Abstract
The adapter protein Crk-Like (CrkL) can associate with the Src substrate p130Cas (Cas). The biological role of CrkL downstream of Cas, however, has been largely obscure. Consistent with the ability of CrkL to biochemically associate with Cas, we found that Src triggers translocation of CrkL to focal adhesions (FAs) in a manner dependent on Cas. Forced localization of CRKL to FAs (FA-CRKL) by itself was sufficient to induce activation of Rac1 and Cdc42 and rescued haptotaxis defects of mouse embryonic fibroblasts (MEFs) lacking Src, Yes, and Fyn, three broadly expressed Src family members required for integrin-induced migration. Consistent with Rac1 activation, FA-CRKL induced cotranslocation of a Rac1 activator, Dock1, to focal adhesions. These results therefore indicate a role for CrkL in mediating Src signaling by activating small G proteins at focal adhesions. Furthermore, MEFs lacking CrkL show impaired integrin-induced migration despite expression of a closely related protein, Crk-II, in these cells. These results therefore provide formal evidence that CrkL plays a specific role in integrin-induced migration as a downstream mediator of Src.
Crk-Like (CrkL) is a member of the Crk adapter protein family, consisting of one SH2 and two SH3 domains. Among many proteins known to associate with CrkL (and Crk), p130Cas (Cas) has been implicated in Src signaling (36). In normal cells, Cas is distributed predominantly in the cytosol with a small fraction localized to focal adhesions where interaction of the cell with the extracellular matrix takes place through heteromeric transmembrane proteins known as integrins (31). Activated Src induces translocation of Cas to podosome-like abnormal focal adhesions (31). It has been shown that Cas plays an essential role in Src signaling since mouse embryonic fibroblasts (MEFs) lacking Cas are resistant to activated Src-induced anchorage-independent growth (19). In normal cells, focal adhesion kinase (Fak) and Src cooperate to phosphorylate Cas upon integrin binding to the extracellular matrix (35, 44). While another Src family member, Fyn, also associates with Cas (37), overall tyrosine phosphorylation of Cas largely relies on Src (14, 47).
Consistent with the implicated role of Src family kinases in integrin-mediated signaling, MEFs lacking Src, Yes, and Fyn—the three most widely expressed members of the Src family (SYF cells)—display severe reduction in global tyrosine phosphorylation of many Src substrates, including Cas, that the extracellular matrix protein fibronectin induces in normal cells (25). Whereas SYF cells are capable of migrating toward platelet-derived growth factor, they show impaired motility toward fibronectin (haptotaxis). The recent finding that catalytic activity of Src is necessary for proper haptotaxis provides further evidence that involvement of Src substrates is essential in this process (7). Since Cas-deficient cells show similar motility defects (18), these findings suggest that a pathway or pathways downstream of Cas mediate Src-dependent adhesion signaling induced by integrins.
As Cas is a major CrkL binding protein, we explore a role for CrkL in integrin-induced signaling downstream of Src in the present work. We show that one important function of Src family kinases is to induce translocation of CrkL to focal adhesions through Cas. By taking advantage of MEFs isolated from CrkL-deficient mice (12), we show that CrkL plays an essential role in integrin signaling. Since Crk and CrkL can associate with similar if not identical sets of proteins in vitro, the functions of these proteins have been thought to be largely overlapping (10). On the other hand, whereas Crk-II, a broadly expressed isoform of the Crk gene most closely related to CrkL, is not transforming in fibroblasts (28), overexpression of CrkL transforms cells in vitro and in vivo (17, 41), thus suggesting a functional difference between these two closely related proteins. Crk-II has been implicated in the pathway from CED-2 to CED-10 originally identified in Caenorhabditis elegans due to close similarity between CED-2 and Crk-II (33). Our studies described in this paper provide formal evidence that CrkL, rather than Crk-II, plays an essential role in linking integrin signaling to this evolutionally conserved pathway downstream of Src and Cas.
MATERIALS AND METHODS
Subcellular targeting constructs of CRKL.
Focal adhesion-targeted CRKL (FA-CRKL) was generated by inserting in frame a human CRKL coding sequence into the BamHI site immediately 5′ of the translation start site of green fluorescent protein (GFP)-LIM in a modified pGreenLantern-1 expression vector (27). The stop codon of CRKL cDNA was replaced with a BglII site by PCR mutagenesis by using Pfu DNA polymerase (Stratagene) prior to subcloning. The Cfr10I-Cfr10I (nucleotides 560 to 752), DraI-AluI (nucleotides 941 to 1049), or AvaIII-BalI (nucleotides 1127 to 1327) fragment was removed to generate CRKL(ΔSH2), CRKL(ΔSH3n), or CRKL(ΔSH3c), respectively. The number of nucleotides referred here corresponds to that of human CRKL (GenBank accession no. X59656). Full-length CRKL and CRKL(K39) cDNA fragments were gifts from Brian J. Druker (16). Mitochondrial surface-targeted CRKL (CRKL-GFP-mito) was generated by fusing CRKL-GFP (without a stop codon) in frame with the transmembrane region of the Listeria protein ActA (residues 612 to 633), which directs the fusion protein to the outer surface of mitochondria (a gift from Frank B. Gertler) (2). For permanent expression, vectors were linearized and transfected into cells by electroporation or using Effectene (Qiagen) as described in the manufacturer's protocol. In some experiments, retrovirus was used for permanent expression as described below. For transient expression, intact plasmids were transfected into cells using Effectene.
MEFs.
Primary MEFs were isolated from embryonic-day-12.5 Crkl−/− and wild-type embryos. Some cells used in this study were spontaneously immortalized by regular splitting according to the 3T3 protocol. To reintroduce CRKL into Crkl−/− cells, cells were electroporated with a linearized pBABE puro CRKL plasmid for permanent expression. SYF cells and their Src-expressing control cells were gifts from Philippe Soriano (25). MEFs lacking Cas and their Cas-expressing control cells were gifts from Hisamaru Hirai (18). Activated mouse Src in which the regulatory tyrosine residue is replaced with a phenylalanine (SrcY529F) (27) was introduced into Crkas−/−(Cas-deficient) cells and their control cells by pBABE puro SrcY529F retrovirus as prepared below. MEFs lacking Csk as well as Src or Fyn were reported previously (21, 46). Primary MEFs were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (HyClone). All other immortalized cells were maintained in DMEM supplemented with 7.5% Cosmic calf serum (HyClone).
Retroviral expression.
NIH 3T3 cells or SYF cells that express FA-GFP, FA-CRKL, or FA-CRKL(ΔSH3n) were generated by retrovirus infection by using a pBABE puro retroviral vector (30). Ecotropic retrovirus was produced by transient transfection of φNX cells (a gift from G. P. Nolan) with the viral vector as previously described (23). One to 2 days after infection, cells infected with pBABE puro retrovirus were selected with 2 μg of puromycin/ml.
Immunofluorescence staining.
Cells were plated onto fibronectin-coated glass coverslips in a 24-well tissue culture plate and fixed after an indicated period of culture. F-actin was stained with Alexa 594-conjugated phalloidin as previously described (27). Subcellular localization of paxillin, vinculin, and Dock1 was determined with antipaxillin (BD Biosciences) monoclonal antibody, antivinculin (Sigma) monoclonal antibody, or anti-Dock1 polyclonal antibodies (Santa Cruz Biotechnology) followed by Alexa 594 or Cy5-conjugated anti-mouse immunoglobulin G. Subcellular localization of multiple fluoropores (including GFP) was recorded by using a Zeiss axiovert microscope equipped with a charge-coupled device camera.
Cell migration.
Modified Boyden chamber assays were performed to assess cell motility as previously described by using a polyethylene terephthalate (PET) tissue culture insert with 8-μm pores (BD Biosciences) (25). Haptotaxis assays were performed with PET inserts, of which only the lower surface was coated with 5 μg of fibronectin/ml for 2 h. Chemotaxis and chemokinesis assays were performed with PET inserts, of which both the upper and lower sides were coated with fibronectin. For haptotaxis assays, both upper and lower chambers were filled with serum-free DMEM. For chemotaxis and chemokinesis assays, the agent was added to either lower or upper chamber, respectively. After 16 h of serum starvation, cells were harvested with trypsin-EDTA, which was then neutralized by trypsin inhibitor (Sigma). Cells were further diluted in serum-free DMEM (with 1× insulin-transferrin-sodium selenite medium supplement for SYF cells) and plated into the upper chamber. After 3 h, cells were fixed in neutral buffered formalin and those remaining on the upper surface were removed by cotton swabs. The membrane was removed by scalpel, and cells on the lower surface were stained with 4′,6′-diamidino-2-phenylindole. The number of cells that had traversed through the pores to the lower surface of the membrane was counted by the ImageJ program (a Java version of NIH Image) in digital images taken under a fluorescent microscope.
Immunoblot and pulldown assays.
Cells were lysed in radioimmunoprecipitation assay buffer. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis were performed according to a standard protocol. Polyclonal anti-Crk-II or anti-CrkL antibodies (Santa Cruz Biotechnology) were used to detect Crk-II or CrkL protein levels. Monoclonal anti-Ras, anti-Rac1 (BD Biosciences), and polyclonal anti-Cdc42 (Santa Cruz Biotechnology) antibodies were used to detect Ras, Rac1, or Cdc42, respectively. After incubation with secondary antibody conjugated with horseradish peroxidase, blots were developed with a chemiluminescent agent (Pierce). The results of each immunoblot were analyzed by the ImageJ program for quantitation with at least two different exposures to avoid over- or underexposure.
Pulldown assays were performed as previously described (51). Briefly, cell lysates in radioimmunoprecipitation assay buffer (400 μg of proteins) were incubated with 20 μg of recombinant glutathione transferase (GST) fusion proteins (GST-Raf1-RBD for GTP-Ras and GST-Pak-RBD for GTP-Rac1 and GTP-Cdc42) precoupled to glutathione-Sepharose 4B beads (Amersham Pharmacia) at 4°C for 1 h. One-third of precipitated proteins was loaded into each lane to detect GTP-loaded small G proteins bound to the GST fusion protein by immunoblot analysis as described above. To determine the amount of Rac1, Cdc42, or Ras, a total cell lysate containing 10 μg of proteins was loaded into each lane in parallel. Plasmids for GST-Raf1-RBD and GST-Pak-RBD were gifts from Martin Alexander Schwartz and Michiyuki Matsuda, respectively.
RESULTS
Translocation of CrkL to focal adhesions in response to increased activity of Src.
While many CrkL binding proteins have been identified, the biological pathways that CrkL mediates in the cell are still obscure. Previous biochemical analysis has shown that CrkL can physically associate with p130Cas (Cas) and paxillin (5, 38, 39). Since Cas and paxillin are substrates of Src family tyrosine kinases in vivo (25, 46), elevated activity of Src family kinases may affect subcellular localization of CrkL. To test this possibility, we have examined subcellular localization of CrkL tagged with GFP (CRKL-GFP). CRKL-GFP was diffusely distributed in the cytosol in perinuclear compartments and in the nucleus in quiescent wild-type MEFs (Fig. 1A). In contrast, a subset of CRKL-GFP constitutively colocalized to focal adhesions with paxillin in MEFs lacking a major negative regulator of Src family kinases, Csk, in which multiple Src members are therefore activated (21) (Fig. 1A). Similar results were obtained by staining endogenous CrkL in Csk−/− cells (data not shown). On the other hand, CRKL(K39)-GFP, in which the FLVR motif essential for binding to phosphorylated tyrosine residues was mutated to an FLVK sequence, did not localize to focal adhesions in Csk−/− cells (Fig. 1A). These results therefore indicate that Src family kinases can induce translocation of CrkL to focal adhesions in a manner dependent on the SH2 domain of CrkL.
FIG. 1.
Subcellular localization of CrkL in fibroblasts that express active Src. (A) CRKL-GFP or CRKL(K39)-GFP was transiently expressed in wild-type, Csk−/−, Src−/−, Csk−/−, and Fyn−/− Csk−/− MEFs as indicated. Localization of the fusion protein was determined by GFP fluorescence. Antipaxillin was used as a marker for focal adhesions. (B) An activated mouse Src (SrcY529F) was permanently expressed in Crkas−/− cells and their control cells with reintroduced Cas (Crkas−/− + Cas) by pBABE puro retroviral vector. Subcellular localization of endogenous CrkL and vinculin was determined by immunofluorescence staining. F-actin was stained by phalloidin. Arrows indicate aggregation of F-actin, CrkL, and vinculin in podosome-like adhesion structures (overlap of the three colors show large whitish dots).
To assess relative contributions of Src members to CrkL translocation, we examined subcellular localization of CRKL-GFP in MEFs lacking Src or Fyn as well as Csk (Src−/− Csk−/− or Fyn−/− Csk−/−) (46) (Fig. 1A). We found that localization of CRKL-GFP was greatly reduced in Src−/− Csk−/− cells at focal adhesions, although it was still detectable. On the other hand, Fyn−/− Csk−/− cells showed accumulation of CRKL-GFP at focal adhesions. Localization of CRKL-GFP in Src−/− Fyn−/− Csk−/− cells was not overtly different from that in Src−/− Csk−/− cells (not shown). As noted earlier, Cas has been implicated in Src signaling. We therefore examined CrkL localization in Cas-deficient (Crkas−/−) cells as well as control cells with reintroduced Cas (Crkas−/− + Cas) after overexpression of activated mouse Src (SrcY529F) by retrovirus infection. Whereas CrkL accumulated to abnormal focal adhesions called podosomes in control MEFs expressing SrcY529F, the concentration of CrkL at focal adhesions was diminished in Crkas−/− MEFs expressing SrcY529F (Fig. 1B). These results therefore suggest that Src plays a major role in CrkL translocation, dependent largely on the Src substrate Cas, to focal adhesions in embryonic fibroblasts, while other Src family members or potentially other tyrosine kinases may also participate.
Subcellular localization of CrkL in migrating fibroblasts.
In the above experiments, translocation of CrkL to focal adhesions was examined in cells in which Src activity was abnormally induced. To address whether translocation of CrkL occurs in physiological contexts, we next examined normal fibroblastic cells (Fig. 2). Permissive conditions for migration were generated by scraping cells off from a confluent culture to generate a cell-free zone as used in “wound healing” motility assays. As cells migrated into the cell-free zone, CrkL became concentrated at the ruffling edge, where F-actin also accumulated (Fig. 2A). In a large subset of migrating cells (approximately 50%), endogenous CrkL localized to focal adhesions in a limited area of the cell (Fig. 2B). Closer examination of CrkL in these focal adhesions showed that CrkL was concentrated in a region of focal adhesions where paxillin and F-actin overlapped (Fig. 2C). Similar distribution of CrkL within focal adhesions was observed in Csk−/− cells as well as in MEFs expressing SrcY529F (not shown). Thus, translocation of CrkL to focal adhesions and F-actin-rich domains normally occurs during migration, although this may be transient.
FIG. 2.
Subcellular localization of CrkL in migrating cells. (A to C) Subcellular localization of endogenous CrkL, paxillin, and F-actin. NIH 3T3 cells were plated on glass coverslips coated with fibronectin. Photographs of migrating cells were taken 3 h after generation of the cell-free zone. (A) The upper row shows quiescent NIH 3T3 cells; the lower row shows migrating NIH 3T3 cells. (B) The panel shows focal adhesion localization of CrkL in a large subset of migrating NIH 3T3 cells. (C) A higher magnification of the part of the cell highlighted by a white rectangle in panel B. The images are merged for three components: F-actin, CrkL, and paxillin. The panel at the right shows enlarged focal adhesion structures, where whiteness indicates overlap among F-actin, CrkL, and paxillin.
Translocation of CrkL to focal adhesions rescues motility defects of SYF cells.
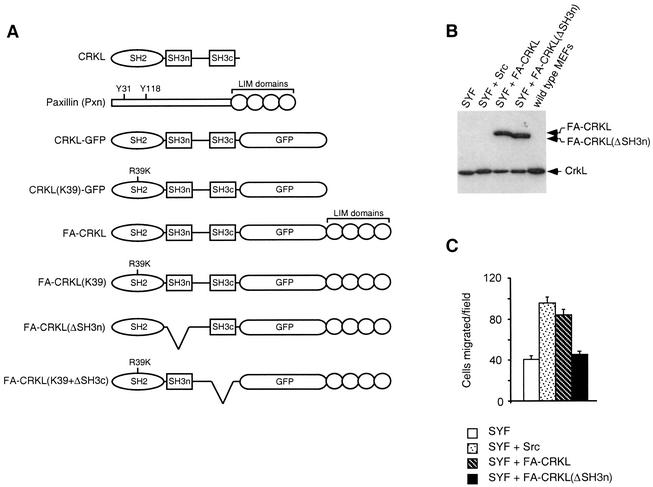
To explore the role that CrkL plays at focal adhesions, we have generated fusion proteins of CrkL (FA-CRKL) with the LIM domains of paxillin, which are essential for focal adhesion localization (3). Figure 3A illustrates the structures of fusion proteins used in this study. These proteins were further tagged by GFP to aid determination of subcellular location of these proteins. As noted above, Src family kinases (namely, Src, Fyn, and Yes) are essential for phosphorylation of focal adhesion proteins such as Cas and paxillin (25). One possible explanation for impaired haptotaxis toward fibronectin in MEFs lacking Src, Yes, and Fyn (SYF cells) may be the inability of these cells to recruit CrkL to focal adhesions in response to fibronectin-integrin binding. To provide evidence for this hypothesis, we expressed FA-CRKL proteins in SYF cells (Fig. 3B). As expected, expression of FA-CRKL (at a level comparable to that for endogenous CrkL) was sufficient for complementing impaired motility of SYF cells in haptotaxis assays (Fig. 3C; Student two-sample t test, P = 0.004 compared to SYF cells) to a degree similar to that of control SYF cells in which wild-type Src had been reintroduced. On the other hand, expression of FA-CRKL lacking the SH3n domain had no detectable effect in these assays. These results therefore indicate that localization of CrkL to focal adhesions is sufficient to override the need for Src family kinases during directional locomotion toward fibronectin (haptotaxis).
FIG. 3.
Subcellular targeting of CRKL to focal adhesions rescues motility defects of SYF cells. (A) The panel illustrates the structures of fusion proteins used in this study. (B) Immunoblot analysis for endogenous CrkL and FA-CRKL proteins expressed in cells used in panel C. (C) Modified Boyden chamber haptotaxis assay. The number of cells (cells migrated/field) that had traversed to reach the lower surface coated with fibronectin through pores in the insert that separated two chambers was determined 3 h after plating cells into the upper chamber. Error bars indicate the standard deviation of the sample data obtained in four individual microscopic fields under a 40× objective lens. Similar results were obtained in three independent experiments.
CrkL is required for haptotaxis toward fibronectin.
To determine whether CrkL is required for cell motility, we then examined MEFs lacking CrkL (Crkl−/− cells). Consistent with the effect of FA-CRKL on cell migration, Crkl−/− cells showed poor haptotaxis toward fibronectin compared to that of wild-type cells and of control Crkl−/− cells in which human CRKL was reintroduced to a level similar to that of wild-type cells (Crkl−/− + CRKL) (Fig. 4A and B; Student two-sample t test, P = 0.0001). Interestingly, however, Crkl−/− cells showed normal chemotaxis toward serum (Fig. 4B). These cells also responded normally to serum for chemokinesis (not shown). A CrkL-related protein, Crk-II, was expressed in Crkl−/− cells with an elevated level (approximately 1.7-fold) compared to that of wild-type cells or of Crkl−/− cells, which express reintroduced CrkL (Fig. 4A). These results therefore indicate that normal haptotaxis requires CrkL, while Crkl−/− cells retain the general machinery of cell locomotion, which can be utilized in serum-stimulated migration.
FIG. 4.
CrkL-deficient cells display haptotaxis defects. (A) Immunoblot analysis for CrkL and Crk-II in MEFs used for panel B. (B) Modified Boyden chamber migration assays. Haptotaxis toward fibronectin was determined as shown in Fig. 3C. Chemotaxis toward serum was determined in an assay similar to the haptotaxis assay except that both sides of the insert were coated with fibronectin with or without fetal bovine serum (10%) (FBS + or −, respectively) included in the lower chamber. Error bars indicate the standard deviation of the sample data obtained in four independent microscopic fields under a 40× objective lens. Three independent experiments yielded similar results.
Translocation of CRKL to focal adhesions results in cytoskeletal reorganization.
To provide insight into the cellular events that CrkL translocation to focal adhesions can induce, we examined the morphology of NIH 3T3 cells that express FA-CRKL (Fig. 5). The majority of cells (approximately 60%) extended multiple membrane protrusions decorated by fan-shaped lamellipodia with permanent expression of FA-CRKL at a level comparable to that of endogenous CrkL (see Fig. 6A for expression level). Figure 5A presents a severely affected case to emphasize characteristic changes that FA-CRKL can induce in the cytoskeleton. FA-CRKL localized to focal adhesions as well as to the edge of lamellipodia, where F-actin also accumulated.
FIG. 5.
FA-CRKL induced cytoskeletal reorganization. (A) Expression of FA-CRKL in NIH 3T3 cells induced multiple membrane protrusions decorated by lamellipodia (arrows). The part highlighted by a white rectangle was enlarged and shown at right. Blue arrowheads indicate FA-CRKL localized to focal adhesions. Green arrowheads indicate FA-CRKL localization at the membrane cortex where F-actin (red arrowheads) also colocalized. Merged color images use red and green to indicate localization of F-actin and FA-CRKL, respectively. (B) Cytoskeletal reorganization induced by transient expression of mutants of FA-CRKL. Localization of F-actin (red) and GFP (green) signals for fusion proteins is shown. Arrowheads indicate membrane protrusions decorated by lamellipodia. (C) Subcellular targeting of CRKL to the outer membrane of mitochondria. Localization of F-actin (red), CRKL-GFP-mito (green), and paxillin (blue) is shown. (D) The incidence of lamellipodial formation was counted after transient expression of each transgene indicated. Percent values indicate the number of NIH 3T3 cells with more than two membrane protrusions decorated by lamellipodia per total transfected (GFP-positive) cells. To assist observation of lamellipodia, cells were stained with phalloidin. Error bars indicate the standard deviation of the sample data collected in three independent sets of samples, each of which was scored with at least 60 cells in random fields under a 40× objective lens. (E) The number of protrusions containing lamellipodia per cell (x axis) was counted in each cell and plotted for occurrence (y axis) in NIH 3T3 cells transiently expressing FA-CRKL, FA-CRKL(ΔSH3n), or FA-GFP. For example, four cells had seven lamellipodium-containing protrusions in the FA-CRKL group. A total of 65 cells was counted for each group. Transient expression shown in panels B to E was performed at a dose of 0.1 μg/well of the expression plasmid, except that the experiments for panel D were performed at a dose of 0.3 μg/well.
FIG. 6.
Activation of small G proteins by FA-CRKL. (A) Immunoblot assay for expression levels of FA-CRKL compared to those of endogenous CRKL in NIH 3T3 cells expressing empty vector (CTR; lane 1), FA-CRKL, or FA-CRKL(ΔSH3n). (B) Pulldown assays for GTP-loaded Rac1, Cdc42, and Ras. Overall levels of these small G proteins were determined in parallel by using total cell lysates from NIH 3T3 cells expressing empty vector (CTR; lane 1), FA-CRKL (lane 2), or FA-CRKL(ΔSH3n) (lane 3) (shown at left). GTP-loaded forms were pulled down as described in Materials and Methods, and their levels were determined by immunoblotting by using specific antibodies to these small G proteins (shown at right). Levels of the GTP-loaded form were compared to total levels (not discriminating among GTP- and GDP-bound forms) of the G protein determined in total cell lysates. Experiments were repeated three times and yielded similar results. (C) The effects of dominant-negative forms of small G proteins were evaluated by counting the number of cells that show multiple membrane protrusions induced by FA-CRKL. Increasing doses of expression plasmids for Rac1 N17, Cdc42 N17, RhoA N17, or Ras N17 were cotransfected into NIH 3T3 cells coexpressing FA-CRKL at a fixed dose of 0.1 μg/well. Cells were stained with phalloidin to aid scoring samples. Values indicate the percentage of cells with lamellipodia among the total number of GFP-positive transfected cells. Error bars indicate the standard deviation of the sample values obtained in three independent sets of samples. (D) Subcellular localization of Dock1 in NIH 3T3 cells that express either FA-CRKL or FA-CRKL(ΔSH3n). Localization of fusion proteins was determined by GFP fluorescence. A part of the FA-CRKL-expressing cell highlighted by the white square was enlarged to show details of colocalization of FA-CRKL with Dock1.
To determine the domains of FA-CRKL necessary for this effect, we then transiently expressed mutants of FA-CRKL in NIH 3T3 cells. FA-CRKL(K39) (with an R39K point mutation that disrupts binding of the SH2 domain to phosphotyrosine residues) still induced cytoskeletal reorganization (Fig. 5B), thus suggesting that, while the SH2 domain plays an essential role in translocation of CrkL to focal adhesions (Fig. 1), it may be dispensable in mediating pathways downstream once localized to focal adhesions. It has been noted that some SH2 domains may mediate protein-protein interactions independently of phosphotyrosine (1, 8). Therefore, the point mutation in the SH2 domain of FA-CRKL(K39) may still allow protein-protein interactions independently of phosphotyrosine. However, another SH2 mutant of FA-CRKL in which the SH2 domain was deleted still induced cellular projections containing lamellipodia in a manner similar to that of FA-CRKL or FA-CRKL(K39) (data not shown). On the other hand, deletion of the SH3n domain from FA-CRKL abolished its ability to induce characteristic protrusions decorated by lamellipodia (Fig. 5B). FA-CRKL(K39+ΔSH3c) in which both the SH2 and SH3c domains were mutated was still capable of inducing cytoskeletal rearrangements (Fig. 5B). Therefore, the SH3n domain appeared to be necessary and sufficient for the effect of FA-CRKL on the cytoskeleton.
While the results mentioned above suggest a role for SH3 binding proteins in cytoskeletal rearrangement at focal adhesions, it is possible that FA-CRKL may sequester SH3 binding proteins from other functional locations important for cytoskeletal organization. We therefore addressed this possibility by targeting of CRKL to another subcellular location, the mitochondrial outer surface with another fusion protein, CRKL-GFP-mito. Unlike FA-CRKL, however, overexpression of CRKL-GFP-mito did not generate membrane protrusions decorated with lamellipodia (Fig. 5C). Therefore, FA-CRKL leads to cytoskeletal reorganization most likely by activation of CRKL SH3n binding proteins at focal adhesions rather than by sequestration of SH3 binding proteins.
We then compared the effects of FA-CRKL with that of CRKL-GFP. While CRKL-GFP induced a slight increase in the number of cells with characteristic membrane protrusions compared to GFP alone (11.3 versus 4.6%; Student t test, P = 0.0053), FA-CRKL was far more effective than was CRKL-GFP (64.3 versus 11.3%; Student t test, P = 0.0011) by transfecting cells at a high dose of 0.3 μg/well of plasmid by Effectene (Fig. 5D). Lower doses of CRKL-GFP resulted in no significant increase in the number of cells with lamellipodia, whereas FA-CRKL induced lamellipodia in approximately 58, 37, and 23% of transfected cells at doses of 0.1, 0.04, and 0.015 μg/well of plasmid, respectively (not shown). Together with the localization control shown in Fig. 5C, these results indicate that focal adhesion localization of CRKL efficiently activates the machinery of cytoskeletal organization. Furthermore, in addition to an increase in the number of cells with lamellipodia, expression of FA-CRKL significantly increased the number of lamellipodia per cell compared to that of control cells (Kolmogorov-Smirnov test for frequency distributions, P ≤ 2.2 × 10−16) (Fig. 5E). On the other hand, expression of FA-GFP or FA-CRKL lacking the SH3n domain showed no overt change in the cytoskeleton. These results therefore confirmed that focal adhesion localization of CRKL efficiently leads to cytoskeletal reorganization.
Translocation of CRKL to focal adhesions activates pathways toward Rac1 and Cdc42.
In order to provide insight into the molecular mechanism of cytoskeletal changes induced by FA-CRKL, we next examined the state of small G proteins that have been implicated in organization of the actin cytoskeleton. We found that levels of GTP-loaded (active) Rac1 and Cdc42 in NIH 3T3 cells expressing FA-CRKL were elevated approximately 2.5- and 2.3-fold compared to that of control NIH 3T3 cells, respectively (Fig. 6B). On the other hand, FA-CRKL expression had little effect on levels of GTP-bound Ras. Consistent with these results, expression of a dominant-negative form of either Rac1 (Rac1 N17) or Cdc42 (Cdc42 N17), but not RhoA (RhoA N17) or Ras (Ras N17), inhibited the effect of FA-CRKL on cytoskeletal reorganization judged by formation of characteristic membrane protrusions in a dose-dependent manner (Fig. 6C). These results therefore indicate that translocation of CRKL to focal adhesions activates pathways toward Rac1 and Cdc42, both of which are necessary for cytoskeletal reorganization.
Among Crk/CrkL SH3 binding proteins, Dock1 (also called Dock180) is an activator of Rac1 (24, 32). Consistent with elevated levels of GTP-loaded Rac1, endogenous Dock1 constitutively localized to focal adhesions in NIH 3T3 cells expressing FA-CRKL (Fig. 6D). On the other hand, overall Rac1 subcellular distribution appeared to be unaffected in these cells (not shown). These results therefore suggest that CrkL translocation leads to Dock1 localization to focal adhesions where Dock1 may activate Rac1.
DISCUSSION
CrkL is a member of the Crk adapter protein family. Crk was originally identified as the cellular homologue of the oncogene product v-Crk in the avian sarcoma virus CT10 (29, 34). Alternative splicing of the cellular Crk gene generates two isoforms, Crk-I and Crk-II (also referred to as isoforms a and b), of which Crk-I is structurally more similar to v-Crk, consisting only of one SH2 domain and one SH3 domain (28). Disruption of the Crk-II isoform in mice causes no overt phenotype, while residual transcription from the mutated Crk locus generates proteins virtually identical to Crk-I (20). It is therefore possible that either or both Crk-I and CrkL may compensate for loss of the Crk-II isoform in these mice.
Unlike the Crk gene, the Crkl locus generates only one protein similar to Crk-II (45). While FA-CRKL was capable of complementing SYF cells in haptotaxis assays, Crkl−/− cells showed defects in haptotaxis but not in cell motility stimulated by serum. While it is possible that Crk expressed in Crkl−/− cells may partially compensate for CrkL deficiency, it should be noted that Crkl−/− cells showed haptotaxis defects despite elevated expression of Crk-II in these cells compared to that in control cells (approximately 1.7-fold compared to that of wild-type cells [Fig. 4A]). These results therefore indicate that CrkL plays a specific role in cell migration induced by integrin signaling. However, our results do not exclude the possibility that Crk may function similarly to CrkL in other types of cells or for other purposes. Since CrkL is not essential for serum-stimulated cell motility, it remains to be determined whether serum-stimulated cell migration relies on Crk or on other pathways independent of either Crk or CrkL.
Fak is another protein tyrosine kinase that can directly interact with Src family kinases through the phosphorylated Y397 residue of Fak (40). Fak also associates with the SH3 domain of Cas (31). Like Src family kinases, it has been reported that Fak is also essential for cell motility induced by fibronectin (6, 42). It is worthwhile, however, to note that, although Fak−/− cells show impaired chemotaxis toward platelet-derived growth factor, SYF cells are capable of migrating toward this growth factor (25, 43). Therefore, Fak may activate a distinct pathway in addition to those dependent on Src family kinases. It remains to be determined whether CrkL may also play a role in Fak or growth factor-mediated cell motility.
The major Crk/CrkL SH3 binding protein, Dock1, can bind to the small G protein Rac1 (24). Although Dock1 does not have a Dbl homology (DH) domain conserved among conventional guanine nucleotide exchange factors for small G proteins, genetic evidence in C. elegans and Drosophila suggests that Dock1 (CED-5 and Mbc in worms and flies, respectively) is a critical component of the Rac1 activation machinery (9, 32, 48). It has been reported that Dock1 also contains a PtdIns (3,4,5)P3 binding site (26). Whereas activated phosphoinositide 3-kinase (PI3K) can induce translocation of endogenous Dock1 to the plasma membrane, it does not increase GTP loading of Rac1 (26). Although “overexpression” of a farnesylated Dock1 mutant has been shown to cause a morphological change (15), these findings suggest that membrane localization of “endogenous” Dock1 per se may not be sufficient for Rac1 activation. On the other hand, expression of FA-CRKL induced cotranslocation of endogenous Dock1 to focal adhesions, which likely explains elevated GTP loading of Rac1 in the cell. It is possible that other pathways that CrkL can mediate may cooperate at focal adhesions for activation of Rac1. In light of the ability of Dock1 to localize to the plasma membrane through the PtdIns(3,4,5)P3 binding site mentioned above, it is noteworthy that PI3K has been identified as a Crk/CrkL SH3 binding protein (10). Thus, CrkL may affect Dock1 localization not only through its direct binding to Dock1 but also through PI3K. This mechanism may explain the localization of FA-CRKL as well as Dock1 to the leading edge of lamellipodia in addition to focal adhesions. Interestingly, despite the ability of Dock1 to associate with Rac1, subcellular distribution of Rac1 was not overtly different between FA-CRKL-expressing cells and their controls (not shown). Therefore, activation of Dock1 and other cofactors may be an important mechanism for Rac1 activation rather than Rac1 translocation.
Recently, it was shown that complex formation between Dock1 and ELMO1 is also necessary for Rac1 activation (4). Genetic evidence from C. elegans suggests an essential role for the pathway of CED-2 (Crk-II), CED-5 (Dock1), and CED-12 (ELMO) to CED-10 (Rac) activation in cell migration as well as engulfment of apoptotic bodies (11, 49, 50). However, involvement of Src-like kinases or Cas in this pathway has not been reported in C. elegans. Furthermore, although Crk-II has been referred to as the CED-2 orthologue (33), our TBLASTN search of the WormBase (Sanger Institute) indicates that amino acid sequence similarities between mouse CrkL and CED-2 are comparable to those of mouse Crk-II and CED-2 (52 versus 62%), while the smallest-sum probability was 2.4 × 10−8 versus 1.9 × 10−6, respectively. Therefore, it is tempting to speculate that, in cells of higher organisms such as mammals in which Crk and CrkL genes are evolved from the common ancestral gene CED-2, CrkL specifically links integrin signaling to this evolutionally conserved pathway downstream of Src and Cas.
While cotranslocation of CrkL and Dock1 to focal adhesions likely explains the mechanism of Rac1 activation, FA-CRKL-induced GTP loading of Cdc42 may involve other mechanisms. It has been reported that Dock1 can activate Rac1 but not Cdc42 in 293T cells (24). It is possible that Cdc42 may be activated by a pathway downstream of Rac1 in embryonic fibroblasts. However, it should be noted that Rac1 and Cdc42 appear to be activated in distinct spatiotemporal patterns in randomly moving HT1080 cells (22). It is not yet clear whether the latter observation can be generalized to other cell types such as MEFs or to cells undergoing integrin-induced directional migration. The mechanisms by which CrkL activates Cdc42 remain to be determined.
Since paxillin is a major component of focal adhesions and is required for general cell motility (13), it is possible that FA-CRKL fusion proteins may influence cell signaling independent of CRKL either by activating LIM domain-dependent functions or by replacing endogenous paxillin from focal adhesions. In addition, one might anticipate that FA-CRKL(ΔSH3n) potentially acts as a dominant- negative protein. However, FA-GFP (fusion of GFP and paxillin LIM domains) or FA-CRKL(ΔSH3n) did not affect the cell morphology or the ability of the cell to migrate toward fibronectin or serum. Also, NIH 3T3 cells expressing a similar fusion of CRKL with the FAT domain of Fak showed cell morphology indistinguishable from that of FA-CRKL (not shown). These observations therefore suggest that these possibilities mentioned above are unlikely at least under the experimental conditions used in our studies, although it is difficult to completely rule them out.
Crkl−/− embryos show developmental defects in migratory stem cell-like populations called neural crest cells in which integrin signaling presumably plays an important role. Our findings in Crkl−/− MEFs may therefore help to explain the phenotype of CrkL-deficient mice. Mouse embryos lacking Src, Fyn, and Yes show a severe phenotype (25) at embryonic day 9.5. Mice lacking Crkas (mouse Cas) die around embryonic day 12.5 due likely to heart failure (19). Since these phenotypes are severer than that of Crkl−/− embryos, it is likely that pathways dependent on CrkL may not represent all signaling pathways that Src family kinases mediate through Cas. Functional and genetic links between Crkl and Crkas as well as Src family members remain to be established in order to determine the molecular mechanism of the Crkl−/− mutant phenotype in vivo.
Acknowledgments
We thank S. Kron for critical reading of the manuscript; B. J. Druker, F. B. Gertler, H. Hirai, M. Matsuda, G. P. Nolan, S. M. Thomas, M. A. Schwartz, and P. Soriano for valuable reagents; and S. Bond for technical assistance at the University of Chicago Digital Microscopy Facility.
This work was supported in part by grants to A.I. from Wendy Will Case Cancer Fund, the American Cancer Society (RPG 00-239-01-CSM), and Department of the Army (DAMD 17-02-1-0639).
REFERENCES
- 1.Anderson, S. M., E. A. Burton, and B. L. Koch. 1997. Phosphorylation of Cbl following stimulation with interleukin-3 and its association with Grb2, Fyn, and phosphatidylinositol 3-kinase. J. Biol. Chem. 272:739-745. [DOI] [PubMed] [Google Scholar]
- 2.Bear, J. E., J. J. Loureiro, I. Libova, R. Fassler, J. Wehland, and F. B. Gertler. 2000. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101:717-728. [DOI] [PubMed] [Google Scholar]
- 3.Brown, M. C., J. A. Perrotta, and C. E. Turner. 1996. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J. Cell Biol. 135:1109-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brugnera, E., L. Haney, C. Grimsley, M. Lu, S. F. Walk, A. C. Tosello-Trampont, I. G. Macara, H. Madhani, G. R. Fink, and K. S. Ravichandran. 2002. Unconventional Rac-GEF activity is mediated through the Dock180 ELMO complex. Nat. Cell Biol. 4:574-582. [DOI] [PubMed] [Google Scholar]
- 5.Butler, A. A., V. A. Blakesley, A. Koval, R. deJong, J. Groffen, and D. Le Roith. 1997. In vivo regulation of CrkII and CrkL proto-oncogenes in the uterus by insulin-like growth factor-I. Differential effects on tyrosine phosphorylation and association with paxillin. J. Biol. Chem. 272:27660-27664. [DOI] [PubMed] [Google Scholar]
- 6.Cary, L. A., D. C. Han, T. R. Polte, S. K. Hanks, and J. L. Guan. 1998. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 140:211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cary, L. A., R. A. Klinghoffer, C. Sachsenmaier, and J. A. Cooper. 2002. SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22:2427-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaldson, L. W., G. Gish, T. Pawson, L. E. Kay, and J. D. Forman-Kay. 2002. Structure of a regulatory complex involving the Abl SH3 domain, the Crk SH2 domain, and a Crk-derived phosphopeptide. Proc. Natl. Acad. Sci. USA 99:14053-14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson, M. R., B. J. Galletta, and S. M. Abmayr. 1997. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J. Cell Biol. 138:589-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feller, S. M. 2001. Crk family adaptors—signalling complex formation and biological roles. Oncogene 20:6348-6371. [DOI] [PubMed] [Google Scholar]
- 11.Gumienny, T. L., E. Brugnera, A. C. Tosello-Trampont, J. M. Kinchen, L. B. Haney, K. Nishiwaki, S. F. Walk, M. E. Nemergut, I. G. Macara, R. Francis, T. Schedl, Y. Qin, L. Van Aelst, M. O. Hengartner, and K. S. Ravichandran. 2001. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 107:27-41. [DOI] [PubMed] [Google Scholar]
- 12.Guris, D. L., J. Fantes, D. Tara, B. J. Druker, and A. Imamoto. 2001. Mice lacking the homologue of the human 22q11.2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nat. Genet. 27:293-298. [DOI] [PubMed] [Google Scholar]
- 13.Hagel, M., E. L. George, A. Kim, R. Tamimi, S. L. Opitz, C. E. Turner, A. Imamoto, and S. M. Thomas. 2002. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 22:901-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamasaki, K., T. Mimura, N. Morino, H. Furuya, T. Nakamoto, S. Aizawa, C. Morimoto, Y. Yazaki, H. Hirai, and Y. Nojima. 1996. Src kinase plays an essential role in integrin-mediated tyrosine phosphorylation of Crk-associated substrate p130Cas. Biochem. Biophys. Res. Commun. 222:338-343. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa, H., E. Kiyokawa, S. Tanaka, K. Nagashima, N. Gotoh, M. Shibuya, T. Kurata, and M. Matsuda. 1996. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol. Cell. Biol. 16:1770-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaney, C., K. Kolibaba, A. Bhat, T. Oda, S. Ohno, S. Fanning, and B. J. Druker. 1997. Direct binding of CRKL to BCR-ABL is not required for BCR-ABL transformation. Blood 89:297-306. [PubMed] [Google Scholar]
- 17.Hemmeryckx, B., A. van Wijk, A. Reichert, V. Kaartinen, R. de Jong, P. K. Pattengale, I. Gonzalez-Gomez, J. Groffen, and N. Heisterkamp. 2001. Crkl enhances leukemogenesis in BCR/ABL P190 transgenic mice. Cancer Res. 61:1398-1405. [PubMed] [Google Scholar]
- 18.Honda, H., T. Nakamoto, R. Sakai, and H. Hirai. 1999. p130(Cas), an assembling molecule of actin filaments, promotes cell movement, cell migration, and cell spreading in fibroblasts. Biochem. Biophys. Res. Commun. 262:25-30. [DOI] [PubMed] [Google Scholar]
- 19.Honda, H., H. Oda, T. Nakamoto, Z. Honda, R. Sakai, T. Suzuki, T. Saito, K. Nakamura, K. Nakao, T. Ishikawa, M. Katsuki, Y. Yazaki, and H. Hirai. 1998. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat. Genet. 19:361-365. [DOI] [PubMed] [Google Scholar]
- 20.Imaizumi, T., K. Araki, K. Miura, M. Araki, M. Suzuki, H. Terasaki, and K. Yamamura. 1999. Mutant mice lacking Crk-II caused by the gene trap insertional mutagenesis: Crk-II is not essential for embryonic development. Biochem. Biophys. Res. Commun. 266:569-574. [DOI] [PubMed] [Google Scholar]
- 21.Imamoto, A., and P. Soriano. 1993. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell 73:1117-1124. [DOI] [PubMed] [Google Scholar]
- 22.Itoh, R. E., K. Kurokawa, Y. Ohba, H. Yoshizaki, N. Mochizuki, and M. Matsuda. 2002. Activation of Rac and Cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 22:6582-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinsella, T. M., and G. P. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405-1413. [DOI] [PubMed] [Google Scholar]
- 24.Kiyokawa, E., Y. Hashimoto, S. Kobayashi, H. Sugimura, T. Kurata, and M. Matsuda. 1998. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 12:3331-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi, S., T. Shirai, E. Kiyokawa, N. Mochizuki, M. Matsuda, and Y. Fukui. 2001. Membrane recruitment of DOCK180 by binding to PtdIns(3,4,5)P3. Biochem. J. 354:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, L., M. Okura, and A. Imamoto. 2002. Focal adhesions require catalytic activity of Src family kinases to mediate integrin-matrix adhesion. Mol. Cell. Biol. 22:1203-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda, M., S. Tanaka, S. Nagata, A. Kojima, T. Kurata, and M. Shibuya. 1992. Two species of human CRK cDNA encode proteins with distinct biological activities. Mol. Cell. Biol. 12:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer, B. J., M. Hamaguchi, and H. Hanafusa. 1988. A novel viral oncogene with structural similarity to phospholipase C. Nature 332:272-275. [DOI] [PubMed] [Google Scholar]
- 30.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamoto, T., R. Sakai, H. Honda, S. Ogawa, H. Ueno, T. Suzuki, S.-I. Aizawa, Y. Yazaki, and H. Hirai. 1997. Requirements for localization of p130cas to focal adhesions. Mol. Cell. Biol. 17:3884-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolan, K. M., K. Barrett, Y. Lu, K. Q. Hu, S. Vincent, and J. Settleman. 1998. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 12:3337-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddien, P. W., and H. R. Horvitz. 2000. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2:131-136. [DOI] [PubMed] [Google Scholar]
- 34.Reichman, C. T., B. J. Mayer, S. Keshav, and H. Hanafusa. 1992. The product of the cellular crk gene consists primarily of SH2 and SH3 regions. Cell Growth Differ. 3:451-460. [PubMed] [Google Scholar]
- 35.Ruest, P. J., N.-Y. Shin, T. R. Polte, X. Zhang, and S. K. Hanks. 2001. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol. Cell. Biol. 21:7641-7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai, R., A. Iwamatsu, N. Hirano, S. Ogawa, T. Tanaka, H. Mano, Y. Yazaki, and H. Hirai. 1994. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 13:3748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai, R., T. Nakamoto, K. Ozawa, S. Aizawa, and H. Hirai. 1997. Characterization of the kinase activity essential for tyrosine phosphorylation of p130Cas in fibroblasts. Oncogene 14:1419-1426. [DOI] [PubMed] [Google Scholar]
- 38.Salgia, R., E. Pisick, M. Sattler, J. L. Li, N. Uemura, W. K. Wong, S. A. Burky, H. Hirai, L. B. Chen, and J. D. Griffin. 1996. p130CAS forms a signaling complex with the adapter protein CRKL in hematopoietic cells transformed by the BCR/ABL oncogene. J. Biol. Chem. 271:25198-25203. [DOI] [PubMed] [Google Scholar]
- 39.Salgia, R., N. Uemura, K. Okuda, J. L. Li, E. Pisick, M. Sattler, R. de Jong, B. Druker, N. Heisterkamp, L. B. Chen, et al. 1995. CRKL links p210BCR/ABL with paxillin in chronic myelogenous leukemia cells. J. Biol. Chem. 270:29145-29150. [DOI] [PubMed] [Google Scholar]
- 40.Schaller, M. D., J. D. Hildebrand, J. D. Shannon, J. W. Fox, R. R. Vines, and J. T. Parsons. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14:1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senechal, K., C. Heaney, B. Druker, and C. L. Sawyers. 1998. Structural requirements for function of the Crkl adapter protein in fibroblasts and hematopoietic cells. Mol. Cell. Biol. 18:5082-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieg, D. J. 1999. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112:2677-2691. [DOI] [PubMed] [Google Scholar]
- 43.Sieg, D. J., C. R. Hauck, D. Ilic, C. K. Klingbeil, E. Schaefer, C. H. Damsky, and D. D. Schlaepfer. 2000. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2:249-256. [DOI] [PubMed] [Google Scholar]
- 44.Tachibana, K., T. Urano, H. Fujita, Y. Ohashi, K. Kamiguchi, S. Iwata, H. Hirai, and C. Morimoto. 1997. Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase. A putative mechanism for the integrin-mediated tyrosine phosphorylation of Crk-associated substrates. J. Biol. Chem. 272:29083-29090. [DOI] [PubMed] [Google Scholar]
- 45.ten Hoeve, J., C. Morris, N. Heisterkamp, and J. Groffen. 1993. Isolation and chromosomal localization of CRKL, a human crk-like gene. Oncogene 8:2469-2474. [PubMed] [Google Scholar]
- 46.Thomas, S. M., P. Soriano, and A. Imamoto. 1995. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature 376:267-271. [DOI] [PubMed] [Google Scholar]
- 47.Vuori, K., H. Hirai, S. Aizawa, and E. Ruoslahti. 1996. Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases. Mol. Cell. Biol. 16:2606-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, Y. C., and H. R. Horvitz. 1998. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 392:501-504. [DOI] [PubMed] [Google Scholar]
- 49.Wu, Y. C., M. C. Tsai, L. C. Cheng, C. J. Chou, and N. Y. Weng. 2001. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev. Cell 1:491-502. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, Z., E. Caron, E. Hartwieg, A. Hall, and H. R. Horvitz. 2001. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev. Cell 1:477-489. [DOI] [PubMed] [Google Scholar]
- 51.Zwartkruis, F. J., R. M. Wolthuis, N. M. Nabben, B. Franke, and J. L. Bos. 1998. Extracellular signal-regulated activation of Rap1 fails to interfere in Ras effector signalling. EMBO J. 17:5905-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]