Abstract
The aims of this study were to investigate the influence of serotonin (5-HT) on the effects of intra-articular injections of glucocorticoid on pain of the temporomandibular joint (TMJ) in patients with inflammatory disorders of the TMJ. The pretreatment synovial fluid 5-HT was negatively, and plasma 5-HT positively, correlated to change in TMJ pain after treatment. The pretreatment plasma 5-HT was positively correlated to change in pressure-pain threshold after treatment. In conclusion, this study shows that local and systemic serotonergic mechanisms partly determine the effect of intra-articular glucocorticoid treatment on TMJ pain in patients with chronic TMJ arthritis of systemic nature, while change in pressure-pain threshold over the TMJ is influenced by systemic serotonergic mechanisms.
INTRODUCTION
Glucocorticoids administered locally or systemically suppress inflammation and pain in rheumatoid arthritis [1, 2]. Glucocorticoids pass through the cellular membrane and bind to glucocorticoid receptors in the cytoplasm. Activated receptors inhibit the expression of genes for proinflammatory cytokines, enzymes, receptors, and adhesion molecules, while increasing the expression of genes coding for antiinflammatory proteins like interleukin-10 and interleukin-1 receptor antagonist [2]. However, there is no report whether glucocorticoid inhibits release of serotonin (5-HT) from, for example, thrombocytes or mast cells or whether local or systemic serotonergic mechanisms influence the response to local glucocorticoid administration of peripheral joint pain.
Treatment with intra-articular glucocorticoid of the temporomandibular joint (TMJ) has proved efficient in pain, tenderness to digital palpation of the lateral aspect of the joint, and mobility 4–6 weeks after administration in patients with rheumatoid arthritis (RA; [1, 3]). Another study reported that only resting pain relief remained after 5 weeks, while pressure-pain threshold and movement pain had returned to the pretreatment level [3]. Glucocorticoids might therefore exert diverse effects on different pain entities [4] such as resting pain, pain on joint movement, tenderness to digital palpation, or pressure-pain threshold, perhaps due to a different molecular mechanism behind each pain entity.
Alstergren et al [5] showed that TMJ synovial fluid level of 5-HT is associated with pain intensity perceived on movement and to decreased mandibular mobility in patients with systemic inflammatory disorders. Voog et al [6] showed that intra-articular injection of the 5-HT3 receptor antagonist granisetron in the TMJ reduces resting as well as movement pain intensity and increases pressure-pain threshold in patients with rheumatoid arthritis. These two studies indicate that peripheral serotonergic mechanisms are involved in the modulation of pain and hyperalgesia/allodynia in systemic inflammatory joint disorders. However, no study has reported the influence of 5-HT level in the TMJ or blood on the effect of local glucocorticoid treatment.
The aims of this study were therefore to investigate the influence of synovial fluid and blood levels of 5-HT on the effects by intra-articular injections of glucocorticoid on pain and allodynia of the TMJ in patients with chronic and systemic inflammatory disorders of the TMJ.
MATERIAL AND METHODS
Patients
One male and nineteen female patients with inflammatory TMJ disorders participated (Table 1). Inclusion criteria were diagnosis of seropositive or seronegative RA (n = 3 and n = 5, respectively), ankylosing spondylitis (n = 3), osteoarthritis (n = 1), psoriatic arthropathy (n = 1), chronic unspecific polyarthritis (n = 2), systemic lupus erythematosus (n = 1), Sjögren's syndrome (n = 1), common variable immunodeficiency (n = 1), or Marfan's syndrome (n = 2). The diagnosis of RA was determined according to the criteria of American College of Rheumatology [7]. The diagnosis of TMJ inflammatory disorder due to polyarthritis was determined according to the diagnostic classification by the American Academy of Orofacial Pain [8]. An exclusion criterion was intra-articular glucocorticoid treatment of the TMJ within 3 months. All patients were referred to the clinic from rheumatologists or general medical practitioners. Eight patients used nonsteroidal antiinflammatory drugs (naproxen), 5 patients oral glucocorticoid (methylprednisolone), 7 patients disease-modifying antirheumatic drugs (sulfazalasine, methotrexate, infliximab), and 4 patients had no current (systemic) medical treatment.
Table 1.
Pretreatment data of 20 patients with chronic inflammatory joint disease subjected to intra-articular glucocorticoid injections. IQR = 25th–75th percentile; n is the number of patients.
| Background variables | Median | IQR | n | |
| Age | (years) | 50 | 18 | 20 |
| Duration of general joint involvement | (years) | 10 | 14 | 20 |
| Duration of local TMJ involvement | (years) | 5 | 11 | 19 |
| Number of involved joints | (1–9) | 6 | 3 | 20 |
| Thrombocyte particle concentration | (109/L) | 289 | 95 | 14 |
The study was approved by the local Ethical Committee at Karolinska University Hospital in Huddinge, Sweden (142/02 and 176/91).
Pain intensity ratings
The patients were asked about pain in nine joint regions besides the TMJ (neck, shoulders, elbows, hands, upper back, lower back, hips, knees, and feet; max score 9).
A 100 mm visual analog scale with end-points denoted “no pain” (0 mm) and “worst pain ever experienced” (100 mm) was used to assess the current TMJ pain intensity on each side at rest and on maximum voluntary mouth opening.
The number of mandibular movements that provoked pain in the TMJ (maximum mouth opening, laterotrusion to both sides, and protrusion) was counted for each side (score 0–4).
Tenderness to digital palpation of the lateral and posterior aspects of the TMJ on each side was assessed. On each side and aspect one unit was scored if the patient reported tenderness and two units if the palpation caused a pain reflex. The total score was also counted (score 0–4).
The pressure-pain threshold was recorded over the palpable lateral pole of the TMJ condyle with the patient's mouth closed and over glabella. The pressure-pain threshold was defined as the minimum pressure needed to evoke a painful sensation recognizable by the subjects. The pressure-pain threshold was assessed by a hand-held electronic pressure algometer (Somedic Production AB, Sollentuna, Sweden) consisting of a pressure transducer probe connected to a pistol grip with a display unit. The tip of the pressure transducer has a flat, circular rubber tip with an area of 1.0 cm2. A linearly increasing pressure rate (50 kPa/s) was applied until the subject responded to the first pain sensation by pressing a button on a device connected to the probe that froze the current pressure-pain threshold level on the display.
Synovial fluid sampling from the temporomandibular joint
TMJ anesthesia was achieved by blocking the auriculotemporal nerve with 2.0 mL 2% lidocaine (Xylocain, Astra-Zeneca, Södertälje, Sweden). The TMJ was punctured with a standard disposable needle (diameter = 0.65 mm) inserted into the posterior part of the upper joint compartment. TMJ synovial fluid samples were obtained by washing the joint cavity with saline using a push and pull technique [9]. The washing solution, consisting of 78% saline (NaCl 9 mg/mL, Pharmacia Upjohn, Uppsala, Sweden) and 22% hydroxocobalamin (Behepan 1 mg/mL; Pharmacia Upjohn), was slowly injected into the posterior part of the upper joint cavity with approximately 1 mL at a time and then aspirated. The total volume of the washing solution injected was 4 mL. The hydroxocobalamin was included in order to determine the volume of synovial fluid recovered in the aspirate by comparing the spectrophotometric absorbance of the aspirate with that of the washing solution. The synovial fluid level was then calculated. Only samples that fulfilled previously established sample quality criteria were included in the statistical analysis [10].
Blood sampling
Venous blood was collected in a sodium citrate tube (0.105 mol/L) to determine the erythrocyte sedimentation rate and in an EDTA tube that was immediately cooled and centrifuged (1500g for 30 minutes at +4°C) and then frozen (−70°C), and later examined for 5-HT in plasma. In addition, venous blood was collected without additives for analysis of serum concentrations of 5-HT, thrombocyte particle count, and C-reactive protein. These tubes were left at room temperature for 60 minutes for coagulation and thereafter centrifuged (1500g for 10 minutes at +4°C). The serum was then removed and frozen (−70°C) until analysis. Time from freezing to analysis varied between 1 and 6 months for the blood samples.
Drug
The acetate ester of methylprednisolone (40 mg/mL) with lidocaine hydrochloride (10 mg/mL) added (Depo-Medrol cum lidocaine; Pfizer AB, Täby, Sweden) was injected in a volume of 0.7 mL into the upper joint compartment.
Treatment and examination schedule
The patients were examined before and after treatment. The median (IQR) interval between the first and second examinations of 38 (52) days. At the first examination, glucocorticoid was injected into the TMJ after the synovial fluid sampling and at the second examination only synovial fluid sampling was performed.
Analysis of serotonin
Serum and plasma 5-HT levels were analyzed by a commercially available EIA-kit (Immunotech A Coulter Company, Marseille, France) with a detection limit of 0.5 nmol/L. The intra-assay coefficient of variation for this assay is less than 9.4 and the interassay less than 9.9% according to the manufacturer.
The concentration of synovial fluid levels of 5-HT was analyzed by using the same kit but it was modified to be applicable at concentrations between 1.6 and 5000 nmol/L. To compensate for hydroxocobalamin interactions with the assays, the synovial fluid aspirates were read against a standard curve with hydroxocobalamin included [9,10]. The small hydroxocobalamin interaction was completely compensated for by this procedure.
Statistical analysis
Synovial fluid samples from both occasions were available from one TMJ on each patient and the clinical and synovial fluid data from this side were used in the analysis. The central tendency and the variation of the variables are presented as median and interquartile range (IQR; 75th–25th percentile). The significances of the differences between the variables before and after treatment were calculated by the Wilcoxon test. Spearman's ranked correlation test was used to calculate the significance of the correlations between the variables. A probability level below .05 was considered as significant.
RESULTS
Table 2 shows the pretreatment and follow-up values of the clinical variables and synovial fluid levels of 5-HT. Erythrocyte sedimentation rate (normal: < 28 mm), C-reactive protein (normal: < 10 mg/L), and thrombocyte particle concentration (normal: 150–400 109/L) were abnormal in 0%, 20%, and 14% of the patients.
Table 2.
General and local (temporomandibular joint; TMJ) disease activity before and 5 weeks after intra-articular administration of glucocorticosteroid in 20 patients with chronic inflammatory joint disease. Pain intensity was assessed with a 100 mm visual analog scale (VAS), the number of mandibular movements that provoked pain in the TMJ (maximum mouth opening, laterotrusion to both sides and protrusion) was counted for each side, tenderness to digital palpation of the lateral and posterior aspects of the TMJ on each side was recorded as one unit if the patient reported tenderness and two units if the palpation caused a pain reflex, and the pressure-pain threshold was recorded over the palpable lateral pole of the TMJ condyle and over glabella; IQR = 25th−75th percentile, n denotes number of patients, % > 0 denotes observations percent exceeding 0, and na denotes not applicable.
| Clinical variables | Pretreatment | Follow-up | |||||||||
| Median | IQR | n | % > 0 | Median | IQR | n | % > 0 | P | |||
| General disease activity | |||||||||||
| General joint pain intensity | (VAS score) | 46 | 33 | 20 | 100 | 30 | 36 | 15 | 87 | — | |
| Erythrocyte sedimentation rate | (mm/first h) | 9 | 11 | 16 | na | 18 | 16 | 15 | na | — | |
| C-reactive protein | (mg/L) | 0 | 0 | 16 | 20 | 0 | 12 | 13 | 38 | — | |
| TMJ pain intensity | |||||||||||
| At rest | (VAS score) | 50 | 41 | 20 | 95 | 25 | 42 | 20 | 80 | .028 | |
| Upon maximal mouth opening | (VAS score) | 51 | 40 | 13 | 100 | 37 | 40 | 13 | 92 | — | |
| Number of painful mandibular movements | 3 | 1 | 20 | 90 | 2 | 2 | 20 | 80 | — | ||
| Tenderness to digital palpation | |||||||||||
| Lateral | — | 1 | 1 | 20 | 95 | 1 | 1 | 20 | 60 | .028 | |
| Posterior | — | 1 | 1 | 20 | 65 | 1 | 1 | 20 | 50 | — | |
| Pressure-pain threshold | |||||||||||
| Temporomandibular joint | (kPa) | 136 | 95 | 20 | na | 141 | 104 | 20 | na | — | |
| Glabella | (kPa) | 240 | 94 | 14 | na | 237 | 119 | 14 | na | — | |
| Serotonin levels | |||||||||||
| Synovial fluid | (nmol/L) | 16 | 126 | 20 | 55 | 13 | 667 | 20 | 65 | — | |
| Serum | (nmol/L) | 898 | 506 | 14 | 100 | 670 | 547 | 11 | 91 | — | |
| Plasma | (nmol/L) | 37 | 23 | 10 | 100 | 17 | 20 | 6 | 100 | — | |
Changes after glucocorticoid treatment in relation to pretreatment serotonin levels
Serotonin was detectable in the synovial fluid from 11 patients before treatment and in 12 after. The pretreatment synovial fluid level of 5-HT was negatively correlated to the change in TMJ pain intensity at rest, which decreased significantly (P = .028), and TMJ pain intensity on maximum mouth opening after treatment, that is, a high pretreatment level of 5-HT was associated with a decrease of TMJ pain intensity at rest (rs = −0.52, n = 20, P = .018; Figure 1a) and on maximum mouth opening (rs = −0.57, n = 13, P = .041; Figure 1b). The pretreatment synovial fluid level of 5-HT was also negatively correlated to the change in synovial fluid 5-HT (rs = −0.55, n = 20, P = .012), which means that high pretreatment 5-HT was associated with decrease of 5-HT after treatment.
Figure 1.
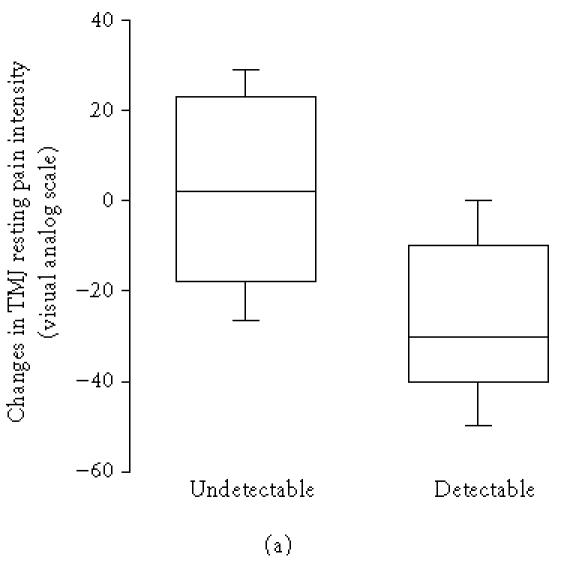
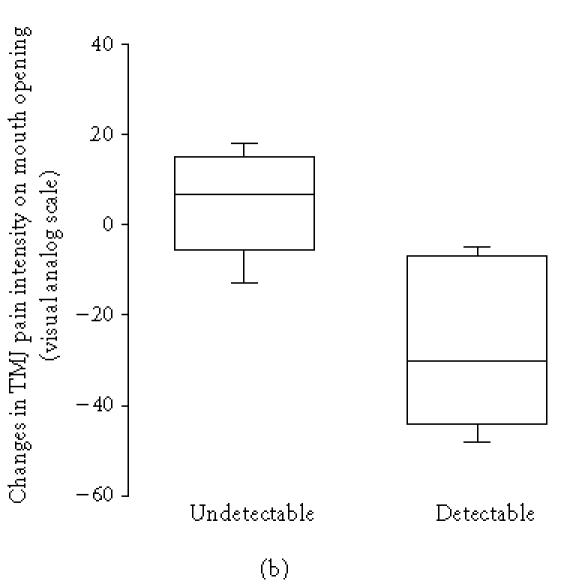
(a) Changes in temporomandibular joint (TMJ) resting pain intensity after intra-articular glucocorticoid treatment in 9 patients with undetectable (< 0.5 nmol/L) and 11 patients with detectable pretreatment levels of serotonin (5-HT) in the TMJ synovial fluid and chronic inflammatory joint disease. There was a negative correlation between 5-HT and change in resting pain after treatment (rs = −0.52, n = 20, P = .018). (b) Changes in TMJ pain intensity on mouth opening in 8 patients with undetectable (< 0.5 nmol/L) and 5 patients with detectable pretreatment levels of 5-HT in TMJ synovial fluid and chronic inflammatory joint disease. There was a negative correlation between 5-HT and change in pain intensity on mouth opening after treatment (rs = −0.57, n = 13, P = .041).
The pretreatment plasma level of 5-HT was above the normal limit (15 nmol/L) in 9 out of 10 patients before treatment and in 3 out of 6 after treatment and it was correlated to the level of C-reactive protein (rs = 0.66, n = 10, P = .038). The pretreatment plasma level of 5-HT correlated to change in TMJ pain intensity at rest (rs = 0.66, P = .040, n = 10; Figure 2a) and pressure-pain threshold after treatment (rs = 0.83, n = 10, P = .003; Figure 2b), that is, a high pretreatment level of 5-HT was associated with an increase of resting pain and pressure-pain threshold of the TMJ. The pretreatment thrombocyte count was likewise positively correlated to change in pressure-pain threshold (rs = 0.59, n = 14, P = .024).
Figure 2.
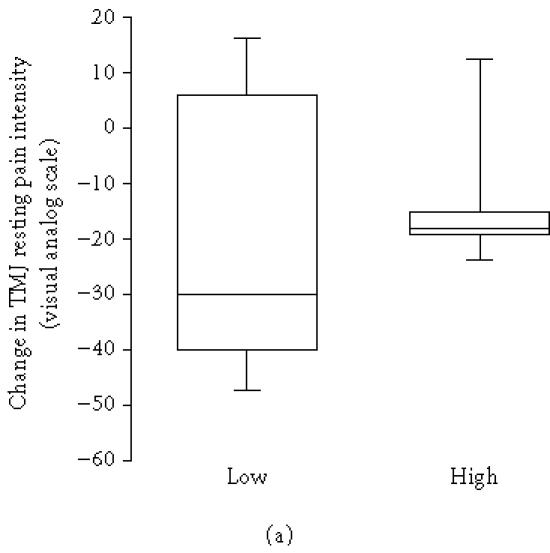
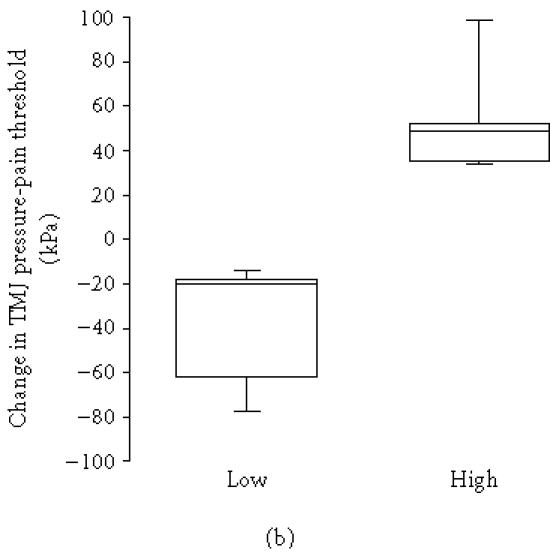
(a) Change in temporomandibular joint (TMJ) resting pain intensity after intra-articular glucocorticoid treatment in 7 patients with lower level than median group (< 37 nmol/L) and 3 patients with higher level than median pretreatment plasma levels (≥ 37 nmol/L) of serotonin (5-HT) in patients with chronic inflammatory joint disease. There was a positive correlation between 5-HT and change in resting pain intensity after treatment (rs = 0.66, n = 10, P = .040). (b) Change in temporomandibular joint (TMJ) pressure-pain threshold after intra-articular glucocorticoid treatment in 5 patients with lower level than median group (< 37 nmol/L) and 5 patients with higher level than median pretreatment plasma levels (≥ 37 nmol/L) of serotonin in patients with chronic inflammatory joint disease. There was a positive correlation between 5-HT and change in pressure-pain threshold after treatment (rs = 0.83, n = 10, P = .003).
Relation between changes after glucocorticoid treatment
The change in serum level of 5-HT was correlated to the change in number of painful mandibular movements (rs = 0.77, n = 9, P = .014).
The change in TMJ resting pain was positively correlated to change in pain intensity on maximum mouth opening (rs = 0.74, n = 13, P = .004). The significant decrease (P = .028) in lateral tenderness to palpation was negatively correlated to the change in pressure-pain threshold over the TMJ (rs = −0.50, n = 20, P = .026; Figure 3a) as well as over the glabella (rs = −0.63, n = 14, P = .016; Figure 3b), that is, a decrease in TMJ tenderness was associated with an increase in pressure-pain threshold at both sites.
Figure 3.
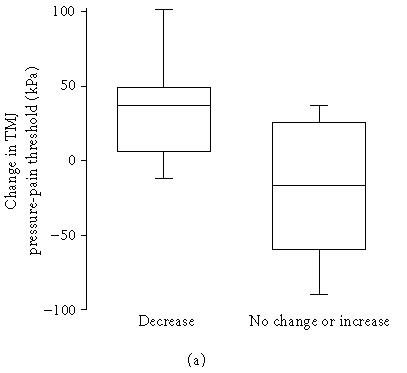
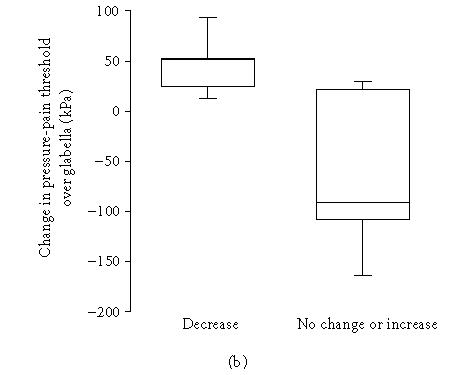
Relation between change in temporomandibular joint (TMJ) tenderness to lateral digital palpation and change in (a) TMJ and (b) glabella pressure-pain thresholds after intra-articular glucocorticoid treatment in patients with chronic inflammatory joint disease. (a) Ten patients had a decrease and 10 had no change or an increase of TMJ tenderness to lateral digital palpation. There was a negative correlation between change in TMJ tenderness to lateral digital palpation and change in TMJ pressure-pain threshold after treatment (rs = −0.50, n = 20, P = .026). (b) Five patients had a decrease and 9 had no change or an increase of TMJ tenderness to lateral digital palpation. There was a negative correlation between change in TMJ tenderness to lateral digital palpation and change in glabella pressure-pain threshold after treatment (rs = −0.63, n = 14, P = .016).
Pretreatment relations
The synovial fluid level of 5-HT was significantly correlated to neither plasma (rs = −0.14, n = 10, P = .680) nor serum level of 5-HT (rs = −0.37, n = 14, P = .190). The serum level of 5-HT was positively correlated to the plasma level of 5-HT (rs = 0.93, n = 9, P < .001).
The serum level of 5-HT was positively correlated to C-reactive protein (rs = 0.67, n = 13, P = .012), while plasma level of 5-HT was positively correlated to C-reactive protein and thrombocyte particle concentration (rs = 0.66, n = 13, P = .038, and rs = 0.68, n = 9, P = .045). C-reactive protein was also correlated to number of painful mandibular movements (rs = 0.64, n = 15, P = .010).
The thrombocyte count was correlated to plasma level of 5-HT (rs = 0.68, n = 9, P = .044).
Tenderness to lateral palpation was negatively correlated to pressure-pain threshold of the TMJ (rs = −0.68, n = 20, P < .001) as well as over the glabella (rs = −0.81, n = 14, P < .001). Pressure-pain threshold of the TMJ was correlated to pressure-pain threshold of the over the glabella (rs = 0.79, n = 14, P < .001).
DISCUSSION
This study shows that the changes in TMJ pain after intra-articular treatment with glucocorticoid are partly determined by pretreatment 5-HT in TMJ synovial fluid and plasma in chronic arthritids of systemic nature, that is, these diseases seem to include local as well as systemic serotonergic mechanisms. It also shows that the changes in pressure-pain threshold over the TMJ are influenced by pretreatment systemic 5-HT.
Patients with detectable pretreatment levels of synovial fluid 5-HT experienced a larger decrease of TMJ pain intensity at rest than those without, and likewise, more relief from TMJ pain on movement. This is supported by Alstergren et al [5] who found that a high synovial fluid level of 5-HT was associated with pain on mandibular movements. Patients with low plasma 5-HT levels had a larger decrease in TMJ resting pain than those with high levels and those with reduced serum levels of 5-HT after treatment had decreased number of painful mandibular movements. Plasma and serum levels of 5-HT were in turn correlated with C-reactive protein before treatment, which indicates that both are associated with the systemic inflammatory activity. In a previous study, high serum level of 5-HT has been associated with painful mandibular movements as well as pain on maximum mouth opening [11]. The findings in this study thus indicate a local as well as systemic influence of 5-HT on the changes in TMJ pain induced by local glucocorticoid treatment. However, the influence of local and systemic 5-HT has opposite directions, that is, detectable synovial fluid 5-HT is associated with pain relief, while high plasma levels of 5-HT are associated with less reduction of pain.
TMJ resting pain decreased significantly in this patient sample after intra-articular injection of glucocorticoid, which has been demonstrated in several other studies [1, 12, 3]. This effect seems to be influenced by free 5-HT in two compartments, that is, synovial fluid and blood. It can be hypothesized that 5-HT in both compartments may independently result in sensitization or activation of nociceptive afferents [ 13] or act more indirectly by activating other cells like T lymphocytes or monocytes/macrophages [14, 15], which in turn release algogenic mediators like TNF-α or IL-1β. Serotonin may also modulate or amplify neurogenic inflammation in chronic pain conditions by the 5-HT3 receptor, which results in local release of, for example, substance P [14, 16]. Intravenous treatment with the 5-HT3 receptor antagonist tropisetron reduced the serum substance P levels in fibromyalgia patients who responded to pain relief, which indicates that systemic serotonergic mechanisms interact with peripheral neurogenic inflammatory activity [17]. Both peripheral and central 5-HT3 receptors are pronociceptive and the peripheral 5-HT3 receptor involves specific primary afferent nociceptors [13].
In addition to the 5-HT3 receptor serotonin may act via other peripheral pain-related receptors in inflammatory conditions. In experimental adjuvant-induced inflammation of the hind paw of the rat, increased 5-HT2A receptor mRNA was expressed in calcitonin gene-related peptide-synthesizing dorsal root ganglion neurons [18]. Systemic administration of 5-HT2A receptor antagonist then produced analgesic effects on thermal hyperalgesia caused by the peripheral inflammation.
The source of synovial fluid 5-HT can be assumed to be thrombocytes or mast cells that accumulate and activate in the arthritic synovial membrane or fluid [19, 20]. The level of free 5-HT in plasma is determined by the equilibrium between free 5-HT and 5-HT stored in circulating thrombocytes as indicated by the relation between thrombocyte particle count and 5-HT in plasma in this study and serum in a previous study [11]. The thrombocytes become activated by systemic inflammation, as indicated by their relation to both C-reactive protein and plasma 5-HT, which increases the level of free 5-HT. The mode of action of glucocorticoid with regard to 5-HT cannot be determined by the data in this study, but glucocorticoid injected into the joint may inhibit release of 5-HT from thrombocytes or mast cells in the synovial membrane. This would result in a decrease of 5-HT in the synovial fluid after treatment, which was not found in this study. Another more likely explanation according to the present results is that the 5-HT is an intermediate mediator and bind to receptors on cells such as T lymphocytes or monocytes/macrophages, which in turn are activated to release inflammatory mediators with algogenic properties. These cells have intracellular glucocorticoid receptors to which glucocorticoids bind and activate to inhibit the expression of genes for, for example, proinflammatory algogenic cytokines [2]. These latter explanations are in agreement not only with the finding that TMJ pain is more reduced if detectable levels of 5-HT are present in the synovial fluid before treatment, but also with the finding that the synovial fluid level per se is not changed by the treatment. The lack of correlation between synovial fluid and plasma 5-HT levels further indicates that the effects of 5-HT in the synovial fluid and unbound 5-HT in the blood, which both may be derived from, for example, activated thrombocytes, are independent. The pain-relieving effect of intra-articular glucocorticoid in the TMJ seems to be reduced if high unbound levels of 5-HT derived from the circulation are available in the synovial tissues, which occurs when the systemic inflammatory activity is high. In this condition, the number of inflammatory cells that can be activated by 5-HT will increase and the injected glucocorticoid may not suffice to block their activity and to provide pain relief for 5 weeks. The higher systemic inflammatory activity, the more important this mechanism will be since 5-HT in plasma is correlated to C-reactive protein. The best prognosis for pain relief by intra-articular glucocorticoid administration, according to this study, can therefore be expected for patients with a combination of high level of 5-HT in synovial fluid and low level in plasma, that is, high local inflammatory activity but low systemic activity.
The changes in pressure-pain threshold of the TMJ seem to be influenced by 5-HT in plasma since a high pretreatment level was associated with increase of the threshold and low level with decrease. The blood level and not the synovial fluid level of 5-HT therefore seems to be a putative predictor for the effect of intra-articular glucocorticoid treatment on pressure-pain threshold over the TMJ. High level of 5-HT in plasma and high systemic inflammatory activity may hence predict increase of pressure-pain threshold and reduced relief of TMJ resting pain as a likely outcome of intra-articular glucocorticoid treatment. These contrasting results indicate different pain mechanisms behind TMJ resting pain and pressure-pain threshold over the TMJ. The relation between changes in pressure-pain threshold over the TMJ and lateral tenderness to digital palpation of the TMJ indicates that lateral tenderness has a partly different mechanism than TMJ resting pain, which is further illustrated by the relation between lateral tenderness of the TMJ and pressure-pain threshold over glabella.
A systemic effect by the intra-articular glucocorticoid treatment cannot be excluded but is probably weak since neither pain in general, pressure-pain threshold of the glabella, erythrocyte sedimentation rate, nor C-reactive protein was significantly changed after treatment.
In conclusion, this study shows that local and systemic serotonergic mechanisms partly determine the effect of intra-articular glucocorticoid treatment on TMJ pain in patients with chronic TMJ arthritis of systemic nature, while change in pressure-pain threshold over the TMJ is influenced by systemic serotonergic mechanisms as part of the systemic inflammatory condition.
ACKNOWLEDGMENTS
This study was financially supported by Grants from Folktandvården Sörmland (FOU), the Swedish Medical Research Council (Grant 10416), the Institute of Odontology, Karolinska Institutet, Sörmlands läns landsting (FOU; Grants 604 and 627), the Swedish National Association against Rheumatism, Signe and Reinhold Sund's Foundation, and the Swedish Dental Association.
References
- 1.Kopp S, Akerman S, Nilner M. Short-term effects of intra-articular sodium hyaluronate, glucocorticoid, and saline injections on rheumatoid arthritis of the temporomandibular joint. J Craniomandib Disord. 1991;5(4):231–238. [PubMed] [Google Scholar]
- 2.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94(6):557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 3.Alstergren P, Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. The effect on joint fluid concentration of neuropeptide Y by intra-articular injection of glucocorticoid in temporomandibular joint arthritis. Acta Odontol Scand. 1996;54(1):1–7. doi: 10.3109/00016359609003501. [DOI] [PubMed] [Google Scholar]
- 4.Taiwo YO, Levine JD. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience. 1992;48(2):485–490. doi: 10.1016/0306-4522(92)90508-y. [DOI] [PubMed] [Google Scholar]
- 5.Alstergren P, Kopp S. Pain and synovial fluid concentration of serotonin in arthritic temporomandibular joints. Pain. 1997;72(1-2):137–143. doi: 10.1016/s0304-3959(97)00022-5. [DOI] [PubMed] [Google Scholar]
- 6.Voog O, Alstergren P, Leibur E, Kallikorm R, Kopp S. Immediate effects of the serotonin antagonist granisetron on temporomandibular joint pain in patients with systemic inflammatory disorders. Life Sci. 2000;22(5):591–602. doi: 10.1016/s0024-3205(00)00965-6. [DOI] [PubMed] [Google Scholar]
- 7.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 8.Okeson JP, Adler RC, Anderson GC, et al. Differential diagnosis and management considerations of temporomandibular disorders. In: Okeson JP, editor. Orofacial Pain: Guidelines for Assessment, Diagnosis and Management. Chicago, Ill: Quintessence; 1996. pp. 113–184. [Google Scholar]
- 9.Alstergren P, Appelgren A, Appelgren B, Kopp S, Nordahl S, Theodorsson E. Measurement of joint aspirate dilution by a spectrophotometer capillary tube system. Scand J Clin Lab Invest. 1996;56(5):415–420. doi: 10.3109/00365519609088796. [DOI] [PubMed] [Google Scholar]
- 10.Alstergren P, Kopp S, Theodorsson E. Synovial fluid sampling from the temporomandibular joint: sample quality criteria and levels of interleukin-1 beta and serotonin. Acta Odontol Scand. 1999;57(1):16–22. doi: 10.1080/000163599429057. [DOI] [PubMed] [Google Scholar]
- 11.Kopp S, Alstergren P. Blood serotonin and joint pain in seropositive versus seronegative rheumatoid arthritis. Mediators Inflamm. 2002;11(4):211–217. doi: 10.1080/09629350290000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopp S, Wenneberg B, Haraldson T, Carlsson GE. The short-term effect of intra-articular injections of sodium hyaluronate and corticosteroid on temporomandibular joint pain and dysfunction. J Oral Maxillofac Surg. 1985;43(6):429–435. doi: 10.1016/s0278-2391(85)80050-1. [DOI] [PubMed] [Google Scholar]
- 13.Zeitz KP, Guy N, Malmberg AB, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22(3):1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farber L, Haus U, Spath M, Drechsler S. Physiology and pathophysiology of the 5-HT3 receptor. Scand J Rheumatol Suppl. 2004;33(119):2–8. [PubMed] [Google Scholar]
- 15.Fiebich BL, Akundi RS, Seidel M, et al. Expression of 5-HT3A receptors in cells of the immune system. Scand J Rheumatol Suppl. 2004;33(119):9–11. [PubMed] [Google Scholar]
- 16.Khalil Z, Helme RD. Serotonin modulates substance P-induced plasma extravasation and vasodilatation in rat skin by an action through capsaicin-sensitive primary afferent nerves. Brain Res. 1990;17(2):292–298. doi: 10.1016/0006-8993(90)91149-b. [DOI] [PubMed] [Google Scholar]
- 17.Stratz T, Fiebich B, Haus U, Muller W. Influence of tropisetron on the serum substance P levels in fibromyalgia patients. Scand J Rheumatol Suppl. 2004;33(119):41–43. doi: 10.1080/03009740410007023. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto K, Imbe H, Morikawa Y, et al. 5-HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain. 2002;99(1-2):133–143. doi: 10.1016/s0304-3959(02)00070-2. [DOI] [PubMed] [Google Scholar]
- 19.Zeller J, Weissbarth E, Baruth B, Mielke H, Deicher H. Serotonin content of platelets in inflammatory rheumatic diseases. Correlation with clinical activity. Arthritis Rheum. 1983;26(4):532–540. doi: 10.1002/art.1780260413. [DOI] [PubMed] [Google Scholar]
- 20.Endresen GK. Evidence for activation of platelets in the synovial fluid from patients with rheumatoid arthritis. Rheumatol Int. 1989;9(1):19–24. doi: 10.1007/BF00270285. [DOI] [PubMed] [Google Scholar]


