Abstract
The rates of germination of Bacillus subtilis spores with l-alanine were increased markedly, in particular at low l-alanine concentrations, by overexpression of the tricistronic gerA operon that encodes the spore's germinant receptor for l-alanine but not by overexpression of gerA operon homologs encoding receptors for other germinants. However, spores with elevated levels of the GerA proteins did not germinate more rapidly in a mixture of asparagine, glucose, fructose, and K+ (AGFK), a germinant combination that requires the participation of at least the germinant receptors encoded by the tricistronic gerB and gerK operons. Overexpression of the gerB or gerK operon or both the gerB and gerK operons also did not stimulate spore germination in AGFK. Overexpression of a mutant gerB operon, termed gerB*, that encodes a receptor allowing spore germination in response to either d-alanine or l-asparagine also caused faster spore germination with these germinants, again with the largest enhancement of spore germination rates at lower germinant concentrations. However, the magnitudes of the increases in the germination rates with d-alanine or l-asparagine in spores overexpressing gerB* were well below the increases in the spore's levels of the GerBA protein. Germination of gerB* spores with d-alanine or l-asparagine did not require participation of the products of the gerK operon, but germination with these agents was decreased markedly in spores also overexpressing gerA. These findings suggest that (i) increases in the levels of germinant receptors that respond to single germinants can increase spore germination rates significantly; (ii) there is some maximum rate of spore germination above which stimulation of GerA operon receptors alone will not further increase the rate of spore germination, as action of some protein other than the germinant receptors can become rate limiting; (iii) while previous work has shown that the wild-type GerB and GerK receptors interact in some fashion to cause spore germination in AGFK, there also appears to be an additional component required for AGFK-triggered spore germination; (iv) activation of the GerB receptor with d-alanine or l-asparagine can trigger spore germination independently of the GerK receptor; and (v) it is likely that the different germinant receptors interact directly and/or compete with each other for some additional component needed for initiation of spore germination. We also found that very high levels of overexpression of the gerA or gerK operon (but not the gerB or gerB* operon) in the forespore blocked sporulation shortly after the engulfment stage, although sporulation appeared normal with the lower levels of gerA or gerK overexpression that were used to generate spores for analysis of rates of germination.
Spores of various Bacillus species can remain dormant for long periods of time but can rapidly “return to life” through the process of spore germination (18, 19, 20, 28). Spore germination is normally triggered by the stereospecific binding of specific low-molecular-weight nutrients to receptors located in the spore's inner membrane (12, 27, 28). These receptors are comprised of three separate proteins, at least two of which appear to be integral membrane proteins (18, 19, 20, 28). In Bacillus subtilis the functional germinant receptors are encoded by three homologous tricistronic operons, gerA, gerB, and gerK (termed gerA operon homologs), in addition to two other homologous operons that are not expressed under normal laboratory sporulation conditions (18, 19, 20, 26, 28). There are multiple similar tricistronic operons in other Bacillus species, as well as in Clostridium species (5, 6, 13, 18, 26, 28). The individual gerA operon homologs encode a receptor for a particular germinant or group of germinants; where this operon has been studied, mutations in any cistron of a gerA operon homolog can destroy that receptor's function (19, 20, 28). In B. subtilis the gerA operon encodes a receptor for l-alanine, while the products of the gerB and gerK operons interact or collaborate in some fashion to provide the germination response to a mixture of asparagine, glucose, fructose, and potassium ions (AGFK). However, any of several specific mutations in the gerB operon allow spores to germinate in d-alanine alone (25). As yet, the structure of these various germinant receptors is not known, but there is evidence that the three proteins encoded by the various gerA operon homologs interact, at least with other proteins encoded by the same gerA operon homolog (19, 20, 26).
Binding of a germinant to its receptor triggers subsequent events in spore germination, including the release of the spore core's large depot (∼10% of the spore's dry weight) of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) and the release of other ions, including H+, Na+, K+, and Ca2+, with the concomitant uptake of some water (26, 28, 34). DPA release is followed by degradation of the spore's peptidoglycan cortex, and cortex hydrolysis is stimulated either indirectly or directly by DPA release (23, 28, 29, 32). While proteins that are likely candidates for DPA channels in the spore's membranes have been identified (35) (these are not the germinant receptors themselves [26]), there is as yet no understanding of how germinant-receptor interaction triggers the earliest events in spore germination, including the release of DPA and cations. Cortex hydrolysis is followed or accompanied by the swelling of the spore core via further water uptake, and this allows the initiation of metabolism (28, 32).
Some information that might bear on the questions of the precise role that the germinant receptors play in spore germination and how they play this role might be obtained if the concentration of specific germinant receptors in spores was increased by a large amount. Analysis of the rates of germination of spores carrying elevated levels of germinant receptors might, for example, then suggest whether these receptors act alone to trigger initial events in spore germination or require direct interaction with other proteins to do this. Such an analysis of spores overexpressing the GerA, GerB, or GerK receptor is the subject of this communication.
MATERIALS AND METHODS
Bacterial strains used.
All B. subtilis strains used are listed in Table 1 and are isogenic except for the differences noted. The parental strain is PS832, which was derived from strain 168. Transformations of Escherichia coli and B. subtilis were as described previously (2, 24, 25). E. coli strains were routinely grown in LB medium (31) supplemented with ampicillin (50 μg/ml). B. subtilis strains were grown in LB medium with antibiotics added as appropriate (100 μg of spectinomycin per ml for spectinomycin resistance [Spr]; 5 μg of erythromycin per ml plus 25 μg of lincomycin per ml for macrolide-lincosamide-streptogramin B resistance [MLSr]; and 5 μg of chloramphenicol per ml for chloramphenicol resistance [Cmr]).
TABLE 1.
B. subtilis strains used
| Strain | Genotype and/or phenotype | Reference, source, or constructiona |
|---|---|---|
| FB10 | gerB* | 25 |
| FB20 | ΔgerA::spc Spr | 26 |
| FB58 | PsspB::gerB Spr | 27 |
| FB60 | ΔgerB::spc Spr | 26 |
| FB68 | ΔgerK::ermC MLSr | 26 |
| PS832 | Wild-type | Laboratory stock |
| PS3415 | PsspB::gerB∗ Spr | pFE135A (26)→FB10 |
| PS3441 | PsspB::gerA MLSr | pPS3439→PS832 |
| PS3461 | PsspB::gerK MLSr | pPS3456→PS832 |
| PS3468 | sspE-lacZ Cmr | pPS918 (9)→PS832 |
| PS3469 | PsspB::gerB sspE-lacZ Cmr Spr | pPS918 (9)→FB58 |
| PS3471 | PsspB::gerB sspE-lacZ Cmr MLSr | pPS918 (9)→PS3441 |
| PS3472 | PsspB::gerK sspE-lacZ Cmr MLSr | pPS918 (9)→PS3461 |
| PS3476 | PsspD::gerA MLSr | pPS3473→PS832 |
| PS3477 | PsspD::gerB Spr | pPS3474→PS832 |
| PS3478 | PsspD::gerK MLSr | pPS3475→PS832 |
| PS3498 | gerB ΔgerK::ermC MLSr | FB68→FB8 |
| PS3499 | gerB∗ ΔgerK::ermC MLSr | FB68→FB10 |
| PS3501 | PsspD::gerA gerB∗ MLSr | PS3476→FB10 |
| PS3502 | PsspD::gerB∗ Spr | pPS3474→FB10 |
| PS3503 | PsspB::gerB∗ ΔgerK::ermC MLSr Spr | FB68→PS3415 |
| PS3521 | gerB∗ ΔgerA::spc Spr | FB20→FB10 |
| PS3556 | PsspB::gerB PsspD::gerK MLSr Spr | PS3431→PS3478 |
| PS3557 | PsspD::gerB PsspD::gerK MLSr Spr | PS3477→PS3478 |
Strains constructed by transformation of plasmid or chromosomal DNA from the strain to the left of the arrow into the strain to the right of the arrow.
Spore preparation.
Spores were routinely prepared at 37°C on 2×SG medium agar plates without antibiotics as described previously (21, 24); in one experiment sporulation was in 2×SG liquid medium. Spores were harvested, stored in a cold room, and cleaned by repeated rounds of centrifugation and washing with water over a period of 1 to 2 weeks (21). Cleaned spores were stored protected from light at ∼6°C in water. All spore preparations used in this work were free (>98%) of growing or sporulating cells or cell debris.
Construction of strains overexpressing the GerA homolog receptors.
The strain overexpressing the wild-type gerB operon under the control of the strong forespore-specific sspB promoter has been described previously (27). The plasmid used to place gerB under PsspB control, pFE135A (27), was also used to transform strain FB10 to Spr. B. subtilis strain FB10 carries a mutant gerB operon, termed gerB*, whose product allows spores to germinate in d-alanine alone (25), and plasmid pFE135A does not carry any gerB sequence that overlaps with the point mutation in the gerB* operon, as this mutation is in the gerBB cistron (25). An Spr transformant was termed PS3415, and its correct construction was confirmed by Southern blot analysis.
To place the gerA operon under PsspB control, a 300-bp region of gerAA (from bp 1 to 300 in the gerAA coding sequence) was amplified by PCR (all primer sequences are available on request); the primers also contained an NdeI site in the upstream primer and a PstI site in the downstream primer. The PCR product was ligated into plasmid pCR2.1 (Invitrogen) and transformed into E. coli to give plasmid pPS3435. The gerAA fragment was excised from pPS3435 by digestion with NdeI and PstI, ligated between the NdeI and PstI sites in plasmid pPS3396 (see below), and cloned in E. coli to give a plasmid, pPS3439, in which PsspB is just upstream of the 5′ end of gerAA. In this construct an additional ATG codon has been added at the 5′ end of gerAA to generate the NdeI site, as gerAA translation normally starts with TTG; the new translation-initiating ATG codon is positioned optimally downstream of the strong ribosome-binding site of sspB. This plasmid was used to transform strain PS832 to MLSr by a single-crossover event in gerAA, giving strain PS3441 in which the gerA operon is under the control of PsspB. The correct construction of this strain was confirmed by Southern blot analysis. Plasmid pPS3396 noted above was constructed by insertion of the ermC gene from plasmid pFE140 (26) on a 1.2-kb KpnI-XbaI fragment between the XbaI and KpnI sites of plasmid pFE133 (27), which carries the PsspB promoter in a pUC19 derivative.
The gerK operon was placed under the control of PsspB by first amplifying the region from bp 1 to 378 of the gerKA coding sequence with an NdeI site on the upstream primer and a PstI site on the downstream primer. The PCR fragment was cloned in E. coli as described above, giving plasmid pPS3454, and the gerKA fragment was excised with NdeI and PstI and cloned between the NdeI and PstI sites of plasmid pPS3396, giving a plasmid, pPS3456, in which gerKA is just downstream of PsspB and is preceded by the strong sspB ribosome-binding site. Plasmid pPS3456 was used to transform PS832 to MLSr, giving strain PS3461, and the correct construction of this strain was verified by Southern blot analysis.
The plasmid to place gerA operon homologs under the control of the promoter of the sspD gene was constructed as follows. A region encompassing 289 bp upstream of sspD (bp −4 to −292 relative to the first nucleotide of the translational start codon [7, 22]) was amplified by PCR with the primers also providing HindIII and NdeI sites at the farthest upstream and less upstream ends, respectively. This fragment encompasses the sspD promoter and at the end adjacent to the sspD coding sequence contains the sspD ribosome-binding site. The primer-provided NdeI site is appropriately spaced relative to this ribosome-binding site such that translation of coding sequences beginning at the ATG in this NdeI site will be efficient. The PCR product was cloned in E. coli as described above, giving plasmid pPS3467. For placement of the gerA operon under PsspD control, the PsspD-containing fragment from pPS3467 was excised as a HindIII-NdeI fragment. This fragment was ligated with plasmid pPS3439 (described above) that had been digested with HindIII and NdeI to remove the PsspB promoter fragment, giving plasmid pPS3473. This plasmid was used to transform B. subtilis strain PS832 to MLSr, giving strain PS3476 in which gerA expression is under the control of PsspD; the correct genomic structure of this strain was confirmed by Southern blot analysis. For placement of gerK under PsspD control, the HindIII-NdeI fragment from plasmid pPS3467 was used to replace the small HindIII-NdeI fragment of plasmid pPS3456, giving pPS3475, and this plasmid was used to transform B. subtilis strain PS832 to MLSr, giving strain PS3478. The correct construction of this strain was confirmed by Southern blot analysis. For placement of gerB under PsspD control, the ∼320-bp XhoI-NdeI fragment from plasmid pPS3467 that contains the sspD promoter and ribosome-binding site was used to replace the XhoI-NdeI fragment containing the sspB promoter in plasmid pFE135A (27), giving plasmid pPS3474. This plasmid was used to transform B. subtilis strain PS832 to Spr, and the correct construction of this strain was again confirmed by Southern blot analysis.
Spore germination.
Prior to spore germination, spores at 2 to 3 mg (dry weight) per ml in water were heat shocked (30 min, 70°C) and then cooled in ice. Spores were routinely germinated at 37°C and optical densities at 600 nm (OD600s) of 0.8 to 1 in 50 mM KPO4 (pH 7.4) with l-alanine or AGFK and in 25 mM Tris-HCl (pH 8.2) with d-alanine (with or without 10 mM d-glucose) or l-asparagine (25, 26). Control incubations with heat-shocked spores incubated at 37°C in the appropriate buffer alone were included for each germination experiment. Spore germination was usually monitored by measurement of the OD600 of the culture (30) over a period of 4 h in a 1-ml cuvette in a Spectronic Genesys 5 spectrophotometer (Milton Roy, Rochester, N.Y.). The maximum germination rate of spores germinating with any given nutrient concentration was calculated from the maximum rate in the fall of the OD600 versus time as determined graphically from the maximum slope of the plot of OD600 values versus time. All values reported are the averages of at least duplicate determinations, generally from two independent spore preparations, and individual values varied by less than 20% from values shown. In all experiments, the maximum rate of germination of spores incubated in buffer alone was only 0.5 to 1% of the maximum rate found with the highest level of various germinants tested (data not shown). Some of the fall in the OD600s of germinating cultures (≤30% monitored by the spectrophotometer noted above [B. Setlow and P. Setlow, unpublished data]) requires the hydrolysis of the spore cortex (14), and this is not the earliest event in the spore germination process (28, 29, 32). The earliest easily measured event in spore germination is the release of various ions, prominent among these being the spore's large depot of the 1:1 chelate of Ca2+-DPA, and DPA release is accompanied by the loss of ∼70% of the total amount of the OD600 that is lost upon spore germination (24, 29, 32; Setlow and Setlow, unpublished). Consequently, measurement of the OD600s of germinating spores, in particular, the maximum rate of the fall of the OD600 at a given germinant concentration, is a simple and reliable method for quantitating and comparing rates of spore germination. However, in some experiments we also monitored spore germination by measuring DPA release. Aliquots of germinating cultures (1 ml) were centrifuged (1 min in a microcentrifuge), and the DPA released into the supernatant fluid was measured by its absorption at 270 nm. In one experiment the spontaneous germination of spores in the absence of germinants was determined by dialysis of spores (OD = 1) at 37°C in the germination buffers; at various times, 150 spores were examined in a phase-contrast microscope to determine the percentage of spores that had germinated. Spores were incubated in dialysis tubing to minimize the possibility that small molecules released from a few spores that germinated would trigger the germination of other spores in the incubation.
Analytical methods.
β-Galactosidase from strains carrying an sspE-lacZ fusion was assayed after cells were made permeable by lysozyme treatment as described previously (17). All β-galactosidase specific activities are expressed in Miller units as described previously (17). Membranes were isolated from dormant spores, GerB and GerA were detected, and their relative levels in spore membranes were determined by Western blot analysis as described previously (27).
RESULTS
Overexpression of germinant receptors and levels of overexpression.
Previous work has shown that the GerB receptor can be overexpressed ∼500-fold in spores when the gerB operon is under the control of the strong forespore-specific sspB promoter (17, 22, 27). This strategy was also applied to the gerB*-encoded receptor, as well as the receptors encoded by the gerA and gerK operons. While the strain overexpressing the gerB* receptor sporulated well and overexpressed GerB* (see below), <0.1% of cells of strains with the gerA and gerK operons under PsspB control completed sporulation (data not shown). Microscopic examination of the gerA and gerK operon strains indicated that the block in their sporulation was after the engulfment stage (data not shown, but we have not studied the precise nature of the sporulation block further). In order to demonstrate the block in sporulation conclusively, we examined the expression of β-galactosidase from an sspE-lacZ fusion, as sspE is expressed in parallel with sspB, as well as the gerA operon and its homologs, in the developing forespore (8, 10, 17, 22). Expression of sspE-lacZ was initially normal in strains expressing gerA and gerK under PsspB control (Fig. 1), indicating that there is no defect in the sporulation of these strains up to and including the time that the synthesis of GerA and GerK begins. However, after reaching levels ∼70% of those in strains carrying gerB or gerB* under PsspB control or with no overexpressed gerA operon homolog, levels of β-galactosidase in strains with gerA or gerK under PsspB control fell rapidly (Fig. 1). In addition, at this time the OD600 of the last-named cultures leveled off and microscopic analyses indicated that the developing forespores were lysing (Fig. 1 and data not shown). In contrast, sspE-lacZ expression and the OD600 continued to increase in cultures of strains containing only sspE-lacZ with or without PsspB-gerB or PsspB-gerB* (Fig. 1 and data not shown). Thus, extremely high levels of GerA or GerK receptors do not allow completion of sporulation, in contrast to the situation with either the GerB or GerB* receptor. Note that levels of β-galactosidase also fell in the strain carrying gerB under PsspB control (Fig. 1), but this was due to the acquisition of lysozyme resistance by the developing forespore (data not shown), as was seen previously (17).
FIG. 1.
OD600 values and levels of β-galactosidase from sspE-lacZ in sporulating cultures of various B. subtilis strains. B. subtilis strains were grown and sporulated in liquid 2×SG medium without antibiotics, the OD600s were measured, and β-galactosidase was extracted and assayed as described in Materials and Methods. Open symbols indicate OD600 values and filled symbols indicate β-galactosidase specific activities for strain PS3471 (PsspB::gerA sspE-lacZ) (triangles) and strain PS3469 (PsspB::gerB sspE-lacZ) (circles). The curves for strains carrying only sspE-lacZ (PS3468) or PsspB::gerK sspE-lacZ (PS3472) were essentially identical (within 20%) to those for the strain with PsspB::gerB sspE-lacZ or PsspB::gerA sspE-lacZ, respectively (data not shown). Values for the strain carrying PsspB::gerB* sspE-lacZ (PS3415) were essentially identical to those for strains PS3468 and PS3469 (data not shown).
Since expression of gerA and gerK under the control of the strong sspB promoter was not compatible with sporulation, we turned to the use of the sspD promoter (22). This promoter is also forespore specific but is only ∼10% as strong as the sspB promoter (4, 17, 22). Strains with the sspD promoter driving expression of the gerA, gerB, gerB*, or gerK operon sporulated normally (data not shown). We were unable to assess the level of overexpression of the gerA and gerK operons with the sspD promoter due to a lack of antisera against the encoded proteins. However, Western blot analyses of proteins from spore inner membranes showed that the sspB promoter led to an ∼200-fold overexpression of GerBA from both the gerB and gerB* operons, similar to what was seen previously (27), but that the sspD promoter led to an ∼20-fold overexpression (Fig. 2 and data not shown). Previous work has also shown that at least some of the GerB receptor overexpressed from PsspB is still functional (27). Note that the overexpressed GerBA in spores was not in inclusion bodies (data not shown), as was also found previously (27). We also found that deletion of the gerA or gerK operon had no effect on levels of GerBA in spores, whether GerBA was overexpressed or expressed only at the low wild-type level (data not shown).
FIG. 2.
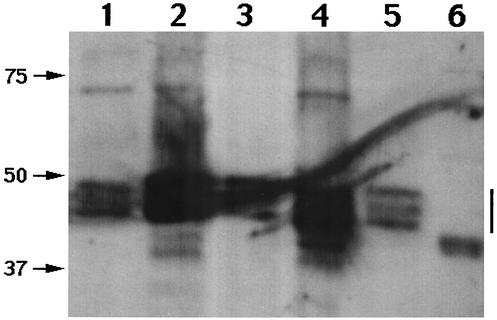
Level of GerBA in spores of various B. subtilis strains. The inner membranes from spores of various strains were isolated, aliquots of membrane protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and GerBA was detected by Western blot analysis as described in Materials and Methods. The amounts and the identities of the membrane samples in the various lanes are 1 mg of wild-type spores (lane 1), 100 μg of PsspB::gerB* spores (strain PS3415) (lane 2), 10 μg of PsspB::gerB* spores (strain PS3415) (lane 3), 1 mg of PsspD::gerB* spores (strain PS3502) (lane 4), 40 μg of PsspD::gerB* spores (strain PS3502) (lane 5), and 1 mg of ΔgerB spores (strain FB60) (lane 6). The numbers and arrows to the left of the figure show the migration positions of molecular mass markers in kilodaltons, and the vertical bar on the right shows the position of the GerBA bands. Note that GerBA gives multiple bands, as seen previously (27).
Effect of elevated levels of the GerA or GerK receptor on spore germination.
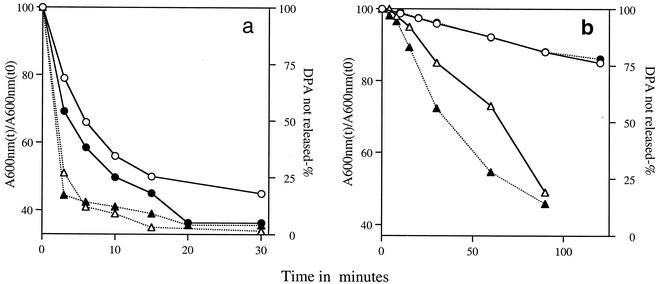
With spores overexpressing the various germinant receptors in hand, we then examined the effects of high levels of germinant receptors on spore germination. Notably, the germination of spores overexpressing GerA was significantly faster in l-alanine than was germination of wild-type spores, particularly at low l-alanine concentrations (Table 2; Fig. 3). This faster germination was seen when spore germination was measured either by the fall in OD600 or by the release of DPA and was particularly obvious when spore germination was carried out at ∼7°C (Fig. 3b). Note that DPA release precedes the full loss in OD600 of germinating cultures, since only ∼70% of the total decrease in OD600 of germinating spores accompanies DPA release (14, 29, 32).
TABLE 2.
Maximum germination rates of spores of various strains as a function of the l-alanine concentrationa
| Spore genotype (strain) | Germination rate (% maximum loss in OD600/h) at indicated l-alanine concn
|
||||||
|---|---|---|---|---|---|---|---|
| 1 μM | 5 μM | 10 μM | 33 μM | 100 μM | 330 μM | 1 mM | |
| Wild-type (PS832) | 1 | 14 | 48 | 210 | 276 | 302 | 307 |
| PsspD::gerA (PS3476) | 15 | 87 | 286 | 575 | 630 | 680 | 750 |
| PsspD::gerB (PS3477) | 51 | 218 | 293 | 310 | 300 | ||
| PsspB::gerB (FB58) | 40 | 210 | 240 | 270 | 275 | ||
| PsspD::gerK (PS3478) | 55 | 192 | 255 | 270 | 275 | ||
Heat-shocked spores of various B. subtilis strains were germinated at 37°C in 50 mM KPO4 (pH 7.4) and various concentrations of l-alanine, the OD600s were measured, and maximum germination rates were calculated as described in Materials and Methods. Use of concentrations of l-alanine up to 10 mM gave no increase in the maximum germination rate over that observed with 1 mM l-alanine.
FIG. 3.
Rate of loss in OD600 values and DPA release during germination of spores with or without elevated levels of the GerA receptor. Spores of strain PS832 (wild type) (○, •) or PS3476 (PsspD::gerA) (▵, ▴) were germinated in 50 mM KPO4 (pH 7.4) and 1 mM l-alanine at either 37°C (a) or 7°C (b), and the OD600 values (○, ▵) and the DPA released (•, ▴) were measured as described in Materials and Methods. Note that not all of the data points taken in the experiment whose results are indicated in panel a are shown. t, time; t0, time zero.
One obvious explanation for the more rapid germination of spores overexpressing the GerA receptor, in particular at low l-alanine concentrations, is that these spores spontaneously germinate much more readily than wild-type spores. However, this was not the case, as <1% of spores of the strain overexpressing GerA exhibited germination when they were incubated for 24 h at 37°C in dialysis tubing in either 50 mM KPO4 (pH 7.4) or 25 mM Tris-HCl (pH 8.2) as described in Materials and Methods; similar results were obtained with spores overexpressing the gerB or gerB* operon from PsspB or overexpressing gerK from PsspD (data not shown).
In contrast to the effects of elevated GerA levels on l-alanine germination, high levels of GerA actually inhibited spore germination in AGFK approximately twofold (Table 3). Spore germination in AGFK requires both the GerB and GerK receptors but not the GerA receptor (18, 19, 20, 28). However, spores in which either GerB or GerK had been overexpressed germinated as did wild-type spores in both l-alanine and AGFK (Tables 2 and 3). Similarly, spores in which GerB and GerK had been overexpressed together (strains PS3556 and PS3557) also exhibited no increase in the rate of AGFK germination when spore germination was measured by either the loss in OD600 or the release of DPA (data not shown). Spores of strains PS3556 and PS3557 also germinated like wild-type spores in l-alanine (data not shown).
TABLE 3.
Maximum germination rates of spores of various strains as a function of the concentration of AGFKa
| Spore genotype (strain) | Germination rate (% maximum loss in OD600/h) at indicated AGFK concnb
|
||||
|---|---|---|---|---|---|
| 1n | 3n | 9n | 27n | 81n | |
| Wild-type (PS832) | 3 | 16 | 90 | 162 | 205 |
| PsspD::gerA (PS3476) | 3 | 7 | 45 | 74 | 105 |
| PsspD::gerB (PS3477) | 3 | 12 | 105 | 180 | 232 |
| PsspB::gerB (FB58) | 2 | 14 | 89 | 155 | 210 |
| PsspD::gerK (PS3478) | 3 | 12 | 78 | 160 | 200 |
Spores of various B. subtilis strains were germinated at 37°C in 50 mM KPO4 (pH 7.4) with various concentrations of asparagine, glucose, and fructose (note that K+ levels were constant), the OD600s were measured, and the maximum germination rates were determined as described in Materials and Methods.
The concentrations of asparagine, glucose, and fructose represented by n are 31 μM, 6 μg/ml, and 6 μg/ml, respectively.
Effect of elevated levels of the GerB or GerB* receptors on spore germination.
As was found with spores with elevated levels of the GerK receptor, spores with elevated levels of the GerB receptor driven by either the sspD or sspB promoter germinated like wild-type spores in both l-alanine and AGFK (Tables 2 and 3). The lack of effect of elevated levels of the GerK or GerB receptor on spore germination, in particular with AGFK, is undoubtedly because both the GerB and GerK receptors (and probably at least one other component [see Discussion]) are needed for germination with AGFK (18, 19, 20).
In order to assess the effects of elevated levels of another single germinant receptor on spore germination, we turned to the GerB* receptor, which is the result of a mutation in the gerB operon that allows this receptor to respond to d-alanine alone (25). Strikingly, spores with higher levels of the GerB* receptor germinated more rapidly in d-alanine than did spores with the wild-type levels of GerB* (Fig. 2; Table 4). Previous work has shown that d-glucose markedly stimulates the germination of spores with the GerB* receptor (25), and germination with d-alanine plus d-glucose was also increased in spores with increased levels of GerB* (Fig. 2; Table 4). The gerB* mutation also allows spore germination in l-asparagine alone (25), and again germination in l-asparagine was more rapid in spores with elevated levels of GerB* than in spores with normal levels (Table 5). The elevated levels of the GerB* receptor caused the greatest increases in rates of spore germination with the lower concentrations of l-asparagine or with the lower concentrations of d-alanine with or without glucose (Tables 4 and 5), similar to what was found with l-alanine germination of spores overexpressing the gerA operon (Table 2).
TABLE 4.
Maximum germination rates of spores of various gerB∗ strains with different concentrations of d-alanine with or without d-glucosea
| Spore genotype (strain) | Germination rate (% maximum loss in OD600/h) at indicated d-alanine concnb
|
||||
|---|---|---|---|---|---|
| 123 μM | 370 μM | 1.1 mM | 3.3 mM | 10 mM | |
| gerB* (FB10) | 1 (11) | 2 (19) | 6 (36) | 15 (60) | 19 (100) |
| PsspD::gerB* (PS3502) | 1 (18) | 7 (48) | 28 (76) | 38 (108) | 51 (139) |
| PsspB::gerB* (PS3415) | 3 (60) | 14 (84) | 47 (120) | 64 (164) | 95 (209) |
Spores of various B. subtilis strains were germinated at 37°C in 25 mM Tris-HCl (pH 8.2) and various concentrations of d-alanine with or without 10 mM d-glucose, the OD600s were measured, and the maximum germination rates were determined as described in Materials and Methods.
Values in parentheses are with 10 mM d-glucose.
TABLE 5.
Maximum germination rates of spores of various gerB* strains at different concentrations of l-asparaginea
| Spore genotype (strain) | Germination rate (% maximum loss in OD600/h) at indicated l-asparagine concn
|
||||
|---|---|---|---|---|---|
| 33 μM | 100 μM | 330 μM | 1 mM | 3 mM | |
| gerB* (FB10) | 3 | 12 | 35 | 78 | 86 |
| PsspD::gerB* (PS3502) | 5 | 21 | 57 | 116 | 130 |
| PsspB::gerB* (PS3415) | 18 | 68 | 125 | 240 | 259 |
Spores of various B. subtilis strains were germinated at 37°C in 25 mM Tris-HCl (pH 8.2) and various concentrations of l-asparagine, the OD600s were measured, and maximum germination rates were determined as described in Materials and Methods. Wild-type spores (strain PS832) do not germinate in l-asparagine alone.
Previous work has indicated that the GerB and GerK receptors can cooperate in some fashion, since germination in AGFK is abolished by mutations in either gerB or gerK (18, 19, 20). Thus, it was of interest to analyze the effect of a gerK mutation on the germination of spores with the gerB* mutation in d-alanine. Loss of the gerK operon did not abolish spore germination in l-asparagine or d-alanine (with or without glucose) but did slightly decrease spore germination rates in these germinants compared to the germination rates of spores carrying either normal or elevated levels of GerB* (Tables 6 and 7). We also found that overexpression of gerA significantly decreased (up to fivefold) the germination rate of gerB* spores with either l-asparagine or d-alanine (with or without glucose) (Tables 6 and 7). In contrast, previous work (24) has shown that the deletion of gerA has no effect on the germination of gerB* spores, and this finding was confirmed in the present work (data not shown).
TABLE 6.
Effect of deletion of gerK or overexpression of gerA on spore germination due to the GerB* receptora
| Spore genotype (strain) | Germination rate (% maximum loss in OD600/h) at the indicated l-asparagine concn
|
||
|---|---|---|---|
| 330 μM | 1 mM | 3 mM | |
| gerB* (FB10) | 30 | 57 | 78 |
| gerB* ΔgerK (PS3498) | 19 | 42 | 52 |
| PsspB::gerB* (PS3415) | 145 | 270 | 294 |
| PsspB::gerK* ΔgerK (PS3503) | 67 | 153 | 205 |
| PsspD::gerA gerB* (PS3501) | 19 | ||
Spores of various B. subtilis strains were germinated at 37°C in 25 mM Tris-HCl (pH 8.2) and various concentrations of l-asparagine, and maximum germination rates were determined as described in Materials and Methods.
TABLE 7.
Effect of deletion of gerK or overexpression of gerA on spore germination due to the GerB* receptora
| Spore genotype (strain) | Germination rate (% maximum loss in OD600/h) at indicated d-alanine concnb
|
||
|---|---|---|---|
| 400 μM | 2 mM | 10 mM | |
| gerB* (FB10) | 1 (17) | 6 (64) | 15 (126) |
| gerB* ΔgerK (PS3498) | <1 (12) | 4 (44) | 9 (84) |
| PsspB::gerB* (PS3415) | 17 (103) | 44 (240) | 78 (300) |
| PsspB::gerB* ΔgerK (PS3503) | 17 (52) | 43 (100) | 84 (165) |
| PsspD::gerA gerB* (PS3501) | 9 (27) | ||
Spores of various B. subtilis strains were germinated at 37°C in 25 mM Tris-HCl (pH 8.2) and various concentrations of d-alanine (with or without 10 mM d-glucose), and maximum germination rates were determined as described in Materials and Methods.
Values in parentheses are with 10 mM d-glucose.
DISCUSSION
In this work we set out to assess the effects of increasing levels of nutrient germinant receptors on rates of spore germination. Previous work has shown that the GerBA protein is present in the spore's inner membrane at a level of ∼25 molecules per spore and that the GerBA level was increased ∼500-fold (27). We presume that the other two proteins encoded by the gerB operon, GerBB and GerBC, are also overexpressed and assembled properly in the spore's inner membrane, as at least some of the overexpressed GerB receptor is functional and there is evidence that at least the GerBA and GerBB proteins physically interact (27). In the present work, we found that putting either gerB or gerB* under the control of PsspB led to an ∼200-fold overexpression of GerBA, while our use of the sspD promoter to drive the expression of gerB or gerB* led to an ∼20-fold overexpression of GerBA. This last number is reasonable, since levels of SspD in spores (and hence presumably sspD expression) are 10 to 15% of those of SspB (4).
The GerA receptor proteins GerAA and GerAC are also in the spore's inner membrane (12), and it is presumed that GerAB is there as well. Levels of gerA expression are ∼15-fold higher than levels of gerB expression based on measurements of the expression levels of similar transcriptional lacZ proteins fused to both genes (8, 10), although there is no knowledge of the level of gerK expression. In contrast to our knowledge of the levels of GerBA in spores of strains overexpressing gerB or gerB*, we have no data on the levels of the GerA or GerK receptor in spores of strains overexpressing gerA or gerK. However, since sporulation was aborted due to forespore lysis with strains expressing gerA or gerK from PsspB and spores from the strain expressing gerA from PsspD germinate more rapidly in l-alanine than do wild-type spores, it seems most likely that the gerA and gerK operons are significantly overexpressed from either PsspD or PsspB.
The reason for the sporulation defect of strains overexpressing gerA or gerK from PsspB is not clear. These strains appear to initiate sporulation normally and progress through forespore engulfment. However, midway through the period when PsspB is active, further development stops and the engulfed forespores lyse within the sporulating cells. The reason for this lysis is also not clear. One possibility is that it is the incorporation of high levels of these membrane proteins into the forespores' inner membranes alone that causes forespore lysis. This is certainly possible, although high levels of some (albeit not all) functional bacterial membrane proteins have been overexpressed in bacteria with no obvious deleterious effects (3, 15, 36). A second possibility is that high levels of the GerA or GerK receptor in the developing forespore somehow trigger premature forespore germination that in turn leads to forespore lysis, much as does the lack of accumulation of DPA by the developing forespore (24). Although expression of gerB or gerB* from PsspB does not cause these effects, perhaps the absolute levels of GerA or GerK accumulated under PsspB control are much higher than levels of GerB accumulated under the control of this promoter. Alternatively, perhaps the GerA and GerK receptors much more readily trigger spore germination either spontaneously or in response to small molecules within the sporulating cell than does the GerB receptor. Indeed, spores containing only the GerA or the GerK receptor germinate quite well in a complex mixture of nutrients, while germination of spores containing only the GerB receptor is reduced up to 100-fold (26). It is also worth noting that expression from PsspB of the two additional B. subtilis gerA operon homologs, yndDEF and yfkQRT, which encode receptors for which no germinant has yet been identified (26), also did not cause any sporulation defect (E. Opoku-Serebuoh, M. Paidhungat, and P. Setlow, unpublished data).
The increases in the germination rates with l-alanine of spores overexpressing gerA or in the germination rate with l-asparagine or with d-alanine (with or without glucose) of spores overexpressing gerB* are further evidence for the crucial role the proteins encoded by these operons play in spore germination and are consistent with their role as receptors whose ligand binding somehow triggers further steps in the spore germination process. It seems very unlikely that the effects on germination rates of spores of strains overexpressing the various GerA homolog receptors are due to general alterations in spore properties as a result of alterations in levels of gene expression during sporulation, since no effects were seen with spores overexpressing GerB. In addition, the effects on spore germination rates were specific to germination with the germinants expected. It is notable that the degree of stimulation in spore germination at concentrations of germinants giving the maximum response was not great: ∼2.5-fold for spores overexpressing gerA and germinating with l-alanine and 2-, 3-, or 5-fold for spores overexpressing gerB* and germinating with d-alanine plus glucose, l-asparagine, or d-alanine, respectively. These increases at maximum germinant concentrations for spores overexpressing gerB* are far below the increases in the levels of GerBA in the inner membranes of spores with gerB* under PsspD or PsspB control. One obvious explanation is of course that much of the overexpressed GerB receptor is not functional, and we cannot eliminate this possibility but reiterate that overexpressed GerBA is localized properly in the spore and that at least some of the overexpressed GerB receptor is functional (27). A second explanation is that much, if not all, of the overexpressed GerA or GerB* receptor is functional but that as germinant receptor concentrations rise, something other than the concentration of these receptors becomes rate limiting for the initial events in spore germination. That this conclusion may indeed be at least partially correct is suggested by analysis of spore germination at germinant concentrations well below those needed to give maximum germination rates. At these lower concentrations, overexpression of either gerA or gerB* gives significantly larger (6- to 15-fold) increases in rates of spore germination. This is the behavior that is expected if initiation of spore germination requires that only a certain number of receptors be occupied by their germinant ligand. At germinant concentrations well below receptor saturation, the absolute number of receptor-germinant complexes should increase as the total number of receptors in the inner membrane increases, assuming that the affinity of the receptors for their ligands is the same for all receptor molecules.
Despite the increases in germination rates of spores overexpressing the GerA or GerB* receptors, the degree of stimulation of spore germination by overexpression of GerB* in particular was well below the increases in levels of GerBA. One explanation for this anomaly is that much (although not all!) of the overexpressed GerB* and other GerA operon homologs are nonfunctional. The covalent addition of diacylglycerol near the N termini of the C proteins of at least the GerA and GerB receptors appears necessary for the function of these receptors (18, 19, 20; M. Paidhungat, B. Setlow, and P. Setlow, unpublished data). Diacylglycerol addition is catalyzed by the lgt (also termed gerF) gene product, and perhaps sufficient Lgt to covalently modify a high percentage of overexpressed GerA homolog receptors is lacking in the developing forespore. If this is the case, it would explain the decreases in the germination rates of gerB* spores in d-alanine or l-asparagine upon overexpression of gerA and the decreases in AGFK germination in otherwise wild-type spores overexpressing gerA. However, if Lgt is limiting for assembly of high levels of functional GerA receptor homologs, then overexpression of either gerB, gerB*, or gerK would also have been expected to significantly reduce spore germination in l-alanine by decreasing the amount of functional GerAC, and this was clearly not the case.
The small increases in the spore germination rates at maximal concentrations of germinants relative to the apparent increases in the levels of overexpressed GerA and GerB* receptors may be because some or even most of the overexpressed receptors are nonfunctional. However, it seems equally likely that as the concentration of GerA homolog receptors is increased, some other component of the initiating event in spore germination becomes rate limiting. Unfortunately, this other component and the initial step triggered or carried out by the germinant-receptor complex are not known. One early event in spore germination is DPA release, a process that will likely require proteins in the inner spore membrane (35), and prior to DPA release there is also the release of monovalent ions, including H+, K+, and Na+ (34). One monovalent ion antiporter has been identified as a protein involved in the germination of B. cereus spores in some fashion (33), but the role of this protein in B. subtilis spore germination has not yet been established. Somehow the various GerA homolog receptors must interact with one or more of these additional inner membrane proteins, with the germinant binding to a GerA homolog receptor triggering the germination cascade. Complicating our understanding of protein-protein interactions in the inner spore membrane is the likely extremely slow movement of proteins in this membrane, as has recently been shown for lipids (A. E. Cowan, D. E. Koppel, B. Setlow, E. Melly, and P. Setlow, unpublished data).
In addition to the evidence for some sort of interaction between different GerA homolog receptors in B. subtilis spores, there is also evidence with spores of other Bacillus species that different GerA homolog receptors must interact, in particular to facilitate spore germination in mixtures of germinants (5, 13). However, precisely what is involved in the interaction between GerA homolog receptors is not clear. For example, do GerA homolog receptors interact to form heterooligomers or homooligomers? Clearly, the GerB receptor can function on its own, when it has acquired the gerB* mutation. However, the decrease in GerB* function in strains carrying a gerK mutation suggests that some type of GerB*-GerK complex may be more efficient in stimulating spore germination with d-alanine or l-asparagine than is the GerB* receptor alone. That there may be some type of interaction between GerA homolog receptors is also consistent with the decrease in the rates of germination with d-alanine or l-asparagine of spores carrying the gerB* mutation and also overexpressing gerA. Perhaps the high level of the GerA receptor in these spores binds the GerB* receptor in some fashion, decreasing the ability of the complexed GerB* to trigger spore germination with d-alanine or l-asparagine. Increased levels of the GerA receptor also decreased AGFK germination of spores with the wild-type GerB receptor, again possibly through the binding of the GerB or GerK receptor by the GerA receptor, thus decreasing the amount of the GerB-GerK receptor complex available for spore germination with AGFK. While the precise interaction of these various GerA homolog receptors is certainly unclear, it seems reasonable to call upon such possible interactions to explain both our and previous data. Indeed, recent work has shown that another type of membrane-localized receptor in bacteria can also form complexes and/or act collaboratively (1, 11, 16). Perhaps the GerA homolog receptors are another example of this phenomenon.
A final point concerns the somewhat surprising result that spores overexpressing both gerB and gerK exhibited no increase in their germination with AGFK. One trivial explanation is of course that functional GerK is actually not significantly overexpressed in our strains, and we currently have no way of ruling out this possibility, although as noted above, this seems unlikely. A second possibility is that none of the overexpressed GerK receptor is functional; again, we cannot rule this out, but as noted above, this is not the case for the overexpressed GerA, GerB, and GerB* receptors. A third possibility is that the rate of spore germination with AGFK is already maximal in wild-type spores and thus that overexpression of GerB and/or GerK cannot increase this rate. While this is certainly possible, spores can germinate faster than the maximum rate seen with AGFK (e.g., with l-alanine [Table 2]). In addition, spores overexpressing the GerB and/or the GerK receptor also exhibited no increase in germination at submaximum AGFK concentrations. Consequently, we favor a fourth possibility, that some component in addition to the GerK and GerB receptors is essential for the triggering of spore germination by AGFK. This idea is consistent with the lack of increase in AGFK germination of spores overexpressing either GerB or GerK, since if either of these receptors is present in an amount smaller than that of the other and only GerB and GerK are needed for germination with AGFK, then overexpression of the normally low-level receptor should have increased the rate of spore germination with this mixture of germinants. The identity of this other component of the AGFK germination pathway is currently unclear, although one possibility is the gerD gene product that appears to be needed primarily for germination in AGFK (20, 28). Clearly, there is still much to learn about the early steps in the germination of spores of Bacillus species.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM 19698 to P.S.).
REFERENCES
- 1.Ames, P., C. A. Studdert, R. H. Reiser, and J. S. Parkinson. 2002. Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA 99:7060-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:74-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auer, M., M. J. Kim, A. Villa, J. Song, X. D. Wang, and D. N. Wang. 2001. High-yield expression and functional analysis of Escherichia coli glycerol-3-phosphate transporter. Biochemistry 40:6628-6635. [DOI] [PubMed] [Google Scholar]
- 4.Bagyan, I., B. Setlow, and P. Setlow. 1998. New small, acid-soluble proteins unique to spores of Bacillus subtilis: identification of the coding genes and studies of the regulation and function of two of these genes. J. Bacteriol. 180:6704-6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlass, P. J., C. W. Houston, M. O. Clements, and A. Moir. 2002. Germination of Bacillus cereus spores in response to l-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148:2089-2095. [DOI] [PubMed] [Google Scholar]
- 6.Clements, M. O., and A. Moir. 1998. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 180:6729-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connors, M. J., J. M. Mason, and P. Setlow. 1986. Cloning and nucleotide sequence of genes for three small, acid-soluble, proteins of Bacillus subtilis spores. J. Bacteriol. 166:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corfe, B. M., A. Moir, D. Popham, and P. Setlow. 1994. Analysis of the expression and regulation of the gerB spore germination operon of Bacillus subtilis 168. Microbiology 140:3079-3083. [DOI] [PubMed] [Google Scholar]
- 9.Fajardo-Cavazos, P., F. Tovar-Rojo, and P. Setlow. 1991. Effect of promoter mutations and upstream and downstream deletions of genes coding for small, acid-soluble spore proteins of Bacillus subtilis. J. Bacteriol. 173:2011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feavers, I. M., J. Foulkes, B. Setlow, D. Sun, W. Nicholson, P. Setlow, and A. Moir. 1990. The regulation of transcription of the gerA spore germination operon of Bacillus subtilis. Mol. Microbiol. 4:275-282. [DOI] [PubMed] [Google Scholar]
- 11.Gestwicki, J. E., and L. L. Kiessling. 2002. Inter-receptor communication through arrays of bacterial chemoreceptors. Nature 415:81-84. [DOI] [PubMed] [Google Scholar]
- 12.Hudson, K. D., B. M. Corfe, E. H. Kemp, I. M. Feavers, P. J. Coote, and A. Moir. 2001. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J. Bacteriol. 183:4317-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ireland, J. A., and P. C. Hanna. 2002. Amino acid- and purine ribonucleotide-induced germination of Bacillus anthracis ΔSterne endospores: gerS mediates responses to aromatic ring structures. J. Bacteriol. 184:1296-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa, S., K. Yamane, and J. Sekiguchi. 1998. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J. Bacteriol. 180:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruse, D., R. Kramer, L. Eggeling, M. Rieping, W. Pfefferle, J. H. Tchieu, Y. J. Chung, M. H. Saier, Jr., and A. Burkovski. 2002. Influence of threonine exporters on threonine production in Escherichia coli. Appl. Microbiol. Biotechnol. 59:205-210. [DOI] [PubMed] [Google Scholar]
- 16.Lamana, A. C., J. E. Gestwick, L. E. Strong, S. L. Borchardt, R. M. Owen, and L. J. Kiessling. 2002. Conserved amplification of chemotactic responses through chemoreceptor signaling. J. Bacteriol. 184:4981-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason, J. M., R. H. Hackett, and P. Setlow. 1988. Studies on the regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores using lacZ gene fusions. J. Bacteriol. 170:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moir, A., B. M. Corfe, and J. Behravan. 2002. Spore germination. Cell. Mol. Life Sci. 59:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moir, A., E. H. Kemp, C. Robinson, and B. M. Corfe. 1994. The genetic analysis of bacterial spore germination. J. Appl. Bacteriol. 76:9S-16S. [PubMed]
- 20.Moir, A., and D. A. Smith. 1990. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 44:531-553. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, England.
- 22.Nicholson, W. L., D. Sun, B. Setlow, and P. Setlow. 1989. Promoter specificity of sigma-G-containing RNA polymerase from sporulating cells of Bacillus subtilis: identification of a group of forespore-specific promoters. J. Bacteriol. 171:2708-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paidhungat, M., and P. Setlow. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 181:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In J. A. Hoch, R. Losick, and A. L. Sonenshein (ed.), Bacillus subtilis and its relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 29.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Muramic acid lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. USA 93:15405-15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romick, T. L., and G. Tharrington. 1997. An automated method for quantifying the l-alanine trigger of Bacillus subtilis spore germination and competitive inhibition by d-alanine. J. Assoc. Rapid Method Autom. Microbiol. 5:215-221. [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 32.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J. Bacteriol. 183:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Southworth, T. W., A. A. Guffanti, A. Moir, and T. A. Krulwich. 2001. GerN, an endospore germination protein of Bacillus cereus, is an Na+/H+-K+ antiporter. J. Bacteriol. 183:5896-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swerdlow, B. M., B. Setlow, and P. Setlow. 1981. Levels of H+ and other monovalent cations in dormant and germinated spores of Bacillus megaterium. J. Bacteriol. 148:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tovar-Rojo, F., M. Chander, B. Setlow, and P. Setlow. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 184:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waditee, R., T. Hibino, T. Nakamura, A. Incharoensakdi, and T. Takabe. 2002. Overexpression of a Na+/H+ antiporter confers salt tolerance on a freshwater cyanobacterium, making it capable of growth in sea water. Proc. Natl. Acad. Sci. USA 99:4109-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]