Abstract
Wolbachia is an intracellular microbe found in a wide diversity of arthropod and filarial nematode hosts. In arthropods these common bacteria are reproductive parasites that manipulate central elements of their host's reproduction to increase their own maternal transmission in one of several ways. Cytoplasmic incompatibility (CI) is one such manipulation where sperm are somehow modified in infected males and this modification must be rescued by the presence of the same bacterial strain in the egg for normal development to proceed. The molecular mechanisms involved in the expression of CI are unknown. Here we show that Wolbachia infection results in increased mRNA and protein expression of the Drosophila simulans nonmuscle myosin II gene zipper. Induced overexpression of zipper in Wolbachia-free transgenic D. melanogaster males results in paternal-effect lethality that mimics the fertilization defects associated with CI. Likewise, overexpression of the tumor suppressor gene, lethal giant larvae [l(2)gl], results in egg lethality and a CI phenotype. Stoichiometric levels of zipper and l(2)gl are required for proper segregation of cellular determinants during neuroblast stem cell division. Taken together these results form the basis of a working hypothesis whereby Wolbachia induces paternal effects in sperm by manipulating the expression of key regulators of cytoskeletal activity during spermatogenesis.
WOLBACHIA'S manipulation of central elements of reproduction has long intrigued evolutionary biologists because of its potential importance as a driving force in directing the evolutionary trajectories of populations (Turelli and Hoffmann 1995), as a possible mechanism of speciation (O'Neill and Karr 1990; Stouthamer et al. 1999; Bordenstein et al. 2001), as a potential tool for the biocontrol of insect pests (Karr 1994; Zabalou et al. 2004; Xi et al. 2005), and as a potential vehicle for the introduction of foreign DNA into natural populations (Turelli and Hoffmann 1999). Estimates of infection rates in arthropods range from 25–75%, making Wolbachia the most widely spread eubacterium known (Werren et al. 1995; Jeyaprakash and Hoy 2000). Maternally inherited, these reproductive parasites have evolved a number of different strategies for manipulating host reproduction that result in their increased transmission through a population. Wolbachia-mediated manipulation of host reproduction includes feminization, male killing, induced parthenogenesis, and cytoplasmic incompatibility (CI), a form of postfertilization reproductive failure in crosses of infected males to uninfected females (O'Neill et al. 1997; Stouthamer et al. 1999).
Because Wolbachia is not present in sperm from infected males and its presence has no effect on the processing of two major male accessory gland proteins (Bressac and Rousset 1993; Snook et al. 2000), it most probably exerts its effect during earlier stages of spermatogenesis. How and where this effect takes place is not known.
Elucidation of the cellular and molecular mechanisms of CI would significantly advance our understanding of how Wolbachia manipulates host reproduction and provide new insights into the mechanisms and dynamics of symbiosis. Wolbachia are obligate endocellular microbes that cannot be cultured outside the host; consequently, little is known about their molecular biology. Because of the presumed effect of Wolbachia in the testis and the lack of specific information on Wolbachia genetics, we chose to focus on the host's response to infection by looking for alterations in host gene activity in infected testes. Here we describe a series of experiments in which we measured differential levels of gene expression to obtain information on the mechanistic basis of CI in male Drosophila. By manipulating gene expression in uninfected males we observed a reduction in egg hatch and a phenotype indistinguishable from CI. Our findings support the general hypothesis that Wolbachia alter the expression of genes essential for normal sperm development in male Drosophila and thereby induce the male component of CI.
MATERIALS AND METHODS
Fly strains used:
Drosophila simulans:
The DSR strain from Riverside, California, (Hoffmann et al. 1986) known to be infected by Wolbachia was obtained from M. Turelli (UC Davis). An uninfected strain (DSRT) was prepared by tetracycline treatment of the DSR strain as described (O'Neill and Karr 1990). Prior to and following all experiments, the infection status of these and the Drosophila melanogaster lines (see below) was determined using the polymerase chain reaction and primers specific for the Wolbachia 16S rDNA gene (O'Neill et al. 1992). D. melanogaster Tempe, a D. melanogaster line collected near Tempe, Arizona, in the spring of 1998, was used as a source of females. These females were found to be infected by Wolbachia and subsequently cleared of infection using tetracycline. hs-zip (long), a hybrid cDNA construct consisting of the hsp70 promoter attached 5′ to a cDNA clone of the zipper gene, was a kind gift of D. Kiehart (Young et al. 1993). hs-l(2)gl, a hybrid cDNA construct consisting of the hsp 70 promoter attached 5′ to a cDNA clone of the l(2)gl gene, was a kind gift of Chris Q. Doe.
cDNA subtraction library preparation and screening:
Testes from DSR and DSRT flies were dissected in sterile Insect Ringer, and RNA and resident mRNA were isolated by binding to streptavidin beads followed by magnetic separation (PolyATract mRNA Isolation System, Promega). First strand cDNA was synthesized from the message isolated from infected testes as per manufacturer's instructions (Universal Riboclone cDNA Synthesis System, Promega). Single-stranded cDNA was hybridized to an excess of similarly prepared mRNA from DSRT testes. Hybridized double-stranded DNA–RNA sequences common to both were separated by hydroxylapatite chromatography and the remaining single-stranded cDNA was used as template for second strand synthesis (Universal Riboclone cDNA Synthesis System, Promega). The resultant double-stranded cDNA was cloned into a lambda gt-11 phage vector and packaged in a commercial packaging extract following manufacturer's protocol (Packagene, Promega). The resultant cDNA library was then screened for those sequences unique to the testes of DSR. cDNA from testes of DSR and DSRT males was radiolabeled, and duplicate filter lifts of the plated library were probed with the respective populations of mRNA. Clones that showed signal when probed with DSR-derived cDNA, but lacked signal when probed with DSRT-derived DNA, were then further investigated. Inserts from candidate clones were subcloned into a plasmid vector and sequenced. Sequencing was carried out using the ABI Prism Cycle Sequencing Dye Terminator Kit and an ABI 3777 automated sequencer. Identification of candidate genes was accomplished by submitting the sequences for a BLAST search (GenBank).
Immunoblotting of testes proteins:
zipper:
Testes from newly eclosed DSR and DSRT males (12–24 hr) were dissected in a drop of Ringer, rinsed once in Ringer, and ground in 50 μl PBS in a 1.5-ml Kontes tube at 40°. After tissue disruption, samples were heated for 2 min at 95° to lyse cells. Protein concentrations were measured using Bio-Rad protein assay kit (Bio-Rad) using BSA as standard. Following solubilization in SDS, samples were boiled in the presence of DTT and equal concentrations loaded onto 4–12% precast Tris-Glycine gels (Novex). Proteins were electrophoresed at 136 V for 2 hr and transferred onto PVDF-Plus transfer membrane (Micron Separations) using a semidry blotting apparatus (Pharmacia). Transfer was carried out over 2 hr at 60 mA constant current. Membranes were blocked for 1 hr in TBST (50 mm Tris–HCl, 100 mm NaCl, 0.1% Triton X-100) containing 5% w/v nonfat dry milk (Carnation), washed three times for 5 min in TBST and incubated with an anti-zipper antibody (Kiehart and Feghali 1986) diluted 1:2500 in TBST for 1 hr. The membrane was then washed three times for 5 min in TBST, followed by incubation for 1 hr in an alkaline phosphatase-conjugated goat anti-rabbit IgG (Jackson) diluted 1:2500. Anti-zipper immunoreactive bands were visualized using BCIP/Tetrazolium solution.
l(2)gl:
Testes of 3-day-old male DSR and DSRT reared at controlled density were dissected into PBS and their testes prepared for Western immunoblotting analysis using the Invitrogen NuPage system (Invitrogen, Paisley, UK). Samples were quantified using the EZQ protein quantitation kit (Molecular Probes, Eugene, OR) and a Fujifilm phosphorimager. Equal amounts of protein were loaded with molecular weight markers and probed with a rat anti-l(2)gl (1:200) antibody and with a mouse anti α-tubulin antibody (Sigma) used as a loading control. Alkaline phosphatase-conjugated secondary antibodies were used in conjunction with ECF Western blotting reagent (Amersham Bioscieces, Little Chalfont, UK). A Fujifilm phosphorimager was used to acquire 14-bit data images (used to quantify the relative amount of the protein of interest on the blot and ensure the loading controls are equal). For both immunoassays band intensity was quantified using AIDA software (Raytest, Straubenhardt, Germany); indicated values are normalized using α-tubulin as an internal control for equal loading.
CI measurement in hs-zip and hs-l(2)gl:
We used a sublethal heat treatment of 36° for 1 hr/day during larval development sufficient to induce zipper overexpression. The heat-shock regime was optimized for zip overexpression and larval viability. The protocol relied on the minimal heat-shock regimen needed to elevate Zipper levels to near those observed in DSR, while minimizing effects on viability. We found that a 1-hr heat treatment of third instar larvae at 36° resulted in sufficient Zipper increases while resulting in <25% pupal lethality. The same regimeregimen was used for the hs-l(2)gl flies. All experiments used males or females that were grown under standard conditions as described (Snook et al. 2000) except as follows: first instar crawling larvae were transferred to fresh cornmeal/agar food vials (50 larvae/vial), treated at 36° for 1 hr, and returned to 25°. Daily 1-hr heat treatments continued until eclosion when males were separated into fresh food vials and maintained for 24 hr. Males received a final heat treatment 6–12 hr prior to mating, were allowed to recover for 6 hr, and were mated to uninfected wild-type females (females were aged for 1 week prior to mating). Control experiments were performed using the same cohort of first instar larvae without heat treatment or heat-treated wild-type first instar larvae. Additional heat-treatment controls, using both wild-type D. melanogaster and D. simulans males, were also performed (not shown). In all cases male viability was the same in all control samples as determined by Mann-Whitney statistical tests. Measurement of CI was carried out as described (Snook et al. 2000). CI is reported as the fraction of unhatched eggs, calculated as the number of unhatched eggs/total number of eggs (Table 1). Nonparametric Mann–Whitney statistical analyses were performed using Statview (Abacus).
TABLE 1.
Effect of Zipper and Lgl on CI expression
| Cross | Treatment | Eggs | Females | Proportion unhatched | Comparison |
|---|---|---|---|---|---|
| 1 | hszipper heat | 2912 | 22 | 0.75 ± 0.05 | 1 vs. 2 (P < 0.01) |
| 2 | hszipper no heat | 2654 | 17 | 0.22 ± 0.07 | |
| 3 | wt heat | 1812 | 10 | 0.14 ± 0.047 | 1 vs. 3 (P < 0.01) |
| 4 | wt no heat | 2199 | 15 | 0.19 ± 0.034 | 3 vs. 4 (NS) |
| 5 | hslgl heat | 2356 | 31 | 0.66 ± 0.15 | 5 vs. 6 (P < 0.01) |
| 6 | hslgl no heat | 2100 | 25 | 0.1 ± 0.063 | |
| 7 | wt heat | 4988 | 29 | 0.26 ± 0.04 | 5 vs. 7 (P < 0.01) |
| 9 | wt no heat | 3666 | 18 | 0.22 ± 0.077 | 7 vs. 9 (NS) |
Incompatibility in single pair crosses between treated males (treatment) and non-heat-treated 3-day-old virgin hszipper and hslgl males and Wolbachia-free wild-type virgin females aged 2–5 days old. Crosses in which no eggs hatched were assumed to be due to the females being unfertilized and were not included in the analysis. Eggs were removed, counted daily, and then scored for hatching 12–24 hrs later. The proportion of eggs hatching was scored over 4 days. NS, not significant; wt, wild type.
Test for rescue of incompatibility:
The CI measurement assay using the hs-zip flies was repeated, except this time larvae heat treated from the third instar were used to test whether the developmental stage at which zipper expression is increased affected the proportion of unhatched eggs. We additionally tested whether this incompatibility could be rescued by the presence of Wolbachia in the egg by crossing heat-treated hs-zip males with Wolbachia-infected females and measuring CI as previously described.
Tissue fixation, staining, and confocal microscopy:
Embryos were fixed and stained using the DROP 1.1 anti-sperm tail antibody as described (Karr 1991) and nuclei stained with Propidium Iodide (Molecular Probes) following treatment with 2 mg/ml RNAase (Promega). Confocal optical sections were obtained using either a Zeiss LSM 510 or a Leica DM IRB microscope. Images were projected along the z-axis and displayed using associated Zeiss image analysis software. Images were annotated and printed using Photoshop 7.0 (Adobe).
RESULTS
Identification of host genes with altered expression due to Wolbachia infection:
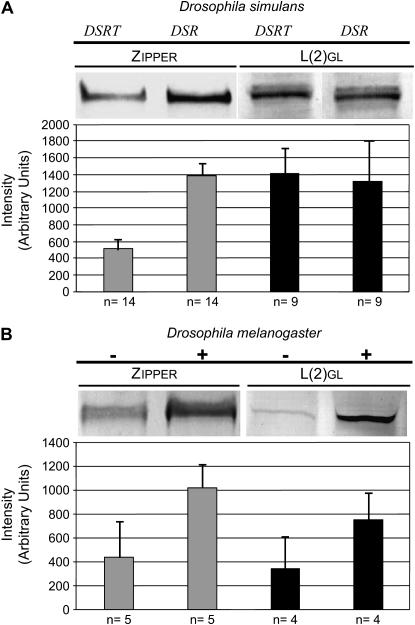
The screening of a subtraction cDNA library prepared from mRNA of infected and uninfected D. simulans Riverside males (DSR and DSRT, respectively) (Hoffmann et al. 1986; O'Neill and Karr 1990) identified seven candidate DSR cDNA clones of which one was represented twice and the others were either very short unidentified sequences or vector sequences. Subsequent DNA sequencing and database searches identified the two positive clones as the Drosophila nonmuscle myosin II gene zipper. Alignment of 660 nucleotides shows only two changes resulting in two substitutions in the inferred amino acid sequence. Thus, Wolbachia-infected testes overexpress zipper mRNA relative to genetically identical uninfected flies. In concert with the mRNA results we found increased levels of zipper protein on immunoblots of testis extracts (Figure 1A).
Figure 1.
Western blot analysis of expression of zipper and l(2)gl in testes of 3-day-old Drosphila. (A) l(2)gl and zipper expression in D. simulans (Riverside) infected (DSR) and uninfected with Wolbachia (DSRT). (B) Effect of heat treatment (indicated +/−) on expression of l(2)gl and zipper in transgenic D. melanogaster carrying a construct with the indicated gene linked to a heat-shock promoter. Band intensity was quantified using AIDA software (Raytest). Values were normalized using α-tubulin as an internal control for equal loading (not shown). Bars show normalized mean band intensity values (± 1 SE). Sample size (n) is shown below each bar. The bands shown represent typical results.
Phenotypic analysis of zipper overexpression in D. melanogaster:
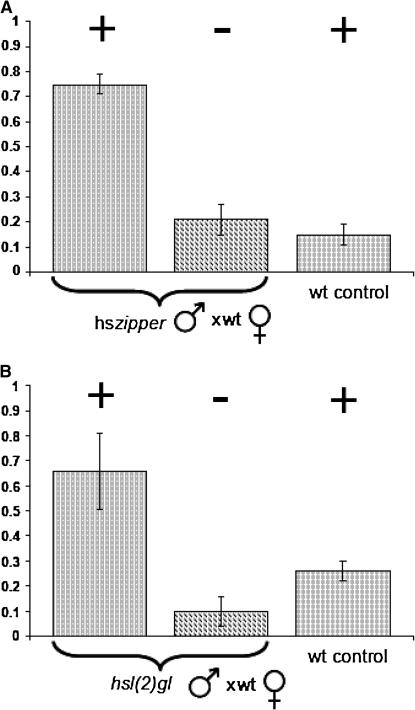
Increased levels of zipper mRNA and protein in infected D. simulans led us to examine the effect of zipper overexpression in uninfected D. melanogaster lines. Heat-shock treatment of an hs-zip line (see materials and methods) resulted in increased levels of Zipper in the testes (Figure 1B) with little overall effect on viability and sperm production (not shown). However, when heat-treated hs-zip males were crossed to wild-type females the proportion of unhatched eggs was significantly higher than in crosses with non-heat-treated hs-zip males or heat-treated wild-type males (Figure 2A, Table 1). We conclude that, with regard to egg-hatch rates, increasing Zipper levels in males resulted in a CI-like phenotype.
Figure 2.
Proportion of eggs unhatched in crosses between wild-type D. melanogaster females and heat-treated Wolbachia-free transgenic D. melanogaster males. + or − sign indicates presence or absence of heat-shock regime during development. (A) Overexpression of zipper. (B) Overexpression of l(2)gl.
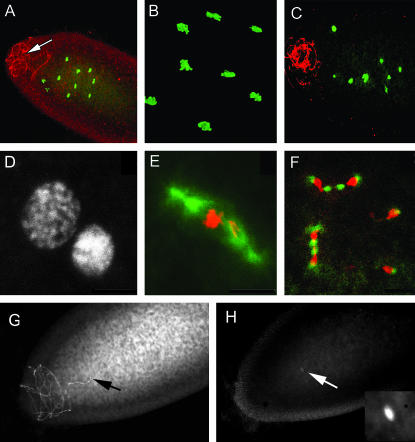
CI in D. simulans has a well-characterized early lethal embryonic phase (Lassy and Karr 1996). We therefore examined early development in fertilized eggs from these crosses (Figure 3). Normal zygote development is characterized by synchronized and evenly spaced nuclear divisions (Foe and Alberts 1983; Zalokar and Erk 1976) clearly observed in eggs fertilized by control and non-heat-treated hs-zip males (Figure 3, A and B). Conversely, heat-treated transgenic hs-zip males displayed numerous chromosomal abnormalities (Figure 3, C–F). These early postfertilization anomalies are very similar to those observed in incompatible CI crosses previously described in D. simulans (Lassy and Karr 1996) and D. melanogaster (Clark et al. 2005). An unambiguous assay for fertilization is visualization of the sperm tail localized in the anterior region of the egg (Karr 1991) (Figure 3, A and G, arrow) eliminating the trivial possibility that heat treatment rendered males sterile or otherwise incapable of producing fertilization-competent sperm. We therefore conclude that increasing Zipper levels in males leads to fertilization defects similar to those observed in CI crosses.
Figure 3.
Expression of CI in hs-zip flies and hs-l(2)gl flies. Confocal images of fertilized eggs visualized by indirect immunofluorescence using an anti-sperm tail primary antibody (A, C, G), a DNA-specific green dye (A–C), propidium iodide (D–F and H), and an anti-α-tubulin antibody (green) (E and H). (A and B) Control crosses displaying normal egg development when fertilized by (A) non-heat-treated males (arrow, sperm tail) or (B) wild-type males. (C–F) Fertilization defects observed in crosses using heat-treated hs-zip males. (G and H) Typical examples of the early lethal phenotype observed in an incompatible cross between a heat-treated hs-lgl male and a wild-type female (H).
Late overexpression of zipper reduces penetrance of the CI-like phenotype:
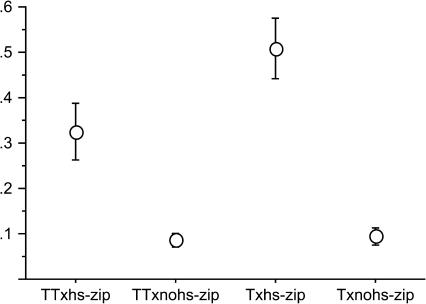
Overexpression of zipper from the third larval instar resulted in weaker CI effect compared to when zipper was overexpressed from the first instar onward (Figure 2A, Figure 4). This indicates that the strength of the effect may increase with earlier overexpression and/or depends on total time over which zipper is overexpressed in the testes.
Figure 4.
Proportion of eggs hatching in crosses between wild-type D. melanogaster females, infected (T) and uninfected (TT) with Wolbachia and hs-zip males, heat treated during development from the third larval instar, or not heated (nohs).
The CI phenotype cannot be rescued by Wolbachia in the egg:
In contrast to expectations, the presence of Wolbachia in the egg did not rescue the CI-like phenotype (Figure 4); the cross between hs-zip males and Wolbachia-infected wild-type females resulted in even stronger incompatibility than crosses with uninfected females (Figure 4). This result was consistently observed in repeat experiments and clearly indicates additive or synergistic interactions of unknown character.
Overexpression of zipper results in a spermatogenesis phenotype:
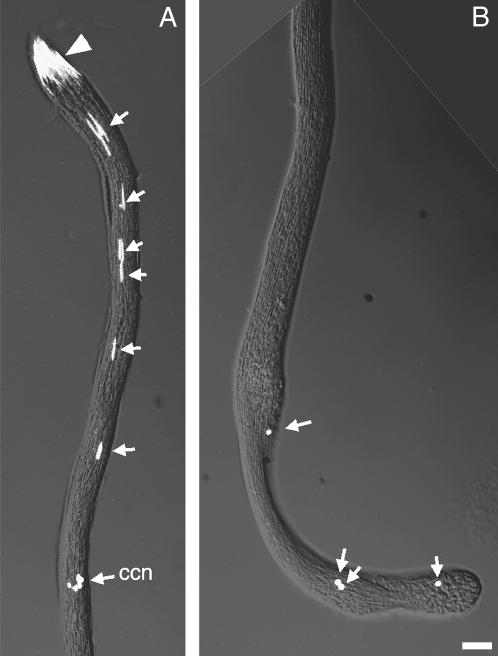
In addition to causing a CI phenotype in fertilized eggs, overexpression of zipper during spermatogenesis resulted in minor defects in developing spermatid bundles. This defect was most easily seen in elongating bundles where the normally apically localized sperm nuclei were found scattered along the length of the cyst (Figure 5). Although not obvious in this particular projection of the data stack, cyst cell nuclei are easily distinguishable by their unique morphology and their location at the periphery of the spermatid bundle. Therefore, the other elongated nuclei observed (Figure 5, arrows) are presumed sperm nuclei that have become displaced from the apical region during elongation. These defects are present prior to spermatid individualization and were not observed in non-heat-treated hs-zip males or in heat-treated wild-type males (data not shown).
Figure 5.
Aberrant sperm cyst from a heat-treated hs-zip male. Two ends of the same elongated, pre-individualized sperm cyst. (A) Spermatid nuclei are normally all apically located in a bundle of nuclei (arrowhead). Abnormal spermatid nuclei are also seen scattered along the length of the cyst (A, arrows). Abnormal spermatid nuclei are less needle-like farther from the apical nuclei bundle (B, arrows). ccn, cyst cell nuclei. Bar, 10 μm.
Overexpression of l(2)gl also results in a CI-like phenotype:
The product of l(2)gl, Lgl (also known as P127), is known to physically interact with Zipper in the specification of cell polarity during germ-line stem cell divisions and mutations in zip strongly suppress the l(2)gl mutant phenotype (Strand et al. 1994a; Peng et al. 2000). We therefore examined the effect of overexpressing l(2)gl in males from a transgenic line carrying a wild-type copy of l(2)gl driven by an hsp70 promoter. First, we examined the endogenous level of l(2)gl in the testes of DSR and DSRT males and found them to be indistinguishable (Figure 1A). We then determined that a heat-shock regime similar to that used for hs-zip resulted in increased Lgl in the testes (Figure 1B). We then measured egg viability in crosses between heat-shocked males and control (uninfected) wild-type females (Figure 2B, Table 1). Again, highly significant reduction in egg hatch was observed, suggesting that overexpression of l(2)gl in males similarly resulted in a CI phenotype. Finally, fertilization defects were also observed similar to those observed in both DSR-incompatible and heat-treated hs-zip crosses (Figure 3, G and H).
DISCUSSION
Zipper overexpression results in a paternal effect:
Under the conditions used, transient zipper overexpression is not lethal and results in mature and motile sperm capable of fertilization. These sperm, although able to penetrate the egg and activate development, are, like sperm from DSR males, incapable of supporting zygote formation and subsequent nuclear divisions. This phenotype is similar to both a paternal effect gene, ms(3)K81 (Yasuda et al. 1995; Loppin et al. 2005), and Wolbachia-induced CI (Lassy and Karr 1996). These results suggest that increased zipper expression during spermatogenesis is causally linked to the expression of CI in D. simulans. How does increased zipper expression in the testes result in defects in fertilization and early egg development? zipper has been extensively studied over the past decade where its action in important morphological events during early embryogenesis is well known (Kiehart and Feghali 1986; Kiehart 2003), although the role of cytoplasmic myosin in spermatogenesis and fertilization is not known and has yet to be specifically examined (D. Kiehart, personal communication). However, heterozygous zip1/+ males generate fully mature, fertilization-competent sperm that do not exhibit either a CI phenotype or reduction in egg viability (M. Clark and T. L. Karr, unpublished observations), suggesting, as expected for a recessive gene, that reduction in Zipper is not detrimental to sperm function and zygote viability. Therefore the effect appears specific to overexpression of Zipper. Further studies on the mechanism by which increased Zipper results in a paternal effect that mimics CI is currently under study in our laboratory. As discussed below, one avenue of exploration involves interactions with the tumor suppressor gene l(2)gl, a gene intimately associated with zipper function (Strand et al. 1994b; Barros et al. 2003).
Males overexpressing zipper were also shown to have weakly penetrant effects on spermatid development (Figure 5). Although the role of zipper in this process is yet to be determined, another closely related myosin, myosin IV (jaguar), does have an essential function (Tuxworth and Chia 2003) during spermatid individualization, and zipper is present in individualization cones (data not shown). It therefore remains to be determined if zipper function during these stages of development is the site of action that elicits CI.
Wolbachia does not rescue Zipper-induced paternal effect.
Curiously, the presence of Wolbachia did not rescue egg lethality in our experiments. In fact, Wolbachia resulted in measurably higher levels of egg lethality suggesting synergistic effects. There are two possible explanations for these findings. First, although counterintuitive, the result may indicate negative interactions not previously observed that reflect inherent differences in the CI modification/rescue systems used by these two species. Second, the heat-treatment regimens used to elevate Zipper in D. melanogaster could only approximate Zipper levels in the DSR lines. Additionally, inherent differences in endogenous Zipper levels and any differential effects of elevated Zipper in these two species may further confound interpretation of the results. The more straightforward approach to measure CI using hs-zip D. simulans transgenic flies, although technically more challenging, is currently planned.
A working model for CI in D. melanogaster:
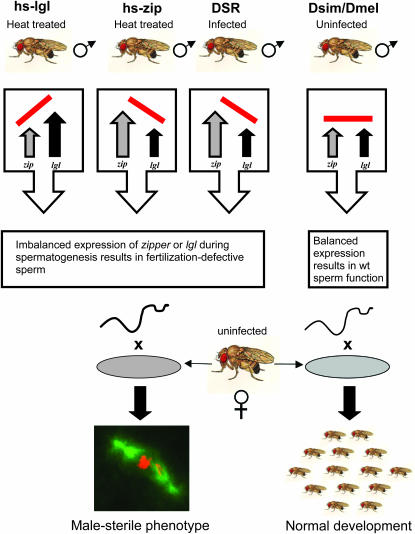
Zipper and Lgl perform crucial functions during embryonic development that may provide clues to their role in sperm dysfunction we have described. They both clearly have a central role in the asymmetric neuroblast divisions in the embryo. Neuroblasts divide asymmetrically to generate a series of smaller, more differentiated ganglion mother cells (GMCs). During each asymmetric division, several proteins/mRNAs are specifically partitioned into the GMC, and this process of protein localization requires the activity of l(2)gl (Peng et al. 2000). The products of l(2)gl and zipper physically interact (Strand et al. 1994b), and mutations in zipper strongly suppress the l(2)gl phenotype, suggesting that the l(2)gl phenotype is due to an imbalance in levels of Zipper protein (Ohshiro et al. 2000; Peng et al. 2000). By analogy, the imbalance in the levels of Zipper in Wolbachia-infected males observed may disrupt protein localization during the asymmetric divisions of the spermatogonial stem cells, leading to similar mislocalization in important determinative factors and ultimately to defective sperm. Our finding that l(2)gl overexpression also results in a CI phenotype is consistent with the hypothesis that balanced expression of zipper and l(2)gl are required for normal sperm development and an imbalance results in modified sperm leading to CI (Figure 6). We note that perturbations in the levels of Zipper and Lgl result in subtle cellular phenotypes as in both instances the affected stem cells go on to produce either neurons (pathfinding defects) or motile sperm (fertilization defect) that fail to function later in development.
Figure 6.
Hypothesis that zipper overexpression causes developmental abnormalities in sperm leading to CI in males.
One major deficiency in this model is that it fails to explain why the modification cannot be rescued by the presence of Wolbachia in the egg. In fact, contrary to expectations, the presence of Wolbachia in D. melanogaster females results in a significant reduction in egg viability (Figure 4). These experiments were highly repeatable and indicate a more complex scenario for the role of these genes in the mechanism of CI; they do, however, demonstrate an interaction of Wolbachia with this system. This surprising result may be related to the well-known differences in the levels of CI expression between D. simulans and D. melanogaster (Hoffmann et al. 1986; Hoffmann and Turelli 1988) making comparisons of the zipper effect problematical. Further studies aimed at overexpressing zipper in D. simulans are currently underway in which a more direct test of rescue can be performed.
Wolbachia-induced overexpression of host gene expression may be an effective strategy used by Wolbachia to affect sperm function during fertilization. Two mechanisms not necessarily mutually exclusive, induced or enhanced transcription and/or mRNA stabilization, could result in the overexpression observed. As such, Wolbachia-induced host gene expression represents a unique form of neomorphic mutation and raises many important questions about the nature of incompatibility and host/symbiosis. For example, does zipper-induced reproductive failure suggest a model for multiple incompatibility types like those previously observed in D. simulans (O'Neill and Karr 1990) and Nasonia (Breeuwer and Werren 1990)? Perhaps different strains of Wolbachia will likewise be associated with overexpression of different specific host genes in the testis.
Acknowledgments
The hs-l(2)gl line was a generous gift of Chris Q. Doe (University of Oregon); the hs-zip line was generously provided by Dan Kiehart (Duke University). This work was generously funded by the National Science Foundation USA (MCB-0135166), the Royal Society, and the Biotechnology and Biological Sciences Research Council UK.
References
- Barros, C. S., C. B. Phelps and A. H. Brand, 2003. Drosophila nonmuscle myosin II promotes the asymmetric segregation of cell fate determinants by cortical exclusion rather than active transport. Dev. Cell. 5: 829–840. [DOI] [PubMed] [Google Scholar]
- Bordenstein, S. R., F. P. O'Hara and J. H. Werren, 2001. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409: 707–710. [DOI] [PubMed] [Google Scholar]
- Breeuwer, J. A., and J. H. Werren, 1990. Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature 346: 558–560. [DOI] [PubMed] [Google Scholar]
- Bressac, C., and F. Rousset, 1993. The reproductive incompatibility system in Drosophila simulans: DAPI-staining analysis of the Wolbachia symbionts in sperm cysts. J. Invertebr. Pathol. 61: 226–230. [DOI] [PubMed] [Google Scholar]
- Clark, M. E., C. L. Anderson, J. Cande and T. L. Karr, 2005. Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics 170: 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe, V. E., and B. M. Alberts, 1983. Studies of nuclear and cytoplasmic behavior during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J. Cell Science 61: 31–70. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A. A., and M. Turelli, 1988. Unidirectional incompatibility in Drosophila simulans: inheritance, geographic variation and fitness effects. Genetics 119: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A., M. Turelli and G. M. Simmons, 1986. Unidirectional incompatibility between populations of Drosophila simulans. Evolution 40: 692–701. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash, A., and M. A. Hoy, 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 9: 393–405. [DOI] [PubMed] [Google Scholar]
- Karr, T., 1991. Intracellular sperm/egg interactions in Drosophila: A three-dimensional structural analysis of a paternal product in the developing egg. Mech. Dev. 34: 101–112. [DOI] [PubMed] [Google Scholar]
- Karr, T. L., 1994. Giant steps sideways. Curr. Biol. 4: 537–540. [DOI] [PubMed] [Google Scholar]
- Kiehart, D. P., 2003. Myosins motor Miranda. Mol. Cell 12: 1346–1347. [DOI] [PubMed] [Google Scholar]
- Kiehart, D. P., and R. Feghali, 1986. Cytoplasmic myosin from Drosophila melanogaster. J. Cell Biol. 103: 1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassy, C. W., and T. L. Karr, 1996. Cytological analysis of fertilization and early embryonic development in incompatible crosses of Drosophila simulans. Mech. Dev. 57: 47–58. [DOI] [PubMed] [Google Scholar]
- Loppin, B., D. Lepetit, S. Dorus, P. Couble and T. L. Karr, 2005. Origin and neofunctionalization of a Drosophila paternal effect gene essential for zygote viability. Curr. Biol. 15: 87–93. [DOI] [PubMed] [Google Scholar]
- O'Neill, S. L., R. Giordano, A. M. Colbert, T. L. Karr and H. M. Robertson, 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 89: 2699–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, S. L., A. A. Hoffmann and J. H. Werren, 1997. Influential Passenger: Inherited Microorganisms and Arthropod Reproduction. Oxford University Press, New York.
- O'Neill, S. O., and T. L. Karr, 1990. Bidirectional cytoplasmic incompatibility between conspecific populations of Drosophila simulans. Nature 348: 178–180. [DOI] [PubMed] [Google Scholar]
- Ohshiro, T., T. Yagami, C. Zhang and F. Matsuzaki, 2000. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 408: 593–596. [DOI] [PubMed] [Google Scholar]
- Peng, C. Y., L. Manning, R. Albertson and C. Q. Doe, 2000. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 408: 596–600. [DOI] [PubMed] [Google Scholar]
- Snook, R. R., S. Y. Cleland, M. F. Wolfner and T. L. Karr, 2000. Offsetting effects of Wolbachia infection and heat shock on sperm production in Drosophila simulans: analyses of fecundity, fertility and accessory gland proteins. Genetics 155: 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer, R., J. A. Breeuwer and G. D. Hurst, 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 53: 71–102. [DOI] [PubMed] [Google Scholar]
- Strand, D., R. Jakobs, G. Merdes, B. Neumann, A. Kalmes et al., 1994. a The Drosophila lethal(2)giant larvae tumor suppressor protein forms homo-oligomers and is associated with nonmuscle myosin II heavy chain. J. Cell Biol. 127: 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand, D., I. Raska and B. M. Mechler, 1994. b The Drosophila lethal(2)giant larvae tumor suppressor protein is a component of the cytoskeleton. J. Cell Biol. 127: 1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M., and A. A. Hoffmann, 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140: 1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M., and A. A. Hoffmann, 1999. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol. Biol. 8: 243–255. [DOI] [PubMed] [Google Scholar]
- Tuxworth, R., and W. Chia, 2003. Asymmetric cell division: Miranda chauffeured by Jaguar? Mol. Cell 11: 288–289. [DOI] [PubMed] [Google Scholar]
- Werren, J. H., W. Zhang and L. R. Guo, 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. R. Soc. Lond. B Biol. Sci. 261: 55–63. [DOI] [PubMed] [Google Scholar]
- Xi, Z., C. C. Khoo and S. L. Dobson, 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310: 326–328. [DOI] [PubMed] [Google Scholar]
- Yasuda, G. K., G. Schubiger and B. T. Wakimoto, 1995. Genetic characterization of ms (3) K81, a paternal effect gene of Drosophila melanogaster. Genetics 140: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, P. E., A. M. Richman, A. S. Ketchum and D. P. Kiehart, 1993. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 7: 29–41. [DOI] [PubMed] [Google Scholar]
- Zabalou, S., M. Riegler, M. Theodorakopoulou, C. Stauffer, C. Savakis et al., 2004. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA 101: 15042–15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalokar, M., and I. Erk, 1976. Division and migration of nuclei during early embryogenesis of Drosophila melanogaster. J. Microbiol. 25: 97–106. [Google Scholar]