Abstract
Within the area of sex allocation, one of the topics that has attracted a lot of attention is the sex ratio problem. Fisher (1930) proposed that equal numbers of males and females have been promoted by natural selection and it has an adaptive significance. But the empirical success of Fisher's theory remains doubtful because a sex ratio of 0.50 is also expected from the chromosomal mechanism of sex determination. Another way of approaching the subject is to consider that Fisher's argument relies on the underlying assumption that offspring inherit their parent's tendency in biased sex ratio and therefore that genetic variance for this trait exists. Here, we analyzed sex ratio data of 56,807 piglets coming from 550 boars and 1893 dams. In addition to classical analysis of heterogeneity we performed analyses fitting linear and threshold animal models in a Bayesian framework using Gibbs sampling techniques. The marginal posterior mean of heritability was 2.63 × 10−4 under the sire linear model and 9.17 × 10−4 under the sire threshold model. The probability of the hypothesis p(h2 = 0) fitting the last model was 0.996. Also, we did not detect any trend in sex ratio related to maternal age. From an evolutionary point of view, the chromosomal sex determination acts as a constraint that precludes control of offspring sex ratio in vertebrates and it should be included in the general theory of sex allocation. From a practical view that means that the sex ratio in domestic species is hardly susceptible to modification by artificial selection.
THE theory of sex allocation predicts, given that an organism reproduces sexually, how it should allocate resources to male and female functions and constitutes one of the most popular topics in evolutionary biology. It involves many related questions such as parental investment or mating systems and there is a vast array of theoretical and empirical literature on these predictions (Charnov 1982; Frank 1990). Here we focus on one of the topics that has attracted a lot of attention within the area of sex allocation: how individuals adjust the proportion of their offspring that are male (the sex ratio problem). Fisher (1930) proposed that equal numbers of males and females have been promoted by natural selection and it has an adaptive significance. His argument is that in a population with a biased sex ratio, parents with a genetic tendency to produce more progenies of the rarer sex attain a higher average number of grandchildren, and therefore the biased sex ratio will disappear.
In mammals, males are the heterogametic (XY) and females are the homogametic (XX) sex. Therefore when male and female gametes unite randomly, half of the resulting zygotes will be XY and differentiate as males and the other half will be XX and differentiate as females. As a consequence the expected primary sex ratio, defined as the proportion of males at conception, is also expected to be 0.50 although with a substantial variance corresponding to the binomial distribution. Because the same sex ratio (0.50) is expected from both the mechanistic chromosomal sex determination and the adaptive explanation, the empirical success of Fisher's theory remains in doubt.
Another way of approaching the subject is to consider that Fisher's argument about adaptive sex ratio relies in the underlying assumption that offspring inherited their parent's tendency in biased sex ratio and therefore requires the existence of genetic variance for this trait. Then, a critical question is whether, in addition to the binomial variance, there is genetic variance for the sex ratio considered as a quantitative trait. It is well known that genetic variation is ubiquitous for almost any trait we can think of (Lynch and Walsh 1998). If such variation exists there will be an opportunity to change the sex ratio by natural selection.
Moreover, there is a practical aspect in the sex ratio issue. Attempts to manipulate sex ratio have a long history in animal breeding because it is economically advantageous to increase the proportion of males in meat production breeds or to decrease it in dairy or egg production breeds. A possibility is to practice artificial selection for sex ratio but the magnitude of response depends critically on the existence of genetic variance for this trait. If that is not the case, this possibility would be seriously compromised.
The existence of genetic variance for sex ratio has been questioned in birds and mammals, both in domestic (Hohenboken 1981) and in wild species (Charnov 1982; Clutton-Brock and Iason 1986; West et al. 2002). One of the problems is the difficulty of statistical detection of variance above the binomial variance due to chromosomal segregation. This is usually done by looking for heterogeneity among families. However, in recent years there has been a spectacular development of mixed-model and Bayesian methodology for analyzing quantitative traits. Some of these developments are well established in animal breeding and are starting to be popular in evolutionary biology (Blasco 2001; Sorensen and Gianola 2002; Kruuk 2004). In this article, we apply diverse statistical methods including sophisticated Bayesian methodology to a vast number of data on sex ratio coming from a strain of Iberian pigs to estimate the heritability of sex ratio.
An important contribution to the sex allocation theory was Trivers and Willard's (1973) prediction that if one sex gains more than the other from extra parental investment, parents with relatively more resources to invest (parents in good condition) will bias their investment toward the sex with the greater reproductive returns. Thus, for sexually dimorphic polygynous mammals, mothers in good condition to provide care to progeny should produce offspring with male-biased sex ratio, while mothers with lower maternal ability should produce female-biased sex ratio. The notion of maternal condition has also been widely discussed and it has been related to many factors such as social rank, age, parity order, or maternal nutrition (Clutton-Brock and Iason 1986).
In Trivers and Willard (1973) there is a correlation between the fitness differences within a sex and the mean fitness across families. Thus, the relevant factor on which selection is supposed to act is not the mean sex ratio produced by individuals, but rather the reaction norm that relates the sex ratio to some environmental or intrinsic factor. Here, the genetic determinism to be investigated would be in relation to the adjustment of the sex ratio rather than the sex ratio itself: the plasticity of this adjustment should be heritable. This topic is not addressed in this article but we do investigate if any trend in the relation of sex ratio with both paternal and maternal age can be inferred using these pig data and the Bayesian methodology.
MATERIALS AND METHODS
Material:
Data have been collected from Iberian pigs of the Torbiscal line, which is the result of blending of four ancient Portuguese and Spanish strains of this breed (Rodrigáñez et al. 2000). The sex of 56,807 piglets, born from 1964 to 2004 in 6775 litters proceeding from 550 boars and 1893 dams, has been recorded. The overall sex ratio or proportion of males at birth, counting both live and dead piglets, was 0.519. The complete genealogy of all the pigs is available.
Statistical methods:
Between-boars heterogeneity analysis:
We have used three methods to test if the observed variance between sires 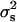 differs from a binomial distribution with the same overall sex ratio. The first method is a simple proof of χ2 of heterogeneity between sires. The component of variance between sires can be estimated, according to Robertson and Lerner (1949), as
differs from a binomial distribution with the same overall sex ratio. The first method is a simple proof of χ2 of heterogeneity between sires. The component of variance between sires can be estimated, according to Robertson and Lerner (1949), as
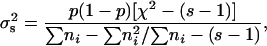 |
where p is the overall sex ratio, s is the number of sires, and ni is the number of progenies of the ith sire.
The second method to estimate 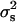 is a simplified maximum-likelihood (SML) method developed by Robertson (1951). It consists of calculating the statistic
is a simplified maximum-likelihood (SML) method developed by Robertson (1951). It consists of calculating the statistic 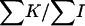 , where
, where
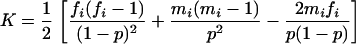 |
and
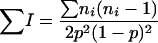 |
and mi and fi are, respectively, the number of male and female offspring of the ith sire. This statistic estimates 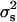 and under the null hypothesis (
and under the null hypothesis (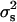 = 0) it is normally distributed
= 0) it is normally distributed 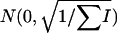 .
.
The third method, proposed by Bar-Anan and Robertson (1975) considers that if the offspring of a given sire has a sex ratio of pi based on n progenies, this gives an estimate of 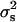 of
of 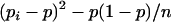 and this estimate has a variance of
and this estimate has a variance of 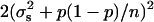 if we assume normal distributions. They then use the reciprocal of this variance as the correct weight to be given to each squared deviation. The process then proceeds by iteration from an initial value of zero.
if we assume normal distributions. They then use the reciprocal of this variance as the correct weight to be given to each squared deviation. The process then proceeds by iteration from an initial value of zero.
Because there are some doubts about how to assess the statistical significance of the results, the empirical P-values were obtained by simulation of the null hypothesis of a binomial distribution with 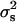 = 0.
= 0.
Between-age classes:
In addition to the χ2 of heterogeneity and SML methods previously described we also test the hypothesis of a linear trend in the sex ratio of the offspring when the age of parents increases. Following Snedecor and Cochrane (1967) the slope b is estimated as
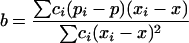 |
with
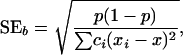 |
where ci is the number of observations per age class and x is the age average. Armitage (1955) showed that there is a simple relationship between the χ2 of heterogeneity and the linearity test,
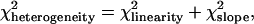 |
where 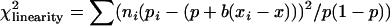 and
and 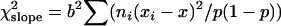 with m − 1, m − 2, and 1 d.f., respectively, being m the number of age classes.
with m − 1, m − 2, and 1 d.f., respectively, being m the number of age classes.
Bayesian analysis with a linear animal model:
A univariate linear model with repeated measures for each sire was used,
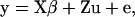 |
where 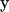 is the vector of observations (1 = male and 0 = female),
is the vector of observations (1 = male and 0 = female), 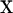 and
and 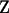 are known incidence matrices,
are known incidence matrices, 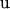 is the vector of additive genetic effects,
is the vector of additive genetic effects, 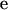 is the vector of residuals, and
is the vector of residuals, and 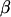 is the vector of location parameters or location effects (age of the dam). A similar univariate linear model with repeated measures for each dam and the age of dam as location effect was also fitted.
is the vector of location parameters or location effects (age of the dam). A similar univariate linear model with repeated measures for each dam and the age of dam as location effect was also fitted.
The Bayesian analyses were performed using Gibbs sampling techniques (Sorensen and Gianola 2002) to obtain inferences on the parameters of interest: variance components and heritability 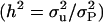 . The convergence was assessed by the double-chain method (Johnson 1996; García-Cortés et al. 1998). For each analysis, a single Gibbs chain of 6,412,800 samples was obtained, discarding the first 12,800 (warm-up) and saving only one sample from each 640 iterations. The effective number of samples was calculated from the estimated autocorrelations using time series methods (Geyer 1992) and ranged from 1500 to 2000 for the different models, resulting in low values of Monte Carlo standard errors. Flat priors were used for all the parameters. From the saved samples the usual statistics for location (posterior mean, mode, and median) were calculated. Credible intervals of the marginal posterior density were calculated as the 95% highest posterior density interval (95% HPD) and the 95% interval (0, k) containing the 95% of the probability area. These intervals reflect better than the standard deviation the dispersion of posterior distributions that can be heavily asymmetric.
. The convergence was assessed by the double-chain method (Johnson 1996; García-Cortés et al. 1998). For each analysis, a single Gibbs chain of 6,412,800 samples was obtained, discarding the first 12,800 (warm-up) and saving only one sample from each 640 iterations. The effective number of samples was calculated from the estimated autocorrelations using time series methods (Geyer 1992) and ranged from 1500 to 2000 for the different models, resulting in low values of Monte Carlo standard errors. Flat priors were used for all the parameters. From the saved samples the usual statistics for location (posterior mean, mode, and median) were calculated. Credible intervals of the marginal posterior density were calculated as the 95% highest posterior density interval (95% HPD) and the 95% interval (0, k) containing the 95% of the probability area. These intervals reflect better than the standard deviation the dispersion of posterior distributions that can be heavily asymmetric.
Bayesian analysis with a threshold animal model:
Traits with binary or “all or none” responses are very common in quantitative genetics and are usually analyzed using a threshold model. In this model, the discrete response is related to a hypothetical unobservable underlying continuous variable often called liability (after Falconer 1981). For example, if there are two categories (male and female) the observation would be in the second class if it exceeds the threshold. Although this threshold is fixed, the distribution of the underlying variable could have different means for each sire and therefore the sex ratio of the offspring would be different for different sires.
A univariate linear model for the liability of each record was used,
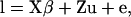 |
where 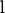 is the vector of unobservable liabilities of each record,
is the vector of unobservable liabilities of each record, 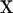 and
and 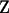 are known incidence matrices relating, respectively, location parameters (
are known incidence matrices relating, respectively, location parameters (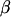 ) and random effects (
) and random effects (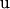 ) to
) to 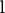 ,
, 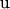 is the vector of additive genetic effects of sires,
is the vector of additive genetic effects of sires, 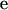 is the vector of random residuals, and
is the vector of random residuals, and 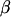 is the vector of location effects (age of dam). The observed categorical data y will be 1 = male or 0 = female according to the conditional probability
is the vector of location effects (age of dam). The observed categorical data y will be 1 = male or 0 = female according to the conditional probability 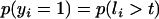 , where t is the fixed threshold. A similar threshold linear model with repeated measures for each dam and age of dam as location effect was also fitted.
, where t is the fixed threshold. A similar threshold linear model with repeated measures for each dam and age of dam as location effect was also fitted.
The testing of the Bayesian hypothesis of heritability being null is not a simple question. Recently, García-Cortés et al. (2001) developed a method to use Bayes' factors for testing whether the heritability is null. They assume flat unbounded prior distributions for the location effects and the prior distribution for the heritability is given by
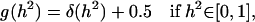 |
where 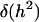 stands for the Dirac delta function. Under these assumptions the probability of the hypothesis of zero heritability from the conventional marginal posterior
stands for the Dirac delta function. Under these assumptions the probability of the hypothesis of zero heritability from the conventional marginal posterior 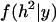 is
is
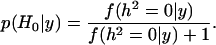 |
Although the use of Bayes' factors with improper priors is questionable, in our case the relevant variable, that is, the heritability has a well-defined prior probability despite that it is a ratio of two unbounded variables. The impropriety has been hidden rather than eliminated but this is enough for our purposes (García-Cortés et al. 2001).
A single Gibbs sampler chain of 101,300 cycles was implemented and the first 1300 were rejected as burn-in. The burn-in length was determined by running an extra chain with different starting values and the same sequence of random numbers (Johnson 1996; García-Cortés et al. 1998). After burn-in, the marginal posterior density for the heritability was obtained after averaging the full conditional distributions obtained in each cycle.
RESULTS
Between-boars heterogeneity analysis:
The number of progenies per boar ranged from 1 to 928, and the sex ratio of the diverse sire families ranged from 0 to 0.526. Assuming that sires may control the sex ratio of their offspring, the variance of these sex ratios will be a measure of the phenotypic variance once we have discounted the binomial variance inherent to the trait.
The simple proof of χ2 of heterogeneity, when information on all the 550 boars was used, gave a χ2-value of 628.59 (P < 0.01), but decreased to a value of χ2 = 521.41 (P < 0.03) when only the 464 boars with a number of offspring >20 piglets were considered. The statistical significance disappeared when using only the 336 boars with >40 offspring (χ2 = 366.25, P < 0.12), bringing the estimate of the variance between sires to 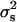 = 3.55 × 10−4. The values of the statistics
= 3.55 × 10−4. The values of the statistics 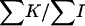 and
and 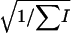 that estimate
that estimate 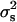 and its standard deviation were, respectively, 1.10 × 10−4 and 0.92 × 10−4 (P < 0.12). Finally, the procedure of Bar-Anan and Robertson (1975) provided estimated values of
and its standard deviation were, respectively, 1.10 × 10−4 and 0.92 × 10−4 (P < 0.12). Finally, the procedure of Bar-Anan and Robertson (1975) provided estimated values of 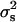 and its standard deviation of 24.45 × 10−4 and 188.36 × 10−4, respectively (P < 0.45).
and its standard deviation of 24.45 × 10−4 and 188.36 × 10−4, respectively (P < 0.45).
The empirical P-values obtained by simulation of the null hypothesis were P < 0.01, P < 0.11, and P < 0.24 for the χ2, SML, and Bar-Anan and Robertson methods, respectively.
Between-age classes:
The ages of dams (in semesters) were grouped in 17 classes, with ranks from 24 to 9449 observations and from 0.44 to 0.54 for the sex ratio values. No heterogeneity was found among dams' age classes in both the χ2 of heterogeneity (χ2 = 13.62, P < 0.63) and the SML method (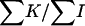 = −0.12 × 10−4,
= −0.12 × 10−4, 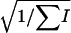 = 0.18 × 10−4, P < 0.76). The regression coefficient and its standard error were, respectively, b = 2.76 × 10−4 and SEb = 3.79 × 10−4, and the partition of the χ2 of heterogeneity results in a value of
= 0.18 × 10−4, P < 0.76). The regression coefficient and its standard error were, respectively, b = 2.76 × 10−4 and SEb = 3.79 × 10−4, and the partition of the χ2 of heterogeneity results in a value of  = 13.09 and
= 13.09 and 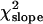 = 0.53.
= 0.53.
The ages of boars (in semesters) were grouped in 13 classes, with a very unbalanced number of observations (from 61 to 12,586) and a wide range of sex ratio values (from 0.46 to 0.64). No heterogeneity was found among boars' age classes in both the χ2 of heterogeneity (χ2 = 15.63, P < 0.21) and the SML method (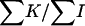 = −0.64 × 10−6,
= −0.64 × 10−6, 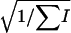 = 1.60 × 10−5, P < 0.48). The regression coefficient on the age class number and its standard error were b = 1.44 × 10−4 and SEb = 5.2 × 10−4, respectively, and the partition of the χ2 of heterogeneity results in values of
= 1.60 × 10−5, P < 0.48). The regression coefficient on the age class number and its standard error were b = 1.44 × 10−4 and SEb = 5.2 × 10−4, respectively, and the partition of the χ2 of heterogeneity results in values of  = 15.56 and
= 15.56 and 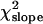 = 0.08.
= 0.08.
Bayesian analysis with the linear animal model:
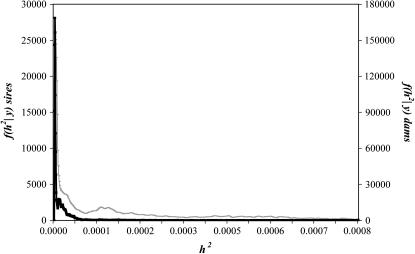
The posterior means of the additive 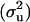 and phenotypic
and phenotypic 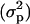 variances were 6.57 × 10−5 and 0.25, respectively, under the sire linear model. The posterior distribution of heritability is plotted in Figure 1. The posterior mean, median, mode, and the 95% interval (0, k) of this distribution were, respectively, 2.63 × 10−4, 1.10 × 10−4, 2.01 × 10−6, and [0, 1.06 × 10−3]. The effects of the mother age classes on the offspring's sex ratios are given in Table 1. An alternative model with the sire age as location effect was also considered. The effects of the sire age classes were irrelevant, and the posterior mean and 95% interval (0, k) of the heritability were 2.97 × 10−4 and [0, 9.75 × 10−4].
variances were 6.57 × 10−5 and 0.25, respectively, under the sire linear model. The posterior distribution of heritability is plotted in Figure 1. The posterior mean, median, mode, and the 95% interval (0, k) of this distribution were, respectively, 2.63 × 10−4, 1.10 × 10−4, 2.01 × 10−6, and [0, 1.06 × 10−3]. The effects of the mother age classes on the offspring's sex ratios are given in Table 1. An alternative model with the sire age as location effect was also considered. The effects of the sire age classes were irrelevant, and the posterior mean and 95% interval (0, k) of the heritability were 2.97 × 10−4 and [0, 9.75 × 10−4].
Figure 1.
Marginal posterior density of the heritability of sex ratio under the linear animal model inferred from sires (thin line) or from dams (thick line).
TABLE 1.
Effects of the classes of mother age on the sex ratio (sire linear model) and on the liability (sire threshold model)
| Classes of mother age (yr) | Linear model
|
Threshold model
|
|||
|---|---|---|---|---|---|
| No. piglets | Mean (×10−2) | 95% HPD (×10−2) | Mean (×10−2) | 95% HPD (×10−2) | |
| 1–1.5 | 9205 | 0.00 | 0.00 | ||
| 1.5–2 | 9449 | 0.77 | −0.70/2.27 | 1.84 | −1.74/5.43 |
| 2–2.5 | 8705 | 0.34 | −1.17/1.83 | 0.69 | −3.07/4.44 |
| 2.5–3 | 7746 | 0.87 | −0.65/2.43 | 2.18 | −1.61/5.97 |
| 3–3.5 | 6193 | 1.04 | −0.61/2.67 | 2.60 | −1.46/6.66 |
| 3.5–4 | 4658 | 0.99 | −0.91/2.70 | 2.41 | −2.07/6.89 |
| 4–4.5 | 3512 | 1.58 | −0.40/3.56 | 3.94 | −1.02/8.89 |
| 4.5–5 | 2554 | 1.74 | −0.51/3.97 | 4.30 | −1.26/9.85 |
| 5–5.5 | 1840 | 0.74 | −1.82/3.26 | 1.86 | −4.56/8.29 |
| 5.5–6 | 1238 | 3.29 | 0.24/6.28 | 8.06 | 0.40/15.73 |
| 6–6.5 | 771 | 0.20 | −3.58/3.97 | 0.52 | −9.01/10.06 |
| 6.5–7 | 426 | −2.11 | −7.07/2.85 | −5.57 | −18.12/6.98 |
| 7–7.5 | 285 | 0.06 | −5.98/5.99 | 0.01 | −14.89/14.92 |
| 7.5–8 | 109 | −7.11 | −16.70/2.73 | −18.06 | −42.72/5.95 |
| 8–8.5 | 54 | 8.16 | −5.58/21.82 | 20.59 | −14.24/55.41 |
| 8.5–9 | 38 | 1.60 | −14.64/17.84 | 3.89 | −37.06/44.85 |
| 9–9.5 | 24 | 3.10 | −17.46/23.62 | 7.42 | −44.26/59.06 |
The posterior distribution of heritability when a model with repeated measures for each dam and age of dam as effect was fitted is also plotted in Figure 1. Under this model the estimate of heritability as the posterior mean was even lower: 3.04 × 10−5 (95% interval (0, k) = [0, 1.2 × 10−4]).
Bayesian analysis with a threshold model:
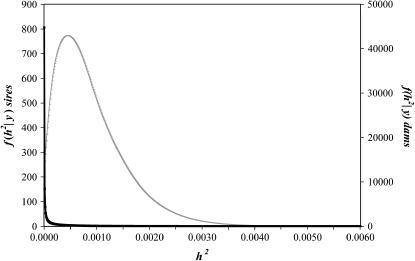
The marginal posterior density of the heritability for the sire model is presented in Figure 2. The marginal expectation was 9.17 × 10−4 and the 95% interval (0, k) was [0, 2.3 × 10−4]. The marginal posterior density at zero was 227.3, which represents the corresponding Bayes factor 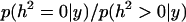 = 227.3, which resulted in
= 227.3, which resulted in 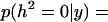 0.996. Mothers' age effects were also very small as in the previous analysis (Table 1).
0.996. Mothers' age effects were also very small as in the previous analysis (Table 1).
Figure 2.
Marginal posterior density of the heritability of sex ratio under the threshold animal model inferred from sires (thin line) or from dams (thick line).
The dam threshold linear model with the age of dam as location effect gives a value of posterior mean heritability of 2.28 × 10−4 with a 95% interval (0, k) of [0, 0.8 × 10−4]. The Bayes factor in favor of the hypothesis of zero heritability was 447.5 and the probability of the heritability being null was 0.998.
DISCUSSION
In most typical chromosomal sexual determination the expected sex ratio at conception is 0.50. This is also the expected value if the sex ratio were a trait optimized by natural selection as Fisher suggested. For this reason the validation of Fisher's theory would not be straightforward. More important is that all the arguments regarding sex ratio theory assume that genetic variance for sex ratio exists. Strictly, Fisher's model is about the way in which selection would act on the genetic variance for sex ratio if it were present. It could be argued that natural selection might have led to the fixation of alleles that produce an equal sex ratio and therefore genetic variance is not present in the current population. However, this argument seems far fetched. First, the input of mutational variance for quantitative traits is substantial, 0.001 times the environmental variance. Second, it is very hard to find any traits where genetic variance is absent (Lynch and Walsh 1998).
Furthermore, the first requisite for the existence of genetic variance is that there is phenotypic variance. But even the detection of phenotypic variance is not an easy task because we must discount the binomial variance inherent to chromosomal segregation. In a simulation study, Dobao et al. (1982) concluded that data from 160 half-sib families of size 500 or 60 half-sib families of size 1000 would be required for detecting values of variances between sires of ∼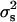 = 0.015. These numbers are very difficult to gather except in livestock populations.
= 0.015. These numbers are very difficult to gather except in livestock populations.
The values of 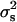 estimated from our data with the χ2, SML, and Bar-Anan and Robertson methods were 3.55 × 10−4, 1.10 × 10−4, and 0.92 × 10−4, with empirical levels of significance of P < 0.01, P < 0.11, and P < 0.24, respectively. Indeed, the close agreement with the expectation from binomial sampling was surprising, because at least some small environmental differences in sex-differential fetal mortality among progenies would be expected (Krackow 1995). The results are similar to the previous finding of Dobao et al. (1982), in Torbiscal and other related strains of Iberian pigs. They found values of
estimated from our data with the χ2, SML, and Bar-Anan and Robertson methods were 3.55 × 10−4, 1.10 × 10−4, and 0.92 × 10−4, with empirical levels of significance of P < 0.01, P < 0.11, and P < 0.24, respectively. Indeed, the close agreement with the expectation from binomial sampling was surprising, because at least some small environmental differences in sex-differential fetal mortality among progenies would be expected (Krackow 1995). The results are similar to the previous finding of Dobao et al. (1982), in Torbiscal and other related strains of Iberian pigs. They found values of 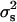 ranging from 0.66 to 7.01 (×10−4). Switonski (1979) also did not find heterogeneity in data of 47,234 piglets from 153 sires and Hohenboken (1981) quotes earlier studies in pigs with the same negative result. In cattle, Bar-Anan and Robertson (1975) and Skjervold and James (1972) estimated the variance between sires (
ranging from 0.66 to 7.01 (×10−4). Switonski (1979) also did not find heterogeneity in data of 47,234 piglets from 153 sires and Hohenboken (1981) quotes earlier studies in pigs with the same negative result. In cattle, Bar-Anan and Robertson (1975) and Skjervold and James (1972) estimated the variance between sires (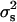 ) as 2.26 ± 0.76 (×10−4) and 3.66 ± 0.44 (×10−4), indicating real differences among families attributed to differential survival of zygotes of both sexes. In poultry, Merat (1970) and Foster and McSherry (1980) found heterogeneity between the offspring of different sires but it was probably an artifact due to the nonrandomization of the sexing process. The early reviews by Williams (1979), Charnov (1982), and Clutton-Brock and Iason (1986) found that heritable differences are small or absent in wild birds and mammals, and this has been also found in more recent studies by Krackow (1995), Hardy (1997), and West et al. (2002). Finally, as Maynard Smith (1980) pointed out there is no evidence of genetic variance of the sex ratio in man, despite massive amounts of data, and second, the sex ratio of domestic poultry and cattle remains obstinately unchanged despite great economic advantages in biased sex ratio.
) as 2.26 ± 0.76 (×10−4) and 3.66 ± 0.44 (×10−4), indicating real differences among families attributed to differential survival of zygotes of both sexes. In poultry, Merat (1970) and Foster and McSherry (1980) found heterogeneity between the offspring of different sires but it was probably an artifact due to the nonrandomization of the sexing process. The early reviews by Williams (1979), Charnov (1982), and Clutton-Brock and Iason (1986) found that heritable differences are small or absent in wild birds and mammals, and this has been also found in more recent studies by Krackow (1995), Hardy (1997), and West et al. (2002). Finally, as Maynard Smith (1980) pointed out there is no evidence of genetic variance of the sex ratio in man, despite massive amounts of data, and second, the sex ratio of domestic poultry and cattle remains obstinately unchanged despite great economic advantages in biased sex ratio.
In this study we have introduced some additional analysis on the basis of more sophisticated models than those used previously. The first is a sire linear animal model solved in a Bayesian framework via Gibbs sampling. This allows us to have a more complete description of the marginal posterior distribution of the heritability of sex ratio inferred from the sex of each sire's progeny (Figure 1), which has a very low mean value (2.63 × 10−4) with the probability of the heritability being <1.06 × 10−3 being 0.95. The second is a sire threshold animal model, also solved via Monte Carlo Markov chain methods, that leads to a heritability value of 9.17 × 10−4 on the underlying scale (Figure 2). Variance component estimation assuming a threshold model always results in greater values than using conventional linear models. Testing the hypothesis of zero heritability does not have a clear and accepted method. The asymptotic properties of the likelihood-ratio test fail at the lower bound of the parametric space (0, 1), and on the other hand several methods based on calculating the Bayes factor have not been generally accepted. Here we follow the reparameterization proposed by García-Cortés et al. (2001) that allows a probability to be assigned to the hypothesis that h2 = 0. The value of this probability is indeed very high: p(h2 = 0|y) = 0.996. We have emphasized the calculation of heritability estimate from boar sex ratio because male sperm are the essential determinants of zygote sex. However, dams might influence the sex determination through sperm competition or early selective abortions that do not affect fitness. When we fit similar dam models we obtain an even lower heritability value, with a probability of being zero of 0.998.
In our case the overall population sex ratio at birth was 0.519, slightly biased as happens in most mammals because of higher male mortality before the end of parental care. In humans where there are the most data, this bias is even more pronounced. Such bias is consistent with Fisherian expectation, which is for the total investment in males and females to be equal at the end of the period of parental investment, and a biased sex ratio in favor of males is expected to compensate for the higher male mortality before the end of parental care. However, the absence of genetic variation for sex ratio leads us to favor a nonadaptive hypothesis to justify this bias, such as differences in mortality between sexes or special physiological properties of the reproductive system (Krackow 1995). In humans, for example, when fertilization occurs early in the menstrual cycle the odds of male conception increase (James 1986).
The mother's age is one of the factors, together with social rank, parity, nutrition, or stress that have been related to “maternal condition” (Clutton-Brock and Iason 1986). In a recent metaanalysis of 37 studies of 18 ungulate species, Sheldon and West (2004) show that studies using behavioral dominance as a measure of maternal condition lead to a substantial correlation between maternal condition and sex ratio (r = 0.17–0.25), whereas studies that used morphological or physiological measures of condition provide little or no evidence for this relationship (r = 0.05–0.06). One of the studies included is Meikle et al. (1996), who found a higher proportion of males piglets (0.59) born to high-ranking sows and a low proportion of males (0.42) born to low-ranking sows. However, Mendl et al. (1995) did not find this effect studying the same relationship but with a different protocol. In any case in the study of Meikle et al. (1996) there was no change in the proportion of sons born with increasing parity or maternal age.
Life-history theory generally predicts that the reproductive effort of multiparous females increases with age and parity. However, the phenotypic trajectory along the time of measures of reproductive effort shows different patterns. In some mammal species, litter size, birth weight, and neonatal survival remain more or less constant, but in other cases these traits increase first and decline at the end of the reproductive period. Hewison et al. (2002) review 16 studies in ungulate species (not including pigs) specifically tested for maternal age effects on sex ratio but do not find any evidence for a significant relationship. They argue that the use of age as a proxy for maternal condition is problematic because of the conflicting effects of increasing condition with age (e.g., because of social rank or experience) but declining condition due to senescence.
In Iberian pigs, Rodríguez et al. (1994) found that the litters and individual piglets heaviest at weaning are those born in the second parity and, thereafter, a negative effect of parity on piglet and litter weight is observed. More recently, Fernández et al. (2005) from analyses fitting random regression models described a similar negative relationship between litter weight at weaning (maternal ability) and parity order. Although the analyzed population of Iberian pigs fulfills all the assumptions of the Trivers and Willard hypothesis, we have not found any relationship between sex ratio and either the paternal or the maternal age in any of the analyses realized. Sex ratio in Iberian pigs is not dependent on the sow's ability to rear piglets.
In summary, for a majority of animals the mechanism of male or female heterogamety poses a powerful constraint in the ability to control sex ratio at conception and suggests that this trait differs from most metrical traits in its underlying genetic control. From a practical point of view that means that the sex ratio is hardly susceptible to modification by artificial selection. Even if such genetic variation exists its magnitude would be so small that the number of individuals needed to evaluate the trait would make it difficult to include in breeding plans. Techniques that try to increase X- or Y-bearing sperm by mechanical, chemical, or immunological treatments of semen (Habermann et al. 2005) or by reproductive methodologies such as the time of insemination (Gutierrez-Adán et al. 1999) would be of more practical value. From an evolutionary point of view, the chromosomal sex determination acts as a constraint that precludes control of offspring sex ratio in vertebrates and it should be included in the more general theory of sex allocation (Charnov 1982). For example, Maynard Smith (1980) has argued that an appropriate model for the evolution of sexual allocation in the higher vertebrates is to assume that the primary sex ratio is fixed at unity, parents can recognize the sex of individual offspring, and the returns (in offspring fitness) are different for the two sexes. He shows that for this model it is an evolutionary stable strategy to invest differently in sons and daughters.
Acknowledgments
We thank the staff of Centro de Investigación Agropecuaria “Dehesón del Encinar” (Oropesa, Spain) for careful data recording. This manuscript was improved by comments from Ben Sheldon (University of Oxford) and B. Villanueva (Scottish Agricultural College, Edinburgh). Research was funded by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria grant C03-022.
References
- Armitage, P., 1955. Test for linear trends in proportions and frequencies. Biometrics 11: 375–386. [Google Scholar]
- Bar-Anan, R., and A. Robertson, 1975. Variation in sex ration between progeny groups in dairy cattle. Theor. Appl. Genet. 46: 63–65. [DOI] [PubMed] [Google Scholar]
- Blasco, A., 2001. The Bayesian controversy in animal breeding. J. Anim. Sci. 79: 2023–2046. [DOI] [PubMed] [Google Scholar]
- Charnov, E. L., 1982. The Theory of Sex Allocation. Princeton University Press, Princeton, NJ.
- Clutton-Brock, T. H., and G. R. Iason, 1986. Sex ratio variation in mammals. Q. Rev. Biol. 61: 339–374. [DOI] [PubMed] [Google Scholar]
- Dobao, M. T., J. Rodrigáñez, L. Silió and M. A. Toro, 1982. Sex ratio variation in Iberian pig, pp. 537–542 in 2nd World Congress on Genetics Applied to Livestock Production, Vol. 8. Wageningen Academic Publishers, Madrid.
- Falconer, D. S., 1981. Introduction to Quantitative Genetics. Longman, New York.
- Fernández, A., M. C. Rodriguez, J. Rodrigáñez and L. Silió, 2005. Genetic evaluation of mothering ability for multiple parities in Iberian pigs, p. 344 in 56th Annual Meeting of the European Association for Animal Production, Vol. 11. Neografis, Uppsala, Sweden.
- Fisher, R. A., 1930. The Genetical Theory of Natural Selection. Oxford University Press, Oxford.
- Foster, W. H., and D. M. G. McSherry, 1980. A search for genetic variation in the sex ration at hatching. Br. Poult. Sci. 21: 131–134. [DOI] [PubMed] [Google Scholar]
- Frank, S. A., 1990. Sex allocation theory for birds and mammals. Annu. Rev. Ecol. Syst. 21: 13–55. [Google Scholar]
- García-Cortés, L. A., M. Rico and E. Groeneveld, 1998. Using coupling with the Gibbs sampler to assess convergence in animal models. J. Anim. Sci. 76: 441–447. [DOI] [PubMed] [Google Scholar]
- García-Cortés, L. A., C. Cabrillo, C. Moreno and L. Varona, 2001. Hypothesis testing for the genetic background of quantitative traits. Genet. Sel. Evol. 33: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer, C. J., 1992. Practical Markov chain Monte Carlo. Stat. Sci. 7: 473–511. [Google Scholar]
- Gutierrez-Adán, A., S. Pérez-Garnelo, J. Granados, J. J. Gaarde, M. Pérez-Guzmán et al., 1999. Relationship between sex ratio and time of insemination according to both time of ovulation and maturational state of oocyte. Zygote 7: 37–43. [DOI] [PubMed] [Google Scholar]
- Habermann, F. A., A. Winter, I. Olsaker, P. Reichert and R. Fries, 2005. Validation of sperm sexing in the cattle (Bos taurus) by dual colour fluorescence in situ hybridization. J. Anim. Breed. Genet. 122: 22–27. [DOI] [PubMed] [Google Scholar]
- Hardy, I. C. W., 1997. Possible factors influencing vertebrate sex ratio: an introductory overview. Appl. Anim. Behav. Sci. 51: 217–241. [Google Scholar]
- Hewison, A. J. M., J. M. Gaillard, R. Blanchard and M. Festa-Bianchet, 2002. Maternal age is not a predominant determinant of progeny sex ratio variation in ungulates. OIKOS 98: 334–339. [Google Scholar]
- Hohenboken, W. D., 1981. Possibilities for genetic manipulation of sex ration in livestock. J. Anim. Sci. 52: 265–277. [DOI] [PubMed] [Google Scholar]
- James, W. H., 1986. Hormonal control of sex ratio. J. Theor. Biol. 118: 427–441. [DOI] [PubMed] [Google Scholar]
- Johnson, V. E., 1996. Studying convergence of Markov chain Monte Carlo algorithms using coupled sample paths. J. Am. Stat. Assoc. 91: 154–166. [Google Scholar]
- Krackow, S., 1995. Potential mechanisms for sex ratio adjustment in mammals and birds. Biol. Rev. 70: 235–241. [DOI] [PubMed] [Google Scholar]
- Kruuk, L. E. B., 2004. Estimating genetic parameters in natural populations using the ‘animal model’. Philos. Trans. R. Soc. Lond. B 359: 873–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, M., and B. Walsh, 1998. Genetics and Analysis of Quantitative Traits. Sinauer Associates, Sunderland, MA.
- Maynard Smith, J., 1980. A new theory of sexual investment. Behav. Ecol. Sociobiol. 7: 247–251. [Google Scholar]
- Meikle, D. B., L. C. Drickamer, S. H. Vessey, R. D. Arthur and T. L. Rosenthal, 1996. Dominance rank and parental investment in swine (Sus scrofa domesticus). Ethology 102: 969–978. [Google Scholar]
- Mendl, M., A. J. Zanella, D. M. Broom and C. T. Whittemore, 1995. Maternal social status and birth sex ratio in domestic pigs: an analysis of mechanism. Anim. Behav. 50: 1361–1370. [DOI] [PubMed] [Google Scholar]
- Merat, P., 1970. Proportion des sexes chez les volailles: variation dans la descendance du meme pere suivant la date d'eclosion. Ann. Génét. Sel. Anim. 2: 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, A., 1951. The analysis of heterogeneity in the binomial distribution. Ann. Eugen. 16: 1–15. [DOI] [PubMed] [Google Scholar]
- Robertson, A., and I. M. Lerner, 1949. The heritability of all-or-none traits: viability of poultry. Genetics 34: 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigáñez, J., M. A. Toro, C. Rodríguez and L. Silió, 2000. Alleles survival from Portuguese and Spanish strains in Iberian pig. Options Méditerranéennes 41: 57–61. [Google Scholar]
- Rodríguez, C., J. Rodrigáñez and L. Silió, 1994. Genetic analysis of maternal ability in Iberian pigs. J. Anim. Breed. Genet. 111: 220–227. [DOI] [PubMed] [Google Scholar]
- Sheldon, B. C., and A. West, 2004. Maternal dominance, maternal condition and offspring sex ratio in ungulate mammals. Am. Nat. 163: 40–54. [DOI] [PubMed] [Google Scholar]
- Skjervold, H., and J. W. James, 1972. Causes of variation in the sex ratio in dairy cattle. Z. Zuchtungsbiol. 95: 293–305. [Google Scholar]
- Snedecor, G. W., and W. G. Cochrane, 1967. Statistical Methods, Ed. 6. Iowa State University Press, Ames, IA.
- Sorensen, D., and D. Gianola, 2002. Likelihood, Bayesian and MCMC Methods in Quantitative Genetics. Springer-Verlag, New York.
- Switonski, M., 1979. Estimation of boar and sow influences on sex ratio in progeny. Rocz. Acad. Roln. W. Pozamann 101: 177–184. [Google Scholar]
- Trivers, R. L., and D. E. Willard, 1973. Natural selection of parental ability to vary the sex ratio of offspring. Science 179: 90–92. [DOI] [PubMed] [Google Scholar]
- West, S. A., S. E. Reece and B. C. Sheldon, 2002. Sex ratios. Heredity 88: 117–124. [DOI] [PubMed] [Google Scholar]
- Williams, G. C., 1979. The question of adaptive sex ratio in outcrossed vertebrates. Proc. R. Soc. Lond. Ser. B 205: 567–580. [DOI] [PubMed] [Google Scholar]