Abstract
In C. elegans, DOG-1 prevents deletions that initiate in polyG/polyC tracts (G/C tracts), most likely by unwinding secondary structures that can form in G/C tracts during lagging-strand DNA synthesis. We have used the dog-1 mutant to assay the in vivo contribution of various repair genes to the maintenance of G/C tracts. Here we show that DOG-1 and the BLM ortholog, HIM-6, act synergistically during replication; simultaneous loss of function of both genes results in replicative stress and an increase in the formation of small deletions that initiate in G/C tracts. Similarly, we demonstrate that the C. elegans orthologs of the homologous recombination repair genes BARD1, RAD51, and XPF and the trans-lesion synthesis polymerases polη and polκ contribute to the prevention of deletions in dog-1 mutants. Finally, we provide evidence that the small deletions generated in the dog-1 background are not formed through homologous recombination, nucleotide excision repair, or nonhomologous end-joining mechanisms, but appear to result from a mutagenic repair mechanism acting at G/C tracts. Our data support the hypothesis that absence of DOG-1 leads to replication fork stalling that can be repaired by deletion-free or deletion-prone mechanisms.
MAINTAINING the integrity of DNA during replication is essential for genome stability. Replication forks can stall as a result of lesions induced by exogenous agents, such as UV light or hydroxyurea (Warner and Hobbs 1969; Edenberg 1976), or as a result of endogenous causes, such as proteins bound to the DNA or secondary structures in DNA that impede the movement of the replisome (Hwang and Kornberg 1990; Trinh and Sinden 1991). It has been suggested that guanine-rich DNA can form G-quadruplex structures within single-stranded DNA during replication, creating blockages that can stall or collapse replication forks (Woodford et al. 1994; Arthanari and Bolton 2001). The importance of preventing the formation of such G-rich secondary structures during replication was demonstrated by the identification of the similar helicase-like-proteins, DOG-1 (for deletions of guanine), which functions to prevent deletions in polyG/polyC tracts (G/C tracts) in Caenorhabditis elegans (Cheung et al. 2002), and RTEL (for regulator of telomere length), which is proposed to function in the resolution of G-rich DNA structures occurring at telomeres in mice (Ding et al. 2004). Recently, the human gene most similar to dog-1, BRIP1, was found to be defective in a subset of patients with Fanconi anemia (Levitus et al. 2005; Levran et al. 2005).
In dog-1 mutants, de novo deletions with a typical size of several hundred nucleotides were shown to occur, initiating in G/C tracts >18 nucleotides in length (Cheung et al. 2002). Because the deletions always initiated near the 3′-end of the G tract and extended upstream for various distances, Cheung et al. (2002) proposed that DOG-1 is required to unwind secondary structures that form in single-stranded G-rich DNA during lagging-strand DNA synthesis. In total, there are nearly 400 G/C tracts of 18 or more consecutive guanines in the C. elegans genome (http://www.wormbase.org). Although G/C tracts are less common in other organisms, regions of G-rich DNA with the potential to form secondary structures like G-quadruplex are prevalent in the human genome (Todd et al. 2005) and occur in telomeres, at recombination hotspots, and in gene promoters, among other sites (Simonsson 2001). If only a portion of G/C tracts form secondary structures in C. elegans, DOG-1 would have a large number of targets. While dog-1 mutants display a mutator phenotype and a slightly reduced brood size, they are otherwise viable and appear healthy. This raises the question of why loss of DOG-1 function does not have more severe effects on the animal. Presumably, there are other proteins or repair pathways that can act to maintain G/C tracts in the absence of DOG-1.
In addition to DOG-1, several other proteins have been implicated in the processing of G-rich DNA secondary structures such as G-quadruplex DNA. The RecQ helicases, BLM and WRN, mutated in Bloom's and Werner's syndromes, respectively, have been shown to unwind G-quadruplex DNA in vitro (Sun et al. 1998; Mohaghegh et al. 2001; Huber et al. 2002). It has also been suggested that BLM can restart replication forks by catalyzing reverse branch migration of the collapsed fork, preventing the need for additional repair mechanisms (Karow et al. 2000). Evidence from budding yeast has shown that the RecQ helicase Sgs1p can stabilize the polymerase at stalled replication forks (Bjergbaek et al. 2005) and has the ability to resolve recombination-dependent intermediates that occur at damaged replication forks (Liberi et al. 2005). The C. elegans BLM ortholog, HIM-6, has also been implicated in repair, where it has been suggested to function downstream of RAD-51 (Wicky et al. 2004; Grabowski et al. 2005). A recent study has shown that HIM-6 functions in actively dividing cells, including the mitotic germ nuclei and in early embryonic cells (Kim et al. 2005), although the exact mechanism by which it functions is unclear. In the absence of DOG-1, HIM-6 might unwind secondary structures directly or resolve secondary structures through RAD-51-dependent or -independent mechanisms.
If the absence of DOG-1 leads to replication fork stalling, several other proteins might be involved in G/C tract maintenance through various repair pathways. Stalled replication forks can be restarted by homologous recombination, involving the RecA homolog RAD51 (Broomfield et al. 2001; Michel et al. 2001; McGlynn and Lloyd 2002; Lundin et al. 2003; West 2003; Saleh-Gohari et al. 2005). This mechanism might be similar to that described for bacteria, in which it has been demonstrated that a stalled fork can reverse to form a structure similar to a Holliday junction, which is a substrate for homologous recombination (McGlynn and Lloyd 2001; Michel et al. 2001). Trans-lesion synthesis by alternative polymerases can also allow replication past lesions, such as secondary structures, that might otherwise block the fork (Lehmann 2002). Either of these mechanisms might be a means for repair at G/C tracts in dog-1 animals.
To gain insights into the role of HIM-6 and other mechanisms that maintain genome fidelity during replication, we have used the C. elegans dog-1 mutant and measured the frequency of animals with small (<500 bp) G/C tract deletions at tracts that are prone to deletions. Here we demonstrate that, in addition to the BLM ortholog HIM-6, components of recombinational repair and trans-lesion synthesis are required to maintain G/C tracts in the absence of DOG-1.
MATERIALS AND METHODS
Nematode strains:
All strains were maintained as described (Brenner 1974). The strains used include: VC13 dog-1 (gk10), CB1479 him-6 (e1423), VC193 him-6 (ok412), VC174 wrn-1(gk99), VC655 brd-1 (gk297), TJ1 cep-1 (gk138), CB1487 him-9 (e1487), TG9 dpy-13(e184) rad-51 (lg8701)/nT1[let-?(m435)], RB864 xpa-1 (ok698), RB873 lig-4 (ok716), and RB964 cku-80 (ok861). To prevent the accumulation of mutations in the mutator background, dog-1(gk10) was routinely crossed to N2 males and the dog-1 deletion was resegregated multiple times. In each case, the homozygous presence of the gk10 deletion was verified by PCR.
Fitness assay:
Animals of genotypes dog-1(gk10) and him-6(ok412) and dog-1(gk10); him-6(ok412) were plated individually and maintained at either 20° or 25°. For each generation, a single L4 stage animal was transferred to a fresh plate. A line was scored as inviable when the parent worm was sterile or laid only inviable embryos.
SYTO12 staining:
L4 animals were staged 24 hr before staining. On the day of staining, 1-day-old adults were picked into 50 μl of 33 μm SYTO12 (Molecular Probes, Eugene, OR) and incubated for 3 hr in the dark. After incubation, animals were destained by allowing them to feed on NGM OP50 seeded plates for 1 hr. Animals were placed in levamisole on agarose pads for viewing. The number of SYTO12-stained bodies per gonad arm was counted using a Zeiss Axioscope fluorescent microscope with ×40 objective. All values reported are ± standard error of the mean.
DAPI staining:
Staged 1-day-old adults were picked into 10 μl of M9 buffer in a watch glass and 200 μl of 150 nm 4′,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis) in ethanol was added. The animals were placed in the dark and allowed to incubate for 1 hr. Animals were destained in M9 buffer overnight at 4° in a humid chamber. DAPI-stained animals were viewed at ×40 magnification on a Zeiss Axioscope fluorescent microscope. A Retiga 2000R camera (Qimaging) and Openlab 4.0.2 software (Improvision) were used to image the germlines of the animals. Using the measure tool in Adobe Photoshop 7.0, the width of individual nuclei in the mitotic region (extending from the distal tip to the first transition zone nuclei) of each gonad arm was measured. For each mitotic region, 10 nuclei were measured and the single largest nucleus was measured.
Measurement of G/C tract deletions:
The occurrence of G/C tract deletions in all single, double, and triple mutants was measured by PCR amplification of the G/C tract within the vab-1 gene on chromosome II. L4 stage animals of the genotype of interest were picked to fresh plates 24–36 hr before DNA preparation. DNA of individual worms was prepared with lysis buffer (10 mm Tris-HCl, 50 mm KCl, 2.5 mm MgCl2, 0.45% NP40, 0.45% Tween20, 0.01% gelatin, 100 μg/ml Proteinase K) and incubated at −70° for 10 min, at 56° for 1 hr, and then at 95° for 15 min. G/C tracts were amplified in each animal by PCR using a set of nested primers. External primer sequences were (external left) 5′-cgattccaacaattggtaaatacc-3′ and (external right) 5′- aatatttgctaaacctattgttgcc-3′. The external PCR program was 94° for 4 min followed by 34 cycles of 94° for 30 sec, 58° for 30 sec, and 72° for 1 min 30 sec, and a final elongation step of 72° for 10 min. One microliter of DNA from the external reaction was used as the template for a second internal PCR. Internal primer sequences were (internal left, IL) 5′- cgacgaaaaatgcagaatttggc-3′ and (internal right, IR) 5′-aggtgtgtgtgcatacctccg-3′. The internal PCR program was the same as the external program, except primers were annealed at 62° and the extension time was 1 min. PCR products were run on 1% agarose gels and stained with SYBR Green (Molecular Probes) for nucleic acid visualization. Gels were imaged using a Gel Doc 2000 (Bio-Rad, Hercules, CA). Animals scored as positive usually contained one or more deletion bands in addition to the wild-type band. Rarely, we also observed individuals in which no wild type band was present. Selected deletions were sequenced (Nucleic Acid and Protein Services, University of British Columbia) to confirm specific amplification of the sequence of interest and the presence of deletions initiating at the G/C tract.
A second G/C tract was tested to determine whether the increased deletion formation observed in the double and triple mutants was specific to the vab-1 G/C tract; this second G/C tract is located within the cosmid R144 on chromosome III. The R144 G/C tract was PCR amplified using the primer sequences (R144-L) 5′-catatggattggcatgtgaagca-3′ and (R144-R) 5′-ctgcctacagtagtctttgcg-3′. The R144 G/C tract PCR program was 94° for 4 min followed by 34 cycles of 94° for 30 sec, 62° for 30 sec, 72° for 1 min 30 sec, and a final elongation step of 72° for 10 min. R144 G/C tract PCR products were visualized in the same manner as those for the vab-1 G/C tract. We observed a 4.4-fold increase in deletions in dog-1; him-9 animals (18 deletions in 125 individuals) compared to dog-1 animals (4 deletions in 123 individuals), indicating that the increased instability is not specific to the vab-1 G/C tract, but also occurs at other G/C tracts.
RNA interference:
Single colonies of Escherichia coli containing the F53A3.2 (polη) and F22B7.6 (polκ) RNA interference (RNAi) constructs were grown overnight in LB medium with 50 μg/ml ampicillin and 12.5 μg/ml tetracycline. Sixty microliters of the culture was added drop-wise to NGM plates containing 50 μg/ml ampicillin, 12.5 μg/ml tetracycline, and 0.5 mm IPTG. The next day, L4 stage animals were placed on the plates to feed and were transferred to fresh plates every 2 days. F1 progeny were picked to fresh plates at the L4 stage and were lysed 24 hr later for G/C tract PCR.
RESULTS
dog-1; him-6 animals display reduced fitness:
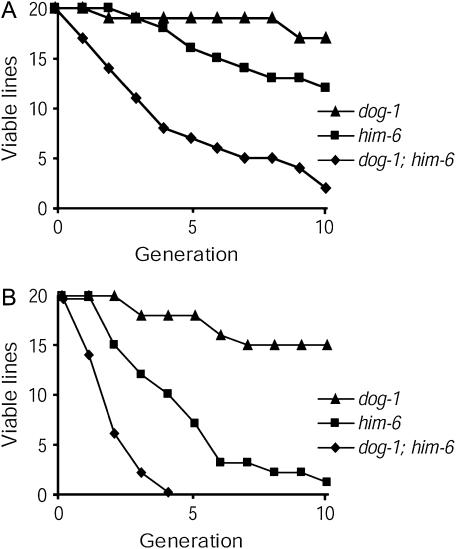
Because BLM and WRN have been shown to unwind G-quadruplex DNA in vitro (Sun et al. 1998; Mohaghegh et al. 2001; Huber et al. 2002), we first tested whether loss of function of the C. elegans BLM ortholog, him-6 (Wicky et al. 2004), or of the WRN homolog, wrn-1 (Lee et al. 2004), would exacerbate the phenotype of dog-1 mutant animals. dog-1; wrn-1 animals had normal viability; however, dog-1; him-6 animals displayed reduced viability. We assayed the fitness of dog-1; him-6 double mutants by maintaining the animals at 20° and 25°, as higher temperatures have been shown to intensify the effects of genome stability mutants in C. elegans (Ahmed and Hodgkin 2000). At 20°, 18 of 20 lines of dog-1; him-6 animals had become sterile by the 10th generation (Figure 1A). The decline in viability was exacerbated when dog-1; him-6 animals were grown at 25°, as all 20 lines had become sterile by the 4th generation (Figure 1B). A lesser decline in viability was observed for him-6 animals in which 19 of 20 lines became sterile by the 10th generation at 25° in accordance with data from Grabowski et al. (2005). Thus, dog-1; him-6 double mutants exhibit reduced fitness that is much more severe than that of either of the single mutants.
Figure 1.
Number of viable lines of dog-1 and him-6 single mutants and dog-1; him-6 double mutants at each generation while being maintained at (A) 20° and (B) 25°.
him-6 synergizes with dog-1:
To examine these double mutants in more detail, we scored the progeny of dog-1; him-6 and dog-1; wrn-1 animals for viability, embryonic lethality, brood size, and the frequency of spontaneous males (which are XO compared to XX hermaphrodites). The high incidence of male (Him) phenotypes is a result of frequent losses of a whole or part of one X chromosome and is often observed in DNA damage checkpoint or repair mutants (Van Haaften et al. 2004). dog-1; wrn-1 animals had normal viability and did not have elevated levels of embryonic lethality or males (data not shown). However, dog-1; him-6 double mutants had significantly fewer viable progeny than him-6 animals (t-test P = 2.45−10) and fewer viable progeny than would be expected for an additive effect of the two genes (average number of viable progeny observed was 27, compared to 46 expected; Table 1). The average brood size of the double mutants was no different from the expected (average brood size observed was 185 compared to 194 expected; Table 1), and the reduction in viable progeny could be accounted for by increased embryonic lethality (average percentage of embryonic lethality observed was 72% compared to 53% expected; Table 1). In dog-1; him-6 broods we observed a significantly higher percentage of male progeny than in him-6 broods (t-test P = 2.89−7); the incidence of males was also higher than that expected for an additive effect of the two genes (average percentage of males observed was 20% compared to 13% expected; Table 1).
TABLE 1.
Phenotype of N2, dog-1, him-6, and dog-1; him-6 animals
| Genotype | % of viable progeny | Total brood size | % of embryonic lethality | % of males |
|---|---|---|---|---|
| N2 (n = 15) | 99.9 ± 0.05 | 271 ± 13 | 0.1 ± 0.05 | 0.2 ± 0.07 |
| dog-1 (n = 37) | 97.1 ± 0.9 | 239 ± 7 | 2.9 ± 0.9 | 0.2 ± 0.07 |
| him-6 (n = 43) | 46.9 ± 2.0 | 220 ± 11 | 53.1 ± 2.0 | 12.6 ± 0.6 |
| dog-1; him-6 (n = 41) | 27.6 ± 1.8 | 185 ± 13 | 72.5 ± 1.8 | 19.7 ± 1.1 |
All values are ± standard error of the mean. n, the number of parent worms whose progeny were scored.
Apoptosis is elevated in dog-1 and him-6 animals, but is no higher in dog-1; him-6:
To further examine the dog-1; him-6 animals, we used the vital dye SYTO12 to observe levels of apoptosis in wild type, dog-1, him-6, and dog-1; him-6 double mutants. In N2 (wild-type) animals, we observed an average of 0.8 ± 0.2 (n = 44 gonad arms) SYTO12-stained corpses per gonad arm, while in dog-1 animals, we observed an average of 3.3 ± 0.2 (n = 77) corpses per gonad arm. To determine whether the elevated apoptosis observed in dog-1 mutants was the result of DNA damage, we constructed a dog-1 cep-1 double mutant and SYTO12 stained these animals. CEP-1 is the C. elegans p53 homolog that is required for DNA-damage-induced apoptosis (Derry et al. 2001; Schumacher et al. 2001). Loss of CEP-1 abrogated the increased apoptosis observed in dog-1 mutants (0.8 ± 0.1 corpses per gonad arm; n = 49). This indicates that the increased apoptosis in dog-1 animals is dependent on the DNA damage checkpoint protein CEP-1. We observed that him-6 animals had an average of 4.7 ± 0.3 (n = 47) SYTO12-stained corpses per gonad arm, similar to findings reported by Grabowski et al. (2005). Kim et al. (2005) have shown that the elevated levels of apoptosis in him-6(e1104) animals are also dependent on the DNA damage checkpoint. In dog-1; him-6 animals, we observed an average of 4.0 ± 0.3 (n = 46) corpses per gonad arm, indicating that no more cells are undergoing apoptosis in the double mutant than in either of the single mutants.
dog-1; him-6 animals have enlarged mitotic nuclei in the germline:
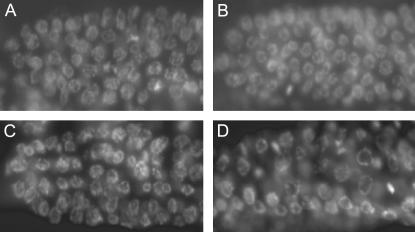
To further investigate the basis of the dog-1; him-6 phenotype, we used DAPI to visualize DNA in the dog-1; him-6 germline mitotic zone. In C. elegans, the germline nuclei undergo mitosis in a syncytium in the distal region of the gonad. These nuclei are spatially separated from the more proximal germ nuclei undergoing the early stages of meiosis by a region known as the transition zone, in which nuclei transitioning from mitosis to meiosis have a characteristic crescent shape. When genotoxic stress is present in the mitotic zone, the damage checkpoint will arrest nuclei, presumably to allow repair to occur (Gartner et al. 2000). This S-phase arrest is characterized by enlarged nuclei and an overall reduction in the number of nuclei within the germline mitotic region (Ahmed et al. 2001; Garcia-Muse and Boulton 2005). We did not observe a severe mitotic arrest in dog-1;him-6 animals, in which there are only a few very large nuclei that occupy the space of the entire mitotic zone, such as the arrest observed in atl-1 mutants after treatment with genotoxic agents (Garcia-Muse and Boulton 2005). However, mitotic nuclei were clearly enlarged in dog-1; him-6 animals when compared to N2, dog-1, or him-6 (Figure 2). To quantify the size of germline mitotic nuclei in dog-1; him-6 animals, we measured the average diameter of nuclei within the mitotic regions of N2 animals, dog-1 and him-6 single mutants, and dog-1; him-6 double mutants. N2 and dog-1 animals had similar average mitotic nuclei sizes (Table 2; t-test P = 0.30). Consistent with him-6 having a role in replication, him-6 animals had a significantly larger average mitotic nuclei size than N2 animals (Table 2; P = 0.00086). However, the average size of nuclei in the mitotic region of dog-1; him-6 animals was much larger than that in N2 animals or in either of the single mutants (Table 2; P = 1.66e−18, P = 1.37e−17, P = 5.76e−15 in t-tests with N2, dog-1, and him-6, respectively). When we measured the diameter of the single largest nucleus in each mitotic zone to determine the average largest nucleus size, N2 and dog-1 animals had similar average largest nucleus sizes (Table 2; P = 0.14). him-6 animals had a slightly larger average largest nucleus size (Table 2; P = 0.0013 in t-test with N2). Again, the average size of the largest nucleus in dog-1; him-6 mitotic regions was much larger than that in N2 animals or in dog-1 or him-6 single mutants (Table 2; P = 1.60e−13, P = 1.96e−12, P = 2.60e−11 in t-tests with N2, dog-1, and him-6, respectively). We interpret the larger size of mitotic nuclei in dog-1; him-6 animals as a response to replicative stress in dog-1; him-6 double mutants.
Figure 2.
Mitotic regions of DAPI-stained (A) N2, (B) dog-1, (C) him-6, and (D) dog-1; him-6 animals. The distal tip of the mitotic region is oriented toward the left.
TABLE 2.
Germline mitotic nuclei size in N2, dog-1, him-6, and dog-1; him-6 animals
| Genotype | Average nuclei size (μm) | Average largest nucleus size (μm) |
|---|---|---|
| N2 (n = 26) | 3.14 ± 0.10 | 4.04 ± 0.08 |
| dog-1 (n = 26) | 3.21 ± 0.09 | 4.19 ± 0.06 |
| him-6 (n = 24) | 3.38 ± 0.10 | 4.40 ± 0.07 |
| dog-1; him-6 (n = 23) | 4.29 ± 0.14 | 6.12 ± 0.15 |
Ten nuclei were measured per mitotic zone. All values are ± standard error of the mean. n, the number of mitotic zones in which nuclei were measured.
Loss of function of him-6 and dog-1 elevates the number of animals with G/C tract deletions:
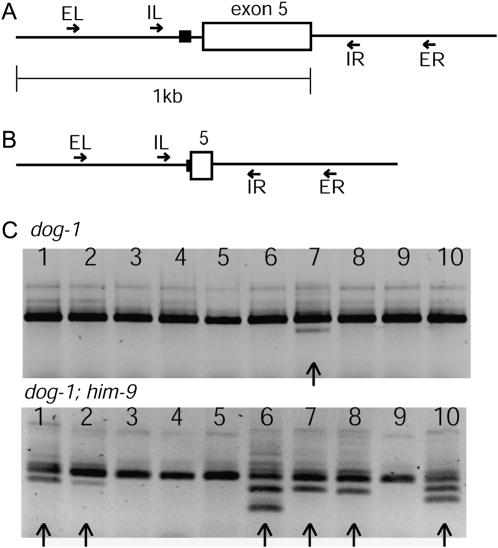
Previously, DOG-1 was shown to function in the maintenance of G/C tracts, as deletions initiating at these tracts were observed in dog-1 mutants (Cheung et al. 2002). We next examined whether the decreased viability and replicative stress in dog-1; him-6 animals might be explained by an overlapping function of DOG-1 and HIM-6 in processing G-rich DNA during replication. If this were the case, we would expect to see G/C tract deletions in him-6 animals and an increased number of deletions in dog-1; him-6 animals compared to dog-1 single mutants. Our assay for G/C tract deletions is PCR based, using sets of nested primers to ensure specific amplification of the G/C tract of interest. For the assay, we chose the G/C tract adjacent to exon 5 of the vab-1 gene located on chromosome II (Figure 3, A and B), which was shown to delete in some dog-1 animals (Cheung et al. 2002). Whole single animals were used for the assay, and as animals have differences in germline proliferation that are dependent on age, we staged the animals such that G/C tract amplification was performed on 1-day-old adults. By this means, we could sample approximately the same number of cells in each animal. We consistently detected deletions of the vab-1 G/C tract in 11% of dog-1 animals assayed, thus allowing us to identify increases or decreases in the number of animals with deletions of this particular G/C tract (Figure 3C). On the basis of the lack of genetic interaction between dog-1 and wrn-1, we did not expect to observe an elevated number of animals with deletions in dog-1; wrn-1 double mutants compared to dog-1 single mutants. dog-1; wrn-1 animals showed only a small increase in the number of animals with deletions relative to dog-1 mutants (Table 3). When we tested him-6 mutants for G/C tract deletions, none were detected, suggesting that HIM-6 does not function specifically to unwind G-rich DNA secondary structures in the presence of DOG-1. However, the number of dog-1; him-6 animals with deletions was increased 3.2-fold over the number of dog-1 animals with deletions (Table 3). This suggests that in the absence of dog-1 function, HIM-6 prevents the formation of deletions initiating in G/C tracts.
Figure 3.
(A) Magnification of the vab-1 gene, showing exon 5, which is nearest the G/C tract. The solid box indicates the position of the G/C tract. EL and ER represent the external left and right PCR primers; IL and IR represent the internal left and right PCR primers. (B) A typical G/C tract deletion. The deletion begins 5′ of exon 5 and extends part way through the exon, bringing the primers closer together. (C) Products of nested PCRs performed on 10 single dog-1 animals (top) and dog-1; him-9 animals (bottom). Arrows indicate lanes where deletion bands are present. In some cases, multiple deletion bands are present in a single animal.
TABLE 3.
Deletions observed in the vab-1 G/C tract in various repair mutants in the dog-1 background
| Genotype | No. of animals assayed | No. with deletions | % of animals with deletions | P-value in t-test with dog-1 | Fold increase relative to dog-1 |
|---|---|---|---|---|---|
| dog-1 | 228 | 26 | 11.4 | 1.0 | |
| wrn-1 | 108 | 2 | 1.9 | ||
| dog-1; wrn-1 | 103 | 20 | 19.4 | 0.32 | 1.7 |
| him-6 | 100 | 0 | 0 | ||
| dog-1; him-6 | 157 | 58 | 36.9 | 0.0094a | 3.2 |
| dpy-13 rad-51 | 102 | 1 | 0.98 | ||
| dog-1; dpy-13 rad-51 | 179 | 84 | 46.9 | 0.00012a | 4.1 |
| him-9 | 108 | 0 | 0 | ||
| dog-1; him-9 | 178 | 65 | 36.5 | 0.0027a | 3.2 |
| dog-1; him-9; dpy-13 rad-51 | 108 | 57 | 52.8 | 0.17b | 4.6 |
| dog-1; him-9; him-6 | 272 | 116 | 42.6 | 0.66b | 3.7 |
| brd-1 | 101 | 2 | 2.0 | ||
| dog-1; brd-1 | 121 | 44 | 36.4 | 0.0018a | 3.2 |
| dog-1; him-9; brd-1 | 100 | 42 | 42.0 | 0.98b | 3.7 |
| cep-1 | 102 | 0 | 0 | ||
| dog-1; cep-1 | 107 | 33 | 30.8 | 0.0040a | 2.7 |
| polη (RNAi) | 100 | 0 | 0 | ||
| dog-1; polη (RNAi) | 108 | 28 | 25.9 | 0.0020a | 2.3 |
| polκ (RNAi) | 121 | 1 | 0.82 | ||
| dog-1; polκ (RNAi) | 101 | 27 | 26.7 | 0.025a | 2.3 |
Number with deletions is the number of individual animals that showed one or more deletions in the vab-1 G/C tract as determined by PCR.
Statistically significant P-value in t-test with dog-1.
P-value in t-test with dog-1; him-9. t-Tests against other applicable double mutants did not give significant P-values.
RAD-51 and HIM-9/XPF prevent small deletions initiating at G/C tracts in dog-1 animals:
The finding that absence of HIM-6 in the dog-1 mutant background leads to an increase in the number of animals with G/C tract deletions led us to question whether other pathways function to prevent G/C tract deletions. To test if homologous recombination repair might be involved in the maintenance of G/C tracts in dog-1 mutants, we constructed dog-1; dpy-13 rad-51 mutants. The dpy-13 mutation was used as a phenotypic marker to identify rad-51 homozygotes. RAD-51 is the C. elegans homolog of the bacterial RecA protein, which has a crucial role in homologous recombination (Rinaldo et al. 1998). In C. elegans, rad-51 has a maternal-effect lethal phenotype. Therefore, we selected dpy-13 rad-51 homozygotes that had segregated from dpy-13 rad-51 heterozygotes and assayed for deletions in these animals. Deletions were very rare in dpy-13 rad-51 animals. The number of animals with deletions in dog-1; dpy-13 rad-51 mutants was increased more than fourfold over the number in dog-1 single mutants (Table 3).
We also assayed for deletions in him-9 and dog-1; him-9 double mutants. him-9 is the ortholog of the human XPF gene (N. J. O'Neil, unpublished data), which is implicated in homologous recombination in yeast and in gene targeting in mammalian cells, as well as in crosslink repair (Klein 1988; Schiestl and Prakash 1988, 1990; Sargent et al. 2000; Niedernhofer et al. 2001, 2004). No deletions were observed in him-9 single mutants. The number of animals with deletions in dog-1; him-9 double mutants was increased 3.2-fold over that in dog-1 single mutants (Table 3). These results imply that both HIM-9 and RAD-51 function in the maintenance of G/C tracts in dog-1 animals. While these data support a role for homologous recombination in repair at G/C tracts in dog-1 animals, this pathway apparently is not sufficient to prevent deletions altogether.
To test whether RAD-51 and HIM-9 function in the same pathway, we constructed a dog-1; him-9; dpy-13 rad-51 mutant and assayed the occurrence of vab-1 G/C tract deletions. These mutants exhibited a number of deletions similar to those in dog-1; dpy-13 rad-51 animals (Table 3), indicating that RAD-51 and HIM-9 function in the same pathway. Furthermore, we questioned whether HIM-6 might also function in homologous recombination or act in a separate pathway. We constructed dog-1; him-9; him-6 triple mutants and found that the number of animals with deletions was increased relative to both dog-1; him-9 and dog-1; him-6 (Table 3). However, the variability of the number of animals with deletions in individual experiments with dog-1; him-9; him-6 ranged from 25.7 to 68.4%, resulting in a high t-test value. The variation in the number of animals with deletions measured in the dog-1; him-9; him-6 triple mutant was much greater than in any other single, double, or triple mutant. Because of this, it was difficult to assess whether HIM-9 and HIM-6 function in the same or different pathways to suppress G/C tract deletions in dog-1 mutants.
BRD-1 prevents G/C tract deletions in dog-1 animals:
BRCA1 and BARD1 have been implicated in the recombinational response to stalled replication forks in mammalian cell lines (Scully et al. 1997, 2000). We investigated whether brd-1, the ortholog of human BARD1 (Boulton et al. 2004), might be involved in repair at G/C tracts in dog-1 mutants. We measured the number of animals with deletions in dog-1; brd-1, which was elevated 3.2-fold over the number of dog-1 animals with deletions (Table 3). We attempted to construct a dog-1; brd-1; dpy-13 rad-51 mutant to determine whether or not brd-1 was epistatic to rad-51, but these animals were not viable. However, in dog-1; brd-1; him-9 triple mutants, the number of animals with G/C tract deletions was not significantly different from the number with deletions in either the dog-1; him-9 or the dog-1; brd-1 strain (Table 3). On the basis of these data, we did not take the inviability of the dog-1; brd-1; dpy-13 rad-51 mutant to indicate that BRD-1 and RAD-51 do not function together in recombinational repair; rather, the checkpoint or other functions of BRD-1 might be required in the absence of RAD-51. These results, taken with others, suggest that BRD-1, RAD-51, and HIM-9 function in recombinational repair at G/C tracts in dog-1 animals.
More G/C tract deletions are observed in dog-1 cep-1 animals:
Evidence from mammalian cell lines has shown that p53 localizes to sites of stalled replication forks, where it is proposed to modulate recombinational repair (Yang et al. 2002; Sengupta et al. 2003). Thus, we tested the effect of the absence of the C. elegans p53 ortholog CEP-1 on deletions in the dog-1 background. The number of animals with deletions observed in dog-1 cep-1 double mutants was elevated 2.7-fold over the number with deletions in dog-1 animals (Table 3). This result could suggest that CEP-1 is also involved in G/C tract maintenance in the absence of DOG-1. However, we cannot rule out the possibility that the increase in the number of animals with deletions might be related to reduced apoptosis of cells with deletions in the absence of CEP-1.
Polymerases η and κ are required for deletion-free repair of G/C tracts:
It is known that polymerases alternative to the typical replicative polymerases are frequently involved in lesion bypass to allow continued replication past various types of DNA damage (Lehmann 2002; Prakash et al. 2005). In particular, polη and polκ have been suggested to accumulate at replication forks stalled by DNA damage (Kannouche et al. 2001, 2003; Bergoglio et al. 2002). We questioned whether these polymerases might also function at G/C tracts in dog-1 mutants. Because currently no trans-lesion synthesis mutants are available in C. elegans, we used RNAi to knock down polη and polκ in the dog-1 background and then assayed for the presence of G/C tract deletions. Deletions were rarely observed in polη or polκ (RNAi) control animals, but were observed in 25.9% of dog-1; polη (RNAi) and 26.7% of dog-1; polκ (RNAi) animals; both of these were significant increases over the number of dog-1 animals with deletions (Table 3). We would have tested a third trans-lesion synthesis polymerase, rev-1, which preferentially incorporates a C opposite template G (Prakash et al. 2005) and is also implicated at stalled forks (Mukhopadhyay et al. 2004); however, the embryonic lethal rev-1(RNAi) phenotype prevented the application of the assay.
Absence of nucleotide excision repair or nonhomologous end joining does not affect G/C tract deletion formation:
We questioned whether nucleotide excision repair (NER) or nonhomologous end joining (NHEJ) might play roles in either preventing or causing deletions at G/C tracts in the absence of DOG-1. We assayed the role of NER in the dog-1 background using an xpa-1 mutant, which is the ortholog of human XPA (Park et al. 2002). XPA has an essential role in NER. We constructed dog-1 xpa-1 double mutants and found that these animals did not exhibit a number of deletions significantly different from dog-1 single mutants (15.8%; P = 0.70). To test whether or not NHEJ might be active at G/C tracts in the dog-1 background, we utilized lig-4 and cku-80, which are homologs of the mammalian NHEJ genes DNA ligase IV and Ku80 (Daley et al. 2005). In dog-1; lig-4 and dog-1; cku-80 double mutants, the number of animals with deletions was not significantly different from the number observed in dog-1 single mutants (15.1%, P = 0.72; 11.1%, P = 0.95, respectively). These data indicate that neither NER nor NHEJ are key mechanisms for deletion-free repair or deletion formation at G/C tracts. However, this does not preclude the involvement of other components of these pathways in the recombinational repair that is clearly required for genome stability in the absence of DOG-1.
Frequent base-pair mutations flank G/C tract deletions:
During the course of our investigation, we sequenced 30 of the deletions that were detected in our assay (selected sequences are shown in Table 4). Nine of the sequenced deletions contained one or more base-pair mutations within a few bases of the deletion site. In most cases, the mutations were single base-pair changes, but multiple base-pair changes and base-pair deletions were also observed. These base-pair mutations occurred with the same frequency in dog-1 single mutants (4 of 15 deletions) as in recombinational repair mutants in the dog-1 background (5 of 15 deletions). This observation suggests that a mutagenic mechanism is involved in the formation of small deletions in dog-1 single mutants and that the same mechanism acts more frequently when other repair pathways, such as recombinational repair, are impaired.
TABLE 4.
Selected sequences of vab-1 G/C tract deletions
| Genotype of animal | Deletion sequence left of break | Deletion sequence right of break |
|---|---|---|
| Wild type | 28705gaaaccccccccccccccccccccat | 28950tggtatcaaaacgtctggcgagtgcg |
| dog-1 | gaaaccccccccc(233-bp deletion) | tagtatcaaaacgtctggcgagtgcg |
| Wild type | 28705gaaaccccccccccccccccccccat | 28872gaatggttcgtgtattccgaatgcgt |
| dog-1 | gaaac (163-bp deletion) | gaatggttcgtgtattccgaatgcgt |
| Wild type | 28705gaaaccccccccccccccccccccat | 28821tacttccgaccacaagttaccgggcc |
| dog-1; brd-1 | gaaaccccc (108-bp deletion) | tac--c-gaccacaagttaccgggcc |
| Wild type | 28705gaaaccccccccccccccccccccat | 28816cttgtctacttccgaccacaagttac |
| dog-1; brd-1 | gaaacccccccc (101-bp deletion) | gttgtctacttccgaccacaagttac |
| Wild type | 28705gaaaccccccccccccccccccccat | 29048agaaaattatacaactatgcctgaat |
| dog-1; him-9 | gaaacccccc (334-bp deletion) | agaaaattatacaactatgcctgaat |
| Wild type | 28705gaaaccccccccccccccccccccat | 28812aacttgtctacttccgaccacaagtt |
| dog-1; him-9 | gaaacccccccc (96-bp deletion) | aacttgtctacttccgaccacaagtt |
| Wild type | 28705gaaaccccccccccccccccccccat | 28834caagttaccgggccaaaagagactga |
| dog-1; dpy-13 rad-51 | gaca (126-bp deletion) | caagttaccgggccaaaagagactga |
| Wild type | 28705gaaaccccccccccccccccccccat | 28985gtggatattctcaaattgccgattcg |
| dog-1; dpy-13 rad-51 | gaaacccccc (271-bp deletion) | gtggatattctcaaattgccgattcg |
Genotype of animal indicates that from which vab-1 G/C tract deletion was isolated. Wild-type sequence is shown above for each deletion. Deletion sequence is shown below with deletion size in parentheses. Superscripts indicate the sequence location within the cosmid. Mismatches are italicized.
DISCUSSION
In this study we have used the dog-1 mutant to develop an in vivo assay to measure the effects of different repair mutants at G-rich DNA. We demonstrate that him-6, rad-51, him-9/XPF, and brd-1/BARD1, which are implicated in recombinational repair, and the trans-lesion synthesis polymerases polη and polκ promote deletion-free repair in the dog-1 mutant. In the absence of any of these proteins, deletions occur more frequently at G/C tracts in the dog-1 background.
DOG-1 is likely to function during replication:
Genomic instability such as that observed in the dog-1 mutant can have several causes, such as defects during replication or transcription. For example, Chavez and Aguilera (1997) showed that blocks in transcriptional elongation in yeast HPR1 mutants are responsible for genomic instability associated with deletions between direct repeats. More recently, Li and Manley (2005) reported that genome stability can be affected by the absence of the protein-splicing factor ASF/SF2 because RNA:DNA hybrids form on the nontemplate strand of transcribed genes, leading to rearrangements. Several lines of evidence suggest that DOG-1 functions during replication and that the deletions observed in dog-1 mutants occur during replication and are not transcription based. First, microarray analysis showed that dog-1 was expressed at 7.67-fold higher levels in wild-type animals compared to glp-4 animals (which lack virtually all germline cells), indicating that dog-1 expression is enriched in the germline, where cells in the mitotic region are constantly undergoing replication (Reinke et al. 2000). Second, the observed deletions always initiate in the G/C tract and extend in the same direction, suggesting a mechanism for deletion formation that could be related to lagging-strand replication (Cheung et al. 2002). Importantly, deletions occur at G/C tracts located at intergenic sites as well as within introns, suggesting that the deletions are not dependent on transcription (Cheung et al. 2002). We have shown that loss of both DOG-1 and HIM-6 results in elevated levels of embryonic lethality in the progeny of dog-1; him-6 animals. An interaction between dog-1 and him-6 would be expected if these two genes have some parallel or overlapping function. Similar to dog-1, him-6 has an elevated expression in the germline (Reinke et al. 2000), and Kim et al. (2005) have shown that it functions in cells undergoing active replication and division, including the mitotic germline stem nuclei and early embryonic cells. Further supporting a role for DOG-1 during replication, we observed enlarged germline mitotic nuclei in dog-1; him-6 animals, suggesting replicative stress. Finally, we have demonstrated that recombination repair and trans-lesion synthesis polymerase genes, which are known to function during replication (Lehmann 2002; Barbour and Xiao 2003; Lundin et al. 2003; Prakash et al. 2005; Saleh-Gohari et al. 2005), play an important role in preventing G/C tract deletions in the dog-1 mutant. Thus, it is probable that the dog-1-dependent deletions are formed during replication.
Homologous recombination and trans-lesion synthesis are required for genome stability in dog-1 animals:
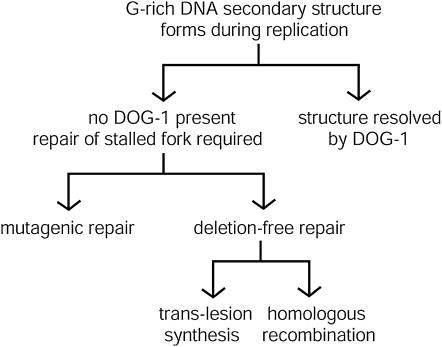
Several reports have indicated that homologous recombination functions at stalled or collapsed replication forks to carry out error-free repair (Barbour and Xiao 2003; Lundin et al. 2003; Saleh-Gohari et al. 2005). Our data show that homologous recombination-associated proteins function in C. elegans at G/C tracts in dog-1 mutants. Absence of the RAD51, XPF, and BARD1 homologs RAD-51, HIM-9, and BRD-1 in the dog-1 background led to an increase in the number of animals with G/C tract deletions, suggesting that these three proteins promote deletion-free repair at G/C tracts (Figure 4). Our epistasis experiments place brd-1, rad-51, and him-9 in the same repair pathway.
Figure 4.
Model of repair pathways functioning downstream of replication blockages in dog-1 mutants. G-rich DNA can form secondary structures when the DNA is single stranded during lagging-strand DNA synthesis. Normally, these structures are unwound by DOG-1. In the absence of DOG-1, repair of the stalled replication fork is required. This repair can be through deletion-free or mutagenic mechanisms. In deletion-free repair, BRD-1, RAD-51, and HIM-9 carry out homologous recombinational repair. HIM-6 might be involved in recombinational repair or it might resolve the replication fork to prevent the need for other repair mechanisms. Trans-lesion synthesis also carries out deletion-free repair. Deletions that are generated at G/C tracts in the absence of DOG-1 are formed through an unknown mutagenic repair process.
We also tested whether trans-lesion synthesis is required for genome stability in dog-1 mutants and found that knockdown of either polη or polκ in the dog-1 background increased the number of animals with deletions. A recent report has shown that polη, in addition to functioning in trans-lesion synthesis, interacts with RAD51 and has the ability to extend D-loops as part of the reinitiation of DNA synthesis by homologous recombination repair (Kawamoto et al. 2005; Mcllwraith et al. 2005). Thus, the role of polη in dog-1 mutants might be related to its function in homologous recombination. It is possible that the increase in the number of animals with deletions that we observed in dog-1; polη (RNAi) and dog-1; polκ (RNAi) animals was not as high as the number seen in homologous recombination mutants in the dog-1 background because we used RNAi to knock down these polymerases. Therefore, low levels of polη and polκ might have been present. It is also possible that there is some degree of redundancy between polη and polκ in dog-1 mutants. Because DOG-1 appears to function during replication and both homologous recombination and trans-lesion synthesis are required for genome stability in the absence of DOG-1, we suggest that persisting G/C tract secondary structures can lead to replication fork stalling in dog-1 mutants. DOG-1 could be the primary means for preventing replication fork stalling by unwinding secondary structures and, in the absence of DOG-1, recombinational repair or trans-lesion synthesis can repair or restart the fork (Figure 4). Mutagenic repair, which results in small deletions, occurs at a low frequency in the absence of DOG-1, but when both DOG-1 and recombinational repair or trans-lesion synthesis are impaired, the deletion-generating mechanism functions more frequently.
HIM-6 could function in fork restart or recombinational repair:
The role for HIM-6 at the replication fork is not clearly defined, as HIM-6 might function either to unwind G/C tract secondary structures or to resolve stalled forks through a RAD-51-dependent or -independent mechanism in dog-1 mutants. In vitro, BLM preferentially unwinds G4 DNA, suggesting that it might prevent replication fork stalling by unwinding these structures in vivo (Sun et al. 1998; Mohaghegh et al. 2001; Huber et al. 2002). Since we did not observe any G/C tract deletions in him-6 animals, we conclude that, in the presence of DOG-1, HIM-6 does not play a key role in unwinding G/C tract secondary structures. However, it is possible that HIM-6 might unwind these structures when DOG-1 is absent. It has also been proposed that BLM plays a role in replication fork restoration by catalyzing branch migration to prevent the need for recombinational repair (Karow et al. 2000). In addition, BLM has been shown to interact with RAD51 (Wu et al. 2001) and can act on recombination intermediates such as Holliday junctions in vitro (Karow et al. 2000; Wu and Hickson 2003). Thus, it has been proposed that BLM resolves these intermediates in a manner that suppresses hyperrecombination (Karow et al. 2000; Wu and Hickson 2003). Wicky et al. (2004) proposed a similar role for HIM-6 in C. elegans, suggesting that it functions downstream of RAD-51. We found that the number of G/C tract deletions in dog-1; him-6 animals was increased, indicating that deletion-free repair is compromised in the double mutants. We observed dog-1; him-9; him-6 triple mutants to have highly variable viability and numbers of animals with deletions, in contrast to other single, double, and triple mutants. As a result of this variability, we cannot conclusively assign a role to HIM-6. HIM-6 might function downstream of RAD-51 to resolve G/C tract repair intermediates through homologous recombination. Another possibility is that HIM-6 functions in a separate pathway that promotes fork restoration independently of recombinational repair in dog-1 mutants.
The C. elegans p53 ortholog, cep-1, and deletions in the dog-1 background:
In C. elegans, as in mammals, CEP-1/p53 is required for DNA-damage-induced apoptosis (Derry et al. 2001; Schumacher et al. 2001). Recent mammalian studies have suggested that p53 can also modulate homologous recombination repair during replication (Sengupta et al. 2003; Sengupta and Harris 2005). This modulation of repair might be related to the ability of p53 to recognize mismatches within a heteroduplex, and thereby suppress erroneous repair (Dudenhoffer et al. 1998; Yun et al. 2004), or to its ability to regulate the processing of Holliday junctions by BLM (Yang et al. 2002). We observed a 2.7-fold increase in the number of dog-1 cep-1 animals with deletions compared to dog-1 animals. This could be interpreted to suggest that CEP-1 promotes maintenance of G/C tracts in dog-1 mutants. However, we have also shown that CEP-1-mediated apoptosis is increased in dog-1 mutants. Another possible interpretation is that we observed more deletions in dog-1 cep-1 animals because CEP-1 was not present to activate apoptosis in cells with DNA damage resulting from absence of DOG-1. Therefore, we might have sampled individual animals containing cells with deletions that would normally have undergone apoptosis, thus leading to higher numbers of animals with deletions. We do not believe that this is the case because, if it were, we should have observed a notable increase in germline deletions in which no wild-type PCR band was visible (i.e., all cells in the entire animal having the same deletion), compared to animals that have mixed populations of deletions and wild-type sequence. However, we cannot rule out the possibility that reduced apoptosis is the reason for the elevated frequency of dog-1 cep-1 animals with deletions.
Loss of NER or NHEJ does not affect deletion formation:
Neither NER nor NHEJ are required for preventing deletions when DOG-1 is absent since the number of animals with deletions did not increase when essential elements of these mechanisms were knocked out. Although NER and NHEJ do not appear to play important roles at G/C tracts in dog-1, components of the NER and NHEJ pathways might function to maintain genomic stability in dog-1 animals. It is well established that certain components of repair pathways can function in different repair mechanisms. For instance, XPF/Rad10 is a component of the NER pathway, but is also involved in the trimming of nonhomologous ends from double-strand breaks during synthesis-dependent strand annealing in yeast (Ivanov and Haber 1997) and crosslink repair in mammalian cell lines (Hoy et al. 1985; Kuraoka et al. 2000). Our finding that absence of the C. elegans XPF homolog HIM-9 in the dog-1 background elevates the frequency of animals with G/C tract deletions, but that absence of XPA-1 does not change the frequency of animals with deletions, illustrates that, while NER is not specifically involved in G/C tract maintenance, other components of the NER pathway are involved. Although neither NER nor NHEJ appear to be the main mechanisms for preventing deletions at G/C tracts, it is entirely possible that other components of these pathways do function in repair at G/C tracts through other pathways such as recombinational repair.
Other potential mechanisms for repair at G/C tracts:
Although we have identified homologous recombination involving RAD-51, HIM-9, BRD-1, and possibly HIM-6, as well as trans-lesion synthesis as mechanisms for repair of forks stalled by G/C tract secondary structure in dog-1 mutants, there are additional pathways that could also be involved. Kai et al. (2005) described a recombination pathway dependent on the fission yeast RAD51 homolog, rhp51, which generated deletions between short direct repeats in replication mutants, indicating that RAD51-dependent repair is not always error free. Our data show that, with respect to G/C tracts, RAD-51 carries out deletion-free repair, since the number of animals with deletions was increased in the absence of RAD-51. However, Kai et al. (2005) also reported that in fission yeast replication mutants, Mus81 is involved in the generation of deletions. Another recent article suggests a model for repair of replication defects in Saccharomyces cerevisiae, where parallel pathways are implicated involving either Rad51-independent Mus81 or Rad51-dependent Sgs1 (Ii and Brill 2005). We have shown that the Sgs1 homolog HIM-6 is involved in G/C tract stability in dog-1 mutants, and it is very possible that another pathway for repair that involves MUS-81 in C. elegans exists.
We sequenced 30 of the deletions obtained in our assay and found that about one-third of these had base-pair mutations within a few bases of the deletion site. The base-pair mutations occurred with equal frequency in dog-1 single mutants and in recombinational repair mutants in the dog-1 background, suggesting that the deletions are formed by the same mechanism in dog-1 mutants as in the absence of both DOG-1 and homologous recombination. The deletions might occur by the same mutagenic mechanism when secondary structures persist due to the absence of DOG-1 and recombinational repair and/or trans-lesion synthesis are unable to repair the stalled fork. Deletions could be formed if a gap in the lagging strand occurs opposite the secondary structure, followed by joining of the ends opposite the gap. Ii and Brill (2005) showed that RNase H2, which is involved in the removal of RNA primers during Okazaki fragment processing, also plays an important role in genome stability by acting in parallel to Mus81 and Rad51. It will be interesting to determine whether a similar mechanism is involved in the generation of small deletions in dog-1 mutants, particularly since DOG-1 has been proposed to act specifically on the lagging strand (Cheung et al. 2002).
Here we have shown that HIM-6, recombinational repair, and trans-lesion synthesis are required to prevent small deletions occurring at G/C tracts in the absence of DOG-1. When any of HIM-6, RAD-51, HIM-9, BRD-1, polη, or polκ are impaired in the dog-1 background, animals exhibit an increase in small deletions initiating in G/C tracts. This study underscores the utility of C. elegans for the study of DNA repair, as individual animals can be assayed to measure the contribution of various repair pathways to genome stability, and highlights the multiple layers of repair that are present in cells to maintain genome integrity.
Acknowledgments
The work presented here was stimulated by collaboration with Iris Cheung and Peter Lansdorp. We thank Carrie Wong and Shir Hazir for technical support; the Riddle lab for the use of their fluorescent microscope; David Baillie for insightful discussion; and Peter Lansdorp, Mike Schertzer, Di Chen, and the Rose lab members for helpful comments on the manuscript. Strains were provided by the Caenorhabditis elegans Genetic Centre, which is supported by the National Institutes of Health Centre for Research Resources. Several of the strains used in this study were generated by the International C. elegans Gene Knockout Consortium. This work was financially supported by a Natural Sciences and Engineering Research Council grant to A.R.; J.Y. has a scholarship from the Natural Sciences and Engineering Research Council and a fellowship from the Michael Smith Foundation for Health Research.
References
- Ahmed, S., and J. Hodgkin, 2000. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature 403(6766): 159–164. [DOI] [PubMed] [Google Scholar]
- Ahmed, S., A. Alpi, M. O. Hengartner and A. Gartner, 2001. C. elegans RAD-5/CLK-2 defines a new DNA damage checkpoint protein. Curr. Biol. 11: 1934–1944. [DOI] [PubMed] [Google Scholar]
- Arthanari, H., and P. H. Bolton, 2001. Functional and dysfunctional roles of quadruplex DNA in cells. Chem. Biol. 8: 221–230. [DOI] [PubMed] [Google Scholar]
- Barbour, L., and W. Xiao, 2003. Regulation of alternative replication bypass pathways at stalled replication forks and its effects on genome stability: a yeast model. Mutat. Res. 532: 137–155. [DOI] [PubMed] [Google Scholar]
- Bergoglio, V., C. Bavoux, V. Verbiest, J. S. Hoffmann and C. Cazaux, 2002. Localisation of human DNA polymerase kappa to replication foci. J. Cell Sci. 115: 4413–4418. [DOI] [PubMed] [Google Scholar]
- Bjergbaek, L., J. A. Cobb, M. Tsai-Pflugfelder and S. M. Gasser, 2005. Mechanistically distinct roles for Sgs1p in checkpoint activation and replication fork maintenance. EMBO J. 24: 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton, S. J., J. S. Martin, J. Polanowska, D. E. Hill, A. Gartner et al., 2004. BRCA1/BARD1 orthologs required for DNA repair in Caenorhabditis elegans. Curr. Biol. 14: 33–39. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomfield, S., T. Hryciw and W. Xiao, 2001. DNA postreplication repair and mutagenesis in Saccharomyces cerevisiae. Mutat. Res. 486: 167–184. [DOI] [PubMed] [Google Scholar]
- Chavez, S., and A. Aguilera, 1997. The yeast HPR1 gene has a functional role in transcriptional elongation that uncovers a novel source of genome instability. Genes Dev. 11: 3459–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, I., M. Schertzer, A. Rose and P. M. Lansdorp, 2002. Disruption of dog-1 in Caenorhabditis elegans triggers deletions upstream of guanine-rich DNA. Nat. Genet. 31: 405–409. [DOI] [PubMed] [Google Scholar]
- Daley, J. M., P. L. Palmbos, D. Wu and T. E. Wilson, 2005. Nonhomologous end joining in yeast. Annu. Rev. Genet. 39: 431–451. [DOI] [PubMed] [Google Scholar]
- Derry, W. B., A. P. Putzke and J. H. Rothman, 2001. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294: 591–595. [DOI] [PubMed] [Google Scholar]
- Ding, H., M. Schertzer, X. Wu, M. Gertsenstein, S. Selig et al., 2004. Regulation of murine telomere length by rtel: an essential gene encoding a helicase-like protein. Cell 117: 873–886. [DOI] [PubMed] [Google Scholar]
- Dudenhoffer, C., G. Rohaly, K. Will, W. Deppert and L. Wiesmuller, 1998. Specific mismatch recognition in heteroduplex intermediates by p53 suggests a role in fidelity control of homologous recombination. Mol. Cell. Biol. 18: 5332–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg, H. J., 1976. Inhibition of DNA replication by ultraviolet light. Biophys. J. 16: 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Muse, T., and S. J. Boulton, 2005. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 24: 4345–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner, A., S. Milstein, S. Ahmed, J. Hodgkin and M. O. Hengartner, 2000. A conserved checkpoint pathway mediates DNA damage–induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 5: 435–443. [DOI] [PubMed] [Google Scholar]
- Grabowski, M. M., N. Svrzikapa and H. A. Tissenbaum, 2005. Bloom syndrome ortholog HIM-6 maintains genomic stability in C. elegans. Mech. Ageing Dev. 126: 1314–1321. [DOI] [PubMed] [Google Scholar]
- Hoy, C. A., L. H. Thompson, C. L. Mooney and E. P. Salazar, 1985. Defective DNA cross-link removal in Chinese hamster cell mutants hypersensitive to bifunctional alkylating agents. Cancer Res. 45: 1737–1743. [PubMed] [Google Scholar]
- Huber, M. D., D. C. Lee and N. Maizels, 2002. G4 DNA unwinding by BLM and Sgs1p: substrate specificity and substrate-specific inhibition. Nucleic Acids Res. 30: 3954–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, D. S., and A. Kornberg, 1990. A novel protein binds a key origin sequence to block replication of an E. coli minichromosome. Cell 63: 325–331. [DOI] [PubMed] [Google Scholar]
- Ii, M., and S. J. Brill, 2005. Roles of SGS1, MUS81, and RAD51 in the repair of lagging-strand replication defects in Saccharomyces cerevisiae. Curr. Genet. 48: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, E. L., and J. E. Haber, 1997. DNA repair: RAD alert. Curr. Biol. 7: R492–R495. [DOI] [PubMed] [Google Scholar]
- Kai, M., M. N. Boddy, P. Russell and T. S. Wang, 2005. Replication checkpoint kinase Cds1 regulates Mus81 to preserve genome integrity during replication stress. Genes Dev. 19: 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannouche, P., B. C. Broughton, M. Volker, F. Hanaoka, L. H. Mullenders et al., 2001. Domain structure, localization, and function of DNA polymerase eta, defective in xeroderma pigmentosum variant cells. Genes Dev. 15: 158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannouche, P., A. R. Fernandez de Henestrosa, B. Coull, A. E. Vidal, C. Gray et al., 2003. Localization of DNA polymerases eta and iota to the replication machinery is tightly co-ordinated in human cells. EMBO J. 22: 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow, J. K., A. Constantinou, J. L. Li, S. C. West and I. D. Hickson, 2000. The Bloom's syndrome gene product promotes branch migration of Holliday junctions. Proc. Natl. Acad. Sci. USA 97: 6504–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto, T., K. Araki, E. Sonoda, Y. M. Yamashita, K. Harada et al., 2005. Dual roles for DNA polymerase eta in homologous DNA recombination and translesion DNA synthesis. Mol. Cell 20: 793–799. [DOI] [PubMed] [Google Scholar]
- Kim, Y. M., I. Yang, J. Lee and H. S. Koo, 2005. Deficiency of Bloom's syndrome protein causes hypersensitivity of C. elegans to ionizing radiation but not to UV radiation, and induces p53-dependent physiological apoptosis. Mol. Cells 20: 228–234. [PubMed] [Google Scholar]
- Klein, H. L., 1988. Different types of recombination events are controlled by the RAD1 and RAD52 genes of Saccharomyces cerevisiae. Genetics 120: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka, I., W. R. Kobertz, R. R. Ariza, M. Biggerstaff, J. M. Essigmann et al., 2000. Repair of an interstrand DNA cross-link initiated by ERCC1-XPF repair/recombination nuclease. J. Biol. Chem. 275: 26632–26636. [DOI] [PubMed] [Google Scholar]
- Lee, S. J., J. S. Yook, S. M. Han and H. S. Koo, 2004. A Werner syndrome protein homolog affects C. elegans development, growth rate, life span and sensitivity to DNA damage by acting at a DNA damage checkpoint. Development 131: 2565–2575. [DOI] [PubMed] [Google Scholar]
- Lehmann, A. R., 2002. Replication of damaged DNA in mammalian cells: new solutions to an old problem. Mutat. Res. 509: 23–34. [DOI] [PubMed] [Google Scholar]
- Levitus, M., Q. Waisfisz, B. C. Godthelp, Y. de Vries, S. Hussain et al., 2005. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat. Genet. 37: 934–935. [DOI] [PubMed] [Google Scholar]
- Levran, O., C. Attwooll, R. T. Henry, K. L. Milton, K. Neveling et al., 2005. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat. Genet. 37: 931–933. [DOI] [PubMed] [Google Scholar]
- Li, X., and J. L. Manley, 2005. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell 122: 365–378. [DOI] [PubMed] [Google Scholar]
- Liberi, G., G. Maffioletti, C. Lucca, I. Chiolo, A. Baryshnikova et al., 2005. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev. 19: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin, C., N. Schultz, C. Arnaudeau, A. Mohindra, L. T. Hansen et al., 2003. RAD51 is involved in repair of damage associated with DNA replication in mammalian cells. J. Mol. Biol. 328: 521–535. [DOI] [PubMed] [Google Scholar]
- McGlynn, P., and R. G. Lloyd, 2001. Rescue of stalled replication forks by RecG: simultaneous translocation on the leading and lagging strand templates supports an active DNA unwinding model of fork reversal and Holliday junction formation. Proc. Natl. Acad. Sci. USA 98: 8227–8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn, P., and R. G. Lloyd, 2002. Recombinational repair and restart of damaged replication forks. Nat. Rev. Mol. Cell Biol. 3: 859–870. [DOI] [PubMed] [Google Scholar]
- Mcllwraith, M. J., A. Vaisman, Y. Liu, E. Fanning, R. Woodgate et al., 2005. Human DNA polymerase eta promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol. Cell 20: 783–792. [DOI] [PubMed] [Google Scholar]
- Michel, B., M. J. Flores, E. Viguera, G. Grompone, M. Seigneur et al., 2001. Rescue of arrested replication forks by homologous recombination. Proc. Natl. Acad. Sci. USA 98: 8181–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohaghegh, P., J. K. Karow, R. M. Brosh, Jr., V. A. Bohr and I. D. Hickson, 2001. The Bloom's and Werner's syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 29: 2843–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay, S., D. R. Clark, N. B. Watson, W. Zacharias and W. G. McGregor, 2004. REV1 accumulates in DNA damage-induced nuclear foci in human cells and is implicated in mutagenesis by benzo[a]pyrenediolepoxide. Nucleic Acids Res. 32: 5820–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer, L. J., J. Essers, G. Weeda, B. Beverloo, J. de Wit et al., 2001. The structure-specific endonuclease Ercc1-xpf is required for targeted gene replacement in embryonic stem cells. EMBO J. 20: 6540–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer, L. J., H. Odijk, M. Budzowska, E. van Drunen, A. Maas et al., 2004. The structure-specific endonuclease Ercc1-xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol. Cell. Biol. 24: 5776–5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. K., J. S. Yook, H. S. Koo, I. S. Choi and B. Ahn, 2002. The Caenorhabditis elegans XPA homolog of human XPA. Mol. Cells 14: 50–55. [PubMed] [Google Scholar]
- Prakash, S., R. E. Johnson and L. Prakash, 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74: 317–353. [DOI] [PubMed] [Google Scholar]
- Reinke, V., H. E. Smith, J. Nance, J. Wang, C. Van Doren et al., 2000. A global profile of germline gene expression in C. elegans. Mol. Cell 6: 605–616. [DOI] [PubMed] [Google Scholar]
- Rinaldo, C., S. Ederle, V. Rocco and A. La Volpe, 1998. The Caenorhabditis elegans RAD51 homolog is transcribed into two alternative mRNAs potentially encoding proteins of different sizes. Mol. Gen. Genet. 260: 289–294. [DOI] [PubMed] [Google Scholar]
- Saleh-Gohari, N., H. E. Bryant, N. Schultz, K. M. Parker, T. N. Cassel et al., 2005. Spontaneous homologous recombination is induced by collapsed replication forks that are caused by endogenous DNA single-strand breaks. Mol. Cell. Biol. 25: 7158–7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent, R. G., J. L. Meservy, B. D. Perkins, A. E. Kilburn, Z. Intody et al., 2000. Role of the nucleotide excision repair gene ERCC1 in formation of recombination-dependent rearrangements in mammalian cells. Nucleic Acids Res. 28: 3771–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R. H., and S. Prakash, 1988. RAD1, an excision repair gene of Saccharomyces cerevisiae, is also involved in recombination. Mol. Cell. Biol. 8: 3619–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R. H., and S. Prakash, 1990. RAD10, an excision repair gene of Saccharomyces cerevisiae, is involved in the RAD1 pathway of mitotic recombination. Mol. Cell. Biol. 10: 2485–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, B., K. Hofmann, S. Boulton and A. Gartner, 2001. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11: 1722–1727. [DOI] [PubMed] [Google Scholar]
- Scully, R., J. Chen, R. L. Ochs, K. Keegan, M. Hoekstra et al., 1997. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell 90: 425–435. [DOI] [PubMed] [Google Scholar]
- Scully, R., N. Puget and K. Vlasakova, 2000. DNA polymerase stalling, sister chromatid recombination and the BRCA genes. Oncogene 19: 6176–6183. [DOI] [PubMed] [Google Scholar]
- Sengupta, S., and C. C. Harris, 2005. p53: traffic cop at the crossroads of DNA repair and recombination. Nat. Rev. Mol. Cell Biol. 6: 44–55. [DOI] [PubMed] [Google Scholar]
- Sengupta, S., S. P. Linke, R. Pedeux, Q. Yang, J. Farnsworth et al., 2003. BLM helicase-dependent transport of p53 to sites of stalled DNA replication forks modulates homologous recombination. EMBO J. 22: 1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsson, T., 2001. G-quadruplex DNA structures—variations on a theme. Biol. Chem. 382: 621–628. [DOI] [PubMed] [Google Scholar]
- Sun, H., J. K. Karow, I. D. Hickson and N. Maizels, 1998. The Bloom's syndrome helicase unwinds G4 DNA. J. Biol. Chem. 273: 27587–27592. [DOI] [PubMed] [Google Scholar]
- Todd, A. K., M. Johnston and S. Neidle, 2005. Highly prevalent putative quadruplex sequence motifs in human DNA. Nucleic Acids Res. 33: 2901–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh, T. Q., and R. R. Sinden, 1991. Preferential DNA secondary structure mutagenesis in the lagging strand of replication in E. coli. Nature 352: 544–547. [DOI] [PubMed] [Google Scholar]
- van Haaften, G., R. H. Plasterk and M. Tijsterman, 2004. Genomic instability and cancer: scanning the Caenorhabditis elegans genome for tumor suppressors. Oncogene 23: 8366–8375. [DOI] [PubMed] [Google Scholar]
- Warner, H. R., and M. D. Hobbs, 1969. Effect of hydroxyurea on replication of bacteriophage T4 in Escherichia coli. J. Virol. 3: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, S. C., 2003. Molecular views of recombination proteins and their control. Nat. Rev. Mol. Cell Biol. 4: 435–445. [DOI] [PubMed] [Google Scholar]
- Wicky, C., A. Alpi, M. Passannante, A. Rose, A. Gartner et al., 2004. Multiple genetic pathways involving the Caenorhabditis elegans Bloom's syndrome genes him-6, rad-51, and top-3 are needed to maintain genome stability in the germ line. Mol. Cell. Biol. 24: 5016–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford, K. J., R. M. Howell and K. Usdin, 1994. A novel K(+)-dependent DNA synthesis arrest site in a commonly occurring sequence motif in eukaryotes. J. Biol. Chem. 269: 27029–27035. [PubMed] [Google Scholar]
- Wu, L., and I. D. Hickson, 2003. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426: 870–874. [DOI] [PubMed] [Google Scholar]
- Wu, L., S. L. Davies, N. C. Levitt and I. D. Hickson, 2001. Potential role for the BLM helicase in recombinational repair via a conserved interaction with RAD51. J. Biol. Chem. 276: 19375–19381. [DOI] [PubMed] [Google Scholar]
- Yang, Q., R. Zhang, X. W. Wang, E. A. Spillare, S. P. Linke et al., 2002. The processing of Holliday junctions by BLM and WRN helicases is regulated by p53. J. Biol. Chem. 277: 31980–31987. [DOI] [PubMed] [Google Scholar]
- Yun, S., C. Lie-A-Cheong and A. C. Porter, 2004. Discriminatory suppression of homologous recombination by p53. Nucleic Acids Res. 32: 6479–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]