Abstract
The Cpx pathway is a two-component signal transduction system that senses a variety of envelope stresses, including misfolded proteins, and responds by upregulating periplasmic folding and trafficking factors. CpxA resides in the inner membrane and has both kinase and phosphatase activities. CpxR, the response regulator, mediates a response by activating transcription of stress-combative genes. Signal transduction is subject to feedback inhibition via regulon member CpxP and autoamplification. Recently, it was shown that the Cpx pathway is also upregulated when cells adhere to hydrophobic surfaces and that this response is dependent on the outer membrane lipoprotein NlpE. Here we show that while NlpE is required for induction of the Cpx pathway by adhesion, induction by envelope stress and during growth is NlpE independent. We show that while all of the envelope stresses tested induce the Cpx pathway in a manner that is dependent on the periplasmic domain of CpxA, induction during growth is independent of CpxA. Therefore, we propose that the Cpx pathway can sense inducing cues that enter the signaling pathway at three distinct points. Although CpxP is not required for induction of the Cpx pathway, we show that its activity as a negative regulator of CpxA is inactivated by envelope stress. Moreover, the cpxP promoter is more inducible than any other regulon member tested. Consistent with these results, we suggest that CpxP performs a second function, most likely that of a chaperone. Finally, we show that two Cpx-regulated genes are differentially upregulated in response to different envelope stresses, suggesting the existence of three stress-responsive systems.
In gram-negative bacteria, the cell envelope, which consists of the outer membrane, the inner membrane, and the periplasm, comes into direct contact with a variety of environments and is consequently involved in a number of adaptive responses. In order to preserve envelope integrity, at least two stress response pathways in Escherichia coli sense and respond to environmental stress. The σE and Cpx signal transduction pathways both control the synthesis of proteins involved in repairing damaged proteins in the envelope; however, each pathway has unique inputs and outputs (6, 24, 30, 33). It is thought that the σE pathway monitors assembly of outer membrane β-barrel proteins, whereas the primary functions of the Cpx pathway are to maintain surface structures such as pili (19) and to detect the attachment of the bacterial cell to surfaces (19, 29).
The Cpx pathway is a prototypical two-component signal transduction system. The input signal is transduced across the inner membrane by the sensor kinase CpxA. CpxA consists of a periplasmic domain and a conserved cytoplasmic signaling domain separated by two transmembrane α-helices (44). It is thought that upon detection of envelope stress, CpxA autophosphorylates by using ATP at a conserved histidine and then transfers this phosphate to a conserved aspartate in the amino-terminal domain of the response regulator CpxR. CpxR-phosphate (CpxR∼P) then activates transcription of genes whose products are involved in protein physiology in the envelope (33). Known Cpx regulon members include the disulfide oxidase DsbA, the protease and chaperone DegP, the peptidyl-prolyl isomerase PpiA, and Spy, a periplasmic protein of unknown function (8, 30, 31). Recent work revealed that the Cpx regulon may contain >100 members (14).
The Cpx regulon is positively autoregulated and is feedback inhibited by regulon member CpxP, a small periplasmic protein (13, 32). It is thought that CpxP negatively regulates by interacting with the periplasmic domain of CpxA, keeping the pathway off. During envelope stress, CpxP is thought to be titrated from CpxA, perhaps by misfolded proteins, allowing for activation of the pathway (32, 34).
NlpE is an outer membrane lipoprotein that upregulates the Cpx pathway when overproduced (40). Adhesion to hydrophobic surfaces activates the Cpx pathway in an NlpE-dependent manner, suggesting that NlpE is an upstream component of the Cpx pathway (7, 29). The role of NlpE in the transduction of other stresses is not known.
A large number of environmental conditions upregulate the Cpx pathway (4, 5, 20, 21, 25, 29, 40). How, then, does the Cpx pathway appropriately sense and respond to diverse stresses? Here we show that induction of Cpx by known envelope stresses requires the periplasmic domain of CpxA, but not NlpE; we argue that all of these stresses present a single periplasmic signal to the Cpx pathway. We also show that CpxP is not required for signal transduction, but its activity as a negative regulator is inactivated under conditions of envelope stress. Moreover, we report that several Cpx-regulated target genes are coregulated by other stress responses.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are described in Table 1. Isogenic fusion strains were constructed by transduction of lacZ transcriptional fusions, which are carried on a λ phage integrated at attB, into MC4100 using P1vir and selecting for Lac+ on lactose M63 minimal agar. Induction of the Cpx pathway by accumulation of the lipid II enterobacterial common antigen (ECA) intermediate was studied in strains containing the rffA::cam and ompR::Tn10 mutations as previously described (4). Induction by PapE expression was studied in strains containing PapE expression plasmid pHJ13 as previously described (20). All strains were constructed by using standard genetic techniques (36).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Strain construction | Source or reference |
|---|---|---|---|
| Strains | |||
| MC4100 | F−araD139 Δ(argF-lac) U169 rpsL150 (Strr) relA1 flbB5301 deoC1 ptsF25 rbsR | 36 | |
| TR50 | λRS88 [cpxP′-lacZ+] | 34 | |
| PAD282 | MC4100 λRS88 [cpxP′-lacZ+] | MC4100 × TR50 | This study |
| PAD501 | PAD282(pHJ8) | This study | |
| PAD726 | PAD282(pMal-p2) | This study | |
| PAD727 | PAD282(pMCP) | This study | |
| PAD728 | PAD282(pMCPss) | This study | |
| TR70 | cpxR1::spc | 31 | |
| PAD292 | PAD282 cpxR1::spc | PAD282 × TR70 | This study |
| PAD81 | cpxRM199A zii::Tn10 | This study | |
| PAD293 | PAD282 cpxRM199A zii::Tn10 | PAD282 × PAD81 | This study |
| WBS262 | nlpE::spc | 39 | |
| PAD485 | PAD282 nlpE::spc | PAD282 × WBS262 | This study |
| PAD486 | PAD485(pHJ8) | This study | |
| CLC393 | cpxP::kan | Laboratory stock | |
| PAD488 | PAD282 cpxP::kan | PAD282 × CLC393 | This study |
| TR212 | cpxA24 zii::Tn10 | Laboratory stock | |
| PAD455 | PAD282 cpxA24 zii::Tn10 | PAD282 × TR212 | This study |
| PAD504 | PAD455(pHJ8) | This study | |
| TR235 | λRS88 [cpxR′-lacZ+] | 32 | |
| PAD279 | MC4100 λRS88 [cpxR′-lacZ+] | MC4100 × TR235 | This study |
| PAD290 | PAD279 cpxRM199A zii::Tn10 | This study | |
| TR49 | λRS88 [degP′-lacZ+] | 34 | |
| PAD280 | MC4100 λRS88 [degP′-lacZ+] | This study | |
| SP969 | λ RS88 [yihE-dsbA′-lacZ+] | 6 | |
| PAD281 | MC4100 λRS88 [yihE-dsbA′-lacZ+] | This study | |
| TR530 | λRS88 [spy′-lacZ+] | 31 | |
| PAD283 | MC4100 λRS88 [spy′-lacZ+] | This study | |
| PAD593 | MC4100 λRS88 [ppiA′-lacZ+] | This study | |
| Plasmids | |||
| pMal-p2 | Cloning vector for making periplasmic MBP fusion proteins; parent of pMCP and pMCPss | New England Biolabs | |
| pMCP | Overexpression plasmid for periplasmic MBP-CpxP fusion protein | 31 | |
| pMCPss | Overexpression plasmid for tethered MBP-CpxPA24D fusion protein | 31 | |
| pHJ8 | IPTG-inducible PapG expression plasmid | 20 | |
| pGS284 | Cloning vector for allelic exchange; parent of pCpxRM199A and pCpxRD51A | 38 | |
| pCpxRM199A | Plasmid used for recombination of cpxRM199A at the cpxR locus | This study | |
| pCpxRD51A | Plasmid used for recombination of cpxRD51A at the cpxR locus | This study |
Media and chemicals.
All strains were grown in Luria-Bertani (LB) broth or agar at 37°C unless otherwise indicated. cpxA* alleles confer a variety of pleiotropic phenotypes, including resistance to amikacin. Strains containing these alleles linked to zii::Tn10 were maintained on LB agar containing amikacin (Sigma) at 3 μg/ml. Plasmid-containing strains were grown in ampicillin (Sigma) at a concentration of 100 μg/ml. For pH induction of the Cpx pathway, LB broth was buffered in sodium phosphate as previously described (5).
Construction of a single-copy ppiA′-lacZ+ fusion.
The ppiA promoter (30) and upstream region were amplified by PCR from MC4100 chromosomal DNA with restriction-tagged primers ppiA5′ (5′ GTGATCTGTTTGAATTCTTTATTGCAA 3′ [BamHI site underlined]) and ppiA3′ (5′ CCCTTTCGGATCCATTGCTGCG 3′ [EcoRI site underlined]). The resulting 281-bp PCR product was purified (Qiagen), digested with EcoRI and BamHI (New England Biolabs), and introduced upstream of the promoterless lacZ gene in pRS415 at the EcoRI and BamHI sites (37). The ppiA′-lacZ+ transcriptional fusion was recombined with λRS45 and integrated into the chromosome at attB as described by Hand and Silhavy (18). The ppiA′-lacZ+ fusion was characterized in wild-type and cpxR::spc and cpxA24 mutant strains to show Cpx regulation. The fusion behaves as predicted by primer extension assays on the ppiA promoter (30).
Construction of the cpxRM199A and cpxRD51A alleles.
pND10, which overexpresses CpxR (3), was mutagenized with the Gene Editor in vitro site-directed mutagenesis system (Promega) in accordance with the manufacturer's instructions to construct the cpxRM199A (Met changed to Ala at position 199) allele with the primer cpxRM199A (5′GGTTGGAAATGTGCGCATCAATAGCGCG 3′). Mutagenesis for the cpxRD51A (Asp changed to Ala at position 51) mutation was carried out with pND10 as the template for the QuikChange site-directed mutagenesis kit (Stratagene) in accordance with the manufacturer's instructions with the primers D51A3 (5′GCATTGATTTACTTTTGCTAGCCGTAATGATGCCG 3′) and D51A4 (5′CGGCATCATTACGGCTAGCAAAAGTAAATCAATGC 3′). The D51A primers also introduce a silent change in the nucleotide sequence that results in the creation of an NheI site.
Allelic exchange.
The mutations in the cpxRM199A and cpxRD51A alleles were confirmed by sequencing and were introduced into pGS284 (38) by using BamHI and PstI sites in pND10 and BglII and NsiI sites in pGS284. Allelic exchange was performed as previously described (38), with the following changes. A 100-μl volume of an overnight culture of MC4100 λRS88 [cpxP′-lacZ+] was mixed with 50 μl of λvir and plated on maltose MacConkey plates to select for spontaneous lamB mutations, which are phenotypically red, to prevent transfer of λpir from the donor strain during conjugation. The S17-1 λpir donor strain (12) containing the pCpxRM199A or pCpxRD51A plasmid was conjugated with MC4100 lamB λRS88 [cpxP′-lacZ+] on LB agar, and exconjugants were isolated by selecting for growth on LB agar containing 25 μg of ampicillin (Sigma) per ml. Following merodiploid resolution, chromosomal isolates containing the cpxRM199A and cpxRD51A alleles at the cpxR locus were screened for the Lac− phenotype on lactose MacConkey agar. The cpxRM199A and cpxRD51A mutations were linked to zii::Tn10 by P1vir transduction. The resulting strains were confirmed by sequencing.
β-Galactosidase assays.
Single colonies of each strain to be analyzed were inoculated into 5-ml cultures of LB broth with the appropriate antibiotics overnight at 37°C with aeration. On the next day, cultures were subcultured 1:100 into fresh LB broth or sodium phosphate-buffered LB broth and grown for 2.5 h at 37°C with aeration. Strains containing isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible plasmids were induced with 0.5 mM IPTG at 1 h post subculturing. The strains were then grown for an additional 2.5 h at 37°C with aeration. β-Galactosidase assays were performed with microtiter plates as previously described (31). Relative β-galactosidase units were determined by the rate (in units of optical density at 420 nm [OD420] per minute) divided by the OD600 times the volume assayed (in milliliters). Each figure depicts an individual experiment and shows the standard deviation. Each experiment was repeated at least three times.
Western blot analysis.
Following induction of the Cpx pathway by alkaline pH (see above), the OD600 was determined. The reciprocal of the OD600 was used to determine the sample volume, normalizing the number of cells per milliliter. According to this calculation, the appropriate culture volume was harvested by centrifugation, resuspended in 50 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer, and lysed by boiling for 5 min. Samples (15 μl) were electrophoresed on a sodium dodecyl sulfate-12% polyacrylamide gel, transferred to nitrocellulose, and subjected to Western blot analysis. Maltose-binding protein (MBP)-CpxR antiserum (34) was used at a 1:5,000 dilution. β-Galactosidase antiserum (5′-3′ Inc.) was used at a 1:5,000 dilution. Enhanced-chemiluminescence Western blotting reagents were purchased from Amersham Life Sciences.
RESULTS
Inputs.
Components of the Cpx pathway reside in each compartment of the bacterial cell. NlpE resides in the outer membrane. CpxP, a negative regulator of the pathway, resides in the periplasm (5). CpxA and CpxR reside in the inner membrane and cytoplasm, respectively (15, 44). Many different envelope stresses, which could affect any of the four bacterial compartments, are known to upregulate the Cpx pathway. Given that members of the signaling cascade exist in all compartments and that stresses that upregulate the pathway may affect each compartment, we hypothesized that potential signals could enter the pathway at different points in the signaling pathway. For example, a stress originating in the outer membrane could enter the pathway at NlpE. A stress originating in the periplasm could enter the pathway downstream of NlpE, at CpxP or at CpxA. Inner membrane stresses could signal through the transmembrane domains of CpxA, while stresses that affect the cytoplasm could signal to the pathway by affecting the phosphotransfer between CpxA and CpxR or by affecting CpxR directly.
Accumulation of misfolded pilus subunits PapG and PapE in the periplasm is known to upregulate the Cpx pathway (20). However, the signals generated by other envelope stresses are less obvious. Accumulation of lipid II ECA intermediate, most likely in the inner membrane, upregulates the Cpx pathway and causes sensitivity to bile salts, suggesting an outer membrane defect (4). Decreased levels of phosphatidylethanolamine upregulate the Cpx pathway in an NlpE-independent manner, causing both inner and outer membrane defects (25). Alkaline pH upregulates the Cpx regulon, but the effects of increased pH on the envelope are unclear (5). EDTA induces the Cpx pathway (data not shown), most likely by disrupting lipopolysaccharide (LPS) in the outer membrane (28). It is possible that either each of these envelope stresses generates a unique signal that is sensed distinctly by the Cpx pathway or all of the stresses cause the same general signal.
(i) Envelope stress activates the Cpx pathway in an NlpE-independent manner.
Since adhesion to hydrophobic surfaces activates the Cpx pathway in an NlpE-dependent manner, one possibility is that adhesion damages the outer membrane and NlpE thus functions as an outer membrane “damage sensor.” It is also possible that adhesion sends a signal to the Cpx pathway that is distinct from that of envelope stress and the role of NlpE in signal transduction is specific to adhesion. In order to elucidate the role of NlpE in signal transduction, wild-type and mutant nlpE strains were tested for Cpx induction in response to a variety of envelope stresses. Using a cpxP′-lacZ+ fusion strain as a reporter, we found that PapG expression in the absence of the PapD chaperone induced the Cpx pathway in an NlpE-independent manner (Fig. 1). The same results were observed for PapE expression, induction by alkaline pH, accumulation of the ECA intermediate lipid II, and exposure to EDTA (data not shown), suggesting that envelope stress is sensed by Cpx in an NlpE-independent manner. We suggest that NlpE does not function to sense damage but rather that NlpE functions specifically in adhesion.
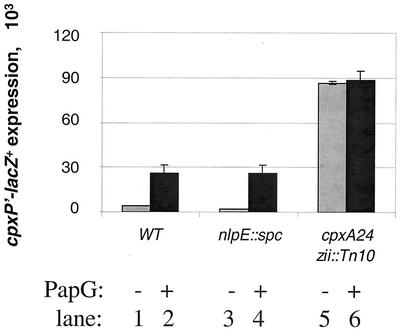
FIG. 1.
PapG expression induces Cpx in a manner that is dependent on the periplasmic domain of CpxA but does not require NlpE. β-Galactosidase activities were determined for PAD501 (lanes 1 and 2), PAD 486 (lanes 3 and 4), and PAD 504 (lanes 5 and 6). pHJ8 is an IPTG-inducible PapG overexpression plasmid (20). zii::Tn10 is commonly used to move Cpx alleles and has no effect on Cpx-mediated signal transduction (31). The cpxA24 strain is signal blind to PapG expression (compare lanes 5 and 6 to lanes 1 and 2). The nlpE null strain is induced to wild-type (WT) levels upon PapG expression (compare lanes 1 and 2 to lanes 3 and 4). All strains were grown and induced as described in Materials and Methods.
(ii) The periplasmic domain of CpxA is required for induction of the Cpx pathway by envelope stress.
The cpxA* mutation, cpxA24, is an in-frame deletion in the periplasmic domain of CpxA. cpxA24 activates the Cpx pathway but, unlike other cpxA* alleles, is not further induced by stress, suggesting that this mutation renders CpxA signal blind (34). By using this signal-blind cpxA* allele, the periplasmic domain of CpxA was shown to be required for induction of the Cpx pathway by NlpE overexpression and for repression of the Cpx pathway by overexpression of CpxP (34). We expected that if all of the stresses present the same signal to the Cpx pathway, then all of the stresses should be sensed in the same way and require the periplasmic sensing domain of CpxA; cpxA24 mutant strains should be blind to envelope stress signals.
In order to determine if induction requires the periplasmic domain of CpxA, the cpxP′-lacZ+ fusion was analyzed by using β-galactosidase assays of wild-type and cpxA24 strains in the presence or absence of PapG expression. As shown in Fig. 1, an intact periplasmic domain of CpxA is required for induction of the Cpx pathway by PapG overexpression. The cpxA24 mutant strain was blind to all of the stresses tested, including alkaline pH, PapE overexpression, NlpE overexpression, and exposure to EDTA (data not shown). These data indicate that an intact periplasmic domain of CpxA is required for induction of the Cpx pathway by envelope stress.
(iii) The periplasmic domain of CpxA is not required for induction of the Cpx pathway during growth.
During the course of these studies, we observed that the Cpx pathway was induced during growth in LB broth and that the induction was not entirely due to pH changes throughout growth. While it was previously reported by De Wulf and Lin that Cpx is induced upon entry into stationary phase in an RpoS-dependent manner in strains derived from MG1655 (13), we found that the induction occurs in an RpoS-independent manner in our strain background (data not shown). We conducted experiments similar to those described above, by using nlpE mutant, cpxP mutant, and cpxA mutant strains to determine how growth is sensed by the Cpx pathway. We found that while cpxP induction was dependent on CpxR, induction was independent of CpxA; strains containing either the cpxA null allele (Fig. 2A) or the cpxA24 allele were still inducible (Fig. 2B). Induction did not require NlpE or CpxP (data not shown). This suggests that induction during growth activates the Cpx pathway downstream of CpxA.
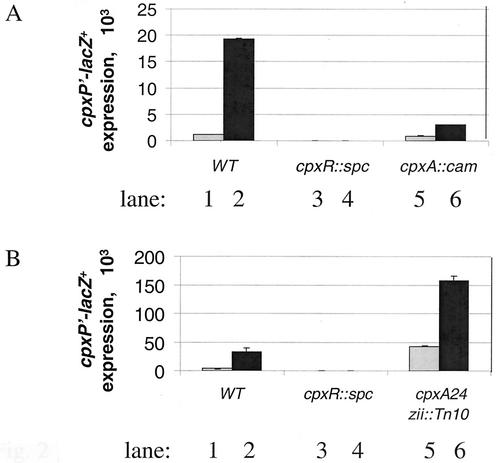
FIG. 2.
Induction of cpxP during growth is CpxA independent. β-Galactosidase activities were measured for PAD282, PAD292, and PAD414 in panel A and for PAD282, PAD292, and PAD455 in panel B. Stationary-phase samples were grown overnight (black bars), subcultured to LB broth, and grown for 2.5 h to mid-log phase (gray bars). The Cpx pathway is induced by growth phase (compare lanes 1 and 2 in panels A and B) in a manner that is dependent on CpxR (lanes 3 and 4 in panels A and B) but not CpxA (compare lanes 5 and 6 to 1 and 2 in panel A). Induction during growth also occurs in the cpxA24 mutant background (compare lanes 5 and 6 to 1 and 2 in panel B). WT, wild type.
Figure 2A and B show that cpxP is strongly induced in wild-type strains during growth. In the cpxA null and cpxA24 mutant strains, this induction is approximately threefold. This difference is explained as follows. In CpxA+ strains, cpxP responds to both growth and the changes in pH that occur during growth in broth. However, in the absence of CpxA, changes in pH are no longer detected and cpxP responds to growth only.
The data in Fig. 2B show that the cpxA24 mutation does not fully induce cpxP. The mutant signal transduction system can still respond to signals that enter the pathway downstream of CpxA.
(iv) Induction by envelope stress is CpxP independent.
We hypothesized that if CpxP were required for induction of the Cpx pathway during envelope stress, cpxP null strains would be signal blind. In order to better determine the role of CpxP in signal transduction, β-galactosidase assays were performed on wild-type and cpxP null strains containing the cpxP′-lacZ+ fusion in response to a variety of envelope stresses. The uninduced level of transcription from the cpxP promoter in the cpxP null strain was twofold higher than the uninduced level of transcription from cpxP in the wild type, consistent with prior experiments showing that CpxP is a negative regulator under noninducing conditions (32). However, upon exposure to alkaline pH, the cpxP null strain was induced to levels comparable to those of the wild-type strain (Fig. 3). The same results were obtained for the other stresses tested, including PapG and PapE expression, exposure to EDTA, and lipid II accumulation (data not shown), indicating that induction of the Cpx pathway by envelope stress is CpxP independent.
FIG. 3.
Induction of Cpx by alkaline pH is CpxP independent. β-Galactosidase activities were determined for PAD282 (lanes 1 and 2) and PAD488 (lanes 3 and 4) in LB broth buffered with sodium phosphate to pH 5.5 (gray bars) and 8.5 (black bars). Under noninducing conditions, β-galactosidase activity is increased approximately twofold in the cpxP null strain compared to that of the wild type (WT), reflecting the role of CpxP as a negative regulator under noninducing conditions (compare lanes 1 and 3). The cpxP null strain is induced to levels comparable to that of the wild-type strain (compare lanes 2 and 4). All strains were grown as described in Materials and Methods.
(v) Envelope stresses inactivate CpxP.
Under conditions that induce the Cpx pathway, there are increased levels of CpxP in the periplasm, as shown by Western blot analysis (data not shown), reflecting the increased cpxP transcription that occurs in response to envelope stress (Fig. 3). Since CpxP is a negative regulator, it is logical to expect that CpxP would not function as a negative regulator under stress conditions. Although CpxP functions as a negative regulator of Cpx under nonstress conditions, there is no evidence for negative regulation during induction by envelope stress, despite high levels of periplasmic CpxP. cpxP null strains are still induced to the same level upon induction by alkaline pH as wild-type strains (Fig. 3).
In support of this, strains overexpressing a periplasmic MBP-CpxP fusion protein or an MBP-CpxPA24D fusion protein tethered to the inner membrane by an uncleaved signal sequence are also inducible by envelope stress. Both Western blot analysis and β-galactosidase assays show that while MBP-CpxP negatively regulates the Cpx pathway under uninduced conditions, the Cpx pathway is still induced in strains overexpressing MBP-CpxP upon treatment with alkaline pH (Fig. 4 and data not shown). Strains harboring pMal-p2 (vector control), pMCP (periplasmic MBP-CpxP), or pMCPss (tethered MBP-CpxPA24D) and the cpxP′-lacZ+ fusion were induced with alkaline pH, and Western blot analysis was performed (see Materials and Methods). The strain containing pMal-p2 represents wild-type induction of the Cpx pathway (Fig. 4, compare βgal lanes 1 and 2). Under uninduced conditions, the levels of β-galactosidase were decreased in strains expressing periplasmic MBP-CpxP or tethered MBP-CpxPA24D compared to the vector control (Fig. 4, compare βgal lane 1 to lanes 3 and 5), demonstrating that both periplasmic MBP-CpxP and tethered MBP-CpxPA24D function as negative regulators under uninduced conditions. However, β-galactosidase levels were greatly increased upon induction of the Cpx pathway in the presence or absence of periplasmic MBP-CpxP or tethered MBP-CpxPA24D overexpression (Fig. 4, compare βgal lane 2 to lanes 4 and 6). Both periplasmic MBP-CpxP and tethered MBP-CpxPA24D fusion proteins were expressed at comparable levels in the presence or absence of stress (Fig. 4, compare MBP-CpxP lanes 3 and 4, and MBP-CpxPA24D lanes 5 and 6), suggesting that alkaline pH does not compromise the stability of the MBP-CpxP fusion proteins. Similar experiments were performed by using PapE expression as the inducing signal and yielded comparable results (data not shown). These data suggest that under conditions that induce the Cpx pathway, MBP-CpxP is not sufficient to prevent induction and therefore does not act as a negative regulator. MBP-CpxP is inactivated by envelope stress. Moreover, since all envelope stresses upregulate CpxP, then all envelope stresses must also inactivate CpxP.
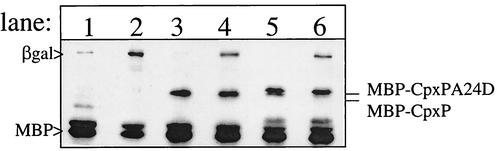
FIG. 4.
CpxP is not a negative regulator under stress conditions. Western blot analysis was performed on PAD726 (lanes 1 and 2), PAD727 (lanes 3 and 4), and PAD728 (lanes 5 and 6) following growth in sodium phosphate-buffered LB broth at pH 5.5 (lanes 1, 3, and 5) or 8.5 (lanes 2, 4, and 6) as described in Materials and Methods. Under noninducing conditions, both MBP-CpxP and MBP-CpxPA24D negatively regulate the Cpx pathway (compare the β-galactosidase level in lane 1 to those in lanes 3 and 5). However, upon induction by alkaline pH, β-galactosidase levels increase in the presence or absence of MBP-CpxP or MBP-CpxPA24D (compare lane 2 to lanes 4 and 6).
Outputs. (i) CpxP is the most highly induced target gene.
Previously, it was observed that the cpxP and degP promoters have different binding affinities for CpxR∼P (T. L. Raivio, personal communication), which implies different sensitivities of these promoters to levels of CpxR∼P. In an effort to further characterize differences in CpxR∼P affinity at Cpx-regulated target genes, induction of Cpx-regulated lacZ transcriptional fusions was analyzed in response to a variety of envelope stresses. When conditions known to induce the Cpx pathway, such as cpxA* alleles, alkaline pH, and NlpE overexpression, were used to induce target gene expression, the fold induction of the cpxP′-lacZ+ transcriptional fusion was greater than the fold induction of the other fusions tested. For example, upon induction by pH 8.5, the cpxP′-lacZ+ fusion was induced approximately 15-fold in a Cpx-dependent manner while other fusions were induced 2- or 3-fold (Fig. 5). In addition, Western blot analysis showed that CpxP protein levels were induced more strongly than CpxR and DegP upon induction by alkaline pH, consistent with fusion analysis (data not shown). These data suggest that cpxP is the most inducible member of the Cpx regulon.
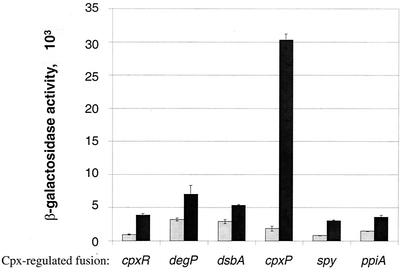
FIG. 5.
CpxP is the most inducible regulon member. β-Galactosidase activities were measured for PAD279 (lanes 1 and 2), PAD280 (lanes 3 and 4), PAD281 (lanes 5 and 6), PAD282 (lanes 7 and 8), PAD283 (lanes 9 and 10), and PAD593 (lanes 11 and 12) in sodium phosphate-buffered LB broth at pHs 5.5 (gray bars) and 8.5 (black bars). cpxP transcription is induced ∼15-fold by alkaline pH, while the other fusions tested were only induced between 2- and 4-fold. All strains were grown as described in Materials and Methods.
(ii) The CpxP promoter has high affinity for CpxR∼P.
CpxR is a member of the OmpR family of winged helix-turn-helix transcription factors. Consequently, OmpR and CpxR are highly homologous response regulators. On the basis of analogy to OmpR, where Asp at position 55 is the putative site of phosphorylation and Val at position 203 is in the recognition helix (23, 35), mutations were made in the putative site of phosphorylation (Asp at position 51 in CpxR) and in the recognition helix (Met at position 199 in CpxR) in CpxR. The cpxRD51A and cpxRM199A mutations were recombined into the chromosome at the cpxR locus by allelic exchange (see Materials and Methods). CpxRD51A and CpxRM199A mutant proteins are stable on the basis of Western blot analysis but are present at levels lower than that of wild-type CpxR (data not shown). The levels of the mutant CpxR proteins appear to be decreased by approximately 50% in these strains, as shown by both Western and cpxR′-lacZ+ fusion analyses. This is expected because we believe that these mutations block the positive feedback loop (13, 32).
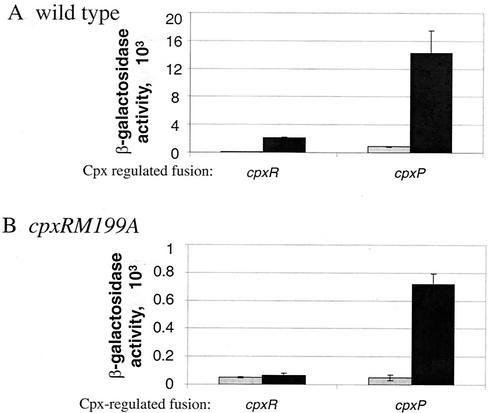
Strains containing either the cpxRD51A mutation or the cpxRM199A mutation and Cpx-regulated lacZ transcriptional fusions were analyzed by using β-galactosidase assays to determine if the CpxR mutant proteins can activate transcription. Strains containing the cpxRD51A mutation behaved like cpxR null strains with all of the Cpx-regulated lacZ fusions tested (data not shown), which, on the basis of analogy with OmpR studies, suggests that phosphorylation at the Asp residue at position 51 is required for transcriptional activation by CpxR. Interestingly, strains containing the cpxRM199A mutation behave like cpxR null strains with respect to all of the Cpx-regulated lacZ fusions tested except for the cpxP′-lacZ+ fusion, where it retains ∼10% of wild-type activity (data not shown). On the basis of analogy with OmpR studies, this suggests that the CpxRM199A mutant protein retains some ability to bind DNA. Moreover, only the cpxP promoter is inducible by alkaline pH in a cpxRM199A-containing strain (compare Fig. 6A and B). Because the mutant protein is leaky and retains some wild-type activity, this suggests that the cpxP promoter has greater affinity for CpxR∼P than the other promoters tested.
FIG. 6.
CpxRM199A can activate transcription at cpxP. β-Galactosidase activities were measured for PAD279 and PAD282 (A) and for PAD290 and PAD293 (B) grown in sodium phosphate-buffered LB broth at pHs 5.5 (gray bars) and 8.5 (black bars). The other target genes tested, including yihE-dsbA, spy, ppiA, and degP, behaved like cpxR (data not shown). All strains were grown as described in Materials and Methods.
(iii) The spy and degP promoters are “differentially upregulated.”
Analysis of Cpx-regulated lacZ transcriptional fusions in the presence and absence of CpxR showed that transcription of cpxP is almost entirely dependent on CpxR∼P, while the other regulon members tested showed between 50 and 70% of the wild-type level of transcription in cpxR null strains. Transcription in the absence of CpxR could suggest either that the cpxR, ppiA, yihE-dsbA, spy, and degP promoters have high levels of basal transcription or that these target genes are also regulated by other signal transduction pathways. We reasoned that genes regulated solely by Cpx would be induced to a comparable degree regardless of the envelope stress tested. On the other hand, target genes that are upregulated to different degrees in response to different stresses must have multiple regulatory inputs. We refer to this idea as differential upregulation.
The Cpx and σE pathways are overlapping, but functionally distinct, envelope stress response pathways, and degP transcription is regulated by both (6, 30). Therefore, we would expect that stresses that induce both pathways would upregulate the degP promoter to a greater degree than stresses that induce the Cpx pathway only. That is, the degP promoter should exhibit differential upregulation. Indeed, the degP promoter was strongly upregulated by both PapG expression (8-fold) and lipid II accumulation (14- to 17-fold) but only moderately induced by the other stresses tested, such as increased pH (2-fold [Fig. 5]). PapG expression and lipid II accumulation have been shown to upregulate both the Cpx and σE pathways (4, 20); pH induces only the Cpx pathway.
We found that the spy promoter was strongly upregulated by expression of both PapG (∼14-fold) and PapE (∼8-fold) but only moderately induced by the other stresses tested, such as lipid II accumulation (2-fold) and alkaline pH (2-fold [Fig. 5]). Because the induction profile of spy varies depending on the stress tested, we suggest that, like degP, spy is differentially upregulated. Moreover, the profile of spy differential upregulation is distinct from that of degP and it has been shown that spy is not regulated by the σE pathway (31). Thus, spy is likely to be coregulated by a third stress-responsive system.
DISCUSSION
The Cpx pathway can sense inducing cues that enter the signaling pathway at three distinct points.
A large number of seemingly distinct stresses upregulate the Cpx pathway. Because components of the Cpx pathway reside in all four cellular compartments, numerous entry points into the signaling pathway could exist (Fig. 7). However, in this report, we have shown that a variety of envelope stresses induce the Cpx pathway in a manner that is dependent on the periplasmic domain of CpxA but not NlpE. That is, a variety of stresses upregulate the Cpx pathway by entering the signaling cascade at a common point, downstream of NlpE, at CpxA.
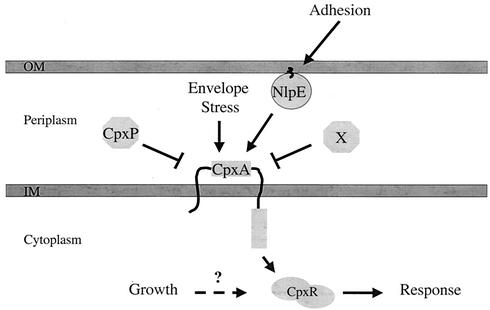
FIG. 7.
CpxRA two-component signal transduction pathway. Components of the Cpx pathway reside in each compartment of the bacterial cell. The Cpx pathway can sense inducing cues that enter the signaling pathway at three distinct points. The mechanism by which growth is sensed remains unclear. X represents the second, as yet unidentified, negative regulator of the Cpx pathway. Note that X could be the periplasmic domain of CpxA. OM, outer membrane; IM, inner membrane.
It is possible that CpxA senses each stress differently or that all of the envelope stresses tested present the same signal to the periplasmic domain of CpxA. Given that the envelope stresses known to induce Cpx have seemingly disparate origins and effects, what does CpxA sense?
Expression of misfolded PapG and PapE pilin subunits have previously been shown to upregulate the Cpx pathway (20), demonstrating that Cpx can sense misfolded envelope proteins. Accumulation of the lipid II intermediate in the ECA biosynthetic pathway is thought to occur in the inner membrane, perhaps affecting the transport of outer membrane proteins and possibly leading to misfolded envelope proteins; the C55 component of lipid II is known to aggregate and behave as a detergent (45). The effect of alkaline pH is unclear, but it is possible that changes in pH result in misfolded envelope proteins. Both phospholipids and LPS are thought to be involved in protein folding in the envelope (1, 9, 10, 26). EDTA is a strong chelating agent and consequently increases the permeability of the outer membrane, displacing LPS with phospholipids in the outer membrane (28). It is therefore possible that both decreased phosphatidylethanolamine and exposure to EDTA could lead to misfolded envelope proteins. We suggest that disparate stresses lead to a common periplasmic inducing signal, misfolded envelope proteins, sensed by the Cpx pathway.
While all of the stresses tested in this study lead to a common periplasmic signal, adhesion and growth phase induction are distinct from envelope stress. Adhesion enters the Cpx pathway upstream of NlpE. Like envelope stress, adhesion does not require CpxP for induction of the Cpx pathway. However, unlike envelope stresses, which induce cpxP transcription ∼8- to 15-fold, adhesion induces cpxP transcription no more than 3-fold (29). The induction of Cpx over the growth curve does not require CpxA since the cpxA24 allele is inducible and not signal blind. Although the precise nature of this induction is unknown, growth phase induction is clearly distinct from both adhesion and envelope stress.
CpxP may act as a periplasmic chaperone.
Our findings that CpxP is greatly induced under conditions of envelope stress and that the activity of CpxP as a negative regulator is inactivated under these conditions strongly suggests that CpxP not only functions as a negative regulator but also performs a second function in the cell. We propose that CpxP may function as a chaperone.
Negative autoregulation by chaperones is a common theme in stress-responsive systems. The heat shock response, which is positively regulated by the alternative sigma factor σ32, is negatively autoregulated by regulon members DnaJ, DnaK, and GrpE (41, 42, 43). DnaK, DnaJ, and GrpE function as a “chaperone machine” (17), sequestering σ32 from core RNA polymerase following clearance of stress (22).
The σE stress response, which is thought to monitor outer membrane β-barrel proteins (19), is negatively autoregulated by regulon members RseA and RseB (11, 27). RseA acts as an anti-sigma factor by complexing with σE and sequestering it from core RNA polymerase. RseB is a periplasmic protein that negatively regulates the pathway by increasing the stability of the σE · RseA complex (2). In strains lacking RseB, the σE regulon is induced approximately twofold, is not completely derepressed (11), and still shows wild-type induction. In this respect, RseB is similar to CpxP. Moreover, RseB has been shown to bind aggregated periplasmic proteins, so it is thought that upon binding misfolded proteins, RseB can no longer interact with the σE · RseA complex, leading to the release and activation of σE (2). Analogously, it is possible that CpxP functions as the primary chaperone for the Cpx pathway and is thus greatly induced by misfolded periplasmic proteins. Misfolded proteins could titrate CpxP from CpxA, allowing for activation of the Cpx pathway.
Another possibility, which is not mutually exclusive with CpxP acting as a chaperone, is that CpxP is involved in shutting off the stress response pathway following the removal of stress. Perhaps the high induction of CpxP results in a periplasmic pool of CpxP that allows for rapid shutoff of the Cpx pathway following stress clearance. Experiments to further distinguish between these possibilities are under way.
CpxP is not the only negative regulator of the Cpx pathway.
In this report, we showed that overexpression of MBP-CpxP (both periplasmic and tethered) is not sufficient to prevent induction of the Cpx pathway under stress conditions. Moreover, induction of the Cpx pathway is comparable in the presence or absence of CpxP. Therefore, CpxP is inactivated during envelope stress, potentially by the signal itself (misfolded envelope proteins).
It was previously shown that spheroplasting induces the Cpx pathway (31). While overexpression of periplasmic MBP-CpxP was insufficient to prevent induction of the Cpx pathway during spheroplasting, tethered MBP-CpxPA24D prevented induction of the pathway (31). Because spheroplasting does not inactivate tethered MBP-CpxPA24D, we suggest that spheroplasting is not an envelope stress and consequently does not result in a stress signal. In genetic studies presented here, we showed that losing CpxP is not sufficient for derepression of the Cpx pathway. This suggests that CpxP cannot be the only negative regulator of the pathway. We propose that induction by spheroplasting results from the loss of CpxP and another, unidentified, periplasmic regulator(s) of the Cpx pathway. This implies that both CpxP and the negative regulator(s) potentially function as sensors for the Cpx pathway.
Moreover, the negative regulators can function independently. For example, during spheroplasting, tethered MBP-CpxP is sufficient for repression (31). Yet, in the absence of CpxP, the Cpx pathway is not fully derepressed, suggesting that another negative regulator(s) keeps the pathway off in the absence of CpxP.
Given that deletions in the periplasmic sensing domain of CpxA activate the Cpx pathway (34), it is also possible that the periplasmic domain of CpxA is the second negative regulator. This would require that spheroplasting affects the integrity of the periplasmic domain of CpxA and that the presence of the tethered MBP-CpxP protein protects this domain.
There are at least three systems that sense and respond to envelope stress.
We have shown that CpxP is solely controlled by the Cpx pathway, while other target genes are subject to multiple regulatory inputs. It was previously shown that degP is regulated by both the σE and Cpx pathways. We found that spy is differentially upregulated by expression of both PapE and PapG, suggesting that spy transcription is induced by yet another stress response pathway that overlaps both the Cpx and σE pathways and responds to misfolded periplasmic proteins. It has been suggested that spy transcription is only partially dependent on the Cpx pathway (31), and it was previously shown that spy is induced by the oxidant indole (16). It seems, therefore, that spy transcription is dependent on another stress response pathway that is induced by indole, PapE and PapG expression.
ppiA, cpxRA, and yihE-dsbA were only moderately induced by all of the stresses tested; these data are consistent with two possibilities. It is possible that these promoters, like cpxP, are solely regulated by Cpx; the fold induction of transcription at these promoters is constant regardless of the stress tested. This suggests that these promoters have a high level of CpxR-independent basal transcription. Another possibility is that these promoters are regulated by other, as yet unknown, regulatory pathways, the inducing cues of which are also unknown.
This method of transcriptional analysis can be used to better define overlap between regulatory pathways at both the input (identification of inducing cues) and output levels. Such experiments could assign inducing cues to multiple regulons. For example, NlpE overexpression and cpxA* alleles are known to specifically upregulate the Cpx pathway. Because NlpE overexpression, cpxA* alleles, and alkaline pH lead to the same profile of target gene upregulation and because alkaline pH does not induce degP or spy transcription differentially, this suggests that alkaline pH does not induce the σE or the other regulatory pathway that induces spy. This method can also be used to place target genes into multiple regulatory pathways, as in the case of spy transcription, and can uncover the existence of regulatory pathways but cannot provide clues as to identity.
Following the submission of the manuscript, Raffa et al. (30a) showed that spy is coregulated by a second stress-responsive system, BaeSR, in response to misfolded PapG, consistent with our predictions.
Acknowledgments
We thank Scott Hultgren for provision of the PapG and PapE expression plasmids. We thank Greg Smith for provision of the pGS284 plasmid and the S17-1λpir E. coli strain and for helpful discussions concerning the allelic exchange procedure. We also thank Tracy Raivio and Kristina Wehr Matthews for cpxRM199A site-directed mutagenesis. We thank Mark Goulian for helpful discussions. We thank members of the Silhavy laboratory for helpful discussions, especially Natividad Ruiz, Daniel Isaac, and Karen Otto, and for critical reading of the manuscript, especially Susan DiRenzo.
This work was supported by National Institutes of Health predoctoral training grant GM07388 to P.A.D. and by National Institute of General Medical Sciences MERIT Award GM34821 to T.J.S.
REFERENCES
- 1.Bogdanov, M., and W. Dowhan. 1998. Phospholipid-assisted protein folding: phosphatidylethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. EMBO J. 17:5255-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collinet, B., H. Yuzawa, T. Chen, C. Herrera, and D. Missiakas. 2000. RseB binding to the periplasmic domain of RseA modulates the RseA-σE interaction in the cytoplasm and the availability of σE RNA polymerase. J. Biol. Chem. 275:33898-33904. [DOI] [PubMed] [Google Scholar]
- 3.Danese, P. N. 1996. Pushing the envelope: extracytoplasmic stress response in Escherichia coli. Ph D. dissertation. Princeton University, Princeton, N.J.
- 4.Danese, P. N., G. R. Oliver, K. Barr, G. D. Bowman, P. D. Rick, and T. J. Silhavy. 1998. Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J. Bacteriol. 180:5875-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese, P. N., and T. J. Silhavy. 1998. CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danese, P. N., and T. J. Silhavy. 1997. The σE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 7.Danese, P. N., and T. J. Silhavy. 1998. Targeting and assembly of periplasmic and outer-membrane proteins in Escherichia coli. Annu. Rev. Genet. 32:59-94. [DOI] [PubMed] [Google Scholar]
- 8.Danese, P. N., W. B. Snyder, C. L. Cosma, L. J. Davis, and T. J. Silhavy. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9:387-398. [DOI] [PubMed] [Google Scholar]
- 9.de Cock, H., M. Pasveer, J. Tommassen, and E. Bouveret. 2001. Identification of phospholipids as new components that assist in the in vitro trimerization of a bacterial pore protein. Eur. J. Biochem. 268:865-875. [DOI] [PubMed] [Google Scholar]
- 10.de Cock, H., and J. Tommassen. 1996. Lipopolysaccharides and divalent cations are involved in the formation of an assembly-competent intermediate of outer-membrane protein PhoE of E. coli. EMBO J. 15:5567-5573. [PMC free article] [PubMed] [Google Scholar]
- 11.De Las Penas, A., L. Connolly, and C. A. Gross. 1997. The σE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σE. Mol. Microbiol. 24:373-385. [DOI] [PubMed] [Google Scholar]
- 12.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 13.De Wulf, P., O. Kwon, and E. C. C. Lin. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 181:6772-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Wulf, P., A. M. McGuire, X. Liu, and E. C. C. Lin. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277:26652-26661. [DOI] [PubMed] [Google Scholar]
- 15.Dong, J., S. Iuchi, H. S. Kwan, Z. Lu, and E. C. C. Lin. 1993. The deduced amino-acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transduction system of Escherichia coli. Gene 136:227-230. [DOI] [PubMed] [Google Scholar]
- 16.Garbe, T. R., M. Kobayashi, and H. Yukawa. 2000. Indole-inducible proteins in bacteria suggest membrane and oxidant toxicity. Arch. Microbiol. 173:78-82. [DOI] [PubMed] [Google Scholar]
- 17.Georgopoulos, C. 1992. The emergence of the chaperone machines. Trends Biochem. Sci. 17:295-299. [DOI] [PubMed] [Google Scholar]
- 18.Hand, N. J., and T. J. Silhavy. 2000. A practical guide to the construction and use of lac fusions in Escherichia coli. Methods Enzymol. 326:11-35. [DOI] [PubMed] [Google Scholar]
- 19.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, C. H., P. N. Danese, J. S. Pinkner, T. J. Silhavy, and S. J. Hultgren. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 16:6394-6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langen, G. R., J. R. Harper, T. J. Silhavy, and S. P. Howard. 2001. Absence of the outer membrane phospholipase A suppresses the temperature-sensitive phenotype of Escherichia coli degP mutants and induces the Cpx and σE extracytoplasmic stress responses. J. Bacteriol. 183:5230-5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberek, K., and C. Georgopoulos. 1993. Autoregulation of the Escherichia coli heat shock response by the DnaK and DnaJ heat shock proteins. Proc. Natl. Acad. Sci. USA 90:11019-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Hackert, E., and A. M. Stock. 1997. Structural relationships in the OmpR family of winged-helix transcription factors. J. Mol. Biol. 269:301-312. [DOI] [PubMed] [Google Scholar]
- 24.Mecsas, J., P. E. Rouviere, J. W. Erickson, T. J. Donohue, and C. A. Gross. 1993. The activity of σE, an Escherichia coli heat-inducible sigma factor, is modulated by expression of outer membrane proteins. Genes Dev. 7:2618-2628. [DOI] [PubMed] [Google Scholar]
- 25.Mileykovskaya, E., and W. Dowhan. 1997. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J. Bacteriol. 179:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millman, J. S., H. Y. Qi, F. Vulcu, H. D. Bernstein, and D. W. Andrews. 2001. FtsY binds to the Escherichia coli inner membrane via interactions with phosphatidylethanolamine and membrane proteins. J. Biol. Chem. 276:25982-25989. [DOI] [PubMed] [Google Scholar]
- 27.Missiakas, D., M. P. Mayer, M. Lemaire, C. Georgopoulos, and S. Raina. 1997. Modulation of the Escherichia coli σE (RpoE) heat shock transcription factor activity by the RseA, RseB, and RseC proteins. Mol. Microbiol. 24:355-371. [DOI] [PubMed] [Google Scholar]
- 28.Nikaido, H. 1996. Outer membrane, p. 29-47. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, second ed., vol. 1. ASM Press, Washington D.C.
- 29.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 99:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pogliano, J., A. S. Lynch, D. Belin, E. C. Lin, and J. Beckwith. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11:1169-1182. [DOI] [PubMed] [Google Scholar]
- 30a.Raffa, R. G., and T. L. Raivio. 2002. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 45:1599-1611. [DOI] [PubMed] [Google Scholar]
- 31.Raivio, T. L., M. W. Laird, J. C. Joly, and T. J. Silhavy. 2000. Tethering of CpxP to the inner membrane prevents spheroplast induction of the cpx envelope stress response. Mol. Microbiol. 37:1186-1197. [DOI] [PubMed] [Google Scholar]
- 32.Raivio, T. L., D. L. Popkin, and T. J. Silhavy. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol. 181:5263-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raivio, T. L., and T. J. Silhavy. 1999. The σE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr. Opin. Microbiol. 2:159-165. [DOI] [PubMed] [Google Scholar]
- 34.Raivio, T. L., and T. J. Silhavy. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:7724-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo, F. D., J. M. Slauch, and T. J. Silhavy. 1993. Mutations that affect separate functions of OmpR the phosphorylated regulator of porin transcription in Escherichia coli. J. Mol. Biol. 231:261-273. [DOI] [PubMed] [Google Scholar]
- 36.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 38.Smith, G. A., and L. W. Enquist. 1999. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J. Virol. 73:6405-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder, W. B. 1995. The secretion related toxicities of LacZ. Ph D. dissertation. Princeton University, Princeton, N.J.
- 40.Snyder, W. B., L. J. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straus, D., W. Walter, and C. A. Gross. 1990. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of σ32. Genes Dev. 4:2202-2209. [DOI] [PubMed] [Google Scholar]
- 42.Tilly, K., N. McKittrick, M. Zylicz, and C. Georgopoulos. 1983. The DnaK protein modulates the heat shock response of Escherichia coli. Cell 34:641-646. [DOI] [PubMed] [Google Scholar]
- 43.Tilly, K., J. Spence, and C. Georgopoulos. 1989. Modulation of stability of the Escherichia coli heat shock regulatory factor σ32. J. Bacteriol. 171:1585-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber, R. F., and P. M. Silverman. 1988. The Cpx proteins of Escherichia coli K12: structure of the CpxA polypeptide as an inner membrane component. J. Mol. Biol. 203:467-478. [DOI] [PubMed] [Google Scholar]
- 45.Ye, X.-Y., M.-C. Lo, L. Brunner, D. Walker, D. Kahn, and S. Walker. 2001. Better substrates for bacterial transglycosylases. J. Am. Chem. Soc. 123:3155-3156. [DOI] [PubMed] [Google Scholar]