Abstract
We used a mouse fetal skin dendritic cell line (FSDC) to study the effect of the strong allergen 2,4-dinitrofluorobenzene (DNFB) on interleukin (IL)-1β release and IL-1β receptor immunoreactivity. Stimulation with DNFB (30 minutes) increased IL-1β release without changing the mRNA levels of the protein. Furthermore, DNFB increased transiently the interleukin-1β-converting enzyme (ICE) activity, as measured with its fluorogenic substrate Z-Tyr-Val-Ala-Asp-AFC. The ICE inhibitor Z-YVAD-FMK prevented the release of IL-1β evoked by DNFB. Incubation of the cells with DNFB (30 minutes) strongly increased IL-1β receptor immunoreactivity. The rapid effect of DNFB on the release of mature IL-1β, without inducing an increase of IL-1β mRNA in FSDC, suggests a posttranslational modification of pro-IL-1β by ICE activity.
INTRODUCTION
The haptens in contact with the skin bind nonspecifically to a multitude of epidermal proteins becoming a complete allergen [1]. Then, skin dendritic cells (DC), namely, Langerhans cells (LC), the most efficient antigen-presenting cells of the epidermis, internalise the hapten-protein immunoreactive complex, probably by endocytosis [2], and process it. LC become activated, migrate to the lymph nodes, and maturate, acquiring a functional state favouring antigen presentation [3]. These events are associated with the secretion of several cytokines (IL-1β, IL-6, IL-12) [4], enhanced expression of functional molecules (MHC-II/I complex, adhesion and costimulatory molecules), and altered antigen uptake, processing, and presenting by LC [3, 5].
IL-1β is required for LC migration induced by chemical allergens [6] and for the contact sensitisation process to occur [7]. The production of this cytokine by LC induces the release of various other cytokines (TNF-α, GM-CSF, IL-1α, IL-6, and IL-10), specially from keratinocytes (KC) [1, 4], that may also contribute to LC activation and migration [4]. In mice, LC are the primary source of IL-1β within the epidermis, and the synthesis of the interleukin is markedly increased in contact hypersensitivity [8], being produced at an early stage, 15 minutes after the binding of a contact sensitiser IL-1β mRNA is upregulated [8]. IL-1β knockout mice manifest impaired contact hypersensitivity to the contact sensitiser trinitrochlorobenzene (TNCB) [7]. Also, topical application of the sensitiser DNFB, dinitrochlorobenzene (DNCB), or TNCB upregulate the level of IL-1β mRNA [8]. Moreover, in vitro, NiCl2 and DNCB significantly increased IL-1β mRNA expression and IL-1β production in cultured human DC [9]. Additionally, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI) induced IL-1β production in monocytes [10] and DNFB, paraphenylene diamine (PPD), and MCI/MI increased IL-1β mRNA in human-blood-derived DC [11].
IL-1β is produced as an inert 31-kd precursor [12] that undergoes enzymatic cleavage to a biologically active form [13]. Conversion of pro-IL-1β into its active form is catalysed by a cysteine protease belonging to the caspase family, the interleukin-1β-converting enzyme (ICE) [14], also termed caspase-1 [15]. In the skin, LC are the primary source of ICE mRNA [16]. IL-1β can bind two receptors, IL-1R type I (IL-1RI) and IL-1R type II (IL-1RII). IL-1β exerts its biological effects on target cells, namely on LC, through the interaction with IL-1RI [17]. IL-1RII is not able to transduce a signal and is referred to as a decoy receptor [18]. Therefore, regulation of IL-1 receptor expression may also have an important function in allergic sensitisation.
In this study, we used a mouse fetal skin dendritic cell line (FSDC), representative of early skin DC precursor [19], to clarify some of the cellular events activated by the sensitiser DNFB in the sensitisation phase of contact hypersensitivity. The results indicate that DNFB induces IL-1R expression and that ICE is involved in DNFB-induced IL-1β secretion in skin DC precursors.
MATERIALS AND METHODS
Reagents
The 2,4-dinitrofluorobenzene (DNFB) and 2,4-dichloronitrobenzene (DCNB) were purchased from Aldrich Chemical Company (Madrid, Spain). The mouse recombinant GM-CSF was from Roche (Carnaxide, Portugal). LPS from Escherichia coli (serotype 026:B26) was obtained from Sigma Chemical Co (Madrid, Spain). The ICE inhibitor CBZ-Tyr-Val-Ala-Asp-FMK (Z-YVAD-FMK), the fluorochrome peptide conjugated Z-Tyr-Val-Ala-Asp-7-amino-4-trifluoromethylcoumarin (AFC-120), and free fluorochrome 7-amino-4-trifluoromethylcoumarin (AFC) were purchased from Enzyme Systems Products (Livermore, Calif). The ELISA kit for mouse IL-1β was obtained from R & D Systems (Minneapolis, Minn). The rat anti-mouse IL-1β receptor monoclonal antibody for immunocytochemistry was obtained from Biotrend (Koln, Germany). The Alexa 488 goat anti-rat IgG conjugate and the ProLong Antifade kit were from Molecular Probes Europe (Leiden, The Netherlands), and the normal goat serum was from Chemicon (Temecula, Calif). Fetal calf serum was from Biochrom (Berlin, Germany) and trypsin from Gibco Invitrogen (Paisley, UK). All other reagents were obtained from Sigma Chemical Co.
Cell culture
The fetal mouse skin dendritic cell line, FSDC, was kindly supplied by Dr G. Girolomoni [19]. The cells were cultured in Iscove's modified Dulbecco's medium, supplemented with 36 mM sodium bicarbonate, 64.4 mM glutamine, 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Measurement of cytokine production
FSDCs (0.2 × 106 cells/well) were cultured in a 48-well microplate and were incubated for 30 minutes in the presence or absence of DNFB (1 μg/mL), DCNB (1 μg/mL), or GM-CSF (10 ng/mL), or for 12 hours in the presence of LPS (40 μg/mL). For testing the effect of the ICE inhibitor Z-YVAD-FMK on IL-1β release (17-kd active form), FSDCs were preincubated for 90 minutes with the ICE inhibitor (100 μM), prior to DNFB stimulation. In all experiments, culture supernatants were collected and centrifuged at 15 800 xg for 5 minutes to precipitate cell debris. Samples were kept at -20°C until used for mouse IL-1β measurement, with an ELISA kit. Procedures were conducted according to the manufacturer's instructions, which specify a detection limit of 10 pg/mL and no measurable cross-reactivity to other cytokines.
Measurement of ICE protease activity in cytosolic fractions
The cells (0.8 × 106 cells/mL) were incubated with the allergen DNFB (1 μg/mL) in a 12-well microplate for the indicated periods of time. Treated and control cells were washed twice with ice-cold phosphate-buffered saline (PBS). The cells were then lysed in 250 μl of lysis buffer (25 mM HEPES, pH 7.5, 5 mM MgCl2, 1 mM EDTA, and 0.1% Triton X-100), containing freshly added 1 mM PMSF, 2.5 μg/mL pepstatin A, and 20 μg/mL benzamidine. Samples were then centrifuged at 13 000 xg, for 15 minutes, at 4°C, and the supernatants were collected and kept at −80°C. Cleared lysates containing 40 μg protein were incubated with reaction buffer (50 mM HEPES, 1% sucrose, and 0.1% CHAPS) containing freshly added 10 mM DTT, and 40 μM of the enzyme substrate Z-Tyr-Val-Ala-Asp-AFC (AFC, 7-amino-4-trifluoromethylcoumarin) for 3 hours at 37°C. Levels of released AFC were measured in a SPEX Fluoromax fluorometer, with excitation at 390 nm and emission at 490 nm. The enzyme activity was calibrated with AFC standard solutions.
Detection of IL-1β receptor by immunocytochemistry
FSDCs were grown on glass coverslips, in 12-well microplates. The cells (0.4 × 106 cells/well) were cultured in the presence or absence of DNFB (1 μg/mL), for 30 minutes, as indicated in “results.” After that, FSDCs were fixed and permeabilised by immersing the coverslips in methanol:acetone (1:1), at −20°C for 10 minutes. Nonspecific binding was blocked by incubation with 0.1% Tween 20/PBS, supplemented with 20% normal goat serum, for 30 minutes at room temperature. Cells were then incubated for 90 minutes at room temperature, with a rat monoclonal antibody directed against both mouse IL-1 types I (IL-1RI) and II (IL-1RII) receptors (7.5 μg/mL). After rinsing with 0.1% Tween 20/PBS, the cells were incubated with Alexa 488-conjugated goat anti-rat immunoglobulin (5 μg/mL), in 0.1% Tween 20/PBS supplemented with 1% normal goat serum, for 1 hour at room temperature. The glass coverslips were rinsed again as before and mounted with ProLong Antifade kit onto a slide. Fluorescence labelling was visualised by confocal microscopy, using an MRC600 confocal imaging system (Bio-Rad Laboratories, Milan, Italy) linked to a Nikon Optiphot-2 fluorescence microscope. A krypton/argon mixed laser was used in combination with a fluorescein filter to examine IL-1β receptor labelling. Image processing included a single-medium filter pass (2 × 2), using confocal assistant software (Bio-Rad Laboratories). Control experiments consisted of processing the same preparations with the omission of the primary antibody and resulted in no specific staining.
RNA extraction
Cells were plated at 2 × 106 cell/well in 6-well microplates, and grown for 24 hours. After stimulation with DNFB for the indicated periods of time, cells were scrapped in TRIzol Reagent (Invitrogen Life Technologies, Paisley, UK). Chloroform was added, and the samples centrifuged at 12 000 xg, at 4°C for 15 minutes, to separate the phases. The aqueous phase was transferred to a fresh tube and the RNA precipitated by mixing with isopropyl alcohol. After centrifugation at 12 000 xg, at 4°C for 10 minutes, the supernatant was removed and the RNA pellet washed with 75% ethanol. The RNA was then resuspended in RNase-free water and stored at −80°C.
Quantitative real-time reverse transcription-PCR
Quantitative fluorescent PCR was performed using the TaqMan system (ABI Prism 7900HT Sequence Detection System; PE Applied Biosystems, Foster City, Calif). Primers and TaqMan probes for mouse IL-1β and 18S ribosomal RNA (rRNA) (used as endogenous control) were obtained from PE Applied Biosystems. First-strand cDNA was synthesised from total RNA using TaqMan reverse transcription reagents (PE Applied Biosystems), as described by the manufacturer's protocol. PCR for IL-1β and 18S rRNA were performed in 25 μL total reaction volumes, with 20 x Target Mix and 2 x TaqMan Universal PCR Master Mix for IL-1β, and 200 nM TaqMan probe, 50 nM forward primers, 50 nM reverse primers, and 2 x TaqMan Universal PCR Master Mix for 18S rRNA endogenous control (PE Applied Biosystems). Thermal cycling was performed with 2 minutes at 50°C for depleting contaminated RNA, and 10 minutes denaturation at 95°C followed by 40 cycles at 95°C for 15 seconds, and 1 minute at 60°C, using the ABI Prism 7700 detection system (PE Applied Biosystems). The relative levels of cDNA generated from cellular RNA were calculated normalising the amount of IL-1β cDNA relatively to 18S rRNA cDNA in each sample.
Data analysis
The results are expressed as mean ± SEM of the indicated number of independent experiments, and statistical analysis was performed using the one-way ANOVA test with the indicated post-test or the unpaired Student t test, as indicated. A difference with P value less than .05 was considered statistically significant.
RESULTS
Stimulation of FSDCs with DNFB increases the release of IL-1β through ICE activation
Since LC-derived IL-1β has an important role in the induction of contact hypersensitivity [11, 20], experiments were conducted in order to determine whether the contact sensitiser DNFB would affect IL-1β secretion in a fetal skin dendritic cell line (FSDC). The cells were stimulated with DNFB (1 μg/mL), for 30 minutes, and IL-1β concentration in culture supernatants was measured with an ELISA kit. DNFB significantly increased the release of IL-1β by FSDCs (Figure 1). Preliminary experiments showed that the concentration of DNFB (1 μg/mL) and the incubation period used (30 minutes) produced a maximal effect on the extracellular accumulation of IL-1β (not shown). In contrast with the effect of DNFB, its inactive analogue DCNB (1 μg/mL) did not induce IL-1β release (Figure 1). These results were compared with the long-term effects of LPS. When the cells were treated for 12 hours with the immunostimulatory molecule LPS, there was also a strong induction of IL-1β secretion (Figure 1).
Figure 1.
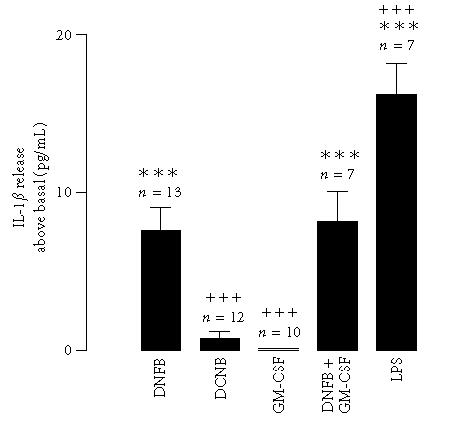
IL-1β release induced by DNFB in a fetal skin dendritic cell line. Cells were stimulated with 2,4-dinitrofluorobenzene (DNFB, 1 μg/mL) or with 2,4-dichloronitrobenzene (DCNB, 1 μg/mL), for 30 minutes. Alternatively, cells were stimulated with GM-CSF (10 ng/mL) alone, or in association with DNFB, for the same time period. As a positive control, the cells were stimulated with LPS (40 μg/mL), for 12 hours. IL-1β was quantified with an ELISA kit. Data express the IL-1β release above the basal secretion. Each value represents the mean ± SEM from the indicated number of experiments, performed in duplicate. Statistical significance was calculated by the one-way ANOVA test with a Bonferroni post-test (∗∗∗ P < .001 as compared to the control; ⩲⩲⩲ P < .001 as compared to stimulation with DNFB).
GM-CSF is a cytokine known to be very important in LC progenitor differentiation, as well as in LC maturation and functional regulation [21]. We have previously shown that FSDCs express functional receptors for GM-CSF [22]. Therefore, we also examined the effect of this cytokine on the contact-sensitiser-induced IL-1β release. Incubation of FSDC with GM-CSF (10 ng/mL) did not cause IL-1β release and did not affect the cytokine release induced by DNFB (Figure 1).
In order to confirm the presence of ICE enzymatic activity that could process IL-1β for secretion, we investigated the effect of different incubation times with DNFB on AFC release from the substrate Z-Tyr-Val-Ala-Asp-AFC. The cells were incubated for 1, 2.5, 15, or 30 minutes in the presence of DNFB (1 μg/mL), and protease activity was measured in the cytosolic extracts. As depicted in Figure 2a, for the cells stimulated for 2.5 or 15 minutes with DNFB, a considerable increase in ICE activity above basal was registered, whereas only a small increase was observed when the incubation times were 1 minute or 30 minutes, as measured by the amount of AFC generated.
Figure 2.
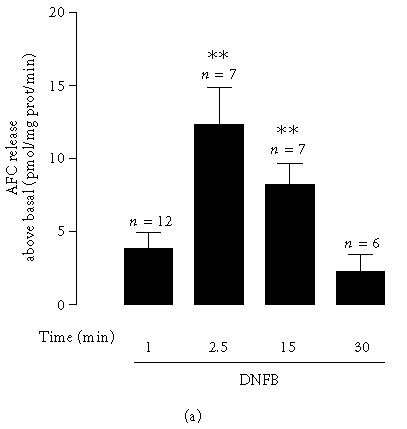
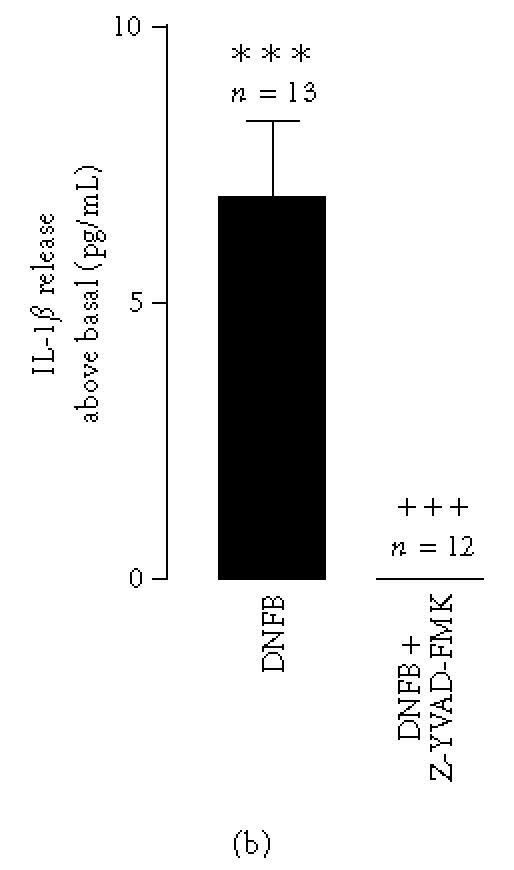
Interleukin-1β-converting enzyme (ICE) protease activity and its role in DNFB-induced IL-1β release. (a) ICE protease activity in cytosolic fractions prepared from cells incubated with DNFB (1 μg/mL), for 1, 2.5, 15, or 30 minutes. Proteolytic activity in cell lysates was measured with the caspase-1 fluorogenic substrate Z-Tyr-Val-Ala-Asp-AFC, as described in “materials and methods.” Results are represented as AFC release above basal, in pmol/mg prot/min. Basal AFC release was 324.2 ± 45.5 pmol/mg protein/min. Data express the mean ± SEM from the indicated number of experiments, performed in duplicate. Statistical significance was calculated by the one-way ANOVA test with a Dunnett post-test (∗∗ P < .01 as compared to the control). (b) IL-1β concentration in the supernatants of fetal skin dendritic cell line. Cells were stimulated with 2,4-dinitrofluorobenzene (DNFB, 1 μg/mL) in the presence or absence of the interleukin-1β-converting enzyme inhibitor, Z-YVAD-FMK (100 μM), for 30 minutes. In this case the cells were preincubated with Z-YVAD-FMK (100 μM), for 90 minutes. IL-1β was quantified with an ELISA kit. Data express the IL-1β release above the basal secretion. Each value represents the mean ± SEM from the indicated number of experiments, performed in duplicate. Statistical significance was calculated by the one-way ANOVA test with a Bonferroni post-test (∗∗∗ P < .001 as compared to the control; ⩲⩲⩲ P < .001 as compared to stimulation with DNFB).
To assess whether there was a role for interleukin-1β-converting enzyme in the DNFB-dependent IL-1β secretion, we employed Z-YVAD-FMK, a peptide inhibitor of ICE. Based on the target sequence in pro-IL-1β (YVHD) [23], pre-incubation of the cells with Z-YVAD-FMK, for 90 minutes, blocked almost completely DNFB-triggered IL-1β release (Figure 2b).
Stimulation of FSDC with DNFB does not increase IL-1β mRNA levels
Since murine LC have been shown to produce most of the IL-1β mRNA during activation with haptens [8], which could account for the IL-1β release detected in this work at 30 minutes stimulation with DNFB, we further investigated the effect of different incubation times (5, 10, 15, 20, or 30 minutes) with DNFB in the level of IL-1β mRNA in FSDC. IL-1β mRNA level was determined in total RNA extracted from cells. As shown in Figure 3, DNFB did not modify IL-1β mRNA expression in FSDC.
Figure 3.
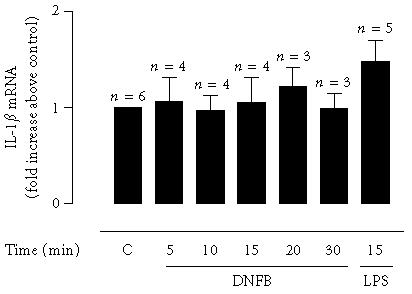
Effect of DNFB on IL-1β mRNA in a fetal skin dendritic cell line. Cells were stimulated with 1 μg/mL of DNFB for the indicated periods of time. Total RNA was then extracted using TRIzol Reagent, as described in “materials and methods,” and cDNA was reverse transcribed from total RNA. Quantitative, fluorescent PCR was performed using the TaqMan system. Relative levels of cDNA were calculated and results expressed as fold increase relatively to the control. Data are expressed as the mean ± SEM from the indicated number of experiments. Statistical significance was calculated by the one-way ANOVA test with a Dunnett post-test (no significance was found).
DNFB increases the IL-1 receptor immunoreactivity
Several reports have shown LC expression of IL-1R in murine epidermis [23, 24, 25]. Moreover, it has also been demonstrated that IL-1β has an autocrine effect on LC, inducing its own production by LC [4, 24]. Based on these findings, we performed subsequent experiments to find out whether the sensitiser DNFB could also be responsible for IL-1β autoregulatory functions in FSDC. When compared with the control cells (Figure 4a), which displayed a faint receptor immunoreactivity, cells incubated for 30 minutes with DNFB (1 μg/mL) and immunostained with an anti-IL-1R antibody that recognises both type I and type II receptors, showed a marked increase in IL-1R immunoreactivity (Figure 4b). The immunoreactivity was more evident in the perinuclear region. In contrast, GM-CSF (10 ng/mL, 12 hours) or DCNB (1 μg/mL) had no significant effect on IL-1R immunoreactivity in FSDC (data not shown). As only IL-1RI mediates the biological activity of IL-1β, we further analysed its immunoreactivity by Western blot. Comparing total extracts of control cells and cells treated with DNFB for 30 minutes, we observed an increase in IL-1RI immunoreactivity, which however did not reach statistical significance (data not shown).
Figure 4.

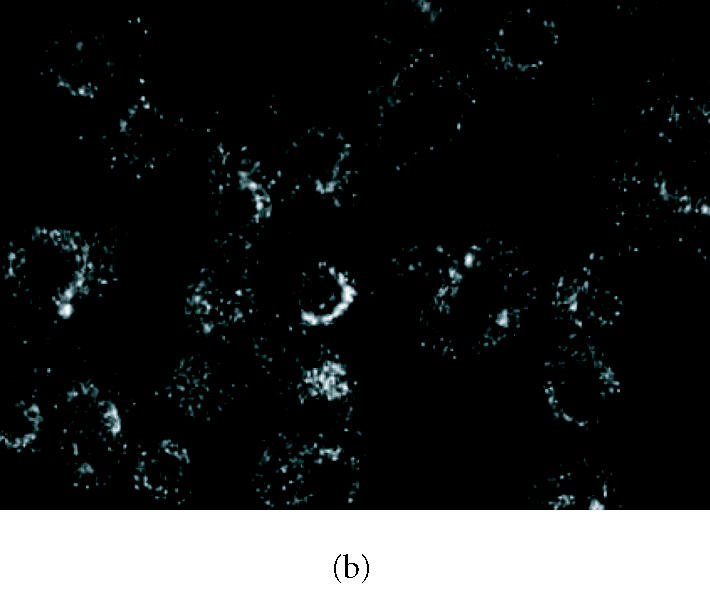
Effect of DNFB on IL-1R immunoreactivity. (b) IL-1R immunoreactivity in FSDCs after exposure to 2,4-dinitrofluorobenzene (DNFB, 1 μg/mL), for 30 minutes. (a) Control cells were maintained in medium alone. Cells were immunostained with an anti-IL-1R type I and type II antibody, as described in “materials and methods.” The figures are representative of the results obtained in three independent experiments.
DISCUSSION
A complex array of cytokines, such as IL-1β, TNF-α, and GM-CSF, is produced by epidermal cells, namely LC and keratinocytes (KC), during the sensitisation phase of allergic contact dermatitis (ACD) [1]. Most data suggest a direct effect of the chemical sensitisers on LC [26, 27, 28]. However, the major cellular events involved in those processes remain largely unknown. In the present work, we used a murine skin-derived dendritic cell line, representative of immature LC [19], to clarify some of the mechanisms involved in the early events of sensitisation, namely, the mechanism involved in IL-1β secretion.
IL-1β, known to cause several modifications in cell phenotype [20, 25], is involved in LC maturation/migration during the process of sensitisation to chemicals applied to the skin. Here, we demonstrated that the strong sensitiser DNFB significantly increased IL-1β release by FSDC (Figure 1). This is in agreement with previous studies showing that skin sensitisers induce IL-1β release in dendritic cells and monocytes [9, 10, 11, 29]. In contrast, the nonsensitiser DCNB had no effect on the secretion of this cytokine (Figure 1), which is in agreement with other studies where the use of this or other nonsensitizers did not upregulate IL-1β protein secretion or mRNA levels on murine epidermis [4, 8].
We also tested the effects of GM-CSF on the IL-1β secretion by FSDC, since the cytokine GM-CSF is also known to be involved in the early stages of ACD [1]. This cytokine, which is overproduced by KC in contact dermatitis [30], constitute a direct or indirect inflammatory stimulus for LC to mature [31]. However, at least in FSDC, GM-CSF alone (at the concentration of 10 ng/mL) did not have any effect on IL-1β production, neither did it significantly increase DNFB-induced IL-1β secretion (Figure 1).
Since ICE, a cytoplasmic cysteine protease, is the major enzyme capable of generating the active and mature form of IL-1β [14, 32] and it is known to be expressed in murine LC cell lines and other DC [16, 33], we investigated if any increase in ICE activity could be implicated in the mechanism of action of this sensitising chemical in FSDC. Using the fluorogenic peptide substrate Z-Tyr-Val-Ala-Asp-AFC to measure ICE activity in cytosolic extracts of the cells incubated with DNFB, we observed that the allergen caused a transient increase of the enzyme activity, which reached its maximum after 2.5 minutes of incubation (Figure 2a). This effect was already evident at 1 minute, and was sustained for 15–30 minutes. We further investigated the role of ICE activity in the sensitiser-evoked IL-1β release, by using a peptide inhibitor that blocks the substrate recognition sequence of that caspase. The effect of DNFB on IL-1β release was completely abolished by the ICE inhibitor (Figure 2b), strongly suggesting that this enzyme is responsible for the IL-1β overproduction induced by DNFB in FSDC. These results are in agreement with those obtained in human keratinocytes stimulated with urushiol [34], in murine macrophages induced by ligands of macrophage scavenger receptor or LPS [35, 36] and in microglial cells stimulated with LPS and ATP [37]. Also, they provide evidence for an important function of the ICE on the IL-1β secretion induced by sensitisers and in the initiation of contact sensitivity. Previous work by Antonopoulos et al [38] showed that caspase-1 is needed for LC migration during skin sensitisation to DNFB and oxazolone. Furthermore, in caspase-1-deficient mice there is no LC depletion after skin application of the sensitisers and they do not develop contact sensitivity. Also, Z-YVAD-FMK, the ICE inhibitor used in the present study, completely prevented LC migration and contact sensitivity, in both in vivo and in vitro models. This effect may be mediated by TNF-α secreted by KC stimulated with the sensitiser, since caspase-1 also plays a role in TNF-α-induced LC migration [38, 39]. In our model, lacking KC, an early induction of ICE in the presence of DNFB, rapidly followed by ICE-mediated IL-1β release, suggests a direct effect of DNFB on DC. This effect of DNFB was specific, since it was not observed in the presence of the tolerogen DCNB.
Our results indicate that DNFB stimulates the release of IL-1β from FSDC due to stimulation of ICE activity. Although LC have been shown to upregulate IL-1β mRNA during sensitisation [8, 11], we could not detect any significant increase of IL-1β mRNA upon stimulation of FSDCs with DNFB (Figure 3). These results suggest that, in FSDCs, the early increase of IL-1β detected is not due to an increase in mRNA production, but rather to ICE activation that converts existing pro-IL-1β into mature IL-1β. Constitutive expression of IL-1β mRNA in DC derived from human blood was reported by others [11], and previous reports detected an increases of IL-1β mRNA in LC, after 15 minutes of stimulation with haptens [8]. Since we observed the release of IL-1β after 30 minutes of stimulation with DNFB, we only investigated putative changes in the mRNA for the interleukin at an early phase of stimulation. In contrast, most of the studies that detected increase of IL-1β mRNA expression and protein release focused on delayed responses (hours to days) [9, 10, 11, 29], which cannot account for the acute effect on IL-1β release detected by us.
We also investigated the effects of DNFB on the IL-1R expression by FSDC. The biological effects of IL-1 are mediated through IL-1R type I (IL-1RI). IL-1R type II (IL-1RII) does not appear to have a signalling function and has been implicated in the downregulation of IL-1β responses [8]. Murine LC cultured for one day express the IL-1RI protein [25], and human LC present low level of IL-1RI and high level of IL-1RII [23]. In our work, we used a monoclonal antibody against both IL-1RI and IL-1RII for immunocytochemistry, and we observed that nonstimulated FSDC presented low levels of IL-1RI/II immunoreactivity. Cell exposure to the allergen DNFB, for 30 minutes, strongly increased the IL-1RI/II immunoreactivity, mainly in the perinuclear region (Figure 4b). A small increase in total IL-1RI protein levels (the biologically active receptor) was observed in FSDC stimulated with DNFB, as determined by Western blot (data not shown).
The effect of DNFB on IL-1β secretion and IL-1R immunoreactivity in FSDC (Figures 1 and 4) suggests that the sensitiser-evoked IL-1β release may have autoregulatory functions on the skin dendritic cells during the very early phase of ACD. This conclusion is corroborated by studies showing that IL-1β injection in mice enhances its own message expression [1], providing evidence for an autocrine loop.
Recently, DNFB was shown to induce a rapid activation of p38 MAPK in dendritic cells [40]. Such activation may be involved in mediating IL-1β release observed in this report, and in human monocyte-derived DC [9] stimulated with contact sensitisers.
In summary, the present study suggests that, in contrast to the nonsensitizer DCNB, the strong contact sensitiser DNFB stimulates a precocious upregulation of IL-1β release by murine skin DC and that an increase in ICE activity is the main mechanism involved in this release. Furthermore, the observed increase in IL-1R receptors suggest that the cytokine may have an autocrine effect on these DC.
ACKNOWLEDGMENT
This work was supported by FCT and FEDER.
References
- 1.Enk AH. Allergic contact dermatitis: understanding the immune response and potential for targeted therapy using cytokines. Mol Med Today. 1997;3(10):423–428. doi: 10.1016/S1357-4310(97)01087-3. [DOI] [PubMed] [Google Scholar]
- 2.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9(1):10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Muller G, Knop J, Enk AH. Is cytokine expression responsible for differences between allergens and irritants? Am J Contact Dermat. 1996;7(3):177–184. [PubMed] [Google Scholar]
- 5.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388(6644):782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 6.Cumberbatch M, Dearman RJ, Groves RW, Antonopoulos C, Kimber I. Differential regulation of epidermal Langerhans cell migration by interleukins (IL)-1alpha and IL-1beta during irritant- and allergen-induced cutaneous immune responses. Toxicol Appl Pharmacol. 2002;182(2):126–135. doi: 10.1006/taap.2002.9442. [DOI] [PubMed] [Google Scholar]
- 7.Shornick LP, De Togni P, Mariathasan S, et al. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzene. J Exp Med. 1996;183(4):1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992;89(4):1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiba S, Manome H, Nakagawa S, et al. p38 mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and 2,4-dinitrochlorobenzene. J Invest Dermatol. 2003;120(3):390–399. doi: 10.1046/j.1523-1747.2003.12065.x. [DOI] [PubMed] [Google Scholar]
- 10.Brand P, Plochmann S, Valk E, et al. Activation and translocation of p38 mitogen-activated protein kinase after stimulation of monocytes with contact sensitizers. J Invest Dermatol. 2002;119(1):99–106. doi: 10.1046/j.1523-1747.2002.01791.x. [DOI] [PubMed] [Google Scholar]
- 11.Pichowski JS, Cumberbatch M, Dearman RJ, Basketter DA, Kimber I. Allergen-induced changes in interleukin 1 beta (IL-1 beta) mRNA expression by human blood-derived dendritic cells: inter-individual differences and relevance for sensitization testing. J Appl Toxicol. 2001;21(2):115–121. doi: 10.1002/jat.742. [DOI] [PubMed] [Google Scholar]
- 12.Hazuda D, Webb RL, Simon P, Young P. Purification and characterization of human recombinant precursor interleukin 1 beta. J Biol Chem. 1989;264(3):1689–1693. [PubMed] [Google Scholar]
- 13.Mosley B, Urdal DL, Prickett KS, et al. The interleukin-1 receptor binds the human interleukin-1-alpha precursor but not the interleukin-1-beta precursor. J Biol Chem. 1987;262(7):2941–2944. [PubMed] [Google Scholar]
- 14.Thornberry NA, Bull HG, Calaycay JR, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 15.Thornberry NA. The caspase family of cysteine proteases. Br Med Bull. 1997;53(3):478–490. doi: 10.1093/oxfordjournals.bmb.a011625. [DOI] [PubMed] [Google Scholar]
- 16.Ariizumi K, Kitajima T, Bergstresser OR, Takashima A. Interleukin-1 beta converting enzyme in murine Langerhans cells and epidermal-derived dendritic cell lines. Eur J Immunol. 1995;25(8):2137–2141. doi: 10.1002/eji.1830250803. [DOI] [PubMed] [Google Scholar]
- 17.Zwijnenburg PJ, van der Poll T, Florquin S, Roord JJ, Van Furth AM. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J Immunol. 2003;170(9):4724–4730. doi: 10.4049/jimmunol.170.9.4724. [DOI] [PubMed] [Google Scholar]
- 18.Neumann D, Kollewe C, Martin MU, Boraschi D. The membrane form of the type II IL-1 receptor accounts for inhibitory function. J Immunol. 2000;165(6):3350–3357. doi: 10.4049/jimmunol.165.6.3350. [DOI] [PubMed] [Google Scholar]
- 19.Girolomoni G, Lutz MB, Pastore S, Assmann CU, Cavani A, Ricciardi-Castagnoli P. Establishment of a cell line with features of early dendritic cell precursors from fetal mouse skin. Eur J Immunol. 1995;25(8):2163–2169. doi: 10.1002/eji.1830250807. [DOI] [PubMed] [Google Scholar]
- 20.Enk AH, Angeloni VL, Udey MC, Katz SI. An essential role for Langerhans cell-derived IL-1 beta in the initiation of primary immune responses in skin. J Immunol. 1993;150(9):3698–3704. [PubMed] [Google Scholar]
- 21.Heufler C, Koch F, Schuler G. Granulocyte-macrophage colony-stimulating factor and interleukin-1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988;167(2):700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz MT, Duarte CB, Goncalo M, Figueiredo A, Carvalho AP, Lopes MC. Granulocyte-macrophage colony-stimulating factor activates the transcription of nuclear factor kappa B and induces the expression of nitric oxide synthase in a skin dendritic cell line. Immunol Cell Biol. 2001;79(6):590–596. doi: 10.1046/j.1440-1711.2001.01041.x. [DOI] [PubMed] [Google Scholar]
- 23.Larregina A, Morelli A, Kolkowski E, Fainboim L. Flow cytometric analysis of cytokine receptors on human Langerhans' cells. Changes observed after short-term culture. Immunology. 1996;87(2):317–325. doi: 10.1046/j.1365-2567.1996.451513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cumberbatch M, Dearman RJ, Kimber I. Characteristics and regulation of the expression on interleukin 1 receptors by murine Langerhans cells and keratinocytes. Arch Dermatol Res. 1998;290(12):688–695. doi: 10.1007/s004030050374. [DOI] [PubMed] [Google Scholar]
- 25.Kampgen E, Koch F, Heufler C, et al. Understanding the dendritic cell lineage through a study of cytokine receptors. J Exp Med. 1994;179(6):1767–1776. doi: 10.1084/jem.179.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz MT, Duarte CB, Goncalo M, Figueiredo A, Carvalho AP, Lopes MC. Differential activation of nuclear factor kappa B subunits in a skin dendritic cell line in response to the strong sensitizer 2,4-dinitrofluorobenzene. Arch Dermatol Res. 2002;294(9):419–425. doi: 10.1007/s00403-002-0342-y. [DOI] [PubMed] [Google Scholar]
- 27.Cruz MT, Duarte CB, Goncalo M, Figueiredo A, Carvalho AP, Lopes MC. The sensitizer 2,4-dinitrofluorobenzene activates caspase-3 and induces cell death in a skin dendritic cell line. Int J Toxicol. 2003;22(1):43–48. doi: 10.1080/10915810305069. [DOI] [PubMed] [Google Scholar]
- 28.Cruz MT, Goncalo M, Figueiredo A, Carvalho AP, Duarte CB, Lopes MC. Contact sensitizer nickel sulfate activates the transcription factors NF-kB and AP-1 and increases the expression of nitric oxide synthase in a skin dendritic cell line. Exp Dermatol. 2004;13(1):18–26. doi: 10.1111/j.0906-6705.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- 29.Aiba S, Terunuma A, Manome H, Tagami H. Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules. Eur J Immunol. 1997;27(11):3031–3038. doi: 10.1002/eji.1830271141. [DOI] [PubMed] [Google Scholar]
- 30.Pastore S, Fanales-Belasio E, Albanesi C, Chinni LM, Giannetti A, Girolomoni G. Granulocyte macrophage colony-stimulating factor is overproduced by keratinocytes in atopic dermatitis. Implications for sustained dendritic cell activation in the skin. J Clin Invest. 1997;99(12):3009–3017. doi: 10.1172/JCI119496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahlenberg JM, Dubyak GR. Differing caspase-1 activation states in monocyte versus macrophage models of IL-1β processing and release. J Leukoc Biol. 2004;76(3):676–684. doi: 10.1189/jlb.0404221. [DOI] [PubMed] [Google Scholar]
- 33.Mutini C, Falzoni S, Ferrari D, et al. Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J Immunol. 1999;163(4):1958–1965. [PubMed] [Google Scholar]
- 34.Zepter K, Haffner A, Soohoo LF, et al. Induction of biologically active IL-1 beta-converting enzyme and mature IL-1 beta in human keratinocytes by inflammatory and immunologic stimuli. J Immunol. 1997;159(12):6203–6208. [PubMed] [Google Scholar]
- 35.Hsu HY, Chiu SL, Wen MH, Chen KY, Hua KF. Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathways. J Biol Chem. 2001;276(31):28719–28730. doi: 10.1074/jbc.M011117200. [DOI] [PubMed] [Google Scholar]
- 36.Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277(25):22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 37.Sanz JM, Di Virgilio F. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J Immunol. 2000;164(9):4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- 38.Antonopoulos C, Cumberbatch M, Dearman RJ, Daniel RJ, Kimber I, Groves RW. Functional caspase-1 is required for Langerhans cell migration and optimal contact sensitization in mice. J Immunol. 2001;166(6):3672–3677. doi: 10.4049/jimmunol.166.6.3672. [DOI] [PubMed] [Google Scholar]
- 39.Cumberbatch M, Bhushan M, Dearman RJ, Kimber I, Griffiths CE. IL-1beta-induced Langerhans' cell migration and TNF-alpha production in human skin: regulation by lactoferrin. Clin Exp Immunol. 2003;132(2):352–359. doi: 10.1046/j.1365-2249.2003.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166(6):3837–3845. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]


