Abstract
DNA methylation is involved in a variety of genome functions, including gene control and chromatin dynamics. MBD1 is a transcriptional regulator through the cooperation of a methyl-CpG binding domain, cysteine-rich CXXC domains, and a transcriptional repression domain. A yeast two-hybrid screen was performed to investigate the role of MBD1 in methylation-based transcriptional repression. We report a mediator, MBD1-containing chromatin-associated factor (MCAF), that interacts with the transcriptional repression domain of MBD1. MCAF harbors two conserved domains that allow it to interact with MBD1 and enhancer-like transactivator Sp1. MCAF possesses a coactivator-like activity, and it seems to facilitate Sp1-mediated transcription. In contrast, the MBD1-MCAF complex blocks transcription through affecting Sp1 on methylated promoter regions. These data provide a mechanistic basis for direct inhibition of gene expression via methylation-dependent and histone deacetylation-resistant processes.
DNA methylation in mammalian cells contributes to genome regulation and normally implicates the formation of transcriptionally inactive chromatin (4, 16). In the nucleus, not only is the DNA methylated, but the methylated DNA must also be interpreted by methyl-CpG binding domain proteins (MBD proteins) (3). There are at least five mammalian MBD proteins: MeCP2, MBD1, MBD2, and MBD3 for transcriptional repression and MBD4 (also known as MED1) for mismatch repair as a thymine glycosylase.
Several transcription repression complexes include the histone deacetylases (HDACs) (11, 42). Hypermethylated DNA usually tends to coexist with hypoacetylated histones on the heterochromatic regions. In fact, MeCP2 and MBD2 interact with a corepressor complex, Sin3, containing HDACs (20, 27, 29). MBD2-MBD3 heterodimer recruits another multifunctional complex, Mi2-NuRD, which possesses both HDAC and chromatin-remodeling activities (43, 49). This combination of Mi2-NuRD and MBD2 may be synonymous with the originally designated MeCP1 complex (17). Recently, Kaiso, which associates with the p120 catenin, was reported as being a new type of methylation-dependent transcriptional repressor, and it is one constituent of the MeCP1 complexes (33). Furthermore, mammalian DNA methyltransferase (DNMT1) not only maintains genome-wide methylation patterns during replication but also forms certain complexes with corepressor DMAP1 and HDACs, with MBD2-MBD3, or with retinoblastoma protein (Rb), E2F1, and HDAC1 (35, 38, 40). A specific HDAC inhibitor, trichostatin A (TSA), has been found to partially relieve transcriptional repression by MeCP2, MBD2, and DNMT1 (20, 27, 29, 38). Nevertheless, these results do raise questions of the essential role of histone deacetylation in methylation-based transcriptional repression. Recent studies have shown that Rb blocks transcription both by recruiting HDAC and by inactivating transcription factors at the promoter (24). As with Rb, MeCP2 has been suggested to repress transcription by an alternative pathway independent of HDACs (21, 41, 48).
Promoter regions of RNA polymerase II (Pol II)-transcribed genes often possess discrete clusters of approximately 1 kb of unmethylated CpG dinucleotides (called CpG islands) (1), whereas the remainder, such as imprinted genes, genes on the inactive X chromosome, and some tissue-specific genes, is densely methylated and repressed. In addition, aberrant methylation patterns in promoter-associated CpG islands cause altered gene expression in human hereditary diseases and cancers (32, 36, 46). Condensed chromatin on methylated promoter regions is likely to interfere with the access of transcriptional activators and coactivators and a set of general transcription factors to their binding sites (23, 37, 47).
Ubiquitous transactivator Sp1 is required for the constitutive and inducible expression of a variety of genes through binding to G-rich elements such as the GC box in the promoter and enhancer (22, 39). Sp1 has distinctive features in gene regulation. First, CpG methylation itself within the GC box does not inhibit the binding ability of Sp1 (18), and the presence of proteins that bind methylated DNA can block the transcription factor (5). Secondly, Sp1 is required to prevent de novo methylation of promoter-associated CpG islands (6, 25), and multiple Sp1 sites direct local demethylation of methyl-CpG dinucleotides in embryonal cells and HeLa cells (12, 34). Thirdly, Sp1 binds general transcription factors such as the TATA-box binding proteins. Despite a great deal of information, little is known about the functional relationship of the DNA methylation system, Sp1, and basal transcription machinery.
Previously, we have presented evidence that MBD1 acts as a transcriptional regulator through the cooperation of MBD, cysteine-rich CXXC domains, and a C-terminal transcriptional repression domain (TRD) (13, 14). The conserved CXXC sequence was originally found in DNMT1 and the Drosophila trithorax group protein ALL-1, but its precise role is still unknown (2). The TRD of MBD1 produces an active transcriptional repression that was initially reported to be partially reversed by the addition of TSA (28). However, MBD1 is not involved in the MeCP1 repressor complex (29). Also, unlike MeCP2 and MBD2, MBD1 is not immunodepleted from HeLa nuclear extracts by anti-HDAC1 antibodies, suggesting that an alternative pathway exists in the repression by MBD1. During investigation of the mechanism of MBD1-dependent transcriptional repression, we found that the repression is resistant to HDAC inhibitors. In this paper, we present evidence demonstrating the importance of a unique mediator, MBD1-containing chromatin-associated factor (MCAF), which binds the TRD of MBD1 to form the repressive complex. Our findings suggest that MBD1 directly prevents transcription from methylated promoters in a histone deacetylation-independent manner, through interacting with MCAF.
MATERIALS AND METHODS
Yeast two-hybrid screening.
Yeast strain CG-1945 carrying pAS2-1-TRD of MBD1 (amino acids 529 to 592 [isoform v1] or 473 to 536 [isoform v3]) (14) was transformed with the HeLa cDNA libraries constructed in pGAD-GH (Clontech). Plasmids harboring cDNA were recovered from the both histidine- and β-galactosidase-positive colonies.
Sequence analysis of MCAF.
The cDNA of MCAF contains the sequence fragments identical to putative p621 (GenBank accession no. AJ242978) and human partial cDNA FLJ10688 (GenBank accession no. AK001550). In addition, MCAF also showed sequence homologies to mouse ATFa-associated modulator (mAM) (GenBank accession no. AJ132702) (10). MCAF shows sequence identities with mAM: 69% (residues 1 to 131), 55% (residues 132 to 561), 76% (562 to 817; domain 1), 77% (residues 818 to 1153), and 98% (residues 1154 to 1270; domain 2). Both domains 1 and 2 of MCAF contained several expressed-sequence-tag clones with homologies in many species, but no other conserved domains have been identified in databases.
Cell culture.
HeLa, U2OS, A549, ASPC, NCI-H1299, and SBC-5 cells and human fibroblasts were cultured in a 1:1 mixture of Dulbecco's modified Eagle's minimum essential medium and Ham's F12 nutrient medium (Invitrogen) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Bio-Whittaker). Schneider cell line 2 (SL2) derived from Drosophila embryos was cultured in Schneider's Drosophila medium with 10% (vol/vol) heat-inactivated fetal bovine serum and 2 mM glutamine.
Transfection and treatment.
HeLa, U2OS, NCI-H1299, and SBC-5 cells were transfected with plasmid DNAs by using a liposome-mediated gene transfer method. The transfected cells were treated with 100 ng of TSA/ml and 1 mM sodium n-butyrate (Wako) for 12 h. Plasmids were transfected into SL2 cells by using a calcium phosphate method.
Plasmids.
The cDNA for MCAF was cloned into pcDNA3 and Drosophila expression vector pAc5.1/V5-His (pAc5.1-MCAF) (Invitrogen). The cDNA for MBD1v1 was ligated into pEGFP-N1 and pDsRed-N1 (Clontech). The pCGN-MBD1v1 and pEGFP-MBD1 (MBD+NLS) were previously described (14). The TRD of MBD1v1 (amino acids 529 to 592) was subcloned into pCMV-GAL4, and point mutants (I576R, L579R, and I576R/L579R) were prepared with a site-directed mutagenesis. The TRD of MeCP2 cDNA (amino acids 207 to 310) was subcloned into pCMV-GAL4.
Protein expression.
The TRD of MBD1v1 cDNA was cloned into pGEX-4T-1 (Amersham Pharmacia) and pRSET (Invitrogen), and three mutant constructs (I576R, L579R, and I576R/L579R) were prepared. The MCAF cDNAs were cloned into pGEX-2TH and pRSET. The Sp1 cDNAs were inserted into pGEX-4T-1.
Antibodies.
The polyclonal antibodies against MCAF were generated by immunizing rabbits against the glutathione S-transferase (GST)-fused MCAF (amino acids 1 to 334). For affinity purification of the antibodies, His-tagged MCAF (1 to 334) was coupled to Affi-Gel 15-activated matrix (Bio-Rad). Antibodies utilized were anti-GAL4, anti-Sp1 (Santa Cruz), anti-FLAG (M5) (Sigma), anti-His (Qiagen), anti-GST (DAKO), anti-hemagglutinin 1 (HA) (Roche), anti-MBD1 (Medical & Biological Laboratories), and anti-acetylated histone H3 and H4 antibodies (Upstate). Western blot and immunofluorescence analyses were carried out as described previously (13).
Immunoprecipitation.
HeLa cells were treated with dimethyl 3,3′-dithiobispropionimidate-2HCl (DTBP) (5 mM) (Pierce) in phosphate-buffered saline, rinsed with an ice-cold buffer (100 mM Tris-HCl, pH 8.0, 150 mM NaCl), and lysed on ice for 30 min with a buffer (1% NP-40, 0.1% sodium dodecyl sulfate, 500 mM NaCl, 10 mM Tris-HCl, pH 7.4, 5 mM EDTA, 1% sodium deoxycholate, 5% glycerol, and protease inhibitors). The cell lysates (60 μg) were incubated with specific antibodies or with control immunoglobulin G (IgG) for 1 h at 4°C. This was followed by incubation for 1 h after adding 30 μl of protein G/A agarose beads (Calbiochem) in a buffer (250 μl) containing 0.2% NP-40, 40 mM Tris-HCl, pH 7.5, 100 mM KCl, 20% glycerol, and protease inhibitors. For immunoprecipitation of endogenous proteins, the DTBP-treated cells were lysed with a hypotonic buffer (0.05% NP-40, 50 mM HEPES, pH 8.0, 10 mM NaCl, 1.5 mM MgCl2, 0.05 mM ZnCl2, 5% glycerol, 1 mM dithiothreitol) supplemented with protease inhibitors and 1 mM sodium orthovanadate for 10 min at 4°C. The nuclei were collected by centrifugation (10,000 × g) at 4°C for 10 min and were resuspended in a hypertonic buffer (1% NP-40, 10 mM Tris-HCl, pH 7.5, 500 mM NaCl, 5 mM EDTA, 5% glycerol, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate, the protease inhibitors, and sodium orthovanadate). After being sonicated and centrifuged for 10 min, supernatants (250 μl) were incubated for 1 h at 4°C with specific antibodies or control IgG and then for 1 h after adding 20 μl of protein A/G agarose beads.
GST pulldown assay.
Bacterially expressed GST and GST fusion proteins (2 μg) were immobilized on glutathione-agarose beads and incubated with His-tagged proteins (2 μg) in a buffer (250 μl) containing 0.1% Triton X-100, 50 mM HEPES, pH 7.4, 100 mM NaCl, 5% glycerol, 2 mM dithiothreitol, and protease inhibitors for 1 h at 4°C. The input indicates 10% of the His-tagged proteins in the reaction mixture.
His-tagged protein affinity chromatography.
His-MCAF Δ3 (2 μg) on nickel-chelating resin (20 μl) was mixed with GST-TRD of MBD1 and GST-Sp1 Δ1 containing the residues 90 to 785 of Sp1 (0 to 2 μg each) in buffer A (9 mM imidazole, 100 mM NaCl, 50 mM NaH2PO4) at 4°C for 60 min. After extensive washes with the buffer, the proteins on the resin were eluted by using buffer A (50 μl) containing 300 mM imidazole.
Luciferase assay.
The luciferase activities were determined as described (13, 14). Values are the means and standard deviations of the results from three independent experiments.
Chemical cross-linking and chromatin immunoprecipitation assay.
Cells (5 × 105) were treated for 30 min on ice with DTBP (5 mM) in phosphate-buffered saline for immunoprecipitations. The cells were rinsed with an ice-cold buffer (100 mM Tris-HCl, pH 8.0, 150 mM NaCl) and cross-linked by addition of 1% formaldehyde for 10 min. Crude cell lysates were sonicated to generate 200- to 1,000-bp DNA fragments in length. Chromatin immunoprecipitation was performed with anti-HA or anti-FLAG antibodies or control IgG according to the manufacturer (Upstate). Specific sequences in the immunoprecipitates were detected by PCR amplification with the p16-W primers (14).
RESULTS
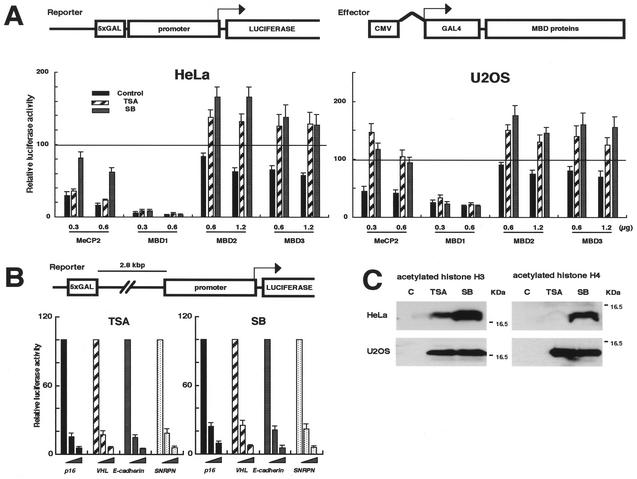
Repression by MBD1 is resistant to HDAC inhibitors.
To investigate the functional relationship between MBD proteins and histone deacetylation in transcriptional silencing, we analyzed their repressive activities under conditions of HDAC inhibition. TRDs of MeCP2 and MBD1 and full-length MBD2A and MBD3 were expressed as a fusion to a DNA binding domain of the yeast GAL4. We examined the effect of these fusion proteins on a luciferase reporter that contains five GAL4 binding elements just upstream of p16, VHL (von Hippel Lindau), E-cadherin, and SNRPN (small nuclear ribonuclear protein N) gene promoters (Fig. 1A). The luciferase activities in combination with insertless GAL4-mock were normalized to 100. All GAL4-fused MBD proteins repressed transcription from VHL promoter in a dose (effector)-dependent manner in both HeLa and U2OS cells (Fig. 1, black bars). The transfected cells were then treated with the HDAC inhibitors TSA and sodium butyrate. The moderate repression by MBD2A and MBD3 was completely reversed by both inhibitors, and the luciferase activities were increased by 1.5- to 2-fold. TRDs of MBD1 and MeCP2 strongly inhibited the promoter activities. The repression by TRD of MeCP2 was also dramatically reversed by both HDAC inhibitors in U2OS cells, while it was diminished significantly by sodium butyrate but less by TSA in HeLa cells. Interestingly, none of these HDAC inhibitors relieved transcriptional inhibition by TRD of MBD1. These data suggest that MBD proteins have different mechanisms underlying gene repression. Very similar results were obtained with the SNRPN promoter (data not shown).
FIG. 1.
MBD1-mediated transcriptional repression is resistant to HDAC inhibitors. (A) Effect of HDAC inhibitors on transcriptional repression by MBD proteins. The reporter constructs contain both the yeast GAL4 DNA binding site (5xGAL) and sequences from the human promoter-associated CpG island upstream of the luciferase cDNA. Effectors express the TRDs of MeCP2 and MBD1 and the MBD2a and MBD3 in a fusion to the GAL4 DNA binding domain. An effector (0.3, 0.6, and 1.2 μg) and the insertless pCMV-GAL4 (0.9, 0.6, and 0 μg) were introduced into HeLa and U2OS cells together with the reporter containing VHL promoter (1.0 μg) and an internal control pRL-CMV (0.02 μg). At 30 h after transfection, the cells were treated for 12 h with the HDAC inhibitors, TSA and sodium butyrate (SB), and with the solvent alone (black). The luciferase activities in combination with pCMV-GAL4 (mock) were normalized to 100 (as indicated by a line). (B) Little influence of HDAC inhibitors on repression by TRD of MBD1 from a distance. GAL4 binding motifs were inserted more than 3 kb upstream of the transcription start site in the reporter constructs. Four gene promoters were used: p16 (black), VHL (hatched), E-cadherin (gray), and SNRPN (white). An effector (0 to 1.0 μg) was transfected into HeLa cells. (C) Induction of acetylated histones H3 and H4 by HDAC inhibitors. Lane C, the solvent alone.
Next, we studied whether the TRDs of MBD1 and MeCP2 can repress transcription from a distance. Five GAL4 binding motifs were inserted more than 3 kb upstream from the transcription start site in the reporter construct (Fig. 1B). TRD of MBD1 completely repressed transcription from a distance in a dose-dependent manner (data not shown), in keeping with the previous report (28), indicating that MBD1 functions regardless of the location relative to core promoter and transcription start site. In contrast, TRD of MeCP2 repressed the promoter activities by about twofold, but the suppressive effect tended to be weakened by the distance (data not shown). Further, we tested the effect of HDAC inhibitors on MBD1-mediated repression from a distance. The transcriptional repression by TRD of MBD1 from a distance was not relieved by the HDAC inhibitors in all the test promoters (Fig. 1B).
To verify the effect of HDAC inhibitors on cellular histones, a Western blot analysis was performed with anti-acetylated histone H3 and H4 antibodies (Fig. 1C). In HeLa cells, TSA induced hyperacetylation of H3 but very weak acetylation of H4, while sodium butyrate hyperacetylated both H3 and H4. The treatment of the cells with higher concentrations of TSA resulted in the acetylation of H4 (data not shown). On the other hand, both histones were equally hyperacetylated by TSA and sodium butyrate in U2OS cells, revealing that HDAC inhibitors act in histone molecule-specific and cell-type-dependent manners. This finding may explain why sodium butyrate, but not TSA, predominantly disturbed repression by MeCP2 in HeLa cells (Fig. 1A) (Discussion). Thus, TRD of MBD1-mediated gene repression is resistant to the HDAC inhibitors. This may contrast with a previous report's findings (28), and we were not able to exclude the possibility that MBD1 cooperates with HDACs in certain circumstances.
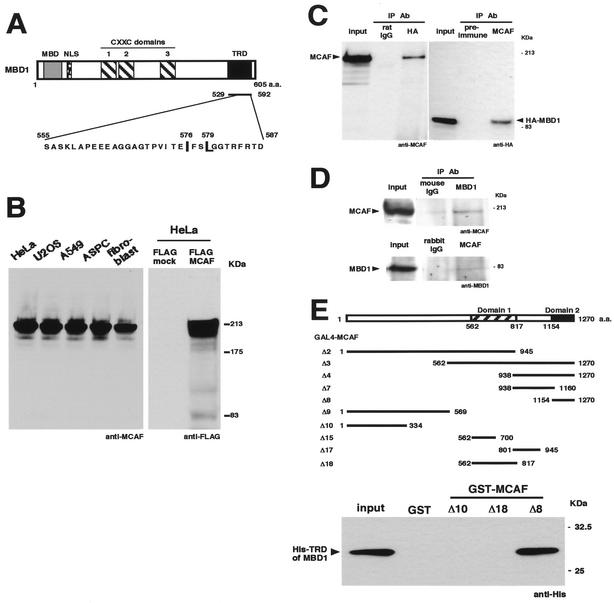
MBD1 interacts with MCAF.
To identify factors that interact with MBD1, we performed a yeast two-hybrid screen by using the TRD as bait (Fig. 2A). From a screening of approximately 7 × 106 independent transformants, we isolated four independent cDNA clones encoding the C-terminal region of a new protein, termed MCAF. We cloned and sequenced a 3,813-bp full-length cDNA that encoded a polypeptide of 1,270 amino acid residues (GenBank accession no. AF425650). When anti-MCAF antibodies were used, Western blot analysis of cultured human cells showed an approximately 200-kDa band of endogenous MCAF (Fig. 2B). FLAG-tagged MCAF was detected with a similar molecular weight in transfected HeLa cells by anti-FLAG antibodies. To check the complex of MBD1-MCAF in the cell, we performed an immunoprecipitation analysis (Fig. 2C). Endogenous MCAF was detected in the immunoprecipitates with HA-tagged MBD1. Likewise, HA-MBD1 was present in the MCAF immunoprecipitates. We further examined the association of these endogenous proteins in HeLa cells without any overexpression (Fig. 2D). The immunoprecipitates with mouse anti-MBD1 monoclonal antibodies contained MCAF. MBD1 was also present in the immunoprecipitates with rabbit anti-MCAF polyclonal antibodies. The result indicated the existence of complexes containing both MBD1 and MCAF in vivo.
FIG. 2.
MBD1 interacts with a transcriptional modulator, MCAF. (A) Structure of MBD1. MBD1 contains an MBD, three cysteine-rich CXXC domains, and a TRD. A yeast two-hybrid screen using the TRD identified MCAF. Two amino acid residues within the TRD (indicated by capital letters) are important for MBD1-dependent repression (Fig. 4). (B) Expression of MCAF. Anti-MCAF polyclonal antibodies and a vector expressing FLAG-tagged MCAF were utilized. (C) Complex formation of MBD1 and MCAF. Endogenous MCAF and HA-tagged MBD1 were immunoprecipitated from HeLa cells. IP, immunoprecipitation; Ab, antibody. (D) Interaction between endogenous MBD1 and MCAF. Mouse anti-MBD1 monoclonal antibodies and rabbit anti-MCAF polyclonal antibodies were used for immunoprecipitation. (E) Biochemical binding assay. MCAF possesses two distinct conserved domains, named domain 1 (Δ18) and domain 2 (Δ8). GST and GST-MCAF (2 μg) were immobilized on glutathione-agarose beads and incubated with His-tagged TRD of MBD1 (2 μg). The input indicates 10% of the indicated proteins in the reaction mixture.
To confirm the direct interaction between MBD1 and MCAF, we produced GST-fused deletion mutants of MCAF (Δ2, Δ3, Δ4, Δ7, Δ8, Δ9, Δ10, Δ15, Δ17, and Δ18), and in vitro pulldown analysis was carried out (Fig. 2E). GST and GST-fused portions of MCAF were immobilized on glutathione-agarose beads and were incubated with His-tagged TRD of MBD1. The TRD of MBD1 bound the minimal C-terminal region of MCAF (Δ8), termed domain 2, in agreement with a yeast two-hybrid screen. Among the mutants, MCAF (Δ3 and Δ4) also showed the positive interactions with TRD of MBD1 (data not shown). In addition, GST-TRD of MBD1 similarly bound His-tagged MCAF (Δ8) (Fig. 4B).
FIG. 4.
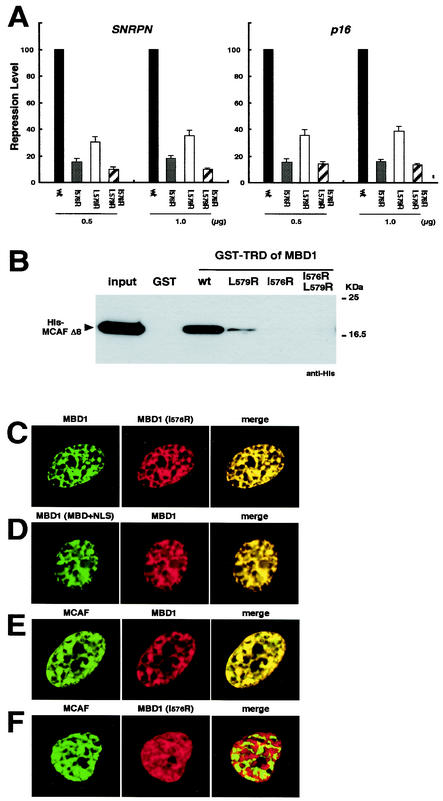
Interaction between MBD1 and MCAF is required for transcriptional repression. (A) TRD mutants of MBD1 reduce the repressive activity. Wild-type (wt) and mutant TRDs of MBD1 in a GAL4 fusion were expressed in HeLa cells. The repression level in combination with wild-type GAL4-TRD of MBD1 was normalized to 100. The amino acid residues in Fig. 2A were subjected to mutagenesis (I576R, L579R, and I576R/L579R). (B) Loss of ability of TRD mutants to bind MCAF. Wild-type and mutant TRDs fused to GST were immobilized and incubated with His-tagged MCAF (Δ8). The input indicates 10% of the protein in the reaction mixture. (C and D) Localization of MBD1 and MBD1 (I576R) mutant in the nucleus. An immunofluorescence analysis of DsRed- or EGFP-fused MBD1 was performed in HeLa cells. MBD1 (MBD+NLS) expresses only the MBD and the nuclear localization signal. (E) Colocalization of MBD1 and native MCAF. (F) Dissociation of MCAF from MBD1 (I576R) mutant.
MCAF is a transcriptional regulator.
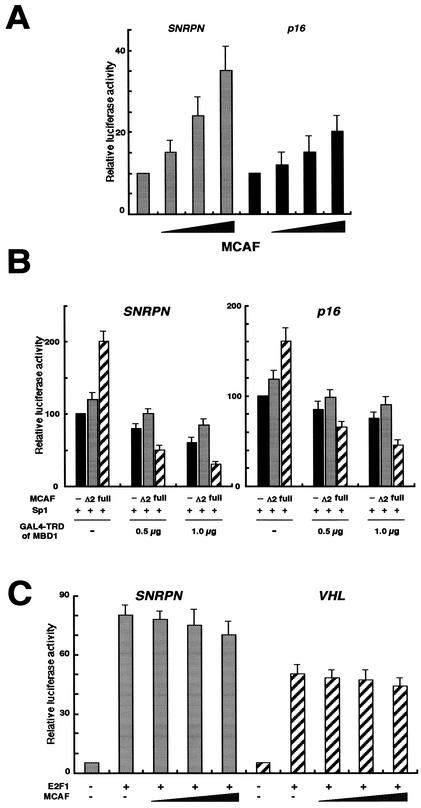
As an important clue for resolving the role of MCAF, MCAF (amino acids 545 to 1058) was found to be identical to the sequence of partially determined p621, which was recently listed as one of the proteins potentially interacting with Sp1 (15). To address a fundamental role of MCAF in transcriptional control, we investigated the effect of MCAF on promoter activities in HeLa cells (Fig. 3A). The SNRPN and p16 promoters were previously shown to contain one and three putative Sp1 binding motifs, respectively (14). The promoter activity of SNRPN is also known to be as strong as that of β-actin. MCAF increased transcription from both promoters in a dose-dependent manner. Next, we examined whether the specific interaction between TRD of MBD1 and MCAF affected an Sp1-activated transcription in Drosophila melanogaster SL2 cells (Fig. 3B). SL2 cells possess a basal transcription apparatus homologous to that of mammalian cells but mostly lack endogenous Sp1 and a DNA methylation system. Transactivation of GAL4 motif-containing reporter constructs by exogenous Sp1 was measured under the coexpression of MCAF and GAL4-TRD of MBD1. To test the need for MCAF for MBD1 (TRD)-mediated repression, we used MCAF Δ2 mutant, which is deficient in association with MBD1 (Fig. 2E). In the absence of MBD1, full-length MCAF augmented transcription by Sp1 and MCAF Δ2 tended to increase luciferase activities to a lesser extent. Further, full-length MCAF enhanced MBD1-dependent repression of the Sp1-activated transcription by approximately 1.5- to 2-fold (Fig. 2E, hatched), whereas the luciferase activities of MCAF Δ2 were the same as the mock transfections (Fig. 2E, gray and black). Thus, MCAF Δ2 lost the ability to enhance Sp1-stimulated transcription and to increase repression by MBD1. The luciferase activities were barely detectable in the absence of Sp1 expression (data not shown). In addition, MCAF did not enhance an E2F1-activated transcription under similar experimental conditions (Fig. 3C). The SNRPN and VHL promoters were previously shown to contain three and one E2F1 binding motifs, respectively (14). These findings suggest that MCAF is a transcriptional positive regulator coupled with Sp1 and that TRD of MBD1 represses an Sp1-activated transcription in association with MCAF.
FIG. 3.
MCAF is a transcriptional regulator. (A) Effect of MCAF on promoter activities. The full length of MCAF (0 to 3 μg) and the insertless pcDNA3 (3 to 0 μg) were introduced into HeLa cells together with the luciferase reporter (1.0 μg) and pRL-CMV (0.02 μg). The luciferase activities in combination with mock vector (1.0 μg) were normalized to 10. (B) Inhibition of an Sp1-activated transcription by TRD of MBD1 and MCAF. Full-length MCAF and Δ2 mutant deficient in association with MBD1 (Fig. 2E) were expressed in D. melanogaster SL2 cells, together with GAL4-TRD of MBD1 and Sp1. GAL4 motif-containing reporter vector (1.0 μg), pPacSp1 (1.0 μg), pAc5.1-MCAF, pAc5.1-MCAF Δ2 and its insertless mock version (1.0 μg), and pAc5.1-GAL4-TRD of MBD1 (0.5 or 1.0 μg) and its mock version (0.5 or 0 μg) were utilized. The repression level in combination with the mock (1.0 μg) was normalized to 100 (black bars), and the relative luciferase activities were corrected by an internal control, pAc5.1-pRL (0.02 μg). (C) MCAF did not enhance an E2F1-activated transcription. Full-length MCAF (0 to 3 μg) and the insertless pcDNA3 (3 to 0 μg) were introduced into the cells together with the SNRPN or VHL luciferase reporter (1.0 μg) and E2F1-expressing vector (1.0 μg).
Interaction between MBD1 and MCAF is necessary for transcriptional repression.
MBD1 possesses a unique TRD sequence in the C-terminal region (Fig. 2A). A previous report showed that two hydrophobic residues, isoleucine-576 (I576) and leucine-579 (L579), are most important for the repressive activities of the TRD of MBD1 (28). To confirm the functional significance of the TRD, we constructed three TRD mutants, which were single or double amino acids converted to arginine (termed I576R, L579R, and I576R/L579R). The repression level of wild-type TRD was normalized to 100. All mutants, in particular I576R and I576R/L579R, abolished the repressive effects on both SNRPN and p16 promoter-driven luciferase reporters containing GAL4 motifs (Fig. 4A). The residue I576 appeared to be critical for repression by the TRD of MBD1. We next analyzed whether these TRD mutations affect the binding affinity between the TRD of MBD1 and MCAF. The wild and mutant types of the TRD of MBD1 were prepared as a GST-fused protein and immobilized on glutathione-agarose beads, followed by incubation with His-MCAF (Δ8) (Fig. 4B). TRD (L579R) bound His-MCAF weakly, whereas both TRD (I576R) and TRD (I576R/L579R) completely lost their binding abilities to MCAF. These data indicate that residue I576 within the TRD is important for the ability of MBD1 to bind MCAF and repress transcription. However, our data did not rule out the possibility that these mutations in TRD of MBD1 disrupt an interaction with another factor.
To determine the specific cooperation of MBD1 and MCAF in the nucleus, we investigated the localization of EGFP- or DsRed-MBD1 relative to MCAF in HeLa cells (Fig. 4C to F). MBD1 showed a punctate distribution with multiple foci in the nuclei, and MBD1 (I576R) colocalized with wild-type MBD1 (Fig. 4C). In addition, MBD1 (MBD+NLS) mutant, which includes only the MBD and nuclear localization signal in the N-terminal region of the protein, also colocalized with full-length MBD1 (Fig. 4D). These results indicate that the region containing the MBD and nuclear localization signal, but not TRD, determines the subnuclear localization of MBD1 in proportion to genome methylation. Both MBD1 and endogenous MCAF colocalized at multiple foci in interphase nuclei, in agreement with in vitro findings (Fig. 4E). On the other hand, the MBD1 (I576R) could not coexist with MCAF (Fig. 4F). Thus, the MBD1 (I576R) lost the ability to interact with MCAF on methylated DNA regions. These results suggest that MBD1-MCAF complex plays a crucial role in forming a methylation-based transcriptionally inactive chromatin.
MBD1-MCAF complex on methylated promoter in living cells.
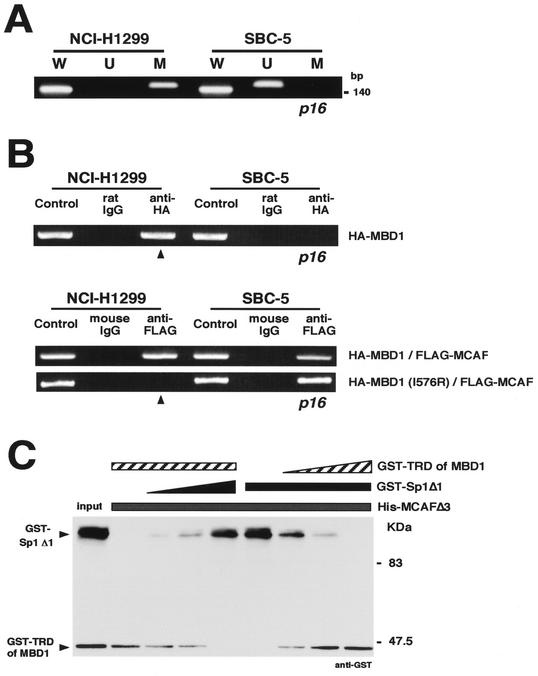
To investigate the association of MBD1 and MCAF with chromosomal gene promoters, we chose the p16 tumor suppressor gene in which hypermethylation of the promoter-associated CpG island causes transcriptional repression in many cancers. The methylation status of the p16 core promoter region was examined by a methylation-specific PCR by using a bisulfite modification of genomic DNAs from human lung cancer cell lines (Fig. 5A). NCI-H1299 cells showed the presence of methylated p16 promoter (Fig. 5A, lane M); meanwhile, the same DNA region was unmethylated in SBC-5 cells (Fig. 5A, lane U). When unmodified DNA templates were used, both cell lines were found to carry the endogenous p16 gene (Fig. 5A, lane W).
FIG. 5.
Association of MBD1 and MCAF on endogenous gene promoter. (A) Methylation-specific PCR. The promoter region of the p16 tumor suppressor gene was studied in human lung cancer cell lines NCI-H1299 and SBC-5. W, U, and M indicate specifically amplified fragments corresponding to unmodified, unmethylated, and methylated sequences, respectively. (B) Link of MCAF by MBD1 to methylated promoter. The cells were treated with a protein-protein cross-linker (DTBP) and formaldehyde. Specific fragments of p16 promoter were detected by PCR amplification by using a set of W primers in the immunoprecipitates. HA-tagged MBD1 was expressed for a chromatin immunoprecipitation with anti-HA antibodies (upper). Wild-type or mutant (I576R) HA-MBD1 was coexpressed together with FLAG-MCAF, followed by the chromatin immunoprecipitation with anti-FLAG antibodies (lower). (C) Competition of MBD1 with Sp1 for binding to MCAF. His-MCAF Δ3 (2 μg) (Fig. 2E) on nickel-chelating resin was incubated with GST-TRD of MBD1 and GST-Sp1 Δ1 containing the residues 90 to 785 of Sp1 (0 to 2 μg each). Bound proteins on the resin were eluted by imidazole. The input indicates 10% of the protein in the reaction mixture.
After these cells were cross-linked with DTBP and then with formaldehyde, immunoprecipitated DNAs were PCR amplified with a set of W primers for p16 promoter sequences. To investigate whether MCAF is linked to the methylated promoter by MBD1 in vivo, HA-tagged MBD1 was expressed in these cultured cells for a chromatin immunoprecipitation with anti-HA antibodies (Fig. 5B). MBD1 was present in the methylated, but not unmethylated, p16 promoter (Fig. 5B, upper panel). Then, to determine the significance of interaction of MBD1 with MCAF, wild and mutant types (I576R) of HA-MBD1 were expressed together with FLAG-MCAF for a chromatin immunoprecipitation with anti-FLAG antibodies (Fig. 5B, lower panel). Wild-type MBD1 linked MCAF to the methylated promoter, while MCAF interaction-deficient MBD1 (I576R) induced the dissociation of MCAF. In addition, MCAF was detected in the unmethylated promoter, regardless of the status of overexpressed MBD1. These data suggest that MBD1 is able to recruit MCAF on the methylated promoter. To further demonstrate whether MBD1 competes with Sp1 for binding to MCAF, immobilized His-MCAF Δ3, which contains both domains 1 and 2 (Fig. 2E), was incubated with GST-TRD of MBD1 and GST-Sp1 Δ1 containing nearly full-length Sp1. Sp1 bound to domain 1 (Δ18), domain 2 (Δ8), and both domains (Δ3) of MCAF (data not shown). The increased amount of Sp1 reduced MCAF-bound MBD1, and the increase of MBD1 oppositely caused the decrease of MCAF-bound Sp1 (Fig. 5C). Taken together, these results suggest that MCAF forms a repressive complex with MBD1, instead of Sp1, on methylated promoters.
DISCUSSION
MBD protein-dependent transcriptional repression.
MBD proteins decipher the epigenetic information of methylation patterns to connect methylated DNA with chromatin components for gene regulation (3, 4). In the present study, TSA induced hyperacetylation of H3 but little acetylation of H4 in HeLa cells, while the acetylation of both histones was increased by TSA in U2OS cells. Butyrate hyperacetylated both H3 and H4 in these two cell lines. Thus, there may be a great diversity in the effectiveness of HDAC inhibitors (26). Under such conditions, transcriptional repression by MBD2 and MBD3 was completely relieved by both HDAC inhibitors. In contrast, repression by MeCP2 was reversed predominantly by butyrate but to a lesser extent by TSA in HeLa cells. This result suggests that MeCP2-dependent repression may basically work together with H4 deacetylation, consistent with a recent report (45). Our studies further emphasized that HDAC inhibition did not affect repression by the TRD of MBD1. Thus, MBD proteins have distinct repression mechanisms based on the involvement of HDACs. Until now, various histone modifications have been identified (19, 42), although information on their relationships with MBD proteins is limited. In addition, the functional redundancy as well as the distinct features of each MBD protein needs to be elucidated, as recently reported by Hendrich et al. (17). There are several lines of evidence showing that either histone deacetylation or DNA methylation, or both, is important for gene inactivation (7, 8) and that the mechanism of transcriptional repression is likely to be dissimilar in each of the inactivated genes on the genome.
We focus on a comparative analysis of functional domains in MBD1 and MeCP2. Ohki et al. (30, 31) and Wakefield et al. (44) characterized the solution structure of the MBDs of MBD1 and MeCP2, respectively, supporting a very similar function of the MBDs in these proteins. Further, TRDs in MBD1 and MeCP2 show no obvious homologies in amino acid sequences, suggesting a different role for these TRDs. For MBD1, the TRD can inhibit the promoter activities from an about 3-kb distance, and this transcriptional repression was not relieved by HDAC inhibition. In contrast, partial reduction of the suppressive effect from a distance was observed in the TRD of MeCP2. The treatment with HDAC inhibitors, however, no longer diminished the repression by MeCP2 from a distance anymore (data not shown). In addition, the CXXC domains of MBD1 might be differentially involved in the gene silencing. Thus, the mechanisms for transcriptional repression by MBD1 and MeCP2 are fundamentally distinct.
MBD1 inhibits transactivation by Sp1 via MCAF.
MBD1 forms a repression complex with MCAF, leading to possible disturbance of Sp1. This complex is likely to inhibit a transcription initiation process, even though MBD1 binds methyl-CpG relatively far from the target promoter, and it may be used for gene silencing less dependent on HDAC activities.
The components of the general transcription machinery bind MCAF (data not shown). In agreement with a coactivator-like activity of MCAF, MBD1-dependent repression was reversed by an excess of MCAF (data not shown). On the other hand, MBD1 may link MCAF to methylated promoters and MBD1-MCAF is likely to affect the formation of a transcriptional preinitiation complex. Thus, MCAF may modulate transcription as either positive or negative mediator in response to genome methylation. In other words, MBD1 seems to abolish the transactivation activity of MCAF to repress transcription. With reference to this issue, it was previously reported that mAM, which shows overall homologies to MCAF, regulates the transcriptional factor ATFa and interacts with general transcription factors (10).
The direct interaction between MCAF and Sp1 is extremely interesting. Courey et al. (9) showed that Sp1 activates transcription synergistically both near and far from the transcription start site in an enhancer-like manner. In addition, Sp1 itself interacts with the basal transcription apparatus, including TFIIB and TBP and the coactivators TAF55, TAF135, and CRSP. Based on our observation that endogenous Sp1 and MCAF coexist in the nucleus (data not shown), Sp1-MCAF is likely to have a transactivation capacity in many gene promoters. Thus, MCAF physically binds Sp1 and the general transcription apparatus like a positive regulator. In contrast, MBD1-MCAF seems to interfere with Sp1-mediated transactivation as well as the transcription preinitiation complexes in methylated DNA regions. These findings may explain why TRD of MBD1 can repress transcription from a distance in an HDAC-independent manner.
Acknowledgments
We thank F. Ishikawa (Kyoto University) for MBD2A and MBD3-expressing plasmids and J. T. Kadonaga (University of California, San Diego) for pPacSp1.
N.F. is a Research Fellow of the Japan Society for the Promotion of Science. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas and by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government (M.N.).
REFERENCES
- 1.Antequera, F., and A. Bird. 1993. Number of CpG islands and genes in human and mouse. Proc. Natl. Acad. Sci. USA 90:11995-11999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bestor, T. H., and G. L. Verdine. 1994. DNA methyltransferases. Curr. Opin. Cell Biol. 6:380-389. [DOI] [PubMed] [Google Scholar]
- 3.Bird, A. 2002. DNA methylation patterns and epigenetic memory. Genes Dev. 16:6-21. [DOI] [PubMed] [Google Scholar]
- 4.Bird, A. P., and A. P. Wolffe. 1999. Methylation-induced repression-belts, braces, and chromatin. Cell 99:451-454. [DOI] [PubMed] [Google Scholar]
- 5.Boyes, J., and A. Bird. 1991. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell 64:1123-1134. [DOI] [PubMed] [Google Scholar]
- 6.Brandeis, M., D. Frank, I. Keshet, Z. Siegfried, M. Mendelsohn, A. Nemes, V. Temper, A. Razin, and H. Cedar. 1994. Sp1 elements protect a CpG island from de novo methylation. Nature 371:435-438. [DOI] [PubMed] [Google Scholar]
- 7.Cameron, E. E., K. E. Bachman, S. Myohanen, J. G. Herman, and S. B. Baylin. 1999. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 21:103-107. [DOI] [PubMed] [Google Scholar]
- 8.Coffee, B., F. Zhang, S. T. Warren, and D. Reines. 1999. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat. Genet. 22:98-101. [DOI] [PubMed] [Google Scholar]
- 9.Courey, A. J., D. A. Holtzman, S. P. Jackson, and R. Tjian. 1989. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell 59:827-836. [DOI] [PubMed] [Google Scholar]
- 10.De Graeve, F., A. Bahr, B. Chatton, and C. Kedinger. 2000. A murine ATFa-associated factor with transcriptional repressing activity. Oncogene 19:1807-1819. [DOI] [PubMed] [Google Scholar]
- 11.Dobosy, J. R., and E. U. Selker. 2001. Emerging connections between DNA methylation and histone acetylation. Cell. Mol. Life Sci. 58:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank, D., I. Keshet, M. Shani, A. Levine, A. Razin, and H. Cedar. 1991. Demethylation of CpG islands in embryonic cells. Nature 351:239-241. [DOI] [PubMed] [Google Scholar]
- 13.Fujita, N., N. Shimotake, I. Ohki, T. Chiba, H. Saya, M. Shirakawa, and M. Nakao. 2000. Mechanism of transcriptional regulation by methyl-CpG binding protein MBD1. Mol. Cell. Biol. 20:5107-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita, N., S. Takebayashi, K. Okumura, S. Kudo, T. Chiba, H. Saya, and M. Nakao. 1999. Methylation-mediated transcriptional silencing in euchromatin by methyl-CpG binding protein MBD1 isoforms. Mol. Cell. Biol. 19:6415-6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunther, M., M. Laithier, and O. Brison. 2000. A set of proteins interacting with transcription factor Sp1 identified in a two-hybrid screening. Mol. Cell. Biochem. 210:131-142. [DOI] [PubMed] [Google Scholar]
- 16.Hendrich, B., and A. Bird. 2000. Mammalian methyltransferases and methyl-CpG-binding domains: proteins involved in DNA methylation. Curr. Top. Microbiol. Immunol. 249:55-74. [DOI] [PubMed] [Google Scholar]
- 17.Hendrich, B., J. Guy, B. Ramsahoye, V. A. Wilson, and A. Bird. 2001. Closely related proteins MBD2 and MBD3 play distinctive but interacting roles in mouse development. Genes Dev. 15:710-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holler, M., G. Westin, J. Jiricny, and W. Schaffner. 1988. Sp1 transcription factor binds DNA and activates transcription even when the binding site is CpG methylated. Genes Dev. 2:1127-1135. [DOI] [PubMed] [Google Scholar]
- 19.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293:1074-1080. [DOI] [PubMed] [Google Scholar]
- 20.Jones, P. L., G. J. Veenstra, P. A. Wade, D. Vermaak, S. U. Kass, N. Landsberger, J. Strouboulis, and A. P. Wolffe. 1998. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19:187-191. [DOI] [PubMed] [Google Scholar]
- 21.Kaludov, N. K., and A. P. Wolffe. 2000. MeCP2 driven transcriptional repression in vitro: selectivity for methylated DNA, action at a distance and contacts with the basal transcription machinery. Nucleic Acids Res. 28:1921-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lania, L., B. Majello, and P. De Luca. 1997. Transcriptional regulation by the Sp family proteins. Int. J. Biochem. Cell Biol. 29:1313-1323. [DOI] [PubMed] [Google Scholar]
- 23.Lu, H., L. Zawel, L. Fisher, J. M. Egly, and D. Reinberg. 1992. Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase II. Nature 358:641-645. [DOI] [PubMed] [Google Scholar]
- 24.Luo, R. X., A. A. Postigo, and D. C. Dean. 1998. Rb interacts with histone deacetylase to repress transcription. Cell 92:463-473. [DOI] [PubMed] [Google Scholar]
- 25.Macleod, D., J. Charlton, J. Mullins, and A. P. Bird. 1994. Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev. 8:2282-2292. [DOI] [PubMed] [Google Scholar]
- 26.Marks, P. A., V. M. Richon, and R. A. Rifkind. 2000. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J. Natl. Cancer Inst. 92:1210-1216. [DOI] [PubMed] [Google Scholar]
- 27.Nan, X., H. H. Ng, C. A. Johnson, C. D. Laherty, B. M. Turner, R. N. Eisenman, and A. Bird. 1998. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393:386-389. [DOI] [PubMed] [Google Scholar]
- 28.Ng, H.-H., P. Jeppesen, and A. Bird. 2000. Active repression of methylated genes by the chromosomal protein MBD1. Mol. Cell. Biol. 20:1394-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng, H. H., Y. Zhang, B. Hendrich, C. A. Johnson, B. M. Turner, B. H. Erdjument, P. Tempst, D. Reinberg, and A. Bird. 1999. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat. Genet. 23:58-61. [DOI] [PubMed] [Google Scholar]
- 30.Ohki, I., N. Shimotake, N. Fujita, J. Jee, T. Ikegami, M. Nakao, and M. Shirakawa. 2001. Solution structure of the methyl-CpG binding domain of human MBD1 in complex with methylated DNA. Cell 105:487-497. [DOI] [PubMed] [Google Scholar]
- 31.Ohki, I., N. Shimotake, N. Fujita, M. Nakao, and M. Shirakawa. 1999. Solution structure of the methyl-CpG-binding domain of the methylation-dependent transcriptional repressor MBD1. EMBO J. 18:6653-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okano, M., D. W. Bell, D. A. Haber, and E. Li. 1999. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99:247-257. [DOI] [PubMed] [Google Scholar]
- 33.Prokhortchouk, A., B. Hendrich, H. Jorgensen, A. Ruzov, M. Wilm, G. Georgiev, A. Bird, and E. Prokhortchouk. 2001. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes Dev. 15:1613-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu, G. Z., and M. Ehrlich. 1999. Demethylation and expression of methylated plasmid DNA stably transfected into HeLa cells. Nucleic Acids Res. 27:2332-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson, K. D., S. Ait-Si-Ali, T. Yokochi, P. A. Wade, P. L. Jones, and A. P. Wolffe. 2000. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat. Genet. 25:338-342. [DOI] [PubMed] [Google Scholar]
- 36.Robertson, K. D., and P. A. Jones. 2000. DNA methylation: past, present and future directions. Carcinogenesis 21:461-467. [DOI] [PubMed] [Google Scholar]
- 37.Roeder, R. G. 1996. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci. 21:327-335. [PubMed] [Google Scholar]
- 38.Rountree, M. R., K. E. Bachman, and S. B. Baylin. 2000. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat. Genet. 25:269-277. [DOI] [PubMed] [Google Scholar]
- 39.Suske, G. 1999. The Sp-family of transcription factors. Gene 238:291-300. [DOI] [PubMed] [Google Scholar]
- 40.Tatematsu, K. I., T. Yamazaki, and F. Ishikawa. 2000. MBD2-MBD3 complex binds to hemi-methylated DNA and forms a complex containing DNMT1 at the replication foci in late S phase. Genes Cells 5:677-688. [DOI] [PubMed] [Google Scholar]
- 41.Wade, P. A. 2001. Methyl CpG-binding proteins and transcriptional repression. Bioessays 23:1131-1137. [DOI] [PubMed] [Google Scholar]
- 42.Wade, P. A. 2001. Transcriptional control at regulatory checkpoints by histone deacetylases: molecular connections between cancer and chromatin. Hum. Mol. Genet. 10:693-698. [DOI] [PubMed] [Google Scholar]
- 43.Wade, P. A., A. Gegonne, P. L. Jones, E. Ballestar, F. Aubry, and A. P. Wolffe. 1999. Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat. Genet. 23:62-66. [DOI] [PubMed] [Google Scholar]
- 44.Wakefield, R. I., B. O. Smith, X. Nan, A. Free, A. Soteriou, D. Uhrin, A. P. Bird, and P. N. Barlow. 1999. The solution structure of the domain from MeCP2 that binds to methylated DNA. J. Mol. Biol. 291:1055-1065. [DOI] [PubMed] [Google Scholar]
- 45.Wan, M., K. Zhao, S. S. Lee, and U. Francke. 2001. MECP2 truncating mutations cause histone H4 hyperacetylation in Rett syndrome. Hum. Mol. Genet. 10:1085-1092. [DOI] [PubMed] [Google Scholar]
- 46.Xu, G. L., T. H. Bestor, D. Bourc'his, C. L. Hsieh, N. Tommerup, M. Bugge, M. Hulten, X. Qu, J. J. Russo, and P. E. Viegas. 1999. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature 402:187-191. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto, S., Y. Watanabe, P. J. van der Spek, T. Watanabe, H. Fujimoto, F. Hanaoka, and Y. Ohkuma. 2001. Studies of nematode TFIIE function reveal a link between Ser-5 phosphorylation of RNA polymerase II and the transition from transcription initiation to elongation. Mol. Cell. Biol. 21:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, F., J. Thiesen, and W. H. Stratling. 2000. Histone deacetylase-independent transcriptional repression by methyl-CpG-binding protein 2. Nucleic Acids Res. 28:2201-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, Y., H. H. Ng, B. H. Erdjument, P. Tempst, A. Bird, and D. Reinberg. 1999. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 13:1924-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]