Abstract
The pssT gene was identified as the fourth gene located upstream of the pssNOP gene cluster possibly involved in the biosynthesis, polymerization, and transport of exopolysaccharide (EPS) in Rhizobium leguminosarum bv. trifolii strain TA1. The hydropathy profile and homology searches indicated that PssT belongs to the polysaccharide-specific transport family of proteins, a component of the type I system of the polysaccharide transport. The predicted membrane topology of the PssT protein was examined with a series of PssT-PhoA fusion proteins and a complementary set of PssT-LacZ fusions. The results generally support a predicted topological model for PssT consisting of 12 transmembrane segments, with amino and carboxyl termini located in the cytoplasm. A mutant lacking the C-terminal part of PssT produced increased amounts of total EPS with an altered distribution of high- and low-molecular-weight forms in comparison to the wild-type RtTA1 strain. The PssT mutant produced an increased number of nitrogen fixing nodules on clover.
The soil bacterium Rhizobium leguminosarum bv. trifolii has the ability to produce an acidic exopolysaccharide (EPS) that plays an important role in symbiotic interaction with clover plants. The EPS of R. leguminosarum is composed of octasaccharide repeating units containing one galactose, five glucoses, and two glucuronic acid residues with acetyl, pyruvyl, and hydroxybutanoyl modifications (20). Similarly to Sinorhizobium meliloti succinoglycan (EPS I), high-molecular-weight (HMW) and low-molecular-weight (LWM) fractions of EPS were identified in culture supernatants of R. leguminosarum (11, 34).
Polysaccharides consisting of repeating units are assembled on a polyisoprenyl-pyrophosphate lipid carrier at the cytoplasmic face of the inner membrane by a sequential transfer of monosaccharides from their nucleotide sugars by the action of specific glycosyltransferases. The oligosaccharides are subsequently translocated, polymerized to an HMW EPS, and transported to the cell surface (57). In R. leguminosarum, assembly of the repeating units is under the control of pssA, pssDE, pssC, pssGHI, and other, as-yet-unidentified, genes encoding glycosyltransferases (5, 22, 26, 46, 53).
Recently, we identified the pssN, pssO, and pssP genes that might be constituents of a type I system involved in the polymerization and export of EPS in R. leguminosarum bv. trifolii TA1 (33, 34). On the basis of computational analysis and sequence similarity to the known proteins, PssP was identified as a member of the membrane-periplasmic auxiliary (MPA1) (42) or polysaccharide copolymerase (PCP2) (39) protein family that are involved in the synthesis of HMW EPS. PssP of R. leguminosarum bv. trifolii TA1 resembles ExoP from S. meliloti, which functions in the synthesis and polymerization of succinoglycan (EPS I) (2). Mutants of R. leguminosarum bv. trifolii and S. meliloti deleted of the pssP or exoP gene, respectively, did not produce the EPS (3, 34). Recently, similarly to other members of the PCP2 family, the autophosphorylating protein tyrosine kinase activity of ExoP, which is essential for EPS I production and size distribution, was shown (41).
The putative PssN protein (33) belongs to the outer membrane auxiliary (OMA) family (42) and, like all members of this family, it contains a sequence for a signal peptidase cleavage. OMA proteins provide the porin-like structure in the outer membrane and, together with MPA1 protein, facilitate translocation of polysaccharide through the cell wall (42).
The function of PssO in R. leguminosarum bv. trifolii EPS biosynthesis is not clear because of a lack of homology to known proteins, but its secondary structure indicates outer-membrane localization. In the bacteria, each OMA-MPA1 pair of proteins functions together with polysaccharide-specific transport (PST) proteins (42). In the case of S. meliloti, the pair of ExoF and ExoP proteins may function together with ExoT, an integral inner membrane PST protein (13, 42).
In the present study, we describe the identification of the pssT gene that is located upstream of the pssNOP gene cluster in R. leguminosarum bv. trifolii TA1. The PssT protein is predicted to be yet another constituent of type I system of polymerization and transport of EPS across the cell wall. Based on the PssT hydrophobicity profile, the outputs of different transmembrane segment (TMS) prediction programs and the “positive inside rule” (55), a secondary structure model was constructed for this protein. The model predicts that PssT contains 12 TMSs with the N and C termini facing the cytoplasm. To determine the actual number of transmembrane spans in PssT protein and its membrane topology, we used a reporter gene fusion approach. This method is based on the observation that the enzyme activities of certain reporter proteins translationally fused to a membrane protein can indicate the subcellular locations of these fusion sites in the hybrid proteins (16, 31, 50). We used alkaline phosphatase (AP), encoded by the phoA gene, which is enzymatically active in the periplasm but not in the cytoplasm (30). In contrast, β-galactosidase, encoded by the lacZ gene, is active in the cytoplasm but not in the periplasm (30). Pairs of PssT-PhoA and PssT-LacZ hybrids with identical fusion joints were analyzed in detail, and the results support the proposed secondary structure model of PssT. Finally, a mutant that synthesized the PssT protein lacking the C-terminal part was constructed. This mutant produced an increased amount of EPS, and the distribution of HMW and LMW forms was altered compared to the wild-type strain. The mutant induced nitrogen-fixing nodules on red clover.
MATERIALS AND METHODS
Bacterial strains, plasmids and culture conditions.
Bacterial strains and plasmids used in the present study are listed in Table 1. R. leguminosarum strains were grown at 28°C in 79CA complete medium (54) with 1% mannitol or 0.5% glycerol as carbon sources. Escherichia coli strains were grown at 37°C in Luria-Bertani (LB) medium (47). Antibiotics for E. coli and R. leguminosarum cultures were used at the following final concentrations: chloramphenicol, 40 μg ml−1; rifampin, 40 μg ml−1; kanamycin, 40 μg ml−1; and ampicillin, 100 μg ml−1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| R. leguminosarum bv. trifolii | ||
| RtTA1 | Wild type; Strr Rifr | 7 |
| RtAH1 | RtTA1 with pssT interrupted by integration of plasmid pAH1; Kmr | This work |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 47 |
| ET8000 | rbs lacZ::IS1 gyrA hutCK | 23 |
| S17-1 | 294 derivative with RP4-2-Tc::Mu-Km::Tn7 chromosomally integrated | 48 |
| JM101 | supE thi Δ(lac-proAB) F′ (traD36 proAB+lacIqlacZΔM15) | 47 |
| Plasmids | ||
| pK19mobGII | pUC19 derivative, lacZα mobgusA; Kmr | 25 |
| M13mp19 | Phage sequencing vector | 47 |
| pSK4158 | ori P15A, promoterless phoA; Cmr | 43 |
| pUCphoA | Derivative of pUC19 and pSK4158; Apr | This work |
| pNM480 | ori ColE1, promoterless lacZY; Apr | 37 |
| pUC98 | pUC19 with 2,728-bp EcoRI-HindIII fragment of RtTA1 containing pssN and fragment of the pssT gene | 33 |
| pLT59 | M13mp19 with 1.9-kb KpnI fragment of RtTA1 containing an incomplete pssT gene | This work |
| pAH1 | pK19mobGII with 1,058-bp EcoRI/KpnI fragment of pLT59 | This work |
| pTA52PHO/LAC | This work | |
| pTV78PHO/LAC | This work | |
| pTA133PHO/LAC | This work | |
| pTA158PHO/LAC | This work | |
| pTA201PHO/LAC | This work | |
| pTA243PHO/LAC | This work | |
| pTA269PHO/LAC | This work | |
| pTA311PHO/LAC | This work | |
| pTA385PHO/LAC | This work | |
| pTL430PHO LAC | This work | |
| pTA455PHO/LAC | This work | |
| pTA480PHO/LAC | This work |
Strr, Streptomycin resistance; Rifr, rifampin resistance; Kmr, kanamycin resistance; Cmr, chloramphenicol resistance; Apr, ampicillin resistance; rbs, ribosome binding site; mob, mobility region.
DNA methods.
Standard techniques were used for plasmid isolation, restriction enzyme digestion, agarose gel electrophoresis, DNA cloning, labeling of DNA, and Southern hybridization (47). Sequencing was performed by using BigDye terminator cycle sequencing kit (Applied Biosystem) and ABI Prism 310 sequencer. Sequence data were analyzed with DNASTAR-Lasergene analysis software. Database searches were done with the BLAST (1) and FASTA (44) programs at the National Centre for Biotechnology Information (Bethesda, Md.) and European Bioinformatic Institute (Hinxton, United Kingdom). Functional and/or structural homology searches were done with PROPSEARCH program (18). The transmembrane helices were predicted with TM-Pred (19), TOPpred (55), DAS (9), HMMTOP v.2.0 (51), TMHMM v.2.0 (38), SPLIT (24), PSORT (40), and VHMPT (29). Most of these tools can be found at ExPASy-Molecular Biology Server (Swiss Institute of Bioinformatics, Geneva, Switzerland). The hydropathy profiles were determined by Kyte and Doolittle plots (27). GOR v.4.0 (12) was used for secondary structure analysis.
Cloning of pssT gene.
The 744-bp EcoRI-KpnI fragment of pUC98 plasmid (33) carrying the 3′ end of the pssT gene was used as a probe in Southern hybridization with RtTA1 genomic DNA digested with several restriction enzymes. The 2.0-kb KpnI genomic fragment was cloned into M13mp19 phage vector resulting in pLT59, containing the 5′ end of pssT gene lacking in pUC98. The 2,370-bp fragment of RtTA1 containing the entire pssT was sequenced.
Mutagenesis of pssT gene.
To construct the pssT gene mutant, the 1,058-bp EcoRI-KpnI fragment of pLT59 was cloned into the pK19mobGII vector resulting in pAH1. Plasmid pAH1 was transferred by conjugation from E. coli S17-1 to R. leguminosarum bv. trifolii TA1. Integration of the hybrid plasmid into the R. leguminosarum bv. trifolii genome by a single-crossover event was selected for by the vector-encoded antibiotic resistance. The Rhizobium mutant obtained by the integration of pAH1 was designated RtAH1. In this mutant, the vector promoter is in an orientation opposite to the transcription of truncated pssT gene. Recombination between pAH1 and the R. leguminosarum bv. trifolii pssT gene was verified by Southern hybridization by using the total DNA of RtAH1 digested with different restriction enzymes and the 1743-bp HindIII-BamHI fragment of pTA480PHO carrying pssT gene as a probe.
Construction of pssT-phoA and pssT-lacZ translational fusions.
For each fusion, an upstream primer preceding the pssT promoter and a specific primer which annealed within the coding sequence of pssT were used to amplify DNA fragments from pLT59 plasmid or genomic DNA of RtTA1. These PCR products were purified, digested with appropriate restriction enzymes, and cloned into phoA (pUCphoA) and lacZ (pNM480) reporter plasmids. To obtain a set of phoA fusion plasmids, the PCR products were digested with HindIII and BamHI and cloned into pUCphoA. To construct the lacZ fusions, the PCR products were digested with BamHI and HindIII and cloned into pNM480. The reporter fusions were selectively created at a number of sites present in the various predicted cytoplasmic and periplasmic loops of the PssT protein. The resulting fusion plasmids, given in Table 1, have been named after the last PssT residue position in the in-frame fusion. The fusion junctions in all of the constructed plasmids were confirmed by DNA sequencing.
Enzyme assays.
AP activities were assessed on LB agar plates by hydrolysis of the chromogenic substrate XP (5-bromo-4-chloro-3-indolylphosphate; 40 μg/ml). Similarly, β-galactosidase activities were assessed on LB agar plates by hydrolysis of the substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 40 μg/ml). AP activities were quantified after cultivation of E. coli ET8000 containing the pssT-phoA gene fusions in LB medium by the method of Manoil (30). β-Galactosidase activities in E. coli ET8000 with pssT-lacZ gene fusions were determined by the method of Miller (36), and activities were expressed in Miller units.
Western immunoblotting.
Whole-cell extracts from E. coli ET8000 transformed with each pssT-phoA fusion construct were prepared from washed stationary-phase cells broken by sonication and 30-μg quantities were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 7.5% polyacrylamide in the running gel. Finally, the proteins were electroblotted to Immobilon-P (Millipore) membranes and probed with rabbit anti-AP antibodies (Polysciences) and AP-conjugated goat anti-rabbit immunoglobulin antibodies (Sigma). The bands were visualized by color development with Nitrotetrazolium Blue and XP.
EPS analysis.
The EPS isolation, column chromatography on BioGel A5m (Bio-Rad), and EPS quantification in culture supernatants were as described earlier (34). The monosaccharide composition of the LMW and HMW EPS fractions was determined as previously described (45).
Plant tests.
Red clover (Trifolium pratense cv. Ulka) seeds were surface sterilized, germinated, and grown as described previously (49). At 4 weeks after the inoculation, plants were harvested and examined for root nodule formation. The nitrogenase activity was measured by acetylene reduction assay according to the method of Hardy et al. (17).
Nucleotide sequence accession number.
The R. leguminosarum bv trifolii TA1 pssT sequence has been submitted to GenBank and is available in the database under the accession number AF402596.
RESULTS
Identification and sequence analysis of the R. leguminosarum bv. trifolii pssT gene.
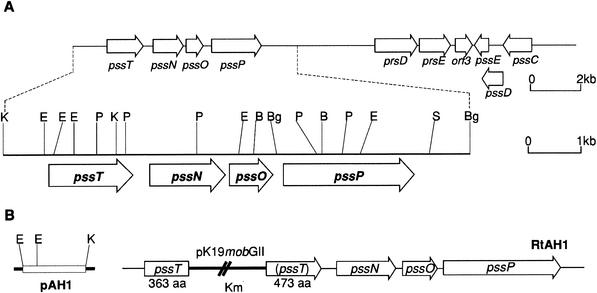
Sequencing of the 2,370-bp genomic fragment of RtTA1 revealed an open reading frame 1,455 bp long in the same orientation as the previously described pssN, pssO and pssP genes (Fig. 1A) (34). This open reading frame, named pssT, has a potential ATG start codon preceded by a putative ribosome-binding site (GGTTG). The predicted protein product of the pssT coding region is composed of 485 amino acids (53.3 kDa). The intergenic region between pssT and pssN is 296 bp long.
FIG. 1.
(A) Physical and genetic map of exo region encompassing the pssTNOP genes of R. leguminosarum bv. trifolii TA1, encoding the putative proteins involved in EPS polymerization and export. (B) On the left, the insert of the pAH1 construct used for integration mutagenesis of R. leguminosarum bv. trifolii TA1 is shown. The resulting genomic structure of RtAH1 mutant is indicated on the right. The inactive part of the pssT gene is in parentheses. The heavy line indicates the vector part of the integrated plasmid. Abbreviations: B, BamHI; Bg, BglII; E, EcoRI; P, PstI; S, SalI; K, KpnI.
A comparison of the deduced PssT amino acid sequence to sequences in the data bases did not reveal a significant homology to any known proteins. After searching the homology on the level of protein secondary structure by using the PROPSEARCH program, we found a structural and/or functional homology of PssT to several bacterial polysaccharide transporters. There is a reliable possibility (80 to 87%) that the PssT protein belongs to a PST superfamily of proteins that are possibly involved in transport of complex polysaccharides (Table 2).
TABLE 2.
Functional and/or structural homologes of the PssT protein of R. leguminosarum bv. trifolii TA1 as determined by the PROPSEARCH program
| Homologous protein (aa)a | Bacterium | Function | Reliability of family identificationb
|
Reference or accession no. | |
|---|---|---|---|---|---|
| Distance | Reliability (%) | ||||
| ExoT (494) | Sinorhizobium meliloti | Succinoglycan (EPS I) transporter | 9.3 | 87 | 13 |
| EpsF (418) | Ralstonia solanacearum | EPS I transporter | 10.24 | 80 | 21 |
| NanT (496) | Escherichia coli | Sialic acid transporter | 10.24 | 80 | P41036 |
| ExoH (370) | Sinorhizobium meliloti | Biosynthesis of succinoglycan (EPS I) | 10.44 | 80 | 13 |
aa, amino acids.
Reliability of family identification as a function of PROPSEARCH distance. A distance between 8.7 and 10.0 translates into a >87% chance that the query sequence and the hit are in the same family (implying in the majority of cases a similar function). Reliability decreases with increases in distance value.
Construction of a topological model for RtTA1 PssT protein.
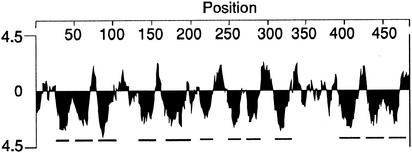
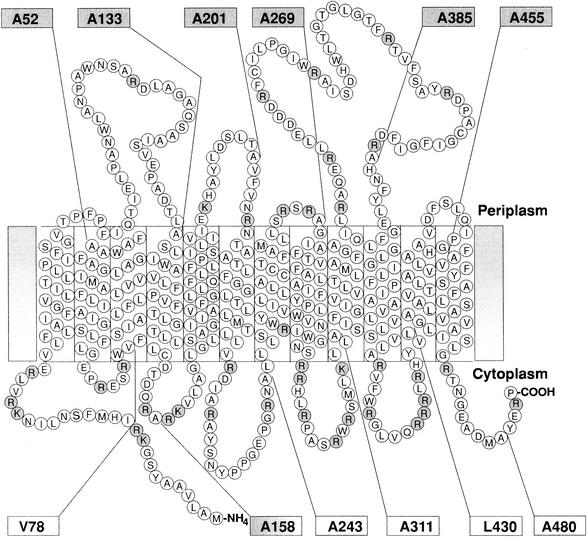
Hydropathy analysis with the Kyte and Doolittle (27) algorithm predicted that PssT possesses extremely hydrophobic stretches that could constitute TMS (Fig. 2). Secondary structure analysis of these putative TMS showed that all of them resemble α-helices, typical for TMSs (12). We have employed different topology prediction programs to generate a topological model for PssT. TOPpred, DAS, HMMTOP, SPLIT, and TMHMM yielded similar results: they all predicted 12 transmembrane helices and an orientation with both the N and C termini in the cytoplasm (NINCIN) as a strongly preferred model. Based on the combined computer results, the hydrophobicity and distribution of positively charged amino acid residues, we proposed the topological model of the RtTA1 PssT protein that is presented in Fig. 3. The 12 transmembrane helices, a large periplasmic loop between TMS9 and TMS10, and both N and C termini located in the cytoplasm characterize this topological model. The 12-TMS helix NINCIN model did not disturb the distribution of positively charged residues along the PssT protein marked in Fig. 3.
FIG. 2.
Hydrophobicity plot of RtTA1 PssT protein as calculated by the method of Kyte and Doolittle (27). The strongly hydrophobic segments are underlined.
FIG. 3.
Proposed topological model for the PssT protein of RtTA1. Positively charged residues are shaded. Shaded boxes indicate positions of PssT-PhoA fusions that gave blue colonies on LB-XP indicator plates and showed high PhoA activity, whereas white boxes correspond to fusions that gave white colonies on indicator plates and showed low AP activity. The numbers in the boxes designate the positions of each fusion within PssT. This topological model was based on VHMPT (i.e., a graphical viewer and editor for helical membrane protein topologies) output.
Testing the model using pssT-phoA gene fusion.
To experimentally verify the topology of PssT in the cytoplasmic membrane, we constructed a series of gene fusions of pssT and phoA. We used a genetic approach described by Boyd et al. (6) in which a series of fusions with residues in the vicinity of C terminus of each predicted hydrophilic region of the protein were constructed. This design should prevent the disruption of known topological determinants, such as positively charged residues, normally located in the internal loops (6). The sites of fusion junctions are shown in Fig. 3. For an increased expression of PssT-PhoA hybrids, pssT was placed under strong lac promoter in a pUCphoA plasmid. The activity of each fusion protein was initially assessed on indicator plates. Bacteria containing the PssT-PhoA fusions displayed an alternating blue and white color corresponding to the fusion point throughout the length of PssT (Table 3). The AP activities were determined for all PssT-PhoA fusions by measuring the rates of hydrolysis of the substrate p-nitrophenyl phosphate. The calculated PhoA activities were relatively high (651 to 6,573 U) in the fusions at amino acid positions A52, A133, A201, A269, A385, and A455 of PssT, indicating that these sites are located in the periplasm (Fig. 3). The PhoA fusions at amino acid positions V78, A243, A311, L430, and A480 of PssT yielded significantly lower activities (55 to 188 U), suggesting that these residues are located in the cytoplasm (Table 3). In all cases, the calculated PhoA activities paralleled the blue-and-white patterns of colonies observed on indicator plates and generally confirmed the topological model proposed for the PssT protein of RtTA1 (Fig. 3). However, in the case of PssT fusion at A158, we observed high AP activity despite the fact that this segment was predicted to be located in the cytoplasm (Table 3). In this hybrid protein, AP is fused to the cytoplasmic domain, inside the stretch of positively charged residues RA*RK (asterisk indicates the fusion point). This type of fusion usually exhibits a much higher AP activity than expected due to a decreased stability of the reporter protein localized in the cytoplasm (50).
TABLE 3.
AP and β-galactosidase activities for the PssT-PhoA and PssT-LacZ hybrid proteinsa
| Position of fusion in PssT | Mean activity (Miller units) ± SD of:
|
|
|---|---|---|
| PhoA | LacZ | |
| No fusionb | 95.66 ± 8 | 2.81 ± 0.29 |
| Ala-52 | 4,191.29 ± 491 | 4.45 ± 2.54 |
| Val-78 | 57.13 ± 17 | 46.08 ± 6.22 |
| Ala-133 | 3,651.29 ± 105 | 1.69 ± 0.92 |
| Ala-158 | 1,322.2 ± 646 | 52.61 ± 6.05 |
| Ala-201 | 6,573.19 ± 616 | 3.91 ± 0.45 |
| Ala-243 | 118.54 ± 52 | 42.81 ± 5.48 |
| Ala-269 | 3,256.6 ± 275 | 3.02 ± 0.72 |
| Ala-311 | 118.07 ± 40 | 58.62 ± 0.85 |
| Ala-385 | 717.48 ± 87 | 4.19 ± 3.82 |
| Leu-430 | 98.52 ± 5 | 55.90 ± 3.76 |
| Ala-455 | 651.05 ± 142 | 3.97 ± 0.04 |
| Ala-480 | 87.74 ± 9 | 1.94 ± 0.18 |
All assays were performed in E. coli strain ET8000 and are averages from at least three independent experiments. Values for PhoA are as defined by Manoil (30).
The PhoA and LacZ activities in ET8000 pUCphoA and ET8000 pNM480, respectively, are given.
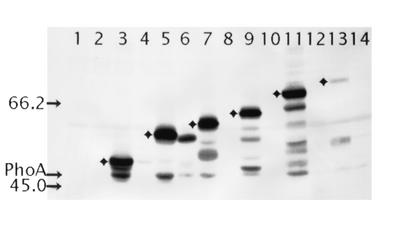
To complement these data, we also used Western blotting with anti-AP antibody to distinguish between the fusions with a periplasmic or cytoplasmic location. Immunoblots show the mixtures of full-length fusion proteins and free PhoA, proportionally the stability of the hybrid proteins. Fusions with a periplasmic PhoA moiety display greater quantities of immunoreactive products in contrast to the cytoplasmic fusions that are poorly or not detected in Western blotting due to rapid degradation of improperly folded PhoA moiety located in the cytoplasm (52). The Western blot (Fig. 4) illustrates that a band of a correct, anticipated size for each fusion protein can be identified, demonstrating that all of the fusions are being expressed. In almost all fusion-bearing strains, we observed a band of 47 kDa (Fig. 4), which corresponds to the size of PhoA and which was not present in both control samples (the extracts of E. coli ET8000 without the plasmid and E. coli ET8000 with pUCphoA). The band intensity of the PssT protein fusion at position A455 is significantly reduced. This may be attributed either to a decreased expression level or to the instability of long polypeptides. Again, as with the AP activity, the band intensity for fusion at A158, showed that the protein accumulated to a much greater extent than other cytoplasmically located fusions. We suggest that an improper translocation of PhoA moiety to the periplasm, rather than periplasmic location of this segment of PssT, caused the increased amount of immunoreactive products for A158 fusion protein. In addition, a smaller than expected size of the A158 hybrid protein, confirms the cytoplasmic location of this fragment of the PssT protein (Fig. 4).
FIG. 4.
Western blot analysis of PssT-PhoA fusion proteins expressed in E. coli ET8000. The bands corresponding to the predicted molecular masses of fusion proteins, with periplasmic location of the PhoA moiety, are marked with diamonds. The sizes (in kilodaltons) of the protein standards are indicated on the left by arrows. The position of 47-kDa band corresponding to the size of the normal PhoA protein is also marked. Lanes: 1, no plasmid; 2, pUCphoA; 3, pTA52PHO; 4, pTV78PHO; 5, pTA133PHO; 6, pTA158PHO; 7, pTA201PHO; 8, pTA243PHO; 9, pTA269PHO; 10, pTA311PHO; 11, pTA385PHO; 12, pTL430PHO; 13, pTL455PHO; 14, pTA480PHO.
Testing the model using LacZ fusions.
To examine the topology of PssT protein by a second method, we used an alternative reporter protein, β-galactosidase. Therefore, we cloned the same set of PCR products into plasmid pNM480, which generates in frame fusions to full-length lacZ (37). The β-galactosidase activities were measured in E. coli ET8000 (Table 3). As previously explained, at any given site, the β-galactosidase fusions were expected to display levels of activity reciprocal to those of PhoA fusions. This was found to be true for 11 of the 12 PssT-LacZ fusions, which displayed activities that were complementing the PhoA fusions, except for fusion A480 (Table 3). We expected this translational fusion to lacZ to be active; however, this was not the case. This fusion, close to the C terminus of PssT, gave no detectable β-galactosidase activity, suggesting that, for some unknown reasons, this construct was not expressing a fusion protein. The fusion at position A158 gives high levels of both AP and β-galactosidase activities. We have reasoned that A158 belongs to the cytoplasmic domain. All of the algorithms used strongly predicted that this segment was located in the cytoplasm, and the presence of three charged residues in this domain favored its cytoplasmic location. Both fusions neighboring A158 (i.e., A133 and A201) showed high AP activity and sharp, intensive bands in Western hybridization with anti-AP antibodies. These results ruled out the periplasmic location of A158 hybrid protein. In addition, the cytoplasmic location of A158 was confirmed by high β-galactosidase activity (Table 3). In conclusion, the data obtained with the PhoA and LacZ fusions generally agreed with the predicted 12-TMS secondary structure model of PssT protein (Fig. 3).
Phenotypic characterization of pssT mutant.
The pssT mutant designated RtAH1 was constructed by integration of pAH1 plasmid carrying a fragment of pssT gene, encoding the PssT protein lacking 11 N-terminal amino acid residues and 121 amino acids residues at the C terminus (Fig. 1B). Integration of the hybrid plasmid into the RtTA1 genome by a single crossover resulted in disruption of the pssT coding region, and the merodiploid strain RtAH1 was created. This mutant produced an incomplete PssT protein (amino acid residues 1 to 363) truncated at the C-terminal part (Fig. 1B).
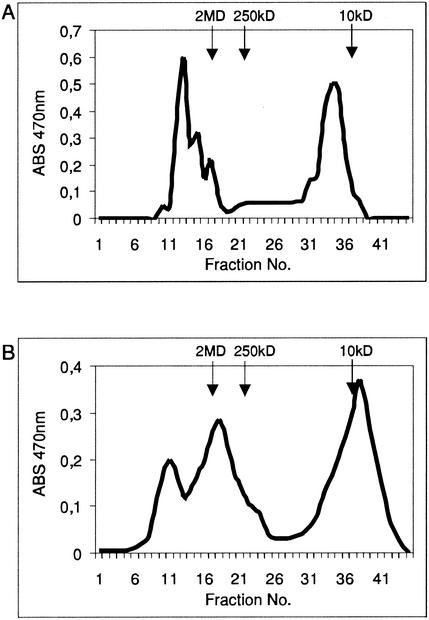
The amount of extracellular EPS in RtAH1 mutant was measured and compared to the amount of EPS produced by wild-type RtTA1 strain. The culture supernatant of the wild-type strain RtTA1 contained 2,687.2 mg of total carbohydrate/liter, whereas the culture supernatant of the pssT mutant contained 3,558.1 mg of total carbohydrate/liter after 4 days of cultivation in 79CA medium with 0.5% glycerol as the carbon source. This finding indicated that production of EPS by pssT mutant was increased to ca. 132.4% of the amount produced by the wild-type strain. Extracellular carbohydrates of the wild-type strain RtTA1 and mutant RtAH1 were fractionated by gel filtration chromatography on a Bio-Gel A5m column. Two fractions of significantly different molecular weights were obtained for the wild-type RtTA1 strain, which represented the HMW EPS and the LMW EPS, the ratio being 49.8:50.2 (Fig. 5A). In the gel filtration chromatography of EPS from RtAH1 mutant, three fractions of different molecular weights were obtained: two fractions of HMW comprising ca. 56.7% and the LMW fraction representing ca. 43.3% of the total EPS (Fig. 5B). To confirm that these three fractions represented the EPS, the monosaccharide composition of the HMW and LMW fractions was determined. The proportion of glucose, glucuronic acid, and galactose was found to be 5:2:1, which is characteristic of EPS of R. leguminosarum bv. trifolii (20). We concluded that the two fractions of HMW and the LMW fraction exclusively contained EPS.
FIG. 5.
Gel filtration chromatography of EPSs produced by R. leguminosarum bv. trifolii wild-type strain RtTA1 (A) and the plasmid integration mutant RtAH1 (B). EPS was fractionated on a Bio-Gel A5m column as described in Materials and Methods. The retention times of dextran blue (2 MDa), dextran T250 (250 kDa), and dextran T10 (10 kDa) molecular mass markers are indicated by arrows.
The R. leguminosarum bv. trifolii wild-type strain RtTA1 and the plasmid integration mutant RtAH1 were each used for the inoculation of 40 Trifolium pratense plantlets. Pink elongated nodules were observed on all plants 4 weeks after the inoculation, but differences in nodule number and nodulation kinetics between the wild-type RtTA1 strain and the RtAH1 mutant were observed. The first nodules became visible on seedlings inoculated with the RtAH1 mutant 2 days earlier than on seedlings inoculated with wild-type RtTA1 strain. At 4 weeks after the inoculation, approximately twice as many nodules appeared on clover inoculated with RtAH1 mutant strain in comparison to the wild-type RtTA1 strain (6.2 ± 2.2 and 3.42 ± 3.3 nodules, respectively). All plants appeared green, indicating nitrogen fixation, and the green wet masses of plants inoculated with RtTA1 and RtAH1 was 30.1 ± 7.3 and 36.0 ± 11.3 mg, respectively. The green mass of clover inoculated with the mutant differed insignificantly from the clover infected with the wild-type RtTA1 strain. The acetylene reduction analysis confirmed the nitrogen fixation in plants inoculated with wild-type strain and RtAH1 mutant (82.6 ± 8.4 and 85.5 ± 3.8 nM ethylene/plant/h, respectively).
DISCUSSION
Recently, we identified three genes, pssN, pssO, and pssP, in R. leguminosarum bv. trifolii TA1 (33) whose products could form the type I secretion system found in gram-negative bacteria, which are involved in the polymerization and export of EPS. The PssP protein could be included in the MPA-1 family and is required for EPS biosynthesis and polymerization (34, 42). PssN has been proposed to function as an OMA protein that provides a porin-like structure in the outer membrane and, together with an MPA1 protein, facilitates the translocation of polysaccharides through the cell wall (42). The type I secretion system usually employs another component, a PST protein, that is located in the cytoplasmic membrane and exhibits a topology typical for this family of proteins, with 12 TMSs (42). We described the identification of the pssT gene that is located upstream of pssNOP gene cluster in R. leguminosarum bv. trifolii TA1. We have also presented a detailed topological and mutational analysis of the PssT protein. Based on the hydrophobicity profile and the homology with other characterized members of the PST family, such as the ExoT protein from S. meliloti (13), a secondary structure model was created for PssT. This model predicts that PssT contains 12 TMSs with N and C termini on the cytoplasmic side of the membrane. The PssT protein was predicted to be a PST constituent of type I system of EPS polymerization and transport across the cell wall.
In order to verify this in silico model, we employed both AP and β-galactosidase as reporters in gene fusion studies. The measured AP activities and the distinction between high- and low-activity PssT-PhoA fusions were large enough to ascribe the cellular location of each fusion junction unambiguously (Fig. 3). However, in the case of a fusion at position A158 of the PssT protein, we observed a high AP activity despite that this segment was predicted to be located in the cytoplasm (Table 3). In establishing a membrane protein topology, individual spanning sequences must both insert into the membrane and anchor the protein stably after the insertion (32). Each membrane-spanning stretch, together with its flanking hydrophilic domain, constitutes a topological determinant, providing both anchoring and orientation of the membrane protein (50). von Heijne (55) has pointed out that orientation of a membrane protein is at least in part determined by positively charged residues located mainly in cytoplasmic loops. In the A158 hybrid protein, the AP is fused to the cytoplasmic domain, inside the stretch of positively charged residues RA*RK (the asterisk indicates the fusion point). Disruption of this strong orientation determinant by the reporter protein probably resulted in PhoA translocation into the periplasm and, thus, high AP activity. This type of fusion usually exhibits much higher AP activities than expected due to a decreased stability of the reporter protein localized in the cytoplasm (50). Nevertheless, we concluded that this protein segment must lie at the cytoplasmic face of the membrane. The complementary data from PssT-LacZ fusion confirmed our expectations, i.e., the A158 fusion protein yielded a high level of β-galactosidase activity. Usually, in a case in which both reporters at the same fusion site display high activities, more significance is given to the AP data because the high activity of PhoA fusions requires an active translocation of the reporter enzyme moiety across the cytoplasmic membrane (52). However, we have reasoned that A158 belongs to a cytoplasmic domain because of the presence of three positively charged residues that favored its cytoplasmic location. In contrast to the cytoplasmic domains, the exported domains of a membrane protein do not seem to have a sequence that determines which side of the membrane they reside on (50). Conflicting results, i.e., high LacZ and PhoA activities at the same fusion site, have been reported in a number of membrane topology studies (10, 15).
Western analysis with anti-PhoA antibodies showed that a band of a correct size for each fusion protein with PhoA in the periplasm could be identified, demonstrating that all fusions were being expressed (Fig. 4). The band intensity for the PssT protein fusion at position A455 is strongly reduced. The activities and expression levels of a periplasmic segment fusion tend to decrease somewhat with length, and long fusions tend to be more toxic for the cells than short ones (30). It cannot be ruled out that a presence of a large hydrophilic domain between TMS9 and TMS10 located in the periplasm might have also contributed to lowering the AP activity of A385 and A455 fusion proteins (Table 3), and a reduced level of expression in the case of A455 fusion. The band intensity for A158 fusion protein (Fig. 4) showed that it accumulated to a much greater extent than other cytoplasmically located fusions. We concluded that it was probably due to the loss of an important orientation determinant in this fusion rather than to the periplasmic location of this segment of PssT. Moreover, the sharp, intensive band observed for both fusions neighboring A158 (i.e., A133 and A201) excluded the periplasmic location of Ala-158. Additionally, the cytoplasmic location of this fusion seems to be confirmed by a high β-galactosidase activity.
Although the data from PssT-PhoA fusions was quite conclusive, we constructed a complementary set of fusions with β-galactosidase. It is known that LacZ is a less reliable reporter than PhoA (16) because it gives false-positive results that confuse the analysis. Such may be because of a failure to obtain a complete translocation of the β-galactosidase due to a competition between the export process and folding of the protein in the cytoplasm (35). The LacZ domain of the fusion protein also shows a tendency to be cleaved off in the cytoplasm, either during synthesis or after insertion of the fusion (52). However, in our studies, LacZ fusions with PssT gave results quite complementary to the ones with PhoA fusions for all but one PssT-LacZ hybrid protein, namely, the A480 fusion (Table 3). This fusion, close to the C terminus of PssT, yielded no detectable β-galactosidase activity, suggesting that, for some unknown reasons, this construct was not expressing a fusion protein. One possible explanation could be that the deletion of a portion of C terminus of PssT tail could interfere with the interactions between hydrophilic domains that are known to be important in the assembly and stability of the final topological structure of certain membrane proteins (50). Despite the lack of LacZ activity of the A480 fusion protein, the properties of A455 PhoA and LacZ fusions (i.e., high AP activity and low β-galactosidase activity, respectively) and the low AP activity of the A480 PhoA fusion seem to confirm the cytoplasmic localization of C terminus of the PssT protein. The data of the PhoA and LacZ fusions supported the predicted topology of PssT protein with 12 TMSs, with both N and C termini located in the cytoplasm (Fig. 3).
The role of the PssT protein in EPS biosynthesis was investigated by plasmid integration mutagenesis. The PssT protein of RtAH1 mutant is truncated after the 363rd amino acid residue, which is located in a large periplasmic loop between TMS9 and TMS10 (Fig. 1B and 3). It could affect the stability and/or assembly of the PssT membrane protein. As mentioned previously, mutant membrane proteins are often subject to rapid proteolysis in bacteria (32). On the other hand, we cannot exclude the possibility that this mutated form of PssT protein is functional, at least to some extent. This mutation led to a significant increase of the level of EPS production, suggesting that PssT could perform a regulatory function in EPS biosynthesis or polymerization. In gel chromatography, the fraction of HMW EPS produced by RtAH1 strain was distributed into two clearly separated fractions of different molecular weights, and the ratio of the HMW EPS to the LMW EPS was increased at the expense of the LMW EPS compared to the EPS produced by wild-type RtTA1 strain (Fig. 5). These data indicate some disturbances in the rate of polymerization of octasaccharide subunits in RtAH1 mutant. The phenotype of PssT mutant with respect to EPS production is opposite to the phenotype of the PssP mutant described earlier (34). EPS isolated from the culture supernatant of the mutant with a disrupted N-terminal PssP domain produced almost exclusively an LMW form of EPS, indicating the importance of PssP in the EPS subunit polymerization. The PssP protein seems to be also required for EPS biosynthesis because the mutant deleted in this gene is deficient in EPS production (34). On the basis of these data, we could suppose that in R. leguminosarum bv. trifolii TA1 the PssT protein, acting in complex with PssP and possibly with the PssN outer membrane protein (34), could be involved in controlling the rate of octasaccharide subunits polymerization and export of the polymer to the cell surface. According to the speculative model for type I polysaccharide export system described by Paulsen et al. (42), we proposed that PssN could interact with a periplasmic loop of the PssP protein (33), whereas the transmembrane regions of PssP associate with the corresponding PST transporter, with the PssT protein facilitating EPS export across the bilayer structure. We hypothesize that the large periplasmic loop between TMS9 and TMS10 in PssT protein could play an important role in this physical association of the constituents of the transport protein complex in the inner and outer membranes. Thus, it cannot be ruled out that the alterations in EPS production and size distribution found in RtAH1 could result from truncating this domain of PssT in the mutant strain.
PssT is functionally and structurally homologous to the ExoT protein from S. meliloti (Table 2). pssT and exoT mutants revealed similar defects in EPS biosynthesis. Both mutants produced increased amounts of HMW EPS at the expense of the LMW form of EPS (14). In addition, the PssT mutant produced significantly increased amounts of EPS. Gonzalez et al. (14) presented evidence that at least three proteins are responsible for the polymerization and export of succinoglycan (EPS I) in S. meliloti. The ExoT protein is involved in the biosynthesis of dimers and trimers of succinoglycan (EPS I), whereas ExoQ inner membrane protein was suggested to be involved in the biosynthesis of HMW EPS I. ExoP, the third component of the biosynthetic machinery, would be essential for biosynthesis of the HMW and LMW forms of EPS I (4, 14).
The ExoH protein of S. meliloti is yet another structural homologue of PssT (Table 2). Since an exoH mutant synthesizes EPS I that lacks succinyl (28), it is possible that the ExoH protein is responsible for the addition of succinyl groups to the octasaccharide subunits (56). Interestingly, the exoH mutant produces increased amount of EPS I that forms mainly an HMW fraction (8).
The effects of pssT mutation on the symbiotic properties of RtAH1 mutant were somewhat surprising. Clover seedlings inoculated with the pssT mutant induced increased the number of nodules in comparison to the parental strain, but the level of nitrogenase activity was essentially the same as in the control plants. It seems likely that the HMW fraction of EPS that is produced in increased amounts by the RtAH1 mutant may play an important role in symbiosis, e.g., in protecting rhizobia against the plant defense response. To our knowledge, a detailed symbiotic characteristic of S. meliloti exoT mutant has not been described to date.
Acknowledgments
We thank M. Merrick (John Innes Centre, Norwich, United Kingdom) for providing the vectors pSK4158 and pNM480 and strain ET8000 used in the present study. We also thank T. Urbanik-Sypniewska for help with the gel filtration techniques.
This study was supported by grant 6P04A 05818 from the Polish Committee for Scientific Research.
A.M. and J.E.K. contributed equally to this study.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Becker, A., K. Niehaus, and A. Pühler. 1995. Low-molecular-weight succinoglycan is predominantly produced by Rhizobium meliloti strains carrying a mutated ExoP protein characterized by a periplasmic N-terminal domain and a missing C-terminal domain. Mol. Microbiol. 16:191-203. [DOI] [PubMed] [Google Scholar]
- 3.Becker, A., and A. Pühler. 1998. Specific amino acid substitutions in the proline-rich motif of the Rhizobium meliloti ExoP protein result in enhanced production of low-molecular-weight succinoglycan at the expense of high-molecular-weight succinoglycan. J. Bacteriol. 180:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, A., and A. Pühler. 1998. Production of exopolysaccharides, p. 97-118. In H. P. Spaink, A. Kondorosi, and P. J. J. Hooykaas (ed.), Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 5.Borthakur, D., R. F. Barker, J. W. Latchford, L. Rossen, and A. W. B. Johnston. 1988. Analysis of pss genes of Rhizobium leguminosarum required for exopolysaccharide synthesis and nodulation of peas: their primary structure and their interaction with psi and other nodulation genes. Mol. Gen. Genet. 213:155-162. [DOI] [PubMed] [Google Scholar]
- 6.Boyd, D., B. Traxler, and J. Beckwith. 1993. Analysis of the topology of a membrane protein by using a minimum number of alkaline phosphatase fusions. J. Bacteriol. 175:553-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravorty, A. K., W. Zurkowski, J. Shine, and B. G. J. Rolfe. 1982. Symbiotic nitrogen fixation: molecular cloning of Rhizobium genes involved in exopolysaccharide synthesis and effective nodulation. J. Mol. Appl. Genet. 1:585-596. [PubMed] [Google Scholar]
- 8.Cheng, H.-P., and G. C. Walker. 1998. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180:5183-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673-676. [DOI] [PubMed] [Google Scholar]
- 10.Danielsen, S., D. Boyd, and J. Neuhard. 1995. Membrane topology analysis of the Escherichia coli cytosine permease. Microbiology 141:2905-2913. [DOI] [PubMed] [Google Scholar]
- 11.Djordjevic, S. P., M. Batley, J. W. Redmond, and B. G. Rolfe. 1986. The structure of the exopolysacchride from Rhizobium sp. strain ANU280 (NGR234). Carbohydr. Res. 148:87-99. [Google Scholar]
- 12.Garnier, J., J.-F. Gibrat, and B. Robson. 1996. GOR secondary structure prediction method version IV. Methods Enzymol. 266:540-553. [DOI] [PubMed] [Google Scholar]
- 13.Glucksmann, M. A., T. L. Reuber, and G. C. Walker. 1993. Genes needed for the modification, polymerization, export and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J. Bacteriol. 175:7045-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez, J. E., C. E. Semino, L.-X. Wang, and L. E. Castellano-Torres. 1998. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 95:13477-13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagting, A., J. van der Velde, B. Poolman, and W. N. Konings. 1997. Membrane topology of the di- and tripeptide transport protein of Lactococcus lactis. Biochemistry 36:6777-6785. [DOI] [PubMed] [Google Scholar]
- 16.Hannessey, E. S., and J. K. Broome-Smith. 1993. Gene-fusion techniques for determining membrane-protein topology. Curr. Opin. Struct. Biol. 3:524-531. [Google Scholar]
- 17.Hardy, R., R. D. Holsten, E. K. Jackson, and C. Burns. 1982. The C2H2-C2H4 assay for N2 fixation. Laboratory and field evolution. Plant Physiol. 43:1185-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobohm, U., and C. Sander. 1995. A sequence property approach to searching protein databases. J. Mol. Biol. 251:390-399. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann, K., and W. Stoffel. 1993. TMbase: a database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 374:166.
- 20.Hollingsworth, R. I., F. B. Dazzo, K. Hallenga, and B. Musselman. 1988. The complete structure of the trifoliin A lectin-binding capsular polysaccharide of Rhizobium trifolii 843. Carbohydr. Res. 172:97-112. [DOI] [PubMed] [Google Scholar]
- 21.Huang, J., and M. Schell. 1995. Molecular characterization of the eps gene cluster of Pseudomonas solanacearum and its transcriptional regulation at a single promoter. Mol. Microbiol. 16:977-989. [DOI] [PubMed] [Google Scholar]
- 22.Ivashina, T. V., M. I. Khmelnitsky, M. G. Shlyapnikov, A. A. Kanapin, and V. N. Ksenzenko. 1994. The pss4 gene from Rhizobium leguminosarum bv. viciae VF39: cloning, sequence and the possible role in polysaccharide production and nodule formation. Gene 150:111-116. [DOI] [PubMed] [Google Scholar]
- 23.Jayakumar, A., I. Schulman, D. McNeil, and E. M. Barnes, Jr. 1986. Role of the Escherichia coli glnALG operon in regulation of ammonium transport. J. Bacteriol. 166:281-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juretic, D., B. K. Lee, N. Trinajstic, and R. W. Williams. 1993. Conformational preference functions for predicting helices in membrane proteins. Biopolymers 33:255-273. [DOI] [PubMed] [Google Scholar]
- 25.Katzen, F., A. Becker, M. V. Ielmini, C. G. Oddo, and L. Ielpi. 1999. New mobilizable vectors suitable for gene replacement in gram-negative bacteria and their use in mapping of the 3′ end of the Xanthomonas campestris pv. campestris gum operon. Appl. Environ. Microbiol. 65:278-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Król, J., J. Wielbo, A. Mazur, J. Kopciñska, B. Lotocka, W. Golinowski, and A. Skorupska. 1998. Molecular characterization of pssCDE genes of Rhizobium leguminosarum bv. trifolii strain TA1: pssD mutant is affected in exopolysaccharide synthesis and endocytosis of bacteria. Mol. Plant-Microbe Interact. 11:1142-1148. [DOI] [PubMed] [Google Scholar]
- 27.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 28.Leigh, J. A., J. W. Reed, J. F. Hanks, A. M. Hirsch, and G. C. Walker. 1987. Rhizobium meliloti mutants that fail to succinylate their calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell 51:579-587. [DOI] [PubMed] [Google Scholar]
- 29.Lin, W.-J., and M.-J. Hwang. 1998. VHMPT: a graphical viewer and editor for helical membrane protein topologies. Bioinformatics 14:866-868. [DOI] [PubMed] [Google Scholar]
- 30.Manoil, C. 1991. Analysis of membrane protein topology using alkaline phosphatase and β-galactosidase gene fusion. Methods Cell Biol. 34:61-75. [DOI] [PubMed] [Google Scholar]
- 31.Manoil, C., and J. Beckwith. 1986. A genetic approach to analyzing membrane protein topology. Science 233:1403-1408. [DOI] [PubMed] [Google Scholar]
- 32.Manoil, C., and B. Traxler. 1995. Membrane protein assembly: genetic, evolutionary, and medical perspectives. Annu. Rev. Genet. 29:131-150. [DOI] [PubMed] [Google Scholar]
- 33.Mazur, A., J. Król, and A. Skorupska. 2001. Rhizobium leguminosarum bv. trifolii pssNOP genes encoding proteins involved in polymerization and translocation of exopolysaccharide. DNA Seq. 12:1-12. [DOI] [PubMed] [Google Scholar]
- 34.Mazur, A., J. Król, J. Wielbo, T. Urbanik-Sypniewska, and A. Skorupska. 2002. Rhizobium leguminosarum bv. trifolii PssP protein is required for exopolysaccharide biosynthesis and polymerization. Mol. Plant-Microbe Interact. 15:388-397. [DOI] [PubMed] [Google Scholar]
- 35.McGovern, K., and J. Beckwith. 1991. Membrane insertion of the Escherichia coli MalF protein in cells with impaired secretion machinery. J. Biol. Chem. 266:20870-20876. [PubMed] [Google Scholar]
- 36.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Minton, N. P. 1984. Improved vectors for isolation of translational lac gene fusions. Gene 31:269-273. [DOI] [PubMed] [Google Scholar]
- 38.Moller, S., M. D. R. Croning, and R. Apweiler. 2001. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 17:646-653. [DOI] [PubMed] [Google Scholar]
- 39.Morona, R., L. Van Den Bosch, and C. Daniels. 2000. Evaluation of Wzz/MPA1/MPA2 proteins based on the presence of coiled-coils regions. Microbiology 146:1-4. [DOI] [PubMed] [Google Scholar]
- 40.Nakai, K., and M. Kanehisa. 1991. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins Struct. Funct. Genet. 11:95-110. [DOI] [PubMed] [Google Scholar]
- 41.Niemeyer, D., and A. Becker. 2001. The molecular weight distribution of succinoglycan produced by Sinorhizobium meliloti is influenced by specific tyrosine phosphorylation and ATPase activity of cytoplasmic domain of the ExoP protein. J. Bacteriol. 183:5163-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulsen, I. T., A. M. Beness, and M. H. Saier. 1997. Computer-based analyses of the protein constituents of transport systems catalysing export of complex carbohydrates in bacteria. Microbiology 143:2685-2699. [DOI] [PubMed] [Google Scholar]
- 43.Paulsen, I. T., M. H. Brown, S. J. Dunstan, and R. A. Skurray. 1995. Molecular characterization of the staphylococcal multidrug resistance export protein QacC. J. Bacteriol. 177:2827-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence analysis. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russa, R., T. Urbanik-Sypniewska, A. S. Shashkov, A. Banaszek, A. Zamojski, and H. Mayer. 1996. Partial structure of lipopolisaccharides isolated from Rhizobium leguminosarum bv. trifolii 24 and its GalA-negative Exo− mutant AR20. Syst. Appl. Microbiol. 19:1-8. [Google Scholar]
- 46.Sadykov, M. R., T. V. Ivashina, A. A. Kanapin, M. G. Shlyapnikov, and V. N. Ksenzenko. 1998. Structural and functional organization of the exopolysaccharide biosynthesis genes in Rhizobium leguminosarum bv. viciae VF39. Mol. Biol. 52:665-671. [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular clonning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 48.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 49.Skorupska, A., U. Biatek, T. Urbanik-Sypniewska, and A. Van Lammeren. 1995. Two types of nodules induced on Trifolium pratense by mutants of Rhizobium leguminosarum bv. trifolii deficient in exopolysaccharide production. J. Plant Physiol. 147:93-100. [Google Scholar]
- 50.Traxler, B., D. Boyd, and J. Beckwith. 1993. The topological analysis of integral cytoplasmic membrane proteins. J. Membr. Biol. 132:1-11. [DOI] [PubMed] [Google Scholar]
- 51.Tusnády, G. E., and I. Simon. 1998. Principles governing amino acid composition of integral membrane proteins: applications to topology prediction. J. Mol. Biol. 283:489-506. [DOI] [PubMed] [Google Scholar]
- 52.van Geest, M., and J. S. Lolkema. 2000. Membrane topology and insertion of membrane proteins: search for topogenic signals. Mol. Biol. Rev. 64:13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Workum, W. A. T., H. C. J. Canter-Cremers, A. H. M. Wijfjes., C. van der Kolk, C. A. Wijffelman, and J. W. Kijne. 1997. Cloning and characterization of four genes of Rhizobium leguminosarum bv. trifolii involved in exopolysaccharide production and nodulation. Mol. Plant-Microbe Interact. 10:290-301. [DOI] [PubMed] [Google Scholar]
- 54.Vincent, J. M. 1970. A manual for the practical study of root nodule bacteria. Blackwell Scientific Publications, Oxford, United Kingdom.
- 55.von Heijne, G. 1992. Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487-494. [DOI] [PubMed] [Google Scholar]
- 56.Wang, L.-X., Y. Wang, B. Pellock, and G. C. Walker. 1999. Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J. Bacteriol. 181:6788-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitfield, C., P. A. Amor, and R. Köplin. 1997. Modulation of the surface architecture of gram-negative bacteria by the action of surface polymer:lipid A-core ligase and by determinants of polymer chain length. Mol. Microbiol. 4:629-638. [DOI] [PubMed] [Google Scholar]