Abstract
Background
Body temperature is a strong predictor of outcome in acute stroke. In a previous randomized trial we observed that treatment with high-dose acetaminophen (paracetamol) led to a reduction of body temperature in patients with acute ischemic stroke, even when they had no fever. The purpose of the present trial was to study whether this effect of acetaminophen could be reproduced, and whether ibuprofen would have a similar, or even stronger effect.
Methods
Seventy-five patients with acute ischemic stroke confined to the anterior circulation were randomized to treatment with either 1000 mg acetaminophen, 400 mg ibuprofen, or placebo, given 6 times daily during 5 days. Treatment was started within 24 hours from the onset of symptoms. Body temperatures were measured at 2-hour intervals during the first 24 hours, and at 6-hour intervals thereafter.
Results
No difference in body temperature at 24 hours was observed between the three treatment groups. However, treatment with high-dose acetaminophen resulted in a 0.3°C larger reduction in body temperature from baseline than placebo treatment (95% CI: 0.0 to 0.6 °C). Acetaminophen had no significant effect on body temperature during the subsequent four days compared to placebo, and ibuprofen had no statistically significant effect on body temperature during the entire study period.
Conclusions
Treatment with a daily dose of 6000 mg acetaminophen results in a small, but potentially worthwhile decrease in body temperature after acute ischemic stroke, even in normothermic and subfebrile patients. Further large randomized clinical trials are needed to study whether early reduction of body temperature leads to improved outcome.
Background
During the first days after stroke, between one and two fifths of the patients develop fever or subfebrile temperatures. [1-4] Increased temperatures have been associated with relatively large infarct volumes, high case fatality, and poor functional outcome, even after adjustment for initial stroke severity [2-6]. The period in which hyperthermia is associated with poor outcome is probably limited to the first 12 or 24 hours from stroke onset.[6,7]
The harmful effects of an early rise in body temperature have been attributed to increased cerebral metabolic demands,[8] changes in the blood-brain barrier permeability, acidosis, and an increased release of excitatory amino acids.[9] In animal models of temporary focal cerebral ischemia, mild intra-ischemic hyperthermia increased infarct volume [10], whereas mild hypothermia reduced infarct size.[11]
The above suggests that pharmacological reduction of temperature in patients with acute ischemic stroke may improve their functional outcome. In a previous randomized trial, we observed that treatment with acetaminophen in a daily dose of 6000 mg after ischemic stroke led to a 0.4°C (95% CI: 0 to 0.8°) lower body temperature at 12 and 24 hours than treatment with placebo; a daily dose of 3000 mg did not result in a significant reduction of body temperature.[12] Other studies in which lower dosages were used yielded promising, but inconclusive results[13,14]. Several studies in adults and children with fever suggest a larger antipyretic effect from ibuprofen than from acetaminophen. [15-17] The effect of ibuprofen on body-core temperature in normothermic and subfebrile patients with stroke, who may have a disturbance of thermoregulation [18], has not been studied.
The aim of our study was to test the effect of high-dose ibuprofen and to confirm the previously observed reducing effect of high-dose acetaminophen on body temperature. After 1 positive and 2 negative trials [12-14], a confirmation of the effect of high dose acetaminophen on body temperature in acute stroke seemed necessary, before we could decide to embark on a larger phase III trial of antipyretic treatment in acute stroke.
Methods
Design
Our study was a three-arm, randomized, double blind, placebo-controlled clinical trial, performed in two university hospitals and in one regional hospital. The protocol for this study has been published.[19]
Patients could be included if they had acute ischemic stroke in the anterior circulation, a body temperature between 36.0°C and 39.0°C, a computed tomography (CT) scan that was compatible with acute ischemic stroke, a focal deficit without rapid improvement, and a possibility to start treatment within 24 h after stroke onset. Patients with a posterior circulation stroke were not included because occasional patients could have severe disturbances of temperature regulation through involvement of the hypothalamus. Patients were excluded if they had severe aphasia, defined as a score of 2 or 3 on question no. 9 of the NIH Stroke Scale (NIHSS) [20] because they could not be expected to understand the information about the study. Other exclusion criteria were: treatment with an NSAID that could not be stopped (low dose aspirin was not an exclusion criterion), hypersensitivity to ibuprofen or acetaminophen, (chronic) liver failure or cirrhosis, (chronic) renal failure, a history of alcohol abuse, active ulcer disease or a history of peptic ulceration or gastro-intestinal hemorrhage in the preceding year, colitis ulcerosa, pregnancy, use of corticosteroids, a severe concomitant medical condition that could affect the assessment of the effect of the study medication on temperature, residual neurological impairment resulting from a previous stroke that might hamper the assessment of functional outcome, and death appearing imminent.
All patients were given verbal and written information about the potential risks and benefits of participation in the study. They gave consent in writing prior to randomization. We obtained the approval of the medical ethics committees of each participating center for the conduct of this study, and we complied with the Helsinki declaration.[21]
The NIH Stroke Scale was used to assess stroke severity at onset, and the modified Rankin scale to determine the patients' pre-stroke functional status. Laboratory investigations included a full blood count, glucose, electrolytes, creatinine, liver functions, and C-reactive protein (CRP).
Body-temperature was measured with a tympanic thermometer at 2-hourly intervals during the first 24 hrs after start of treatment, and thereafter every 12 hours until day 7. Temperatures were taken from the left and the right ear, and averaged. The baseline temperature (at the start of treatment) and temperature at 24 hours after start of treatment were determined both by rectal and tympanic thermometry. Rectal measurements were considered more reliable, and were therefore used for assessment of the primary outcome, body temperature at 24 hours after start of treatment, and for assessment of body temperature at inclusion into the study. Tympanic measurements were used for the other temperature measurements because they were considered less invasive and burdensome to the patients. However, as tympanic measurements are less precise, we used rectal temperatures in our analyses at 0 and 24 hours. Compliance was measured by counting leftover pills, and by a registry of the medication that was actually taken. All patients were treated with low dose (30 mg) aspirin, after a loading dose of 300 mg.
Study medication
Patients were treated for 5 days with acetaminophen or ibuprofen in daily doses of 6 × 1000 mg or 6 × 400 mg, respectively, or with matched placebo. Study drugs were administered through identical capsules. The first dose of medication could be given as an identical suppository. The study medication was provided in white paper boxes, numbered consecutively with a study number.
Randomization and blinding
Treatment allocation was random. The randomization was blocked in lots of six and stratified for the time since onset of stroke (0 to 12 hours versus 12 to 24 hours). A computer-generated list, provided by an independent statistician (PM), containing the medication number and information on the nature of the treatment (placebo, acetaminophen, or ibuprofen) resided only with the independent safety committee and with the study pharmacist. The treatment allocation of any patient could be disclosed before termination of the study if the safety committee deemed this necessary. Local clinical investigators could apply for disclosure of the treatment allocation of an individual patient by the study pharmacist.
Adverse events
Serious adverse events were defined as any potentially life-threatening deterioration in health status within the study-monitoring period (day 0 to day 7). These included any infection, such as pneumonia, sepsis, or urinary tract infection that needed antibiotic treatment according to the judgment of the treating physician, any liver function disturbance (aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), alkaline phosphatase (AF), or total bilirubine levels exceeding twice the local upper limit of normal), gastro-intestinal hemorrhage, and any neurological deterioration (i.e. a decrease in level of consciousness of more than one point on the Glasgow coma scale, or an increase of two or more points on the NIHSS). An independent, unblinded safety and data monitoring committee reviewed all serious adverse events on a two-weekly basis.
Outcomes
The primary outcome was body temperature measured rectally at 24 hours from start of treatment. Secondary outcomes were change from baseline temperature at 1 and 5 days from start of treatment, and time with elevated body temperature (> 37.0°C) (area under the curve) during the first 24 hours and the first five days. The tertiary outcome was functional outcome at 1 month, as determined by the scores on the modified Rankin Scale (mRS) and Barthel Index (BI). The Barthel index is a reliable and valid measure of the ability to perform activities of daily living such as eating, bathing, walking, and using the toilet. Patients able to perform all activities with complete independence are given a score of 20.[22] The modified Rankin scale is a reliable, simplified overall assessment of handicap in which a score of 0 indicates the absence of symptoms and a score of 5, severe disability.[23]
Statistical analysis
The study results were analyzed on an intent-to-treat basis. An "on-treatment" analysis of primary and secondary endpoints was planned. There was no formal interim analysis. The main results of the study are presented as the mean difference in temperature between each treatment group and the placebo group. The precision of these estimates was expressed with 95% confidence intervals, based on the t distribution. No adjustments for multiple comparisons were made. We planned to use multiple linear regression to adjust for possible confounding factors, such as age, stroke type, and stroke severity, but this was not considered necessary.
In order to detect a difference in core body temperature of 0.5°C, with a significance level of α = 0.05, and power 1-β = 0.80, at least 23 patients would be needed in each treatment group; this was rounded to 25. In total, 75 patients were included in this study.[19]
Results
Baseline characteristics
Seventy-five patients were randomized and included in the study between October 2000 and October 2001. Almost half of the patients started treatment within 12 hours after onset of symptoms. Prognostic factors, and other factors that could be related to increased body temperature were evenly distributed amongst the three treatment groups (Table 1). However, patients in the acetaminophen group had a lacunar stroke less often, they had a higher score on the NIHSS and were drowsy more often, and the mean body temperature at the start of treatment was higher than in the placebo group (0.3°C).
Table 1.
Demographics, stroke risk factors and clinical characteristics of the study population.
| Acetaminophen (n = 26) | Ibuprofen (n = 24) | Placebo (n = 25) | |
| Demographics | |||
| Age in years (m, SD) | 69 (16) | 67 (15) | 65 (10) |
| Male sex | 17 (65%) | 16 (67%) | 16 (64%) |
| Risk factors for stroke | |||
| Hypertension | 12 (46%) | 7 (29%) | 8 (32%) |
| Atrial fibrillation | 6 (24%) | 7 (29%) | 1 (4%) |
| Peripheral vascular disease | 4 (15%) | 1 (4%) | 1 (4%) |
| Diabetes mellitus | 1 (4%) | 4 (17%) | 3 (12%) |
| Previous TIA | 5 (19%) | 3 (13%) | 3 (12%) |
| Previous stroke | 3 (12%) | 8 (33%) | 2 (8%) |
| Cigarette smoking | 14 (54%) | 13 (54%) | 16 (64%) |
| Stroke characteristics | |||
| Lowered consciousness level | 5 (19%) | 2 (8%) | 2 (8%) |
| Aphasia | 7 (27%) | 9 (37%) | 6 (24%) |
| NIHSS (m, SD) | 18 (14) | 12 (10) | 14 (11) |
| Lacunar stroke | 8 (31%) | 6 (25%) | 11 (44%) |
| Other clinical characteristics | |||
| Treatment within 0–12 hours | 14 (54%) | 14 (58%) | 12 (48%) |
| Temp. at admission (°C) (m, SD) | 37.2 (0.6) | 37.0 (0.6) | 37.0 (0.5) |
| Temp. at start of treatment (°C) (m, SD) | 37.3 (0.5) | 37.1 (0.7) | 37.0 (0.5) |
Acetaminophen is given in a dose of 1000 mgs, 6 times daily, and ibuprofen in a dose of 400 mg 6 times daily. Values are absolute numbers with percentages of totals in the treatment arms, unless stated otherwise. 'm' indicates mean, 'SD' standard deviation.
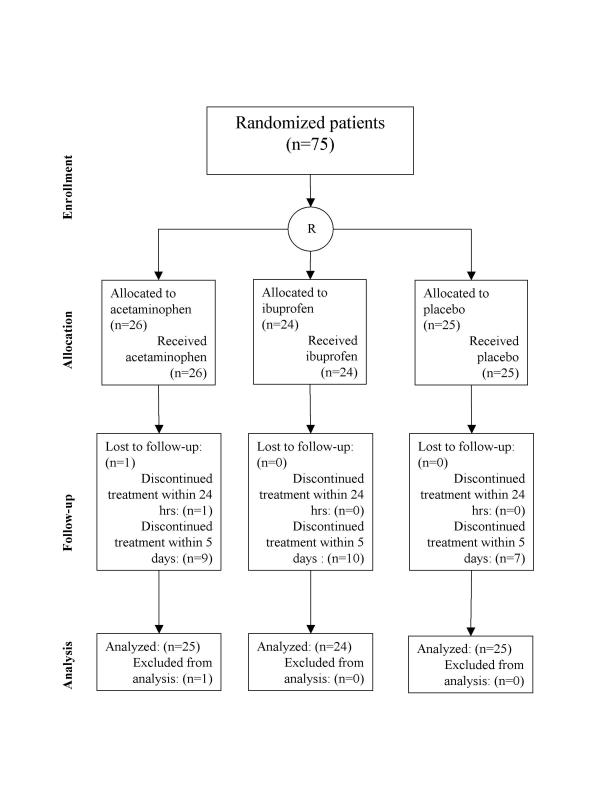
Patient flow
All but one patient were on treatment after 24 hours. Twenty-six patients did not complete the full five-day-treatment schedule (Figure 1). Of the 7 patients in the placebo group who stopped, 4 were discharged within 5 days, 2 withdrew their consent, and 1 patient forgot to take the last 3 doses on day 5. In the ibuprofen group, 6 patients were discharged within 5 days, 2 withdrew consent, and 2 developed a hemorrhagic gastritis. Finally, in the acetaminophen group, one patient, a woman with a severe middle cerebral artery infarction did not complete the first 24 hours of treatment, and died from herniation after hemorrhagic transformation of the infarct. One other patient on acetaminophen died from progressive stroke within the first five days. Four patients were discharged early, one patient had a high serum creatinine, one had slightly elevated transaminases at baseline, which made the local investigator decide to stop the study treatment and finally, one patient withdrew consent. Patients who died within the study period or who had stopped taking their medication, could still be considered as having completed the 28-day outcome assessment.
Figure 1.
Patient flow in the trial.
Outcome
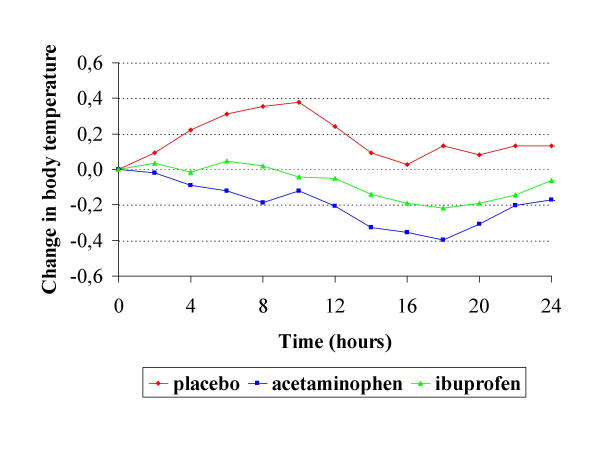
At 24 hours, the mean rectal body temperatures in the three groups did not differ significantly (Table 2). However, compared with placebo, treatment with acetaminophen resulted in a 0.3°C (95% CI: 0.0 to 0.6°C) larger reduction in body temperature in the first 24 hours. This effect occurred early and was stable (Figure 2). In the first 24 hours, ibuprofen had no significant effect on body temperature as compared with placebo. The difference in reduction in body temperature at 24 hours after start of treatment between ibuprofen and acetaminophen was 0.2°C (95% CI: -.2°C to 0.6°C).
Table 2.
Effect of treatment on body temperature (in °C) and outcome. Effects on area under the curve measurements were adjusted for body temperature at start of treatment.
| Acetaminophen N = 25 | Ibuprofen N = 24 | Placebo N = 25 | |
| Temperature at 24 hrs (mean, SD) | 37.2 (0.5) | 37.2 (0.4) | 37.2 (0.5) |
| Difference with placebo (95% CI) | 0 (-.3 to 0.3) | 0 (-.3 to 0.3) | - |
| Change in temperature at 24 hrs, (mean, SD) | -0.1 (0.6) | 0.1 (0.6) | 0.2 (0.5) |
| Difference with placebo (95% CI) | 0.3 (0 to 0.6) | 0.1 (-.3 to 0.4) | - |
| Change in temperature after 5 days in(SD) | 0.3 (1.2) | 0.6 (1.6) | 0.1 (0.6) |
| Difference with placebo (95% CI) | -.2 (-1.0 to 0.7) | -.5 (-1.2 to 0.2) | - |
| Area under the curve (first 24 hours) in hrs × °C (SD) | 888 (12) | 883 (16) | 893 (14) |
| Difference with placebo (95% CI) | 7 (0 to 12) | 7 (2 to 12)) | - |
| Area under the curve (5 days) in hrs × °C (SD) | 4443 (63) | 4367 (128) | 4438 (64) |
| Difference with placebo (95% CI) | 9 (-52 to 34) | 23 (-15 to 62) | - |
| N of patients with Modified Rankin score >2, (%) | 13 (52%) | 9 (38%) | 12 (48%) |
| N of patients with Barthel index <20 (%) | 14 (56%) | 10 (42%) | 14 (56%) |
Figure 2.
Time course of change in tympanic body temperature compared to start of treatment during the first 24 hours of treatment, in the three treatment groups. Discrepancies with Table 2 are caused by rounding and by the use of tympanic measurements.
Body temperature measurements at five days from the start of treatment were available in 52 of the 75 patients. In the 21 patients who died, withdrew consent, or were discharged within 5 days from randomization, no further temperatures could be taken, and in 2 other patients who had discontinued study treatment, temperature measurements were inadvertently stopped. After the first day, mean body temperatures tended to be lower in both groups who were on active treatment (Figure 2). After 5 days, ibuprofen treatment resulted in 0.5°C lower body temperatures, and acetaminophen treatment led to 0.2°C lower body temperatures than placebo treatment, but these differences were not statistically significant (Table 2). In addition, only about half of the patients were still on treatment on day 5.
The comparison between the areas under the temperature curve was hampered by differences in temperature at start of treatment. After adjustment for differences in baseline temperature, the areas for acetaminophen and ibuprofen were significantly smaller than for placebo. Finally, no significant differences in functional outcome were observed between the three regimens (Table 2).
Adverse events
During the treatment period, 1 patients died from herniation and 1 from a a progressive stroke, both were on acetaminophen. One patient (on acetaminophen) developed pneumonia, and two patients (both on ibuprofen) had gastric retention and signs of hemorrhagic gastritis. Liver function disturbances occurred in 3 patients who were on placebo, in 6 patients who used ibuprofen, and in 6 patients who used acetaminophen, resulting in a relative risk of 1.9 (95% CI: 0.5 to 6.7) of acetaminophen compared to the placebo group. According to the local investigators, the liver function disturbances and the gastic disturbances were possibly related to the study treatment.
Discussion
We studied the effect of two common antipyretic agents on body temperature after acute ischemic stroke. Patients on acetaminophen had a more severe stroke at baseline, and had higher body temperatures at the start of treatment. No differences in mean body temperature after 24 hours of treatment (the primary outcome measure) were observed between the three study groups, because of differences in baseline temperature. However, acetaminophen in a daily dose of 6000 mg led to 0.3°C (95% CI: 0.0 to 0.6°C) larger reduction of body temperature than placebo after 24 hours. This effect was similar to the effect that was observed in a previous study.[12] The effect of ibuprofen was smaller and not statistically significant.
There was a trend towards a temperature-reducing effect of ibuprofen at 5 days, but this was based on observations in only about two-thirds of the patients. It is doubtful whether temperature reduction several days after the onset of cerebral ischemia will have potential therapeutic effects.
No statistically significant differences in functional outcome were observed between the treatment groups. This was expected, as our study was not powered for that purpose.
Validity
Although all but one patient completed the first 24 hours of treatment and underwent the primary outcome assessment, only 50 (67%) of the 75 patients completed the 5-day treatment period. This was caused by the general policy to discharge patients as early as possible from the hospital to home.
In this study, we used rectal temperatures as a primary outcome measure, and not intracranial or intravesical temperatures as an approximate to brain temperature. In a study of moderate hypothermia in patients with traumatic brain injury, rectal temperatures remained very close to brain temperatures.[24] In addition, in the Copenhagen study, that demonstrated a convincing relationship between temperature and outcome, only rectal body temperatures were measured.[6]
Our study is not large enough to allow for a direct comparison of the effects of acetaminophen and ibuprofen on body temperature in stroke. Thus, we cannot totally exclude that ibuprofen is more effective than acetaminophen. Treatment with 30 mgs of aspirin daily, after a loading dose of 300 mg could not have confounded our results, because it was given to all patients, and much higher dosages are needed to treat fever.
Other studies
In three other studies, acetaminophen has been used in an attempt to lower body temperature in patients with acute stroke. Koennecke et al. conducted a comparable study and showed that treatment with acetaminophen in a daily dose of 4000 mg resulted in a substantial reduction of the proportion of patients with body temperatures over 37.5°C: 5% of the patients who were treated with a daily dose of 4 g acetaminophen developed body temperatures over 37.5°C, against 36% in the placebo-group.[13] The authors did not report the size of the temperature reduction. Kasner et al. conducted a partly blinded trial of acetaminophen against placebo in 39 patients with hemorrhagic or ischemic stroke, and observed a difference of 0.2 °C in body temperature in favor of active treatment. The patients who received acetaminophen tended to be more often hypothermic (<36.5°C) and less often hyperthermic (>37.5°C), but these results were not statistically significant.[14] In this study, a lower daily dose of acetaminophen (3.9 g) was used than in our study (6 g). In our previous study, we observed that treatment with high-dose, but not low-dose acetaminophen led to a 0.4°C (95% CI: 0 – 0.8°) lower body temperature at and 24 hours than treatment with placebo.[12] The present study confirms this effect.
In a recent study it was shown that the concomitant administration of ibuprofen but not acetaminophen antagonizes the irreversible platelet-inhibiting effect of aspirin.[25] Ibuprofen may therefore limit the already small beneficial effect of aspirin to improve outcome after ischemic stroke.[26] This is an additional reason why acetaminophen may be preferable to ibuprofen for reduction of temperature in patients with acute stroke.
Ethical considerations
Because of the consistently observed relation between hyperthermia and poor outcome after stroke, many physicians want to treat their stroke patients who have a raised temperature with antipyretic drugs.[27,28] However, a causal relationship between hyperthermia and poor outcome has never been demonstrated, and subfebrile temperatures and fever may just be epiphenomena of severe brain damage. In addition, there is no evidence that a pharmacological reduction of increased temperatures will lead to an improved outcome. Preventive administration of antipyretics may even mask infections and lead to delayed treatment with antibiotics. For these reasons, we think that the efficacy of antipyretic drugs to improve outcome after stroke has to be proven in a randomized controlled trial before treatment with these agents is accepted as a standard procedure. For the same reasons, we do not consider it unethical to withhold antipyretics from stroke patients with (sub-)febrile temperatures in the placebo arm of such a trial.
Conclusions
Treatment with a daily dose of 6000 mg acetaminophen results in a small, but potentially worthwhile decrease in body temperature after acute ischemic stroke, even in normothermic and subfebrile patients. Further large randomized clinical trials are needed to study whether this early reduction of body temperature leads to improved outcome. A large, pragmatic multicenter trial of high dose acetaminophen in acute stroke will be launched in 2003.
List of abbreviations
AF – alkaline phosphatase
ALAT – alanine aminotransferase
ASAT – aspartate aminotransferase
BI – Barthel index
CRP – C-reactive protein
CT – computed tomography
MRS – modified Rankin Scale
NIHSS – National institutes of Health Stroke Scale
NSAID – non-steroidal anti-inflammatory drug
Competing interests
None declared.
Authors contributions
DD supervised the study, and contributed to design and data-analysis. EB coordinated data-aquisition, and drafted the manuscript, HW and HG coordinated data-aquisition in the two particitating centers, and contributed in the design of the study and analysis of the data. RM, LK and PK contributed to the design of the study, interpretation of the data analysis and helped writing the manuscript.
All authors read and approved the final manuscript.
Appendix
Study organization
Executive committee: DWJ Dippel, EJ van Breda, RJ Meijer, HB van der Worp, HMA van Gemert. Data-management: EJ van Breda, JHE Hilkemeijer. Statistical analysis: DWJ Dippel. Data monitoring and safety committee: A Algra, (chairman), J van Gijn, PA van Doorn, A Koudstaal. Advisory committee: PJ Koudstaal, LJ Kappelle.Writing committee: DWJ Dippel, EJ van Breda, HB van der Worp, HMA van Gemert, RJ Meijer, LJ Kappelle and PJ Koudstaal. Participating centers: Meander MC Amersfoort, The Netherlands (21 patients): HMA van Gemert, A Hovestad, MBM Vermeulen, JBS Boringa; University Hospital Rotterdam (41 patients): SLM Bakker, F van Kooten, DWJ Dippel, MPJ van Goor, RJ Meijer, D Siepman. University Hospital Utrecht (13 patients): LJ Kappelle, HB van der Worp.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgments
The Stichting Neurovasculair Onderzoek Rotterdam funded this study.
This study benefited greatly from the efforts and critical comments of the nursing staff of the Departments of Neurology of the Meander MC, UMC Utrecht and Erasmus MC.
We thank Paul Janssen, hospital pharmacist, Erasmus MC, and Dr Paul GH Mulder, biostatistician, dept of Biostatistics and Epidemiology of the Erasmus MC, for their help and advice.
Contributor Information
Diederik WJ Dippel, Email: d.dippel@erasmusmc.nl.
Eric J van Breda, Email: breda@neuro.fgg.eur.nl.
H Bart van der Worp, Email: h.b.vandeworp@neuro.azu.nl.
H Maarten A van Gemert, Email: m.gemert@meandermc.nl.
Ron J Meijer, Email: meijer@neur.azr.nl.
L Jaap Kappelle, Email: l.kappelle@neuro.azu.nl.
Peter J Koudstaal, Email: p.j.koudstaal@erasmusmc.nl.
References
- Przelomski MM, Roth RM, Gleckman RA, Marcus EM. Fever in the wake of a stroke. Neurology. 1986;36:427–429. doi: 10.1212/wnl.36.3.427. [DOI] [PubMed] [Google Scholar]
- Castillo J, Martinez F, Leira R, Prieto JM, Lema M, Noya M. Mortality and morbidity of acute cerebral infarction related to temperature and basal analytic parameters. Cerebrovasc Dis. 1994;4:66–71. [Google Scholar]
- Azzimondi G, Bassein L, Nonino F, Fiorani L, Vignatelli L, Re G, et al. Fever in acute stroke worsens prognosis. A prospective study. Stroke. 1995;26:2040–2043. doi: 10.1161/01.str.26.11.2040. [DOI] [PubMed] [Google Scholar]
- Hindfelt B. The prognostic significance of subfebrility and fever in ischaemic cerebral infarction. Acta Neurol Scand. 1976;53:72–79. doi: 10.1111/j.1600-0404.1976.tb04326.x. [DOI] [PubMed] [Google Scholar]
- Castillo J, Davalos A, Marrugat J, Noya M. Timing for fever-related brain damage in acute ischemic stroke. Stroke. 1998;29:2455–2460. doi: 10.1161/01.str.29.12.2455. [DOI] [PubMed] [Google Scholar]
- Reith J, Jorgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347:422–425. doi: 10.1016/S0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- Jorgensen HS, Reith J, Pedersen PM, Nakayama H, Olsen TS. Body temperature and outcome in stroke patients. Lancet. 1996;348:193. doi: 10.1016/s0140-6736(05)66135-1. [DOI] [PubMed] [Google Scholar]
- Nemoto EM, Frankel HM. Cerebral oxygenation and metabolism during progressive hyperthermia. Am J Physiol. 1970;219:1784–1788. doi: 10.1152/ajplegacy.1970.219.6.1784. [DOI] [PubMed] [Google Scholar]
- Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989;20:904–910. doi: 10.1161/01.str.20.7.904. [DOI] [PubMed] [Google Scholar]
- Meden P, Overgaard K, Pedersen H, Boysen G. The influence of body temperature on infarct volume and thrombolytic therapy in a rat embolic stroke model. Brain Res. 1994;647:131–138. doi: 10.1016/0006-8993(94)91407-9. [DOI] [PubMed] [Google Scholar]
- Karibe H, Chen SF, Zarow GJ, Gafni J, Graham SH, Chan PH, et al. Mild intraischemic hypothermia suppresses consumption of endogenous antioxidants after temporary focal ischemia in rats. Brain Res. 1994;649:12–18. doi: 10.1016/0006-8993(94)91043-X. [DOI] [PubMed] [Google Scholar]
- Dippel DWJ, van Breda EJ, van Gemert HM, van der Worp HB, Meijer RJ, Kappelle LJ, et al. Effect of paracetamol (acetaminophen) on body temperature in acute ischemic stroke: a double-blind, randomized phase II clinical trial. Stroke. 2001;32:1607–1612. doi: 10.1161/01.str.32.7.1607. [DOI] [PubMed] [Google Scholar]
- Koennecke HC, Leistner S. Prophylactic antipyretic treatment with acetaminophen in acute ischemic stroke: A pilot study. Neurology. 2001;57:2301–2303. doi: 10.1212/wnl.57.12.2301. [DOI] [PubMed] [Google Scholar]
- Kasner SE, Wein T, Piriyawat P, Villar-Cordova CE, Chalela JA, Krieger DW, et al. Acetaminophen for Altering Body Temperature in Acute Stroke: A Randomized Clinical Trial. Stroke. 2002;33:130–135. doi: 10.1161/hs0102.101477. [DOI] [PubMed] [Google Scholar]
- Kauffman RE, Sawyer LA, Scheinbaum ML. Antipyretic efficacy of ibuprofen vs acetaminophen. Am J Dis Child. 1992;146:622–625. doi: 10.1001/archpedi.1992.02160170102024. [DOI] [PubMed] [Google Scholar]
- Van Esch A, Van Steensel-Moll HA, Steyerberg EW, Offringa M, Habbema JD, Derksen-Lubsen G. Antipyretic efficacy of ibuprofen and acetaminophen in children with febrile seizures. Arch Pediatr Adolesc Med. 1995;149:632–637. doi: 10.1001/archpedi.1995.02170190042007. [DOI] [PubMed] [Google Scholar]
- Autret E, Reboul-Marty J, Henry-Launois B, Laborde C, Courcier S, Goehrs JM, et al. Evaluation of ibuprofen versus aspirin and paracetamol on efficacy and comfort in children with fever. Eur J Clin Pharmacol. 1997;51:367–371. doi: 10.1007/s002280050215. [DOI] [PubMed] [Google Scholar]
- Mackowiak PA. Concepts of fever. Arch Intern Med. 1998;158:1870–1881. doi: 10.1001/archinte.158.17.1870. [DOI] [PubMed] [Google Scholar]
- van Breda EJ, van der Worp HB, Van Gemert HMA, Meijer RJ, Kappelle LJ, Koudstaal PJ, et al. PISA. The effect of paracetamol (acetaminophen) and ibuprofen on body temperature in acute stroke: protocol for a phase II double-blind randomised placebo-controlled trial. BMC Cardiovascular Disorders. 2002;2:7. doi: 10.1186/1471-2261-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. MD State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 1997;349:1569–1581. doi: 10.1016/S0140-6736(97)04011-7. [DOI] [PubMed] [Google Scholar]
- De Keyser J. Antipyretics in acute ischaemic stroke. Lancet. 1998;352:6–7. doi: 10.1016/s0140-6736(05)79507-6. [DOI] [PubMed] [Google Scholar]
- McD Johnston A. Antipyretic therapy is important following stroke. Arch Intern Med. 2000;160:2679–2680. doi: 10.1001/archinte.160.17.2679. [DOI] [PubMed] [Google Scholar]