmidazolium) did not show any inhibition. Akt activation was also analyzed by immunoblotting with an antibody specific to phospho-Akt (T308) (Fig. 10C, upper panel), which monitors the level of activation of this kinase (18). Quantification of the intensity of the bands revealed that inhibitors of Ca2+ reduced the phosphorylation by more than 60% compared to control neurons deprived of serum and KCl (Fig. 10C, lower panel). In contrast, stimulation with 60 mM KCl for 10 min, as well as serum stimulation, enhanced the activation of Akt. Both stimuli applied simultaneously gave similar results. Neurons kept continuously in regular medium supplemented with serum and 25 mM KCl displayed fivefold more activity than did deprived cells. Inhibition of PI3K by LY294002 reduced the phosphorylation of Akt by 50% when compared to the control. Subjected to drugs inhibiting calmodulin (calmidazolium) or calcineurin (cyclosporine), Akt activity was not modified in treated neurons, thus confirming the specificity of the pathways toward Akt. It is noteworthy, however, that inhibiting Ca2+-activated PKC with Gö6976 significantly reduces Akt activity. Taken together, these results show that calmodulin, PKC, and Akt activities depend on Ca2+ influxes in cerebellar granule cells, confirming the results described above.
FIG. 10.
CaMKII, PKC, and Akt kinase activities in neuronal extracts. (A) Calmodulin is activated at a basal level in neurons. Calmodulin activation was indirectly monitored in cerebellar granule neurons by analyzing CaMKII activity. Neuronal extracts were analyzed for CaMKII activity using myelin basic protein as substrate, [γ-32P]ATP, and inhibitors of PKA and PKC. Phosphorylated substrates were retained on a phosphocellulose paper, and the radioactivity was measured. The use of an antagonist of Ca2+ channels (nimodipin) reduced the activity by 50%, while increasing the extracellular concentration of KCl to 60 mM stimulated the activity by 25% (KCl). Calmidazolium, a potent blocker of calmodulin, totally abolished this CaMKII activity. Specific inhibitors for calcineurin (cyclosporine [CsA]), Ca2+-dependent PKCα and PKCβI isozymes (Gö6976), and PI3K (LY294002 [LY]) were also used as additional controls. (B) PKC activity partially depends on Ca2+ influxes in cerebellar granule cells. Neuronal extracts were analyzed for PKC activity using a specific peptide as substrate, [γ-32P]ATP, and inhibitors of PKA and calmodulin. Phosphorylated substrates were retained on a phosphocellulose paper, and the radioactivity was measured. The use of an antagonist of Ca2+ channels (nimodipin) reduced the activity by 50%. PMA stimulated this activity 2.5-fold, while a specific inhibitor of Ca2+-activated PKC (Gö6976) reduced the activity to a basal level. In contrast, inhibitors of calcineurin (CsA), calmodulin (Calmidazolium), and PI3K (LY294002) did not modify PKC activity. (C) Akt activation by Ca2+ influxes in neurons. Akt activation was analyzed by immunoblotting with an antibody specific to phospho-Akt (T308) (upper panel). As a control, total Akt was also determined (middle panel). Quantification of the bandsfrom the upper panel was performed and is represented in arbitrary units (lower panel). Abbreviations: CNTL −, cells were deprived of serum and KCl 2 h before harvesting; Nimo and Bapta, addition of nimodipin and BAPTA-AM, respectively; Serum, FCS and horse serum (each at 5%); KCl, 60 mM KCl (KCl); Serum+KCl, FCS and horse serum (each at 5%) and 60 mM KCl; LY, LY294002; CNTL +, cells incubated in the normal medium containing FCS and horse serum (5% each) and 25 mM KCl. Inhibitors were added 30 min before cells were harvested. Additional controls were performed with specific inhibitors of calmodulin (Calmidazolium), Ca2+-dependent PKCs (Gö6976), and calcineurin (CsA).
Comparison of Ca2+-mediated basal NF-κB activity in neurons to other cell types.
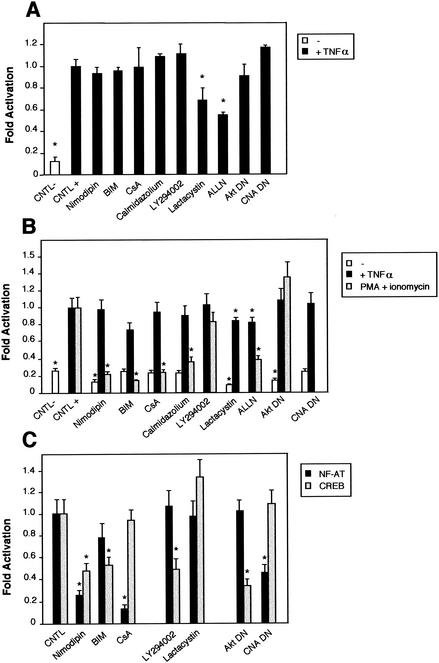
We next determined whether the pathways described previously were specific to the basal NF-κB activity observed in neurons. We therefore tested first if TNF-α-mediated NF-κB activation could be blocked by inhibitors of calmodulin, PKC and Akt pathways in granule cerebellar cells. Neurons were transfected with the NF-κB-responsive (Igκ)3conaluc plasmid, together with the EF1lacZ normalization vector; neurons were treated 48 h later with various inhibitors for 6 h; and the luciferase activity was measured. None of the inhibitors involved in the calmodulin-calcineurin, PKC, or PI3K-Akt pathways were able to inhibit NF-κB activity (Fig. 11A). In contrast, nonspecific inhibitors like lactacystin or ALLN acting on the proteasome inhibited this activity. These results demonstrate that the NF-κB signaling pathways described above are not involved in the activation of NF-κB by TNF-α in cerebellar granule neurons.
FIG. 11.
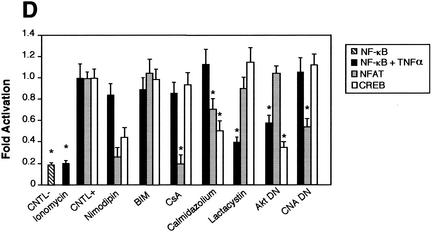
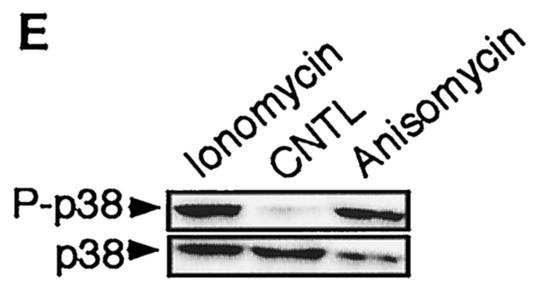
Comparison of the NF-κB activity in neurons with the requirement of Ca2+ in other cells. Cells were transfected with 0.75 μg of either the NF-κB-responsive (Igκ)3conaluc, NF-AT, or CREB-responsive plasmid together with 0.25 μg of EF1lacZ normalization vector; cells were treated for 6 h with various inhibitors or solvents 48 h later; and the luciferase as well as the β-galactosidase activities were measured. Data are the means + SE (error bars) of measures done in triplicate, expressed as fractions of the normalized level of activation obtained with cells in standard conditions, and are representative of three independent experiments. Symbols and abbreviations: *, P < 0.01 (significant difference determined by Student's test, compared to the reference); CNTL -, treatment with solvent; CNTL +, treatment with TNF-α and no inhibitors. (A) Ca2+ is not involved in the activation of NF-κB by TNF-α in cerebellar granule neurons. None of the inhibitors of the calmodulin-calcineurin, PKC, or Akt pathways was able to inhibit NF-κB activity, in contrast to proteasome inhibitors like lactacystin or ALLN. (B) NF-κB activity is not affected by inhibitors of the Ca2+ signaling pathways in Jurkat cells treated with TNF-α but is inhibited when activated by PMA plus calcium ionophores. Forty-eight hours after transfection with (Igκ)3conaluc and EF1lacZ plasmids, cells were exposed to various inhibitors of the Ca2+ to NF-κB signaling pathways described above and to TNF-α or ionomycin plus PMA 30 min later, for 6 h. None of the inhibitors shown to specifically inhibit NF-κB basal activity in neurons was active when these cells were stimulated with TNF-α, in contrast to stimulation with PMA plus ionomycin. Note that inhibitors of the PI3K/Akt pathway, LY294002 and Akt, are ineffective in these cell stimulated with PMA and ionomycin. (C) As controls for the activity of the drugs or the dominant-interfering constructs, two vectors containing the luciferase gene controlled by either NF-AT or CREB transcription factors were transfected into Jurkat cells, together with the normalizing plasmid EF1lacZ. NF-AT is activated through the calcium/calmodulin/calcineurin pathway, and CREB is responsive to the calcium/calmodulin kinases as well as to Akt. Drugs similar to those used in panel B were used for 6 h, 48 h after transfection, and the activities were measured immediately thereafter. (D) Inhibitors of Ca2+ signaling are unable to antagonize NF-κB activation in HeLa cells stimulated by TNF-α. Forty-eight hours after transfection with (Igκ)3conaluc and EF1lacZ plasmids, cells were exposed to various inhibitors of the Ca2+ to NF-κB signaling pathways described above and to TNF-α 30 min later, for 6 h. With the exception of lactacystin and of the expression of a dominant-negative construct of Akt, none of the inhibitors shown to specifically inhibit NF-κB basal activity in neurons was active in these cells. Cells were exposed to ionomycin to show that Ca2+ entry does not stimulate NF-κB activity in these cells. As controls for the efficiency of the pharmacological agents and of the dominant-negative constructs, HeLa cells were transfected in parallel with NF-AT and CREB-responsive reporter vectors. The same parameters as those used for (Igκ)3conaluc were used. (E) p38 kinase is activated by calcium entry in HeLa cells. HeLa cells were treated with anisomycin (1 μM), a specific activator of the p38 pathway, or ionomycin (1 mM), a calcium ionophore, for 1 h. Cellular extracts were subjected to immunoblot analysis with a primary antibody directed against phosphothreonine 180 and phosphotyrosine 182, indicative of p38 activation (P-p38). To check for correct loading, same extracts were analyzed using an antibody directed against the whole p38 (p38).
We then compared the characteristics of NF-κB activation in neurons to that in other types of cells in which calcium has been found to play a role in the activation of signaling pathways. In T lymphocytes, ionomycin, a calcium ionophore, has been shown to synergize with PMA to stimulate NF-κB activation (82, 86). We demonstrated that it was possible to inhibit the activation of NF-κB with PMA plus ionomycin in Jurkat cells by using inhibitors of Ca2+ entry (nimodipin) or of related pathways like calmodulin (calmidazolium), calcineurin (cyclosporine), or PKCs (BIM), but not with LY294002, an inhibitor of PI3K, or a dominant-negative construct for Akt (Fig. 11B). At a basal level, however, this Akt dominant-negative construct could significantly reduce NF-κB basal activity in these T cells (open bar). In contrast, inhibitors of calmodulin, calcineurin, or PKC were unable to inhibit the basal level of NF-κB in Jurkat cells.
To confirm that pharmacological agents or dominant-negative constructs were indeed efficient in this cellular context, we used two vectors containing the luciferase gene controlled by either NF-AT or CREB transcription factors. NF-AT has been shown to be activated through the calcium/calmodulin/calcineurin pathway (72), while CREB is responsive to the calcium/calmodulin kinases as well as to Akt (70, 84). Figure 11C clearly shows that nimodipin, which is blocking L-Ca2+ channels; cyclosporine, which inhibits calcineurin; calmidazolium, an anticalmodulin agent; and a dominant-negative construct for calcineurin inhibit NF-AT-driven luciferase activity as expected, while blocking unrelated pathways (BIM for PKCs and LY294002 for PI3K) had no effect. Similarly, a CREB-responsive reporter vector has its activity inhibited, compared to the control conditions, only by blocking the calmodulin kinases or PI3K/Akt pathways.
To further explore if other cells responding to cytosolic calcium can also trigger the activation of NF-κB, we then analyzed HeLa cells to determine the possible involvement of the different pathways we identified previously in neurons. These cells activate the calcium/calmodulin/CaMKII pathway as well as the p38 mitogen-activated protein (MAP) kinase pathway in response to calcium (61, 65). First, calcium influxes triggered by ionomycin did not elicit NF-κB activation in HeLa cells (Fig. 11D). Second, inhibitors of Ca2+ channels (nimodipin), calmodulin-calcineurin, PKC, Akt, and CaMK kinase (CaMKK) were unable to inhibit the activation of NF-κB by TNF-α, with the exception of an Akt dominant-negative construct in HeLa cells. As controls for the efficiency of the different treatments, the use of a NF-AT-responsive constructs demonstrated that cyclosporine, calmidazolium, and a dominant-negative construct effectively inhibited the Ca2+/calmodulin/calcineurin pathway. Similarly, a CREB-responsive construct clearly showed that inhibitors of the Ca2+/calmodulin/CaMKK, as well as the Akt pathway are efficient in HeLa cells.
To demonstrate that although calcium entry in HeLa cells has no effect on NF-κB activity, it is still effective on other pathways, we used the fact that a MAP kinase-associated protein kinase (also known as PRAK) was shown to be activated by ionomycin in HeLa cells (61). As PRAK is essentially activated by the MAP kinase p38, we checked to see whether it was possible to activate p38 in HeLa cells through the intracellular entry of external calcium provoked by ionomycin. Using antibodies directed against phosphorylated threonine 180 and tyrosine 182 that are phosphorylated upon p38 activation, we showed (Fig. 11E) that Ca2+ was able to activate the p38 pathway but not the NF-κB pathway in HeLa cells. These results led us to conclude that this combination of pathways, involving mainly calmodulin-dependant kinases, PKC and Akt, as well as their specific cross-talks in response to Ca2+ influxes to the cytosol, seem to be more specific to cerebellar granule neurons than to other type of cells, to relay signals from calcium entry to NF-κB.
DISCUSSION
One important issue that remains to be resolved in neuron physiology is how a signal that is released by neurotransmitters binding to their receptors at the synapse is transduced into the nucleus to activate gene transcription. A family of transcription factors, NF-κB, seems to play general as well as specific roles in neurons. Despite the importance of NF-κB in neuron function, little is known about the signaling pathways leading to NF-κB activation. In this study, we have chosen to utilize primary cultures of neonatal cerebellar granule neurons, which require depolarization for survival, to investigate the mechanisms by which Ca2+ stimulates NF-κB-mediated gene expression. Exposure of cerebellar granule neurons to elevated levels of extracellular KCl concentrations depolarizes the plasma membrane and stimulates the opening of L-VSCCs. We first showed that the level of NF-κB activity was proportional to the concentration of extracellular KCl, and then demonstrated that only fast L-VSCCs were involved in this Ca2+-mediated NF-κB activation (Fig. 3B). We then demonstrated that a certain level of intracellular Ca2+ is necessary for the full level of NF-κB basal activity (Fig. 3C). Although the inhibition was partial with BAPTA-AM, a chelating agent of intracellular Ca2+, with 40% residual activity, it was possible to further reduce this activity to the level of the control vector by removing the serum from the medium (data not shown). Calcium is involved in the induction of NF-κB activity following various stimuli. In neurons, activation of NF-κB is triggered by glutamate (30, 32, 43), and in other types of cells, various stresses cause the activation of NF-κB via the mobilization of Ca2+ (27, 33, 34, 40, 64). Moreover, several physiological signals were also shown to mobilize intracellular Ca2+ and activate NF-κB (54, 71, 79, 83), contributing to a growing number of reports involving Ca2+ as a second messenger necessary for the induction of NF-κB. Having shown the role of Ca2+ in the stimulation of NF-κB in primary neurons, we then examined which molecules could further mediate signaling. One of the most important molecules integrating the Ca2+ signal is calmodulin. We found an important decrease in the level of NF-κB constitutive activity when we specifically inhibited calmodulin, at both nuclear translocation and transactivation levels, suggesting the central importance of this molecule in NF-κB activity in neurons (Fig. 4, 8, and 9). Since one of the targets of calmodulin is calcineurin, we used a pharmacological and a molecular approach, which has a low probability of interfering nonspecifically with the same molecules, to demonstrate the requirement of calcineurin for the activation of NF-κB in cerebellar granule neurons (Fig. 5A). Other kinases known to be activated by Ca2+ entry in the cytosol are Ca2+-sensitive PKCs. Using specific inhibitors to block the PKCs and more specifically the Ca2+-sensitive PKCα and PKCβI isozymes (Fig. 6), our results show a down-regulation of NF-κB-mediated transcriptional activation. A variety of extracellular stimuli known to activate PKC also activate NF-κB (8, 9, 33, 49, 50, 68, 85, 94). Recently, it has also been suggested that PKCα as well as the atypical PKCs bind to the IKKs in vitro and in vivo and stimulate the activity of IKKβ but not that of IKKα (47). These data therefore suggest a critical role for the PKC isoforms in the NF-κB pathway. In contrast to synergistic effects previously described in T lymphocytes (26, 82, 86), using specific inhibitors of each pathway (Table 1), we found distinct actions between PKCs and calcineurin in neurons. However, the mechanism whereby calcineurin converge with PKC-dependent pathways to activate IKK complex is still unknown, but it is noteworthy that these molecules do not seem to play a role in p65 transcriptional activity (Fig. 9).
Ca2+ influx through L-VSCCs triggers p21ras (ras) activation within 30 s of stimulation (28). Ras activation is known to stimulate, in particular, PI3K and Akt (21, 24). Akt inhibits cell death pathways by directly phosphorylating and inactivating proteins involved in apoptosis, including Bad, procaspase 9, and members of the forkhead transcription family (18). In addition it can stimulate signaling pathways that upregulate the activity of transcription factors. Recently, Akt has also been shown to be involved in the activation of NF-κB (5, 6, 44, 52, 63, 74, 80, 89). In this study, using a pharmacological and a molecular approach, we demonstrate that ras, PI3K, and Akt are all necessary for the transcriptional activity of NF-κB (Fig. 7). The results presented here confirm and extend the recent data obtained by others concerning a role for PI3K and Akt in the survival of sympathetic and cerebellar granule cells (17, 20, 60, 66). PI3K is also necessary for the phosphorylation and activation of the NF-κB p65 subunit in response to IL-1β (73, 80) or to epidermal growth factor (7). However, these results have not been extended to explain the role of Akt in neurons. Many of the physiological inducers of the PI3K/Akt pathway also stimulate the transcriptional activity of NF-κB (5, 6, 44, 52, 63, 73, 74, 80, 89). Several of these reports suggest that Akt stimulates NF-κB transcriptional activity through direct mechanisms involving IKKs and NF-κB nuclear translocation. However, other authors claim that PI3K or Akt stimulates NF-κB by phosphorylating the transactivating domain of the p65 subunit (6, 52, 80), in particular at serine 529 (89), although it does not necessarily exclude the role of the IKK complex. Here we demonstrate that inhibition of the Akt pathways, using either pharmacological inhibitors of PI3K, or dominant-interfering molecules for the ras/PI3K/Akt pathway, blocks the translocation of NF-κB from cytosol to nucleus (Fig. 8) as well as p65 transactivating activity (Fig. 9).
Inhibition of Ca2+ signaling by deprivation of KCl or other treatments resulted in a decrease in the p65 transactivating activity to 50% of its control value (Fig. 9C). Furthermore, inhibition of calmodulin with two different pharmacological inhibitors resulted in a decreased transcriptional activity of p65 (Table 1; Fig. 9A). In addition, recent reports showed that the CaMKIV also activates p65 directly (39), while CaMKII was found to activate IKK (35). This identified a Ca2+-triggered signaling cascade which may stimulate p65 transactivating potential. We therefore checked to see if CaMKs could establish a link between Ca2+/calmodulin on one hand and p65 on the other hand. Surprisingly, we found that only the dominant-negative mutant for CaMKII inhibits 40% of p65 transactivating activity (Fig. 9B). In contrast, CaMKIV dominant-negative mutants did not interfere with this activity. Another surprise came from the result that only the p65 mutant with a mutation on serine 529 was affected by a dominant-negative CaMKII construct, but not a mutant with a mutation on serine 536, suggesting that CaMKII may exert its action by phosphorylating this last residue, unlike the other kinases. In conclusion, in the present work we show that Ca2+ is necessary for full activity of the p65 transactivation domain (Fig. 9), and that CaMKII is also involved in NF-κB signaling, therefore reinforcing the link between NF-κB and Ca2+.
We were concerned in this report by the fact that several other publications reported the involvement of the transduction pathways discussed above in terms of survival or apoptosis in neurons. High extracellular KCl concentrations are necessary for cerebellar granule neurons to survive, and blocking signals provided by elevation of cytosolic Ca2+ elicits cell death (25). Moreover, it has recently been shown that inhibiting PI3K or Akt in neurons or inhibiting NF-κB in sympathetic neurons leads to cell death (18, 24). We provide several arguments to show that the experimental conditions used in this work avoid significant apoptosis in the cultures. (i) Neurons were analyzed at 4 to 6 DIV, when Ca2+ requirement just begins, instead of at 7 to 8 DIV, when neurons are much more sensitive to changes in their culture conditions. (ii) When studying apoptosis, in order to enhance the effects of the various treatments, serum is usually removed (46, 67, 95), neurons are treated for 24 h, and cellular density is reduced; in the majority of our assays, serum was kept at normal concentrations, neurons were treated with drugs for only 6 h and harvested immediately thereafter, and high cellular densities were used. (iii) All the drugs were tested at their pharmacological concentrations during 6 h, using MTT reduction as an indication of mitochondrial function and cell viability (see Materials and Methods). Except for calmidazolium, whose working concentration was adjusted, none of the inhibitors modified the metabolic activity as revealed by MTT (data not shown). (iv) Control experiments with an SRE controlling a luciferase reporter gene (SRE Luc), used to monitor neuronal functioning, were not altered by the different drugs used in this study (Fig. 2 to 7). (v) All NF-κB-driven luciferase activities were normalized to the β-galactosidase activity controlled by a housekeeping EF1 promoter; a nonspecific loss of activity due to inhibition of protein synthesis would be compensated by the normalization. Altogether, these elements indicate that the inhibition of NF-κB activity in neurons treated with inhibitors is likely to reflect the blockade of specific signal transduction pathways instead of being the consequence of massive apoptosis or necrosis.
Previous studies have focused on elucidating specific pathways leading from signaling events at the plasma membrane to NF-κB activation. To our knowledge, this is the first attempt to simultaneously analyze the integration of different transduction pathways in primary neurons. Our results show that a complex network of signaling molecules contributes to NF-κB activation, following the elevation of intracytoplasmic Ca2+, as described in Fig. 12. This signal then diverges in at least three linear pathways of signal transduction identified in the present work, respectively, involving PKC, ras, and calmodulin. First, PKC may integrate two types of signals—one coming from membrane receptors acting through phospholipase C, like TrkB, TrkC, and p75 expressed in rat cerebellar granule neurons (16) and the second elicited by Ca2+ for Ca2+-sensitive PKCs (38). Atypical PKCs (PKCζ, PKCι, and PKCλ) have been shown in many instances to play a role in modulating the NF-κB pathway, by binding IKKs directly and modulating more specifically IKKβ (47). It is therefore not unlikely that Ca2+-sensitive PKCs could also bind IKKs in neuronal cells. The ras pathway acts similarly, integrating membrane receptor signals through small G proteins and Ca2+ signals through ras-GRF, and depending on the neuronal context, may activate PI3K (20, 60, 81). Subsequent activation of Akt has been reported to stimulate NF-κB activity, although the exact mechanism is not clear: Akt has been shown to enhance the degradation of the IκBs and to associate to the IKK complex in vivo, but it is also involved in the NF-κB transcriptional activity (52, 63, 74). The third transduction pathway analyzed here is initially only concerned with Ca2+ and is constituted by calmodulin, which regulates phosphatases and kinases, among which calcineurin and CaMKII have been identified in pathways leading from Ca2+ to NF-κB in the present work. Recently, it has been shown that CaMKIV stimulates NF-κB transactivation via the phosphorylation of the p65 subunit, although the precise target site is not known (39). CaMKII is also able to stimulate IKK, following a stimulation by T cell receptors/CD3 or by PMA in Jurkat cells (35). In neurons, we found that CaMKII was effectively involved in the transactivation potential of p65 (Fig. 9B).
FIG. 12.
Schematic diagram highlighting the proposed signaling pathways leading from the plasma membrane to nuclear NF-κB in neurons. The signaling pathways originating from intracellular calcium entry diverge into three main directions involving PKC, calmodulin-derived, and ras/PI3K/Akt cascades to refocus in particular on IKK complexes and on p50/p65 dimers. Circled intermediates indicate hypothetical points of convergence of pathways regulated by both growth factors, neurotrophins, and calcium signaling cascades in the cytosol. Dashed lines indicate potential interconnections between the different pathways (see Discussion). InP3R, InP3 receptor.
Cross talk between these pathways is also possible: PKC can be phosphorylated by PI3K (12). Several reports have identified that PKC isozymes are mediators of PI3K-dependent and -independent signaling. In parallel, there are new findings showing PKC-dependent phosphorylation of Akt and modulation of its activity, indicating a cross talk between the two pathways (4). Akt activity can also be modulated by calmodulin through a CaMKK (97). This activation of Akt by CaMKK appears to be important in the protection of neurons from programmed cell death during development. However, we found these two pathways to be independent in cerebellar granule neurons (Table 1). Calcineurin also participates in cross talk occurring in the transmission of the Ca2+ signaling, possibly by binding to PKC through the scaffold protein AKAP79 and synergizing with it (22). At the end of the pathway, the IKK complex, which may act as an integrator, receives signals triggered by Ca2+ and membrane receptors. The ultimate level of integration of the signals is the p50/p65 complex, which is controlled at two levels: the level of translocation from cytosol to nucleus by IKKs—controlled by calmodulin/calcineurin, PKC, and PI3K/Akt kinases or phosphatases—and the transactivation level—controlled by calmodulin/CaMKII and Akt (which may also involve the IKK complex as an integrator) (Fig. 12). Finally, this network seems to be specific to cerebellar granule neurons since no other cell tested displayed the same combination of Ca2+-dependent pathways and cross talk between pathways leading to stimulation of NF-κB basal activity.
Acknowledgments
We thank L. Madrid (University of Virginia), L. Schmitz (Genzentrum München), and B. Keupes and H. Gruffat (Institut für Klinische Molekularbiologie und Tumorgenetik) for providing Gal4p65 constructs, Gal4VP16, and TP1-Gal4-luc expression vectors, respectively; M. Karin (University of California—San Diego) for the pRasDN plasmid; Masto Kasuga (Kobe University) for the kind gift of pGEX-Δp85; Thomas F. Franke (Columbia University) for the generous gifts of myrAkt-HA, HA-Akt (K179 M), and HA-Akt expression vectors; G. R. Crabtree (Stanford University) for pCNA; and Michel Kobr (Ecole Polytechnique Fédérale de Lausanne) for the kind gift of pCNB. We also thank J.-P. Loeffler (Strasbourg University-CNRS) for providing the CaMKIV expression vector and T. Grundström (Umeå University) for the gift of CaMKII wild-type and mutant constructs. We thank Gilles Courtois for valuable discussions during the course of this work, Robert Weil for help with Jurkat cells and for judicious advice in experiments dealing with the p65 transactivating potential, Laurence Arbibe for help and antibodies for Akt kinase activity analysis, Christine Bessia for help with Jurkat cell transfections, and Gillian Butler-Browne for carefully reading the manuscript, and we acknowledge comments provided by Jean-Marie Dupret. We are also grateful to Patrick Vicart for full support during the course of this work.
The work presented here was supported in part by a grant from the Ligue Nationale Française contre le Cancer (Equipe labelisée) to Alain Israël.
REFERENCES
- 1.Arenzana-Seisdedos, F., J. Thompson, M. S. Rodriguez, F. Bachelerie, D. Thomas, and R. T. Hay. 1995. Inducible nuclear expression of newly synthesized IκBα negatively regulates DNA-binding and transcriptional activities of NF-κB. Mol. Cell. Biol. 15:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barger, S. W., and M. P. Mattson. 1996. Induction of neuroprotective κB-dependent transcription by secreted forms of the Alzheimer's beta-amyloid precursor. Brain Res. Mol. Brain Res. 40:116-126. [DOI] [PubMed] [Google Scholar]
- 3.Barkett, M., and T. D. Gilmore. 1999. Control of apoptosis by Rel/NF-kB transcription factors. Oncogene 18:6910-6924. [DOI] [PubMed] [Google Scholar]
- 4.Bauer, B., and G. Baier. 2002. Protein kinase C and AKT/protein kinase B in CD4+ T-lymphocytes: new partners in TCR/CD28 signal integration. Mol. Immunol. 38:1087-1099. [DOI] [PubMed] [Google Scholar]
- 5.Beraud, C., W. J. Henzel, and P. A. Baeuerle. 1999. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-κB activation. Proc. Natl. Acad. Sci. USA 96:429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird, T. A., K. Schooley, S. K. Dower, H. Hagen, and G. D. Virca. 1997. Activation of nuclear transcription factor NF-κB by interleukin-1 is accompanied by casein kinase II-mediated phosphorylation of the p65 subunit. J. Biol. Chem. 272:32606-32612. [DOI] [PubMed] [Google Scholar]
- 7.Biswas, D. K., A. P. Cruz, E. Gansberger, and A. B. Pardee. 2000. Epidermal growth factor-induced nuclear factor κB activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc. Natl. Acad. Sci. USA 97:8542-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonizzi, G., J. Piette, S. Schoonbroodt, M. P. Merville, and V. Bours. 1999. Role of the protein kinase C lambda/iota isoform in nuclear factor-κB activation by interleukin-1α or tumor necrosis factor-alpha: cell type specificities. Biochem. Pharmacol. 57:713-720. [DOI] [PubMed] [Google Scholar]
- 9.Bren, G. D., K. N. Pennington, and C. V. Paya. 2000. PKC-zeta-associated CK2 participates in the turnover of free IκBα. J. Mol. Biol. 297:1245-1258. [DOI] [PubMed] [Google Scholar]
- 10.Cambray-Deakin, M. A. 1996. Cerebellar granule cells, p. 3-13. In J. Cohen and G. P. Wilkin (ed.), Neural cell culture. IRL Press, Oxford, United Kingdom.
- 11.Carmichael, J., W. G. DeGraff, A. F. Gazdar, J. D. Minna, and J. B. Mitchell. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 47:943-946. [PubMed] [Google Scholar]
- 12.Carpenter, C. L., and L. C. Cantley. 1996. Phosphoinositide kinases. Curr. Opin. Cell Biol. 8:153-158. [DOI] [PubMed] [Google Scholar]
- 13.Carroll, J. E., E. F. Howard, D. C. Hess, C. G. Wakade, Q. Chen, and C. Cheng. 1998. Nuclear factor-κB activation during cerebral reperfusion: effect of attenuation with N-acetylcysteine treatment. Brain Res. Mol. Brain Res. 56:186-191. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, B., S. Christakos, and M. P. Mattson. 1994. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron 12:139-153. [DOI] [PubMed] [Google Scholar]
- 15.Clemens, J. A., D. T. Stephenson, E. P. Dixon, E. B. Smalstig, R. E. Mincy, K. S. Rash, and S. P. Little. 1997. Global cerebral ischemia activates nuclear factor-κB prior to evidence of DNA fragmentation. Brain Res. Mol. Brain Res. 48:187-196. [DOI] [PubMed] [Google Scholar]
- 16.Courtney, M. J., K. E. Akerman, and E. T. Coffey. 1997. Neurotrophins protect cultured cerebellar granule neurons against the early phase of cell death by a two-component mechanism. J. Neurosci. 17:4201-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowder, R. J., and R. S. Freeman. 1998. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J. Neurosci. 18:2933-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 19.Delhase, M., N. Li, and M. Karin. 2000. Kinase regulation in inflammatory response. Nature 406:367-368. [DOI] [PubMed] [Google Scholar]
- 20.D'Mello, S. R., K. Borodezt, and S. P. Soltoff. 1997. Insulin-like growth factor and potassium depolarization maintain neuronal survival by distinct pathways: possible involvement of PI 3-kinase in IGF-1 signaling. J. Neurosci. 17:1548-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downward, J. 1998. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10:262-267. [DOI] [PubMed] [Google Scholar]
- 22.Faux, M. C., E. N. Rollins, A. S. Edwards, L. K. Langeberg, A. C. Newton, and J. D. Scott. 1999. Mechanism of A-kinase-anchoring protein 79 (AKAP79) and protein kinase C interaction. Biochem. J. 343:443-452. [PMC free article] [PubMed] [Google Scholar]
- 23.Finkbeiner, S., and M. E. Greenberg. 1996. Ca2+-dependent routes to Ras: mechanisms for neuronal survival, differentiation, and plasticity? Neuron 16:233-236. [DOI] [PubMed] [Google Scholar]
- 24.Franke, T. F., D. R. Kaplan, and L. C. Cantley. 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88:435-437. [DOI] [PubMed] [Google Scholar]
- 25.Franklin, J. L., and E. M. Johnson, Jr. 1992. Suppression of programmed neuronal death by sustained elevation of cytoplasmic calcium. Trends Neurosci. 15:501-508. [DOI] [PubMed] [Google Scholar]
- 26.Frantz, B., E. C. Nordby, G. Bren, N. Steffan, C. V. Paya, R. L. Kincaid, M. J. Tocci, S. J. O'Keefe, and E. A. O'Neill. 1994. Calcineurin acts in synergy with PMA to inactivate IκB/MAD3, an inhibitor of NF-κB. EMBO J. 13:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gewirtz, A. T., A. S. Rao, P. O. Simon, Jr., D. Merlin, D. Carnes, J. L. Madara, and A. S. Neish. 2000. Salmonella typhimurium induces epithelial IL-8 expression via Ca2+-mediated activation of the NF-κB pathway. J. Clin. Investig. 105:79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh, A., and M. E. Greenberg. 1995. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science 268:239-247. [DOI] [PubMed] [Google Scholar]
- 29.Grilli, M., J. J. S. Chiu, and M. Lenardo. 1993. NF-κ-B and rel: participants in a multiform transcriptional regulatory system. Int. Rev. Cytol. 143:1-62. [DOI] [PubMed] [Google Scholar]
- 30.Grilli, M., F. Goffi, M. Memo, and P. Spano. 1996. Interleukin-1α and glutamate activate the NF-κB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J. Biol. Chem. 271:15002-15007. [DOI] [PubMed] [Google Scholar]
- 31.Grilli, M., M. Pizzi, M. Memo, and P. Spano. 1996. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science 274:1383-1385. [DOI] [PubMed] [Google Scholar]
- 32.Guerrini, L., F. Blasi, and S. Denis-Donini. 1995. Synaptic activation of NF-κB by glutamate in cerebellar granule neurons in vitro. Proc. Natl. Acad. Sci. USA 92:9077-9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han, B., and C. D. Logsdon. 2000. CCK stimulates mob-1 expression and NF-κB activation via protein kinase C and intracellular Ca2+. Am. J. Physiol. Cell Physiol. 278:C344-C351. [DOI] [PubMed] [Google Scholar]
- 34.Hsuan, S. L., M. S. Kannan, S. Jeyaseelan, Y. S. Prakash, C. Malazdrewich, M. S. Abrahamsen, G. C. Sieck, and S. K. Maheswaran. 1999. Pasteurella haemolytica leukotoxin and endotoxin induced cytokine gene expression in bovine alveolar macrophages requires NF-κB activation and calcium elevation. Microb. Pathog. 26:263-273. [DOI] [PubMed] [Google Scholar]
- 35.Hughes, K., S. Edin, A. Antonsson, and T. Grundstrom. 2001. Calmodulin-dependent kinase II mediates T cell receptor/CD3- and phorbol ester-induced activation of IκB kinase. J. Biol. Chem. 276:36008-36013. [DOI] [PubMed] [Google Scholar]
- 36.Hunot, S., B. Brugg, D. Ricard, P. P. Michel, M. P. Muriel, M. Ruberg, B. A. Faucheux, Y. Agid, and E. C. Hirsch. 1997. Nuclear translocation of NF-κB is increased in dopaminergic neurons of patients with Parkinson disease. Proc. Natl. Acad. Sci. USA 94:7531-7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Israel, A. 2000. The IKK complex: an integrator of all signals that activate NF-κB? Trends Cell Biol. 10:129-133. [DOI] [PubMed] [Google Scholar]
- 38.Jaken, S. 1996. Protein kinase C isozymes and substrates. Curr. Opin. Cell Biol. 8:168-173. [DOI] [PubMed] [Google Scholar]
- 39.Jang, M. K., Y. H. Goo, Y. C. Sohn, Y. S. Kim, S.-K. Lee, H. Kang, J. Cheong, and J. W. Lee. 2001. Ca2+/Calmodulin-dependant protein kinase IV stimulates nuclear factor-κB transactivation via phosphorylation of the p65 subunit. J. Biol. Chem. 8:20005-20010. [DOI] [PubMed] [Google Scholar]
- 40.Jefferson, K. K., M. F. Smith, Jr., and D. A. Bobak. 1999. Roles of intracellular calcium and NF-κB in the Clostridium difficile toxin A-induced up-regulation and secretion of IL-8 from human monocytes. J. Immunol. 163:5183-5191. [PubMed] [Google Scholar]
- 41.Kaltschmidt, B., M. Uherek, B. Volk, P. A. Baeuerle, and C. Kaltschmidt. 1997. Transcription factor NF-κB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 94:2642-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaltschmidt, C., B. Kaltschmidt, and P. A. Baeuerle. 1993. Brain synapses contain inducible forms of the transcription factor NF-κB. Mech. Dev. 43:135-147. [DOI] [PubMed] [Google Scholar]
- 43.Kaltschmidt, C., B. Kaltschmidt, and P. A. Baeuerle. 1995. Stimulation of ionotropic glutamate receptors activates transcription factor NF-κB in primary neurons. Proc. Natl. Acad. Sci. USA 92:9618-9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kane, L. P., V. S. Shapiro, D. Stokoe, and A. Weiss. 1999. Induction of NF-κB by the Akt/PKB kinase. Curr. Biol. 9:601-604. [DOI] [PubMed] [Google Scholar]
- 45.Kopp, E. B., and S. Ghosh. 1995. NF-κB and rel proteins in innate immunity. Adv. Immunol. 58:1-27. [DOI] [PubMed] [Google Scholar]
- 46.Koulich, E., T. Nguyen, K. Johnson, C. A. Giardina, and S. R. D'Mello. 2001. NF-κB is involved in the survival of cerebellar granule neurons: association of IκB phosphorylation with cell survival. J. Neurochem. 76:1188-1198. [DOI] [PubMed] [Google Scholar]
- 47.Lallena, M. J., M. T. Diaz-Meco, G. Bren, C. V. Paya, and J. Moscat. 1999. Activation of IκB kinase beta by protein kinase C isoforms. Mol. Cell. Biol. 19:2180-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leevers, S. J., B. Vanhaesebroeck, and M. D. Waterfield. 1999. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr. Opin. Cell Biol. 11:219-225. [DOI] [PubMed] [Google Scholar]
- 49.Lin, C. H., S. Y. Sheu, H. M. Lee, Y. S. Ho, W. S. Lee, W. C. Ko, and J. R. Sheu. 2000. Involvement of protein kinase C-gamma in IL-1β-induced cyclooxygenase-2 expression in human pulmonary epithelial cells. Mol. Pharmacol. 57:36-43. [PubMed] [Google Scholar]
- 50.Lin, X., A. O'Mahony, Y. Mu, R. Geleziunas, and W. C. Greene. 2000. Protein kinase C-theta participates in NF-κB activation induced by CD3-CD28 costimulation through selective activation of IκB kinase beta. Mol. Cell. Biol. 20:2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipton, S. A. 1997. Janus faces of NF-κB: neurodestruction versus neuroprotection. Nat. Med. 3:20-22. [DOI] [PubMed] [Google Scholar]
- 52.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol. 20:1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martiny-Baron, G., M. G. Kazanietz, H. Mischak, P. M. Blumberg, G. Kochs, H. Hug, D. Marme, and C. Schachtele. 1993. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 268:9194-9197. [PubMed] [Google Scholar]
- 54.Matsumoto, A., Y. Deyama, A. Deyama, M. Okitsu, Y. Yoshimura, and K. Suzuki. 1998. Epidermal growth factor receptor-mediated expression of NF-κB transcription factor in osteoblastic MC3T3-E1 cells cultured under a low-calcium environment. Life Sci. 62:1623-1627. [DOI] [PubMed] [Google Scholar]
- 55.Matsuoka, Y., Y. Kitamura, M. Okazaki, K. Terai, and T. Taniguchi. 1999. Kainic acid-induced activation of nuclear factor-κB in rat hippocampus. Exp. Brain Res. 124:215-222. [DOI] [PubMed] [Google Scholar]
- 56.Mattson, M. P., C. Culmsee, Z. Yu, and S. Camandola. 2000. Roles of nuclear factor κB in neuronal survival and plasticity. J. Neurochem. 74:443-456. [DOI] [PubMed] [Google Scholar]
- 57.Mattson, M. P., Y. Goodman, H. Luo, W. Fu, and K. Furukawa. 1997. Activation of NF-κB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J. Neurosci. Res. 49:681-697. [DOI] [PubMed] [Google Scholar]
- 58.Meberg, P. J., W. R. Kinney, E. G. Valcourt, and A. Routtenberg. 1996. Gene expression of the transcription factor NF-κB in hippocampus: regulation by synaptic activity. Brain Res. Mol. Brain Res. 38:179-190. [DOI] [PubMed] [Google Scholar]
- 59.Mercurio, F., and A. M. Manning. 1999. Multiple signals converging on NF-κB. Curr. Opin. Cell Biol. 11:226-232. [DOI] [PubMed] [Google Scholar]
- 60.Miller, T. M., M. G. Tansey, E. M. Johnson, Jr., and D. J. Creedon. 1997. Inhibition of phosphatidylinositol 3-kinase activity blocks depolarization- and insulin-like growth factor I-mediated survival of cerebellar granule cells. J. Biol. Chem. 272:9847-9853. [DOI] [PubMed] [Google Scholar]
- 61.New, L., Y. Jiang, M. Zhao, K. Liu, W. Zhu, L. J. Flood, Y. Kato, G. C. N. Parry, and J. Han. 1998. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 17:3372-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Neill, L. A., and C. Kaltschmidt. 1997. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20:252-258. [DOI] [PubMed] [Google Scholar]
- 63.Ozes, O. N., L. D. Mayo, J. A. Gustin, S. R. Pfeffer, L. M. Pfeffer, and D. B. Donner. 1999. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82-85. [DOI] [PubMed] [Google Scholar]
- 64.Pahl, H. L., and P. A. Baeuerle. 1996. Activation of NF-κB by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 392:129-136. [DOI] [PubMed] [Google Scholar]
- 65.Patel, R., M. Holt, R. Philipova, S. Moss, H. Schulman, H. Hidaka, and M. Whitaker. 1999. Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J. Biol. Chem. 274:7958-7968. [DOI] [PubMed] [Google Scholar]
- 66.Philpott, K. L., M. J. McCarthy, A. Klippel, and L. L. Rubin. 1997. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J. Cell Biol. 139:809-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piccioli, P., C. Porcile, S. Stanzione, M. Bisaglia, A. Bajetto, R. Bonavia, T. Florio, and G. Schettini. 2001. Inhibition of nuclear factor-κB activation induces apoptosis in cerebellar granule cells. J. Neurosci. Res. 66:1064-1073. [DOI] [PubMed] [Google Scholar]
- 68.Pieper, G. M., and Riaz-ul-Haq. 1997. Activation of nuclear factor-κB in cultured endothelial cells by increased glucose concentration: prevention by calphostin C. J. Cardiovasc. Pharmacol. 30:528-532. [DOI] [PubMed] [Google Scholar]
- 69.Prasad, A. V., W. H. Pilcher, and S. A. Joseph. 1994. Nuclear factor-κB in rat brain: enhanced DNA-binding activity following convulsant-induced seizures. Neurosci. Lett. 170:145-148. [DOI] [PubMed] [Google Scholar]
- 70.Pugazhenthi, S., A. Nesterova, C. Sable, K. A. Heidenreich, L. M. Boxer, L. E. Heasley, and J. E.-B. Reusch. 2000. Akt/protein kinase B up-regulates bcl2 expression through cAMP-response element-binding protein. J. Biol. Chem. 275:10761-10766. [DOI] [PubMed] [Google Scholar]
- 71.Quinlan, K. L., S. M. Naik, G. Cannon, C. A. Armstrong, N. W. Bunnett, J. C. Ansel, and S. W. Caughman. 1999. Substance P activates coincident NF-AT- and NF-κB-dependent adhesion molecule gene expression in microvascular endothelial cells through intracellular calcium mobilization. J. Immunol. 163:5656-5665. [PubMed] [Google Scholar]
- 72.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 73.Reddy, S. A., J. H. Huang, and W. S. Liao. 1997. Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFκB and AP-1 activation. J. Biol. Chem. 272:29167-29173. [DOI] [PubMed] [Google Scholar]
- 74.Romashkova, J. A., and S. S. Makarov. 1999. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86-90. [DOI] [PubMed] [Google Scholar]
- 75.Rong, Y., and M. Baudry. 1996. Seizure activity results in a rapid induction of nuclear factor-κB in adult but not juvenile rat limbic structures. J. Neurochem. 67:662-668. [DOI] [PubMed] [Google Scholar]
- 76.Sakurai, H., H. Chiba, H. Miyoshi, T. Sugita, and W. Toriumi. 1999. IkB kinases phosphorylate NF-kB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 274:30353-30356. [DOI] [PubMed] [Google Scholar]
- 77.Salminen, A., P. K. Liu, and C. Y. Hsu. 1995. Alteration of transcription factor binding activities in the ischemic rat brain. Biochem. Biophys. Res. Commun. 212:939-944. [DOI] [PubMed] [Google Scholar]
- 78.Schmidt-Ullrich, R., S. Mémet, A. Lilienbaum, J. Feuillard, M. Raphael, and A. Israël. 1996. NF-κB activity in transgenic mice: developmental regulation and tissue specificity. Development 122:2117-2128. [DOI] [PubMed] [Google Scholar]
- 79.Shatrov, V. A., V. Lehmann, and S. Chouaib. 1997. Sphingosine-1-phosphate mobilizes intracellular calcium and activates transcription factor NF-κB in U937 cells. Biochem. Biophys. Res. Commun. 234:121-124. [DOI] [PubMed] [Google Scholar]
- 80.Sizemore, N., S. Leung, and G. R. Stark. 1999. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol. Cell. Biol. 19:4798-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soler, R. M., J. Egea, G. M. Mintenig, C. Sanz-Rodriguez, M. Iglesias, and J. X. Comella. 1998. Calmodulin is involved in membrane depolarization-mediated survival of motoneurons by phosphatidylinositol-3 kinase- and MAPK-independent pathways. J. Neurosci. 18:1230-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steffan, N. M., G. D. Bren, B. Frantz, M. J. Tocci, E. A. O'Neill, and C. V. Paya. 1995. Regulation of IkB alpha phosphorylation by PKC- and Ca2+-dependent signal transduction pathways. J. Immunol. 155:4685-4691. [PubMed] [Google Scholar]
- 83.Sun, L., and G. Carpenter. 1998. Epidermal growth factor activation of NF-κB is mediated through IκBα degradation and intracellular free calcium. Oncogene 16:2095-2102. [DOI] [PubMed] [Google Scholar]
- 84.Sun, P., H. Enslen, P. S. Myung, and R. A. Maurer. 1994. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 8:2527-2539. [DOI] [PubMed] [Google Scholar]
- 85.Tando, Y., H. Algul, M. Wagner, H. Weidenbach, G. Adler, and R. M. Schmid. 1999. Caerulein-induced NF-κB/Rel activation requires both Ca2+ and protein kinase C as messengers. Am. J. Physiol. 277:G678-G686. [DOI] [PubMed] [Google Scholar]
- 86.Trushin, S. A., K. N. Pennington, A. Algeciras-Schimnich, and C. V. Paya. 1999. Protein kinase C and calcineurin synergize to activate IκB kinase and NF-κB in T lymphocytes. J. Biol. Chem. 274:22923-22931. [DOI] [PubMed] [Google Scholar]
- 87.Usachev, Y. M., and S. A. Thayer. 1999. Controlling the urge for a Ca2+ surge: all-or-none Ca2+ release in neurons. Bioessays 21:743-750. [DOI] [PubMed] [Google Scholar]
- 88.Verma, I. M., J. K. Stevenson, E. M. Schwarz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 9:2723-2735. [DOI] [PubMed] [Google Scholar]
- 89.Wang, D., and A. S. Baldwin, Jr. 1998. Activation of nuclear factor-κB-dependent transcription by tumor necrosis factor-alpha is mediated through phosphorylation of RelA/p65 on serine 529. J. Biol. Chem. 273:29411-29416. [DOI] [PubMed] [Google Scholar]
- 90.Weil, R., C. Laurent-Winter, and A. Israel. 1997. Regulation of IκBβ degradation. Similarities to and differences from IκBα. J. Biol. Chem. 272:9942-9949. [DOI] [PubMed] [Google Scholar]
- 91.Whiteside, S. T., J. C. Epinat, N. R. Rice, and A. Israel. 1997. IκB epsilon, a novel member of the IκB family, controls RelA and cRel NF-κB activity. EMBO J. 16:1413-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whiteside, S. T., M. K. Ernst, O. LeBail, C. Laurent-Winter, N. Rice, and A. Israel. 1995. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Mol. Cell. Biol. 15:5339-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whiteside, S. T., and A. Israel. 1997. I κB proteins: structure, function and regulation. Semin. Cancer Biol. 8:75-82. [DOI] [PubMed] [Google Scholar]
- 94.Wooten, M. W., M. L. Seibenhener, G. Zhou, M. L. Vandenplas, and T. H. Tan. 1999. Overexpression of atypical PKC in PC12 cells enhances NGF-responsiveness and survival through an NF-κB dependent pathway. Cell Death Differ. 6:753-764. [DOI] [PubMed] [Google Scholar]
- 95.Yabe, T., D. Wilson, and J. P. Schwartz. 2001. NFκB activation is required for the neuroprotective effects of pigment epithelium-derived factor (PEDF) on cerebellar granule neurons. J. Biol. Chem. 276:43313-43319. [DOI] [PubMed] [Google Scholar]
- 96.Yang, K., X. S. Mu, and R. L. Hayes. 1995. Increased cortical nuclear factor-κB (NF-κB) DNA binding activity after traumatic brain injury in rats. Neurosci. Lett. 197:101-104. [DOI] [PubMed] [Google Scholar]
- 97.Yano, S., H. Tokumitsu, and T. R. Soderling. 1998. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396:584-587. [DOI] [PubMed] [Google Scholar]
- 98.Yu, Z., D. Zhou, A. J. Bruce-Keller, M. S. Kindy, and M. P. Mattson. 1999. Lack of the p50 subunit of nuclear factor-κB increases the vulnerability of hippocampal neurons to excitotoxic injury. J. Neurosci. 19:8856-8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang, S., T. Tobaru, J. A. Zivin, and D. A. Shackelford. 1998. Activation of nuclear factor-κB in the rabbit spinal cord following ischemia and reperfusion. Brain Res. Mol. Brain Res. 63:121-132. [PubMed] [Google Scholar]