Abstract
Pseudohypoaldosteronism type II (PHAII) is an autosomal dominant disorder of hyperkalemia and hypertension. Mutations in two members of the WNK kinase family, WNK1 and WNK4, cause the disease. WNK1 mutations are believed to increase WNK1 expression; the effect of WNK4 mutations remains unknown. The clinical phenotype of PHAII is opposite to Gitelman syndrome, a disease caused by dysfunction of the thiazide-sensitive Na-Cl cotransporter. We tested the hypothesis that WNK kinases regulate the mammalian thiazide-sensitive Na-Cl cotransporter (NCC). Mouse WNK4 was cloned and expressed in Xenopus oocytes with or without NCC. Coexpression with WNK4 suppressed NCC activity by more than 85%. This effect did not result from defects in NCC synthesis or processing, but was associated with an 85% reduction in NCC abundance at the plasma membrane. Unlike WNK4, WNK1 did not affect NCC activity directly. WNK1, however, completely prevented WNK4 inhibition of NCC. Some WNK4 mutations that cause PHAII retained NCC-inhibiting activity, but the Q562E WNK4 demonstrated diminished activity, suggesting that some PHAII mutations lead to loss of NCC inhibition. Gain-of-function WNK1 mutations would be expected to inhibit WNK4 activity, thereby activating NCC, contributing to the PHAII phenotype. Together, these results identify WNK kinases as a previously unrecognized sodium regulatory pathway of the distal nephron. This pathway likely contributes to normal and pathological blood pressure homeostasis.
Introduction
Pseudohypoaldosteronism type II (PHAII) (Online Mendelian Inheritance in Man no. 145260) is an autosomal dominant disease characterized by severe hypertension, hyperkalemia, and sensitivity to thiazide diuretics. Based on results of sodium chloride versus sodium bicarbonate or sulfate infusion, Schambelan postulated that the syndrome results from a “chloride shunt” in the renal distal nephron (1). An increase of distal nephron chloride permeability would both increase Na reabsorption, leading to hypertension, and depolarize the transepithelial voltage, leading to hyperkalemia. This mechanism, however, does not readily explain another important feature of PHAII: marked sensitivity to thiazide diuretics.
Mayan and colleagues reported that thiazide diuretics reduce systolic and diastolic pressure by 45 and 25 mmHg, respectively, in PHAII patients, compared with 13 and 10 mmHg in patients with essential hypertension (2). Therefore an alternative or additional mechanism is required to explain the complete PHAII phenotype. One possible mechanism involves the thiazide-sensitive Na-Cl cotransporter (NCC, also known as NCCT or TSC). The chloride shunt of PHAII could reflect an increase in NCC activity; such an increase could occur either because NCC activity within the distal convoluted tubule (DCT) is increased or because NCC expression extends to segments that normally do not express this transport protein. In either case, an increased fraction of Na reabsorption would traverse an electroneutral pathway, thereby reducing the transepithelial voltage and inhibiting potassium secretion.
Several years ago, we evaluated and excluded NCC as linked to PHAII, at least among those families evaluated (3). Recently, Wilson and colleagues (4) used positional cloning to identify two gene products, WNK1 and WNK4, that are linked to PHAII. Both are members of a recently described (5) protein kinase family known as WNKs, for with no lysine (K), because they lack a lysine within the kinase domain at a location previously thought to be essential for kinase activity. Both WNK1 and WNK4 are highly expressed in kidney (4). Within kidney, WNK1 is expressed in the cytoplasm of DCT, connecting tubule, and cortical and medullary collecting duct cells. WNK4 is also expressed along the distal nephron, but its expression is limited to the DCT, CNT, and cortical collecting duct. WNK4 expression by DCT cells is largely within the tight junction, where it colocalizes with ZO-1, a tight-junction protein (4). In other nephron segments, however, WNK4 expression is also cytoplasmic.
The PHAII-causing WNK1 mutations are deletions within the first intron. Wilson and colleagues (4) reported that these mutations increase WNK1 expression in leukocytes; they postulated that PHAII can result from “gain-of-function” WNK1 mutations that increase WNK1 activity. In contrast, discrete missense WNK4 mutations that cause a phenotypically similar syndrome were identified in the coding region. These mutations occur within short highly conserved regions of WNK4 that lie outside the kinase domain. The authors did not speculate about whether mutations in WNK4 increase or decrease its activity. One mechanism to explain the observed phenotype of PHAII would involve WNK kinases regulating NCC activity. In the present work, we used the Xenopus oocyte expression system to test the hypothesis that WNK1 or WNK4 regulates NCC activity.
Methods
DNA constructs.
Human WNK4 sequence was used to perform a TBLASTN search of the mouse GenBank expressed sequence tag (EST) database. Three unique EST clones were identified and obtained from Research Genetics (Huntsville, Alabama, USA). One clone contained an open reading frame encoding 1,222 amino acids that was greater than 88% identical to human WNK4. WNK4 was amplified from mouse (BALB/c) kidney cDNA by PCR. The resulting product was sequenced, confirming identity with the sequence above, and then subcloned into a Xenopus expression vector, pgh19. This mouse WNK4 sequence was reported to GenBank (accession no. AY 184228). PHAII-causing mutations were introduced into WNK4 using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, California, USA). The fidelity of the mutations was confirmed by sequencing at The Vollum Institute at Oregon Health and Science University.
We previously cloned mouse NCC (6). An N-terminal flag–tagged NCC was generated in pgh19 using PCR and sequenced to confirm the results. Rat WNK1, a generous gift of Melanie Cobb (University of Texas Southwestern Medical School, Dallas, Texas, USA), was subcloned into pgh19. Serum- and glucocorticoid-induced kinase (SGK) was a generous gift of David Pearce, University of California, San Francisco (San Francisco, California, USA).
Generation of anti-WNK4 antibody.
An anti–mouse WNK4 synthetic peptide antibody was generated in rabbits by immunizing with a peptide representing amino acids 644–662 of mouse WNK4 (ASGLSDMGEGGQMRKNPVK). The antibody was produced by Alpha Diagnostic International (San Antonio, Texas, USA).
Sodium uptake.
DNA template (NCC, WNK1, and WNK4) was linearized downstream from the 3′ UTR using NotI or AclI. The DNA was then treated with proteinase K (mCAP mRNA Capping Kit; Stratagene) for 1 hour at 37°C, extracted twice with equal volumes of phenol and chloroform, and then with chloroform alone. The DNA was precipitated in 0.1 volume of 3 M sodium acetate and 100% ethanol (1:25) in dry ice. The DNA was resuspended in RNase-free buffer, and the size and amount were checked by agarose gel electrophoresis. T7 RNA polymerase (mMES-SAGE mMACHINE; Ambion Inc., Austin, Texas, USA) was used to transcribe cRNA’s. Transcript yield, size, and quantity were checked by spectroscopy and RNA agarose gel electrophoresis.
For injection, RNA was heated at 65°C for 2 minutes, quick spun, and aspirated into a pipette. Sorted Xenopus oocytes were injected with 50 nl of water each or 12.5 ng each of NCC, WNK1, WNK4, or SGK cRNA individually or in combination. When multiple cRNA’s were injected into oocytes, 12.5 ng of each was injected, but the total injected volume remained 50 nl. After injection, oocytes were stored in Barth’s storage solution (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES, pH 7.4) for 3–5 days at 18°C, with fresh solution changes daily.
Injected oocytes were transferred to ND96 Cl– free solution (96 mM Na+ isethionate, 2 mM K+ gluconate, 1.8 mM Ca2+ gluconate, 1 mM Mg2+ gluconate, 5 mM HEPES, 2.5 mM Na+ pyruvate, and 5 mg/dl gentamicin, pH 7.4) 24 hours prior to the uptake assay. Thirty minutes prior to the addition of uptake medium, the oocytes were added to ND96 (Cl– and K+ free) with inhibitors (1 mM ouabain, 100 μM amiloride, and 100 μM bumetanide), according to the protocol of Gamba (7). The oocytes were then transferred to isotonic uptake medium (58 mM NaCl, 38 mM N-methyl-D-glucamine, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES, with inhibitors, pH 7.4) and 10 μCi/ml final concentration of 22Na and incubated at 30°C for 1 hour. The oocytes were washed five times with 3 ml ice-cold isotonic uptake medium, lysed in 0.1% SDS, added to 5 ml of liquid scintillant (Hionic-Fluor; Perkin-Elmer Life Sciences, Wellesley, Massachusetts, USA), and counted for 2 minutes in a liquid scintillation counter. Fifteen to thirty oocytes were analyzed for each uptake condition during each experiment. Mean values reflect five to ten repetitions of each experiment (each using 15–30 oocytes per experimental condition).
Immunoblot.
Groups of five oocytes injected 4 days prior with cRNA were transferred to Eppendorf tubes and chilled on ice for 5 minutes. Incubation buffer was then replaced with 500 μl of homogenization buffer (20 mM Tris-HCl, pH 7.6, 100 mM NaCl, and 2% NP-40) and the oocytes were lysed by repeated vortexing and pipeting. The yolk and cellular debris were pelleted at 3,600 g for 10 minutes and the supernatant was centrifuged at 3,600 g three times to remove additional yolk. All centrifugation steps were performed at 4°C. Oocyte homogenate samples were stored at –70°C prior to use.
Immunoblotting was essentially as described previously (6). Oocyte homogenates were solubilized in sample buffer (100 mM Tris-HCl, pH 7.6, 3% glycerol, 2% SDS, and 0.01% β-mercaptoethanol) and heated at 60°C for 10 minutes. The proteins were then separated on a 3–8% NuPAGE Tris-Acetate Gel (Novex no. EA0378; Invitrogen Corporation, Carlsbad, California, USA) and transferred to PVDF paper. The blot was blocked with Tween (Blotto-T) (5% nonfat dried milk in PBS-T) and then incubated with anti-NCC (8) (diluted 1:1,000 in Blotto-T) or anti-WNK4 antibody (diluted 1:1,000). The blot was then washed, incubated with secondary antibody (HRP-conjugated goat anti-rabbit IgG, diluted 1:2,000; Zymed Laboratories Inc., South San Francisco, California, USA). Protein was detected using the ECL Plus kit (Amersham Life Sciences Inc., Arlington Heights, Illinois, USA) according to the manufacturer’s instructions. Detected bands were analyzed densitometrically using NIH’s ImageJ image processing program (available at http://rsb.info.nih.gov/ij/docs/index.html) (8).
Immunofluorescence.
Four days after injection, oocytes were fixed with 3% paraformaldehyde in PBS for 4 hours at 4°C. Cryosections 6 μm thick were placed on chrom alum gelatin-coated glass slides, blocked with PBS buffer containing 0.1% BSA, and incubated with anti–mouse NCC (8) diluted in PBS buffer containing 0.1% BSA and 0.05% Tween 20 (1:100) at 4°C overnight. Sections were rinsed with PBS four times, incubated with FITC-conjugated goat anti-rabbit antibody IgG, H+L (1:200, Zymed Laboratories Inc.) for 1 hour at 22°C, and rinsed with PBS buffer four times. The slides were then viewed on a Zeiss Axiophot phase-contrast microscope (Carl Zeiss Inc., Thornwood, New York, USA). Membrane NCC staining was evaluated by two observers blinded to the injected material.
Immunoprecipitation.
Oocytes injected with flag-tagged NCC were prepared as described above for Western blot. Rabbit anti–mouse NCC (2 μl) was added to oocyte homogenate (100 μl) and incubated at 22°C for 1 hour. Thirty microliters of protein G–Sepharose (Amersham Life Sciences Inc.) was added and incubated at 4°C overnight. The reaction mixture was then centrifuged and the beads were washed five times in homogenizing buffer. Immunoprecipitated NCC protein was eluted from the protein G–Sepharose by heating the mixture to 60°C for 10 minutes with sample buffer containing 2.5% SDS and 2.5% β-mercaptoethanol. Immunoprecipitates were analyzed by SDS-PAGE.
Cell-surface biotinylation.
Cell-surface biotinylation was performed as described (9, 10), with modifications. Oocytes, prepared as above, were washed five times with ND96 and then incubated with 1 mg/ml Sulfo-NHS-LC-Biotin (Pierce Biotechnology Inc., Rockford, Illinois, USA) for 1 hour at 4°C. The mixture was then washed five times, quenched with 100 mM glycine for 1 hour at 4°C, and prepared as described above for immunoprecipitation. ImmunoPure Streptavidin Beads (Pierce Biotechnology Inc.) were added and the mixture was incubated at 4°C for 2 hours. The beads were washed five times and eluted with SDS sample buffer, as above. The precipitated proteins were analyzed by Western blot using the anti–mouse NCC antibody.
Statistics.
Group comparisons were made by unpaired Student t test. When multiple comparisons were made, the Bonferroni correction was used. A value of P < 0.05 was considered significant.
Results
WNK4 cloning and expression in oocytes.
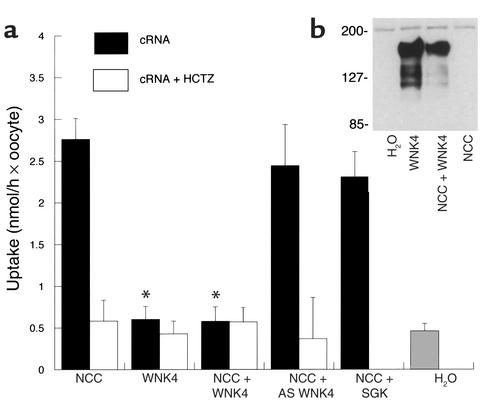
The mouse WNK4 was expressed in oocytes. Immunoblot of oocytes injected with WNK4 cRNA with or without NCC (Figure 1b) showed a protein band of the expected size, whereas oocytes injected with cRNA encoding NCC alone or with water did not.
Figure 1.
(a) Effect of WNK4 on NCC-mediated 22Na uptake, expressed as nmol/h per oocyte. Uptake rates ± SEM of oocytes injected with cRNA encoding NCC, WNK4, NCC + WNK4, NCC + an antisense WNK4 (AS WNK4), NCC + SGK (an unrelated kinase), and water are shown. WNK4 alone has no effect on uptake. Coexpression with WNK4 significantly reduces NCC-mediated Na uptake. Neither antisense WNK4 nor SGK affects NCC activity. *Uptake significantly different from NCC injection alone; P < 0.001. (b) Immunoblot of oocytes probed with anti-WNK4 antibody: oocytes were injected with water or with cRNA encoding WNK4, WNK4 + NCC, or NCC alone. Only oocytes injected with WNK4 cRNA express WNK4 protein. For NCC, WNK4, and NCC + WNK4, there were 12 repetitions of each experiment (15–30 oocytes per experiment). For NCC + antisense WNK4 and NCC + SGK, there were four experiments.Relative molecular weights are shown in kDa. HCTZ, hydrochlorothiazide.
Effects of WNK4 on Na uptake.
Injection of cRNA encoding mouse WNK4 alone did not affect Na uptake (see Figure 1a) compared with water-injected oocytes. As reported previously (6, 11), injection of oocytes with mouse NCC cRNA increased Na uptake five- to tenfold compared with water-injected controls (Figure 1a). Sodium uptake by NCC-expressing oocytes in the presence of 10–5 M hydrochlorothiazide was similar to uptake by water-injected controls. This indicates that NCC induces thiazide-sensitive Na-Cl cotransport (Figure 1a). When cRNA’s encoding both WNK4 and NCC were injected together, Na uptake was significantly lower than when NCC was expressed alone (Figure 1a). In fact, WNK4 reduced the thiazide-sensitive component of Na uptake by more than 85% (Figure 1a). The specificity of this effect was confirmed by determining the effect on NCC-mediated Na uptake of cRNA transcribed in the antisense direction. This antisense WNK4 construct did not affect NCC-mediated Na uptake (Figure 1a). Specificity was further confirmed by testing an unrelated kinase, SGK; SGK did not affect NCC-mediated Na uptake (effects of SGK on NCC activity have not been described previously).
Effects of WNK4 on NCC abundance and processing.
WNK4 might reduce NCC activity by inhibiting NCC synthesis or by disrupting normal NCC processing. We have demonstrated previously that defects in NCC processing in the ER and Golgi complex can be detected by immunoblot of oocytes (6). Wild-type NCC runs as a double band on immunoblots of oocyte membranes. A band near 110 kDa represents the core-glycosylated form of the protein, while a diffuse band near 125–140 kDa represents the mature glycosylated form (6). Mutant NCC proteins that cause Gitelman syndrome, which are misprocessed, are recognized by the “quality control” mechanism of the ER, and do not reach the plasma membrane, run as single core-glycosylated bands on immunoblot. Figure 2a indicates that oocytes expressing NCC alone demonstrate two protein bands, as expected for normally processed NCC. Coexpression of WNK4 with NCC did not significantly affect NCC protein abundance or reduce the relative abundance of the mature glycosylated versus immature (core glycosylated) form of NCC. Values for relative abundance of the core-glycosylated band relative to NCC alone were: NCC plus WNK4, 112% ± 19%; NCC plus both WNK4 and WNK1, 106% ± 21% (P value not significant). This indicates that the effect of WNK4 to reduce NCC activity is not the result of defects in NCC processing in the ER or Golgi complex.
Figure 2.
(a) Immunoblot of immunoprecipitated oocyte membranes probed with anti-NCC antibody. Oocytes injected with NCC, NCC + WNK4, and NCC + WNK4 + WNK1 express the same amount of core-glycosylated protein. The ratio of mature glycosylated to core-glycosylated NCC is the same in all three groups. (b) Immunocytochemistry of oocytes stained with anti-NCC. Representative oocytes injected with NCC, NCC + WNK4, and NCC + WNK4 + WNK1 are shown. (c) Immunoblot of surface-biotinylated NCC. Only the mature glycosylated form of NCC was evident in this blot (data not shown). Coexpression of WNK4 with NCC reduced surface NCC expression by 85% (see Results). Coexpression with WNK1 and WNK4 restored surface NCC levels toward levels observed with NCC alone.
To test whether WNK4 reduces NCC activity by altering NCC trafficking to the plasma membrane, immunocytochemistry was performed. Figure 2b shows a representative photomicrographs of oocytes expressing NCC alone and NCC plus WNK4. Coexpression of NCC with WNK4 reduced NCC expression at the plasma membrane compared with expression of NCC alone. Figure 2c shows an immunoblot of immunoprecipitated surface-biotinylated proteins, probed with anti-NCC. This blot shows that WNK4 reduced surface NCC expression by 85% versus NCC alone (NCC alone, 100%; NCC + WNK4, 15% ± 5%, P < 0.005).
Effects of mutant WNK4.
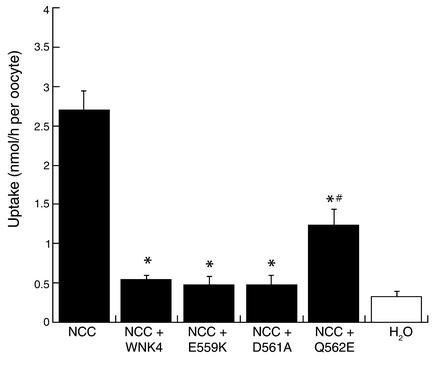
WNK4 mutations cause PHAII. To determine whether PHAII-causing mutant WNK4 proteins inhibit NCC, NCC was injected with mutant WNK4. Figure 3 shows that E559K and D561A maintain nearly full NCC-inhibitory potency. In contrast, the Q562E WNK4 mutation was significantly less effective than wild-type WNK4. At the same molar ratio of WNK4 to NCC, Q562E was approximately half as potent as wild-type WNK4.
Figure 3.
Effect of mutant WNK4 on NCC-mediated 22Na uptake, expressed as nmol/h per oocyte. This figure compares rates of Na uptake (± SEM) of oocytes injected with cRNA encoding NCC and NCC + wild-type WNK4, E559K WNK4, D561A WNK4, and Q562E WNK4. Both wild-type and mutant WNK4 inhibited NCC activity (*P < 0.05 compared with NCC); Q562E reduced uptake significantly less than did wild-type WNK4 (#P < 0.05 compared with NCC+ WNK4). There were six experiments (15–30 oocytes per experiment).
Effects of WNK1 on NCC activity.
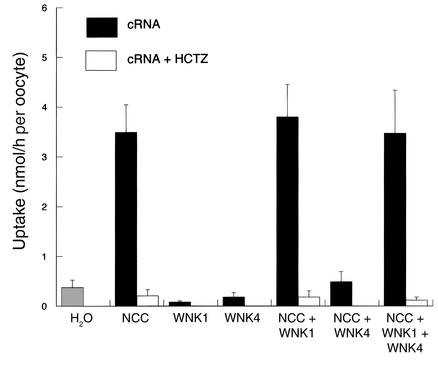
WNK1 did not induce Na uptake by oocytes compared with water-injected controls (see Figure 4). Furthermore, unlike WNK4, WNK1 did not affect NCC-mediated Na uptake; uptake was not different in oocytes expressing NCC with or without WNK1. The experiments shown in Figure 4 replicate the WNK4 effect on NCC activity noted above; when WNK4 was expressed with both WNK1 and NCC, however, Na uptake rates were restored to levels observed in oocytes expressing NCC alone. Thus, WNK1 blocked or attenuated the effect of WNK4 to reduce NCC-mediated Na uptake.
Figure 4.
Effect of WNK1 on NCC-mediated 22Na uptake, expressed as nmol/h per oocyte. This figure compares rates of Na uptake (± SEM) of oocytes injected with cRNA encoding NCC, WNK1, and NCC + WNK1, NCC + WNK4, and NCC + WNK4 + WNK1. *Uptake is significantly different (P < 0.001) from that of oocytes injected with NCC alone. There were seven experiments in each group (15–30 oocytes per experiment).
Immunocytochemistry and analysis of biotinylated NCC (Figure 2) show that NCC coexpression of WNK1 with WNK4 increased surface NCC expression toward levels observed with NCC alone (NCC alone, 100%; NCC + WNK4 + WNK1, 103% ± 16%, P value not significant).
Discussion
PHAII is an autosomal dominant disease of hyperkalemia and hypertension. PHAII patients also demonstrate acidosis, hypercalciuria, reduced bone density, and enhanced sensitivity to thiazide diuretics (2). Thus, the PHAII phenotype is the opposite of the Gitelman syndrome phenotype. Gitelman syndrome is a disease in which NCC mutations (12) disrupt NCC function (6). Recently, mutations in WNK kinases were shown to cause PHAII (4), suggesting that these kinases regulate Na and Cl transport. The current results indicate that WNK kinases regulate NCC activity, defining a novel Na regulatory pathway that may play a central role in controlling sodium and potassium homeostasis in humans.
The thiazide-sensitive Na-Cl cotransporter is expressed at the apical membrane of epithelial cells lining the DCT (13). This transport protein is a member of the cation-chloride cotransporter gene family and reabsorbs 3–7% of filtered NaCl (13). It is the target of thiazide diuretics, drugs that remain the first-line treatment for essential hypertension (14). Gitelman syndrome, an autosomal recessive disease of hypokalemic metabolic alkalosis with hypokalemia (12), is caused by NCC mutations, many of which generate misfolded proteins that do not reach the plasma membrane (6, 15). We have previously investigated NCC (SLC12A3) as a candidate gene for PHAII and did not find linkage (3). The present results implicate NCC in the pathogenesis of PHAII but suggest that its involvement is secondary to regulation by WNK kinases.
When expressed in oocytes with NCC, WNK4 suppressed NCC-mediated Na uptake by more than 85% compared with oocytes injected with NCC alone. This indicates that WNK4 is a potent regulator of NCC activity. Coupled with the observation that WNK4 is expressed along the distal nephron (4), this suggests that WNK4 kinase participates importantly in regulating salt excretion in vivo. The results further suggest that the effect of WNK4 to suppress NCC activity is mediated largely by a reduction in NCC delivery to the plasma membrane. While NCC mutations that cause Gitelman syndrome also reduce plasma membrane NCC delivery, coexpression of WNK4 with NCC did not disrupt processing of nascent NCC. This suggests that WNK4 does not impair NCC folding, but rather interferes with a regulated mechanism for membrane trafficking.
WNK4 has been shown, in preliminary results, to possess kinase activity (16). Thus, one mechanism by which it could inhibit NCC is by phosphorylating the transporter. Gamba and colleagues (17) showed that NCC activity is modestly inhibited by PKC. Yet the effects of PKC are quite modest in comparison with the dramatic effects of WNK4. This suggests that, if direct NCC phosphorylation is responsible for the WNK4 effects, the phosphorylation site may be different from that targeted by PKC. Further experiments will be necessary to determine whether the kinase activity of WNK4 is necessary for its effects on NCC function, but WNK kinases possess non-kinase domains, including the coiled coil domains, that may participate in kinase-independent mechanisms (5). Furthermore, a kidney-specific isoform of WNK1 was recently described that lacks the kinase domain due to alternative splicing (18). The role of this kinase-deficient WNK kinase, versus the kinase-intact WNK kinase used in the current experiments, remains to be determined.
Based on the effects of WNK4 to inhibit NCC activity, we tested whether the mutant WNK4s retain NCC-inhibiting activity. When expressed in Xenopus oocytes, two of three PHAII-causing mutations retain their ability to inhibit NCC activity. A third, however, Q562E, is significantly less potent than wild-type WNK4 at inhibiting NCC activity. A loss of NCC-inhibiting activity would increase distal Na and Cl reabsorption, leading to the PHA phenotype, yet most loss-of-function mutations lead to autosomal recessive, not dominant, disorders. One explanation for an autosomal dominant loss-of-function mutation would be a dominant negative effect. We did not detect a dominant negative effect of the mutant WNK4 proteins in the present experiments (data not shown), although some autosomal dominant transport disorders are not successfully recreated using the oocyte expression system (19). It should also be emphasized that detecting an effect of mutations using the oocyte expression system may not recapitulate a defect that occurs in vivo. Thus, additional experiments will be necessary to identify the mechanism by which WNK mutations cause PHAII.
WNK4 mutations could also lead to the PHAII phenotype by acting on NCC in a tubule segment–specific manner. The mammalian DCT of most species (including human) contains two distinct segments (20, 21). All transepithelial Na reabsorption by DCT1 traverses an electroneutral pathway with Cl. Along DCT2, however, electroneutral Na reabsorption by the NCC operates in parallel with electrogenic Na reabsorption via the epithelial Na channel, ENaC (20, 21). Less NCC is usually expressed along DCT2 than along DCT1, corresponding to lower rates of electroneutral Na reabsorption along the “late” distal tubule (22). WNK4 mutations might relieve constitutive suppression of NCC activity along DCT2, leading to increased Na and Cl transport along this segment. Wilson and colleagues (4) suggested, based on the effect of Na salts to increase K excretion and on the paracellular localization of WNK4 expression, that WNK4 may affect paracellular chloride permeability. The present results do not exclude a role for WNK4 in mediating or regulating paracellular permeability in addition to its effect on NCC activity. In fact, WNK4 could play a role in coordinating transport via the transcellular (NCC) and paracellular (conductive Cl movement) pathways.
WNK1 did not affect NCC activity directly, but instead altered the ability of WNK4 to suppress NCC activity. This indicates a previously unrecognized interaction between WNK kinases. The effect of WNK1 to inhibit WNK4 is nearly as powerful as the effect of WNK4 to inhibit NCC activity. The effect of WNK1 on WNK4 has the net result of increasing NCC activity. Wilson and colleagues (4) reported that PHAII-causing WNK1 mutations increase WNK1 expression. An increase in WNK1 expression would suppress WNK4 activity, activating NCC, and could lead to the PHAII phenotype.
The phosphorylation targets of WNK1 are unknown. While WNK1 may phosphorylate WNK4, recent results suggest that the kidney-specific WNK1 lacks a kinase domain. Xu et al. (23) found that WNK1 contains an autoinhibitory domain between amino acids 510 and 540, centered around two important phenylalanine moieties at 524 and 526. This FXF motif binds to the catalytic domain and inhibits its activity. Both this autoinhibitory domain and the kinase domain of WNK1 are highly conserved in WNK4, raising the possibility that the autoinhibitory domain from WNK1 interacts with WNK4.
WNK1 is activated by hypertonicity (5). Such activation, if it occurs in the distal tubule, may explain the effects of luminal NaCl to alter NCC activity acutely. Increases in luminal NaCl concentration along the DCT lead to rapid increases in NCC activity (22). This occurs despite the fact that the typical luminal concentrations of Na and Cl are well above the Km for the transport protein (24). This suggests that luminal ion concentrations may influence NCC activity allosterically. A hypertonicity-induced stimulation of WNK1 activity could mediate such an effect by increasing NCC activity.
In summary, these results indicate that WNK1 and WNK4 interact to regulate NCC activity in the mammalian distal nephron. They identify a previously unrecognized kinase pathway that may play a central role in controlling extracellular fluid volume. Known regulators of NCC activity may interact with this pathway, a pathway that may also participate in osmolality-induced transport regulation. Mutations in WNK kinases cause PHAII. The mutation Q562E is less active than wild type in inhibiting NCC activity. Thus, WNK mutations probably increase NCC activity, thereby contributing to the PHAII phenotype. While PHAII is rare, a genome-wide screen of hypertensive individuals identified a chromosomal region encompassing WNK4 as an important contributor to blood pressure variability (4, 25). Thus, WNK kinases, and their ability to regulate NCC, may contribute importantly to human blood pressure variation and provide novel targets for pharmaceutical intervention.
Acknowledgments
This work was supported by the NIH National Institute of Diabetes & Digestive & Kidney Diseases (grant R01 DK-51496) and by internal funds provided by Oregon Health and Science University. The authors thank David Cohen, David Rosansky, and Arohan Subramanya for helpful discussions and Melanie Cobb for the WNK1 clone.
Footnotes
See the related Commentary beginning on page 947.
Portions of this work were presented at the Experimental Biology Meeting, April 20–24, 2002, New Orleans, Louisiana, USA, and at the meeting of the American Society of Nephrology, October 30–November 4, 2002, Philadelphia, Pennsylvania, USA, and appear in abstract form (2002. Late Breaking Abstract Book, Experimental Biology 2002, page 17 #LB76; 2002. J. Am. Soc. Nephrol. 13:75A).
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: pseudohypoaldosteronism type II (PHAII); Na-Cl cotransporter (NCC); distal convoluted tubule (DCT); serum- and glucocorticoid-induced kinase (SGK).
References
- 1.Schambelan M, Sebastian A, Rector FC., Jr Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): role of increased renal chloride reabsorption. Kidney Int. 1981;19:716–727. doi: 10.1038/ki.1981.72. [DOI] [PubMed] [Google Scholar]
- 2.Mayan H, et al. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J. Clin. Endocrinol. Metab. 2002;87:3248–3254. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 3.Simon DB, et al. Examination of the thiazide-sensitive Na-Cl cotransporter as a candidate gene in Gordon’s syndrome. J. Am. Soc. Nephrol. 1995;6:632. (Abstr.) [Google Scholar]
- 4.Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 5.Xu B, et al. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 6.Kunchaparty S, et al. Defective processing and expression of the thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman’s syndrome. Am. J. Physiol. 1999;277:F643–F649. doi: 10.1152/ajprenal.1999.277.4.F643. [DOI] [PubMed] [Google Scholar]
- 7.Gamba G, et al. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J. Biol. Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 8.Bostanjoglo M, et al. 11b-hydroxysteroid dehydrogenase, mineralocorticoid receptor and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J. Am. Soc. Nephrol. 1998;9:1347–1358. doi: 10.1681/ASN.V981347. [DOI] [PubMed] [Google Scholar]
- 9.Chiu CS, et al. Number, density, and surface/cytoplasmic distribution of GABA transporters at presynaptic structures of knock-in mice carrying GABA transporter subtype 1-green fluorescent protein fusions. J. Neurosci. 2002;22:10251–10266. doi: 10.1523/JNEUROSCI.22-23-10251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes G, et al. Role of N-linked glycosylation in rat renal Na/Pi-cotransport. J. Biol. Chem. 1994;269:24143–24149. [PubMed] [Google Scholar]
- 11.Gamba G, et al. Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc. Natl. Acad. Sci. U. S. A. 1993;90:2749–2753. doi: 10.1073/pnas.90.7.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon DB, et al. Gitelman’s variant of Bartter’s syndrome, inherited hypokalemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat. Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 13.Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol. Rev. 2000;80:277–313. doi: 10.1152/physrev.2000.80.1.277. [DOI] [PubMed] [Google Scholar]
- 14.Appel LJ. The verdict from ALLHAT—thiazide diuretics are the preferred initial therapy for hypertension. JAMA. 2002;288:3039–3042. doi: 10.1001/jama.288.23.3039. [DOI] [PubMed] [Google Scholar]
- 15.De Jong JC, et al. Functional expression of mutations in the human NaCl cotransporter: evidence for impaired routing mechanisms in Gitelman’s syndrome. J. Am. Soc. Nephrol. 2002;13:1442–1448. doi: 10.1097/01.asn.0000017904.77985.03. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi K, Rai T, Kobayashi K, Uchida S, Sasaki S. Transient expression of WNK 4 in MDCK cells: localization and functional analysis. J. Am. Soc. Nephrol. 2002;13:483A. (Abstr.) [Google Scholar]
- 17.Suastegui R, Martinze F, Plata C, Gamba G. Regulation of the thiazide-sensitive Na-Cl cotransporter by activation of PKA and PKC. J. Am. Soc. Nephrol. 1996;7:1291. (Abstr.) [Google Scholar]
- 18.Xu Q, Modrek B, Lee C. Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res. 2002;30:3754–3766. doi: 10.1093/nar/gkf492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quilty JA, Li J, Reithmeier RA. Impaired trafficking of distal renal tubular acidosis mutants of the human kidney anion exchanger kAE1. Am. J. Physiol. Renal Physiol. 2002;282:F810–F820. doi: 10.1152/ajprenal.00216.2001. [DOI] [PubMed] [Google Scholar]
- 20.Bachmann S, Bostanjoglo M, Schmitt R, Ellison DH. Sodium transport-related proteins in the mammalian distal nephron - distribution, ontogeny and functional aspects. Anat. Embryol. (Berl.). 1999;200:447–468. doi: 10.1007/s004290050294. [DOI] [PubMed] [Google Scholar]
- 21.Loffing J, et al. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am. J. Physiol. Renal Physiol. 2001;281:F1021–F1027. doi: 10.1152/ajprenal.0085.2001. [DOI] [PubMed] [Google Scholar]
- 22.Ellison DH, Velázquez H, Wright FS. Thiazide sensitive sodium chloride cotransport in the early distal tubule. Am. J. Physiol. 1987;253:F546–F554. doi: 10.1152/ajprenal.1987.253.3.F546. [DOI] [PubMed] [Google Scholar]
- 23.Xu BE, et al. Regulation of WNK1 by an autoinhibitory domain and autophosphorylation. J. Biol. Chem. 2002;277:48456–48462. doi: 10.1074/jbc.M207917200. [DOI] [PubMed] [Google Scholar]
- 24.Monroy A, Plata C, Hebert SC, Gamba G. Characterization of the thiazide-sensitive Na(+)-Cl(-) cotransporter: a new model for ions and diuretics interaction. Am. J. Physiol. Renal Physiol. 2000;279:F161–F169. doi: 10.1152/ajprenal.2000.279.1.F161. [DOI] [PubMed] [Google Scholar]
- 25.Levy D, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension. 2000;36:477–483. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]