Abstract
Psoriatic arthritis is a diverse condition that may be characterized by peripheral inflammatory arthritis, axial involvement, dactylitis and enthesitis. Magnetic resonance imaging (MRI) allows visualization of soft tissue, articular and entheseal lesions, and provides a unique picture of the disease process that cannot be gained using other imaging modalities. This review focuses on the literature on MRI in psoriatic arthritis published from 1996 to July 2005. The MRI features discussed include synovitis, tendonitis, dactylitis, bone oedema, bone erosions, soft tissue oedema, spondylitis/sacroiliitis and subclinical arthropathy. Comparisons have been drawn with the more extensive literature describing the MRI features of rheumatoid arthritis and ankylosing spondylitis.
Introduction
Magnetic resonance imaging (MRI) has advanced our understanding of many types of arthritis, both with respect to inflammatory processes and articular damage. Psoriatic arthritis (PsA) has received less research scrutiny than rheumatoid arthritis (RA) in many areas, including imaging [1], but this is likely to change because MRI outcome measures are increasingly being used in clinical trials of new therapeutic agents such as biologics [2]. In this review we summarize the literature describing the MRI features of articular and entheseal disease in PsA and include references to histopathological correlates where this information is available. Very few published studies have concentrated specifically on PsA, and most data come from studies of broader groups of patients with 'inflammatory arthritis' or 'spondyloarthropathies' (SpAs) and from case reports and small case series.
Method
A Medline/Embase search was undertaken from 1966 to July 2005 using the following search words; EXP arthritis, psoriatic, magnetic resonance imaging, magnetic resonance, psoriasis and combinations thereof. A total of 264 hits resulted, and of these 20 reports were regarded as useful (category 1, Table 1) but two could not be obtained from library serial collections or electronically.
Table 1.
Criteria used for Medline/Embase search
| Code | Description | Number of articles |
| 1 | Review: in Medline/Embase and meets criteria for MRI and PsA | 20 |
| 2 | Review: not in Medline/Embase but found via hand search and meets criteria for MRI and PsA | 12 |
| 3 | Discard: MRI but not PsA or PsA but not MRI | 115 |
| 4 | Discard: MRI and PsA but not adults | 9 |
| 5 | Discard: MRI and PsA but review article only | 50 |
| 6 | Discard: English abstract but full text not in English | 29 |
| 7 | Discard: not relevant for other reason | 39 |
MRI, magnetic resonance imaging; PsA, psoriatic arthritis.
Twelve more articles were found by hand searching and were included in the source material for this review. Of the 30 articles finally identified [3-32], 24 dealt with MRI of peripheral joints or entheses and six with axial joints. Table 2 classifies these articles alphabetically (by first author) and describes the type of study, number of patients examined and field strength of MRI machine used. The review has been organised according to the various MRI characteristics of PsA, including synovitis, bone erosion and bone oedema, enthesitis, tenosynovitis and dactylitis, spondylitis/sacroiliitis and subclinical disease.
Table 2.
Source material for review: articles dealing with MRI examination of PsA
| First author [ref.] | Year | Study typea | Description | PsA patients who underwent MRI (n) | MRI field strength (T) |
| Antoni [3] | 2002 | 1 | Open-label study of infliximab in PsA; dynamic MRI of hands or feet | 10 | 1.5 |
| Backhaus [4] | 1999 | 2 | Patients with inflammatory arthritis; MRI of hands | 9 | 0.2 |
| Bollow [5] | 1995 | 2 | SpA patients; MRI of sacroiliac joints | Not stated | 1.5 |
| Bollow [6] | 2000 | 2 | SpA patients; biopsies compared with MRI of sacroiliac joints | 2 | 1.5 |
| Bongartz [7] | 2005 | 5 | Psoriatic onycho-pachydermo periostitis; MRI evaluation | 1 | Not stated |
| Cantini [8] | 2001 | 4 | Distal pitting oedema; MRI evaluation | 7 | 0.5 |
| Cimmino [9] | 2005 | 4 | PsA and RA patients; dynamic MRI of wrists | 15 | 0.2 |
| Giovagnoni [10] | 1995 | 2 | PsA and RA patients; MRI of hands | 28 | 1.0 |
| Godfrin [11] | 2003 | 3 | SpA patients with entheseal pain; MRI of entheses | 5 | 1.5 |
| Jevtic [12] | 1995 | 2 | RA and SpA patients; MRI of hands | 6 | 2.3 |
| Maillefert [13] | 2003 | 3 | Patients with inflammatory arthritis; MRI of hindfoot | 1 | 1.5 |
| McGonagle [14] | 1998 | 2 | SpA and RA patients with knee effusion; MRI evaluation | 3 | 0.5 and 1.5 |
| McGonagle [15] | 2002 | 2 | Patients with plantar fasciitis (SpA and mechanical); MRI of entheses | 4 | 0.5 and 1.5 |
| Melchiorre [16] | 2003 | 2 | PsA and RA patients; MRI of temporomandibular joint | 11 | 0.5 |
| Muche [17] | 2003 | 2 | SpA patients; MRI of sacroiliac joints | 5 | 1.5 |
| Offidani [18] | 1998 | 4 | Patients with psoriasis and no joint pain; MRI of hands | 25 | 1.0 |
| Olivieri [19] | 1996 | 2 | SpA patients with dactylitis; MRI of fingers | Not stated | 0.5 |
| Olivieri [20] | 1997 | 2 | SpA patients with dactylitis of the toes; MRI of toes | 6 | 0.5 |
| Olivieri [21] | 2002 | 2 | PsA patients with dactylitis of the fingers; MRI of fingers | 6 | 1.5 |
| Olivieri [22] | 2003 | 5 | PsA Patient with dactylitis of fingers; MRI of fingers | 1 | Not stated |
| Padula [23] | 1998 | 5 | Patients with dactylitis; MRI evaluation | 2 | Not stated |
| Padula [24] | 1999 | 5 | Patient with psoriasis and tenosynovitis; MRI evaluation | 1 | Not stated |
| Salvarani [25] | 1999 | 5 | Patients with peripheral pitting oedema; MRI evaluation | 2 | Not stated |
| Savnik [26] | 2001 | 2 | Patients with inflammatory arthritis; high field versus low field MRI of wrists and fingers | Not stated | 0.2 and 1.5 |
| Savnik [27] | 2001 | 2 | Patients with inflammatory arthritis (RA and SpA); MRI of wrists and fingers | 8 | 1.5 |
| Savnik [28] | 2002 | 3 | Patients with inflammatory arthritis (RA and SpA); MRI of wrists and fingers, at baseline and after 1 year | Not stated | 0.2 and 1.5 |
| Taylor [29] | 1997 | 5 | PsA patient with enthesitis of elbow; MRI evaluation | 1 | Not stated |
| Tehranzadeh [30] | 2004 | 2 | Patients with inflammatory arthritis; MRI of hand evaluated for large bony lesions | 7 | 1.5 |
| Tuzun [31] | 1996 | 5 | Psoriatic spondylitis mimicking spinal brucellosis; MRI of spine | 1 | Not stated |
| Williamson [32] | 2004 | 2 | PsA patients with clinical features of sacroiliitis; MRI of sacroiliac joints | 68 | 1.0 |
a1, Clinical trial; 2, Observational, cross-sectional; 3, Observational, longitudinal; 4, Case-control; 5, Case report. MRI, magnetic resonance imaging; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SpA, spondyloarthropathy.
Results and discussion
Synovitis
Histopathological studies have suggested that the inflamed synovial membrane of PsA differs in certain subtle ways from rheumatoid synovium with less lining layer hyperplasia, more subsynovial oedema and a greater number of synovial vessels per square millimetre [33]. However, on MRI, PsA synovitis appears indistinguishable from that of RA. Cimmino and coworkers [9] recently used dynamic MRI of the wrist using a 0.2 T dedicated-extremity scanner to address this question. The rate of increase in enhancement following contrast injection did not differ between PsA and RA patients when they were matched for disease activity, but in both groups it was higher than in normal control individuals. The authors concluded that dynamic MRI cannot be used diagnostically to differentiate PsA from RA. Similar findings were described by Antoni and coworkers [3], who used dynamic-enhanced MRI to quantify synovitis in 10 PsA patients before and after infliximab treatment. Contrast enhancement was markedly reduced following anti-tumour necrosis factor-α therapy, consistent with other reports that this cytokine plays a major role in psoriatic skin and joint pathology [34].
Others have measured PsA synovitis on static magnetic resonance scans. Savnik and coworkers [28] used manual outlining of synovial membrane to quantify synovitis in their study of 84 patients with inflammatory arthritis, 18 of whom had reactive arthritis or PsA. MRI of the wrist and finger joints (metacarpophalangeal [MCP], proximal interphalangeal [PIP] and distal interphalangeal [DIP] joints) was performed using 0.2 T and 1.5 T scanners. They noted that the volume of synovial membrane was increased but did not change significantly over 1 year, contrasting with RA patients, in whom it fell in response to therapy. Jevtic and coworkers [12] (using a 2.35 T magnet) described MRI of the finger joints in a group of 13 patients with PsA, three with Reiter's syndrome and 16 with RA. Although in some PsA patients synovitis was observed to conform to a typical rheumatoid pattern, in others there was 'inflamed tissue extending far beyond the joint capsule, involving neighbouring structures such as thickened collateral ligaments and surrounding periarticular soft tissue'. McGonagle and colleagues [35] went on to describe in greater detail the MRI features of enthesitis that may be seen in association with synovitis in PsA (see below), and postulated that true PsA can be distinguished from RA with concomitant psoriasis on these grounds. Examples of PsA synovitis and associated extracapsular/soft tissue lesions are shown in Figures 1 and 2.
Figure 1.
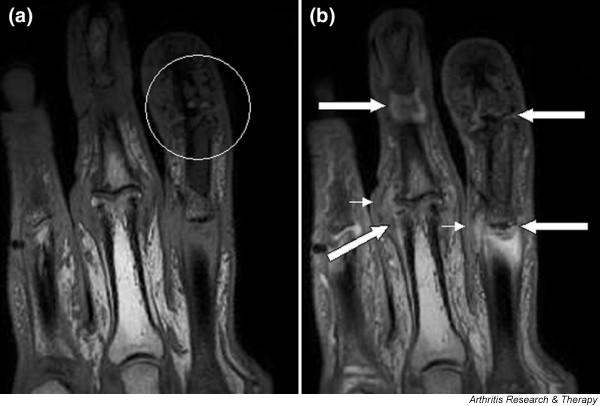
Magnetic resonance images of fingers: psoriatic arthritis. Shown are T1-weighted (a) precontrast and (b) postcontrast coronal magnetic resonance images of the fingers in a patient with psoriatic arthritis. Enhancement of the synovial membrane at the third and fourth proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints is seen, indicating active synovitis (large arrows). There is joint space narrowing with bone proliferation at the third PIP joint and erosions are present at the fourth DIP joint (white circle). Extracapsular enhancement (small arrows) is seen medial to the third and fourth PIP joints, indicating probable enthesitis. Note that this particular slice does not allow optimal visualization of all of the mentioned pathologies.
Figure 2.
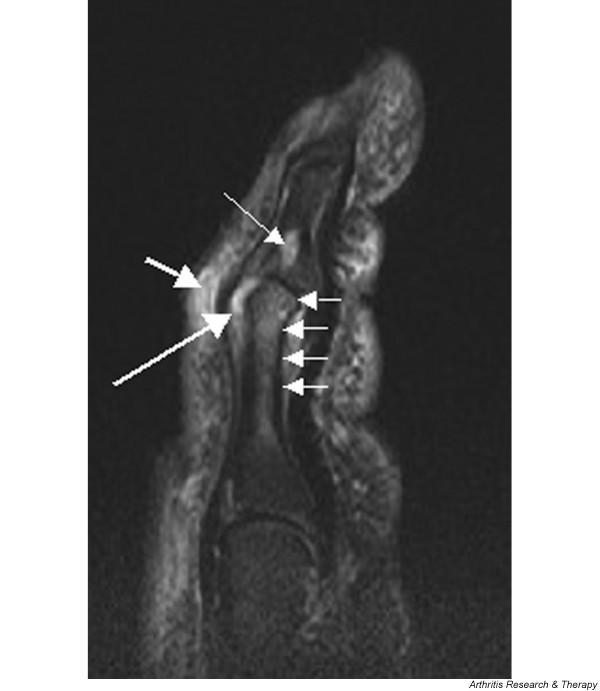
Magnetic resonance image of index finger: psoriatic arthritis (mutilans form). Shown is a T2 weighted fat suppressed sagittal image of the index finger in a patient with PsA (mutilans form). Focal increased signal (probable erosion) is seen at the base of the middle phalanx (long thin arrow). There is synovitis at the proximal interphalangeal joint (long thick arrow) plus increased signal in the overlying soft tissues indicating oedema (short thick arrow). There is also diffuse bone oedema (short thin arrows) involving the head of the proximal phalanx and extending distally down the shaft.
Bone erosions and bone oedema
Although the work conducted by Ritchlin and coworkers [36] recently focused attention on activated osteoclasts in PsA and raised the possibility that a disorder of bone remodelling may underlie this disease, evidence from MRI studies conducted thus far suggests that PsA erosions are rather similar to RA erosions [4,27]. There are differences in terms of distribution, with involvement of non-RA sites such as DIP joints and entheseal insertions [37], but the erosions themselves consist of a break in cortical bone overlying a region of altered signal intensity with definite margins, as described in RA [38]. Also, as in RA, PsA erosions can be large and are frequently not visualized on conventional radiography [30]. The study conducted by Savnik and coworkers [28] in patients with inflammatory arthritis suggested that MRI erosions in PsA patients did not progress over time to the same extent as those in patients with early RA, raising the possibility that PsA bone disease may sometimes be less aggressive. Backhaus and colleagues [4] included 15 PsA patients in their study of MRI, ultrasound and scintigraphy of the finger joints, nine of whom were described as having MRI erosions. Some of these were 'nonenhancing' following contrast injection, suggesting that they may have contained fibrous tissue rather than inflammatory pannus and be relatively 'inactive', but a clear description of these lesions was not given.
As in RA, the histopathological correlate of MRI bone oedema has not been defined in PsA, but Bollow and coworkers [6] found some evidence of osteitis in subcortical bone in their biopsy study of sacroiliac joints in SpA patients (including two with PsA). Bone oedema has been described in PsA, reactive arthritis, ankylosing spondylitis and RA [6,27,39] and is recognized as an ill defined area in the subcortical bone with increased signal on short tau inversion recovery (STIR), T2 weighted with fat saturation (FS) and postcontrast T1 weighted with FS sequences [38]. Savnik and colleagues [27] found MRI bone oedema in PsA patients included in their cohort with early inflammatory arthritis, and observed that the total number of bones affected did not change over 1 year (compared with the RA patients, in whom it increased) [27]. They found examples of PsA bone oedema involving distal, middle and proximal phalanges of the fingers as well as carpal bones, radius and ulna, and described that in various cases it either appeared or disappeared between the baseline and 1 year magnetic resonance examinations. Bone oedema was a strong predictor of bone erosions in their RA group (as was described elsewhere [39]), but this was not specifically demonstrated in PsA joints and further studies are needed to clarify this point.
In their series comparing MRI of the hand in 28 PsA and 18 RA patients, Giovagnoni and coworkers [10] also noted signal change in subchondral bone (bone oedema) in 43% of their patients associated with pronounced periarticular oedema of the soft tissues, spreading to the subcutis, and felt this might constitute a 'psoriatic pattern' on MRI. Jevtic and colleagues [12] described extensive MRI bone oedema involving the proximal phalanx in a patient with Reiter's syndrome (which is of interest because the radiographic features of this condition are said to be identical to those of PsA [40]) and also identified inflammatory changes in overlying soft tissues. McGonagle and coworkers [14] stressed the association between MRI bone oedema and evidence of enthesitis at the knee, and this is described in more detail below. Most recently, in a case report [7], prominent MRI bone oedema was described in a patient with psoriatic onycho-pachydermo periostitis affecting the toes. Both clinical tissue swelling and MRI bone oedema resolved dramatically in response to adalimumab, suggesting an underlying tumour necrosis factor-α mediated process.
Figure 2 shows an example of bone oedema from a patient with the mutilans form of PsA. There is extensive bone oedema involving the head of the proximal phalanx and extending down the shaft of the bone. On the distal aspect of the joint a well circumscribed lesion with increased signal is likely to represent an erosion. Erosions are also apparent in Figure 1 involving PIP and DIP joints.
Enthesitis
Barozzi and coworkers [41] described the MRI features of enthesitis as being characterized by 'swelling [of the entheseal region] and deviation from the normally uniform low signal intensity of tendons and ligaments, [with] distension of adjacent bursae by fluid collection, peritendinous soft tissue swelling and inflammation of bone adjacent to the insertion'. McGonagle and colleagues [14] described these findings in their MRI study of 10 SpA patients (three of whom had PsA) with knee swelling of recent onset. They observed increased signal on T2 weighted images, characterizing 'focal extracapsular fluid/oedema' in entheseal portions of the patellar tendon, the iliotibial band and adjacent to the posterior capsule of the knee. Perientheseal bone marrow oedema was present in six SpA patients, including one with PsA, in whom it involved bone at the tibial plateau as well as bony attachments of the patellar tendon and posterior cruciate ligament. The same group also studied calcaneal enthesopathy in 17 early SpA patients (including four with PsA), and similar findings were described, again often including underlying bone marrow oedema [15]. However, Jevtic and coworkers [12], describing magnetic resonance features of peripheral PsA in the hands, noted that bone oedema was present adjacent to regions of enthesitis in fewer than 50% of patients, indicating that these features are not always observed together.
Godfrin and colleagues [11] described a study of SpA patients (including six with PsA) who had entheseal pain. MRI revealed typical abnormalities at entheses, all of which corresponded to hot spots on radionuclide scans. These authors noted that T2 weighted sequences appeared less sensitive than post-gadolinium FS T1 weighted and STIR sequences for detecting MRI enthesitis. Interestingly, one patient presenting with pelvic pain and MRI changes of enthesitis at the anteroinferior iliac spine subsequently went on to develop full-blown PsA, which is consistent with other reports that isolated entheseal pain may be a first presentation of this disease [29]. Figure 3 shows an example of enthesitis at the Achilles tendon insertion, with erosion and marrow oedema in the adjacent bone.
Figure 3.
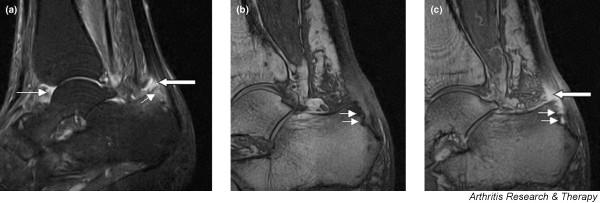
Sagittal magnetic resonance images of ankle region: psoriatic arthritis. (a) Short tau inversion recovery (STIR) image, showing high signal intensity at the Achilles tendon insertion (enthesitis, thick arrow) and in the synovium of the ankle joint (synovitis, long thin arrow). Bone marrow oedema is seen at the tendon insertion (short thin arrow). (b,c) T1 weighted images of a different section of the same patient, before (panel b) and after (panel c) intravenous contrast injection, confirm inflammation (large arrow) at the enthesis and reveal bone erosion at tendon insertion (short thin arrows).
Dactylitis, tendonitis and soft tissue oedema
Common to all forms of SpA but not a feature of RA, dactylitis is one of the more important clinical features that define PsA and is described in about one-third of patients [37]. Olivieri and coworkers have described several series of patients with dactylitis involving the fingers and toes [19-22] and demonstrated that tenosynovitis, usually with effusion, is invariably present. Flexor tendons were more often involved than extensor tendons, and they found concomitant small joint synovitis to be relatively uncommon (ranging from 6% to 27% of cases) and not a 'sine qua non for the sausage-shaped feature' [22]. Following the report of McGonagle and colleagues [14] that suggested that the primary abnormality in PsA was entheseal, this group examined six PsA patients with 11 dactylitic fingers for the presence of enthesitis [22]. Bone oedema was not observed at entheseal insertions of flexor or extensor tendons, but peritendinous soft tissue oedema contributed to digital swelling in a number of more severe cases with what has been described as a 'honeycomb' appearance of the subcutaneous tissues on T2 weighted MRI sequences [8]. Giovagnoni and coworkers [10] also described 'diffuse and pronounced periarticular soft tissue oedema that spread to the subcutis' in the hands of 86% of their PsA patients.
As with other manifestations of PsA including skin disease and arthritis, trauma has been described as a trigger for the development of dactylitis via a 'Koebner-type' phenomenon [24]. A case report described MRI evidence of extensive tenosynovitis and soft tissue oedema involving the fingers and hand, which followed a blow to the hand and eventually settled spontaneously [24]. Figure 4 shows dactylitis in a PsA patient, due to florid flexor tenosynovitis. An example of soft tissue oedema overlying synovitis at a PIP joint is shown in Figure 2.
Figure 4.
Magnetic resonance images of fingers: psoriatic arthritis with dactylitis due to flexor tenosynovitis. Shown are T1 weighted axial (a) precontrast and (b) postcontrast magnetic resonance images of the fingers from a patient with psoriatic arthritis exhibiting flexor tenosynovitis at the second finger with enhancement and thickening of the tendon sheath (large arrow). Synovitis is seen in the fourth proximal interphalangeal joint (small arrow).
Psoriatic arthritis sacroiliitis and spondylitis
MRI is very sensitive for early detection of sacroiliitis in SpA. Williamson and coworkers [32] showed that MRI-diagnosed sacroiliitis was present in 38% of a group of unselected PsA patients and was not necessarily associated with a clinical history of inflammatory back pain or positive sacroiliac provocation tests. The MRI changes described were consistent with the findings presented by Bollow and coworkers [5] and included bone oedema, sacroiliac erosions and the more chronic changes of periarticular fat accumulation and sclerosis. Muche and colleagues [17] conducted a detailed analysis of the MRI features of sacroiliitis in 93 SpA patients, including five with PsA (but their results were not analyzed separately). They confirmed that MRI sacroiliitis was very common in SpA, most often involving the dorsocaudal part of the joint in early disease in which subchondral bone oedema was a frequent finding. Figure 5 shows an example of MRI sacroiliitis in a patient with PsA.
Figure 5.
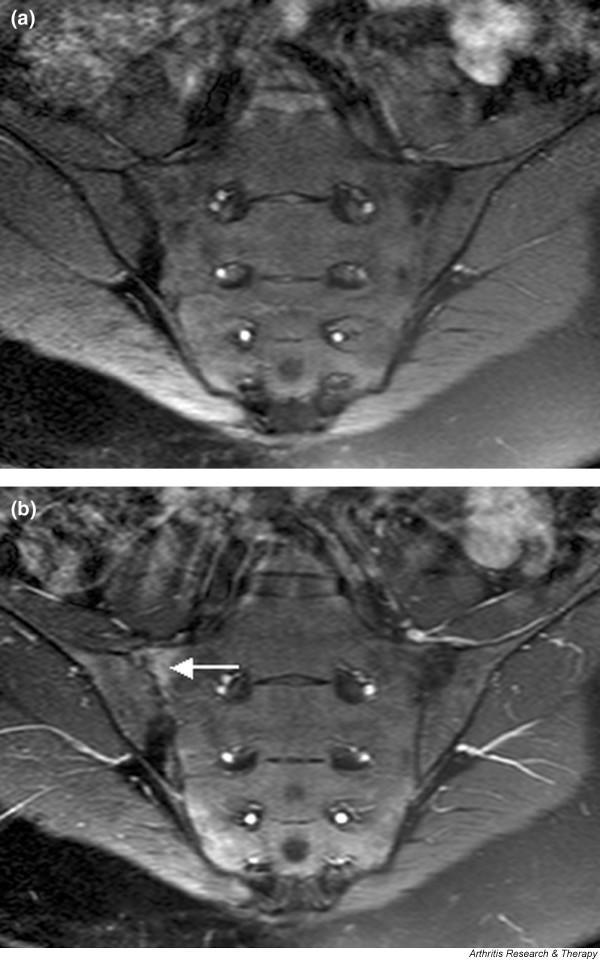
Magnetic resonance images of sacroiliac joints: psoriatic arthritis. Shown are T1-weighted semi-coronal magnetic resonance images through the sacroiliac joints (a) before and (b) after intravenous contrast injection. Enhancement is seen at the right sacroiliac joint (arrow), indicating active sacroiliitis.
There have been no MRI studies of the rest of the spine specifically in PsA, but in ankylosing spondylitis Braun and coworkers [42] determined that MRI bone oedema at vertebral margins was an indicator of disease activity and a predictor of response to infliximab. Radiographically, the spondylitis of PsA and reactive arthritis differs from ankylosing spondylitis in that osteophytes are chunky and asymmetrical [43], and so exploration using MRI would be of interest to determine whether there are disease specific features. Tuzun and colleagues [31] reported a case of psoriatic spondylitis presenting with back pain complicated by disc herniation. MRI findings were florid, with high signal on T2 weighted images involving large areas of the endplates of adjacent vertebral bodies with an appearance suggestive of infection (brucellosis was suspected). These changes resolved following treatment with methotrexate. Figure 6 shows a similar example with extensive bone oedema and erosion at adjacent endplates.
Figure 6.
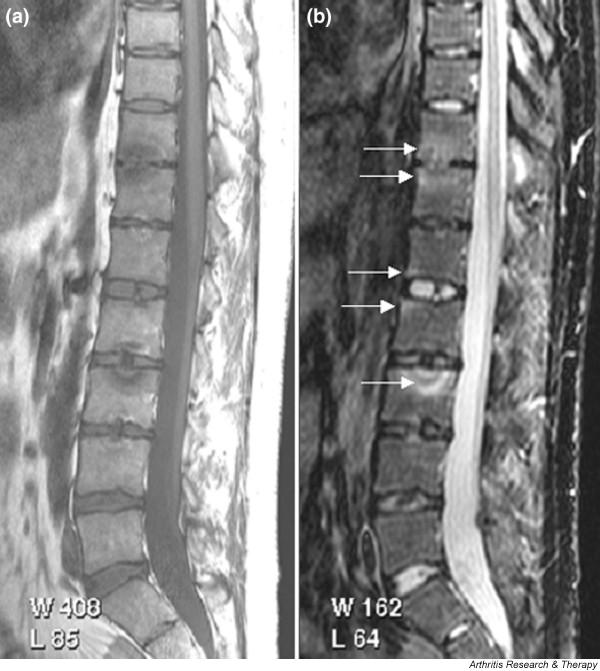
Magnetic resonance images of lumbar and lower thoracic spine: psoriatic arthritis. (a) T1-weighted and (b) short tau inversion recovery (STIR) magnetic resonance images of the lumbar and lower thoracic spine. Signs of active inflammation are seen at several levels (arrows). In particular, anterior spondylitis is seen at level L1/L2 and an inflammatory Andersson lesion at the upper vertebral endplate of L3.
Subclinical disease
Offidani and coworkers [18] investigated a group of 25 patients with psoriasis, but no arthritic symptoms, for possible subclinical MRI evidence of PsA and compared the findings with those in a matched control population. MRI scans from 68% of patients were positive for signs of arthritis including joint or tendon sheath effusions, bone erosions and bone oedema. They concluded that there was a high incidence of 'subclinical arthritis' in patients with psoriasis alone, and these findings agree with those from an earlier scintigraphic study conducted in patients hospitalized with severe psoriatic skin disease [44], which described increased periarticular uptake of isotope at a number of joint regions. An earlier Mayo Clinic study [45] also noted a high incidence of PsA occurring in 39% of patients with psoriasis, and corroboration by the new MRI evidence may lead to estimates of the frequency of PsA being upwardly revised.
Conclusion
PsA shares clinical manifestations with RA and SpA, and this also applies to its MRI features. Peripheral PsA synovitis appears similar to RA synovitis on static and dynamic MRI. Likewise, PsA bone erosions do not have disease specific MRI features, but little is known concerning how they progress over time. MRI bone oedema can involve any of the wrist or finger bones, where it may persist or be transient, but whether it is a predictor of erosions in PsA remains to be determined. With enthesitis, dactylitis and spondylitis, the MRI features of PsA depart from those of RA and conform to the SpA group of disorders. Enthesitis has been described adjacent to peripheral and axial joints, often associated with synovitis and sometimes with bone oedema. Dactylitis has been shown on magnetic resonance scans to be due to tenosynovitis with effusion, sometimes associated with small joint synovitis. Diffuse soft tissue oedema may overlie areas of dactylitis, synovitis, or bone oedema. There are few MRI studies in axial PsA but bone oedema appears to be a sensitive sign of early sacroiliitis. Finally, MRI has revealed evidence of subclinical arthritis in a large proportion of patients with psoriasis alone, suggesting that PsA could be a much more common disorder than was previously suspected.
Abbreviations
DIP = distal interphalangeal; FS = fat saturated; MRI = magnetic resonance imaging; PIP = proximal interphalangeal; PsA = psoriatic arthritis; RA = rheumatoid arthritis; SpA = spondyloarthropathy; STIR = short tau inversion recovery.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
Dr Charlotte Wiell, Copenhagen University Hospitals at Hvidovre and Herlev, Denmark, is acknowledged for her contribution to the image collection presented (Figures 1, 3 and 4).
References
- Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis. 2005;(Suppl II):ii3–ii7. doi: 10.1136/ard.2004.032318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory PA, Gladman DD, Mease PJ. Psoriatic arthritis and imaging. Ann Rheum Dis. 2005;(Suppl II):ii55–ii57. doi: 10.1136/ard.2004.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni C, Dechant C, Lorenz PDH, Wendler J, Ogilvie A, Lueftl M, Kalden-Nemeth D, Kalden JR, Manger B. Open-label study of infliximab treatment for psoriatic arthritis: clinical and magnetic resonance imaging measurements of reduction of inflammation. Arthritis Care Res. 2002;47:506–512. doi: 10.1002/art.10671. [DOI] [PubMed] [Google Scholar]
- Backhaus M, Kamradt T, Sandrock D, Loreck D, Fritz J, Wolf KJ, Raber H, Hamm B, Burmester GR, Bollow M. Arthritis of the finger joints. A comprehensive approach comparing conventional radiography, scintigraphy, ultrasound and contrast-enhanced MRI. Arthritis Rheum. 1999;42:1232–1234. doi: 10.1002/1529-0131(199906)42:6<1232::AID-ANR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Bollow M, Braun J, Hamm B, Eggens U, Schilling A, Konig H, Wolf KJ. Early sacroiliitis in patients with spondyloarthropathy: evaluation with dynamic gadolinium-enhanced MR imaging. Radiology. 1995;194:529–536. doi: 10.1148/radiology.194.2.7824736. [DOI] [PubMed] [Google Scholar]
- Bollow M, Fischer T, Reißhauer H, Backhaus M, Hamm B, Braun J. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis – cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000;59:135–140. doi: 10.1136/ard.59.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongartz T, Harle P, Friedrich S, Karrer S, Vogt T, Seitz A, Muller-Ladner U. Successful treatment of psoriatic onycho-pachydermo periostitis (POPP) with adalimimab. Arthritis Rheum. 2005;52:280–282. doi: 10.1002/art.20763. [DOI] [PubMed] [Google Scholar]
- Cantini F, Salvarani C, Olivieri I, Macchioni L, Niccoli L, Padula A, Falcone C, Boiardi L, Bozza A, Barozzi L, et al. Distal extremity swelling with pitting oedema in psoriatic arthritis: a case-control study. Clin Exp Rheumatol. 2001;19:291–296. [PubMed] [Google Scholar]
- Cimmino MA, Parodi M, Innocenti S, Succio G, Banderali S, Silvestri E, Garlaschi G. Dynamic magnetic resonance imaging in psoriatic arthritis reveals imaging patterns similar to those of rheumatoid arthritis. Arthritis Res Ther. 2004;7:R725–R731. doi: 10.1186/ar1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoni A, Grassi W, Terilli F, Blasetti P, Paci E, Ercolani P, Cervini C. MRI of the hand in psoriatic and rheumatical arthritis. Eur Radiol. 1995;5:590–595. doi: 10.1007/BF00190921. [DOI] [Google Scholar]
- Godfrin B, Laurent Z, Lamboley V, Bertrand-Latour F, Sana N, Fournie B. Spondyloarthropathy with entheseal pain. Joint Bone Spine. 2004;71:557–562. doi: 10.1016/j.jbspin.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Jevtic V, Watt I, Rozman B, Kos-Golja M, Demsar F, Jarh O. Distinctive radiological features of small hand joints in rheumatoid arthritis and seronegative spondarthritis demonstrated by contrast-enhanced (Gd-DTPA) magnetic resonance imaging. Skeletal Radiol. 1995;24:351–355. doi: 10.1007/BF00197064. [DOI] [PubMed] [Google Scholar]
- Maillefert JF, Dardel P, Cherasse A, Mistrih R, Krause D, Tavernier Magnetic resonance imaging in the assessment of synovial inflammation of the hindfoot in patients with rheumatoid arthritis and other polyarthritis. Eur J Radiol. 2003;47:1–5. doi: 10.1016/S0720-048X(02)00065-7. [DOI] [PubMed] [Google Scholar]
- McGonagle DS, Gibbon W, O'Connor P, Green M, Pease C, Emery P. Characteristic magnetic resonance imaging entheseal changes of knee synovitis in spondyloarthropathy. Arthritis Rheum. 1998;41:694–700. doi: 10.1002/1529-0131(199804)41:4<694::AID-ART17>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- McGonagle D, Marzo-Ortega H, O'Connor P, Gibbon W, Pease C, Reece R, Emery P. The role of biomechanical factors and HLA-B27 in magnetic resonance imaging-determined bone changes in plantar fascia enthesopathy. Arthritis Rheum. 2002;46:489–493. doi: 10.1002/art.10125. [DOI] [PubMed] [Google Scholar]
- Melchiorre D, Claderazzi A, Maddali Bongi S, Cristofani R, Bazz-ichi L, Eligi C, Maresca M, Ciompi ML. A comparison of ultrasonography and magnetic resonance imaging in the evaluation of temporomandibular joint involvement in rheumatoid arthritis and psoriatic arthritis. Rheumatol. 2003;42:673–676. doi: 10.1093/rheumatology/keg181. [DOI] [PubMed] [Google Scholar]
- Muche B, Bollow M, Francois RJ, Sieper J, Hamm B, Braun J. Anatomic structures involved in early- and late-stage sacroiliitis in spondyloarthritis. A detailed analysis by contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2003;48:1374–1384. doi: 10.1002/art.10934. [DOI] [PubMed] [Google Scholar]
- Offidani A, Cellini A, Valeri G, Giovagnoni A. Subclinical joint involvement in psoriasis: magnetic resonance imaging and X-ray findings. Acta Derm Venereol (Stockh) 1998;78:463–465. doi: 10.1080/000155598442809. [DOI] [PubMed] [Google Scholar]
- Olivieri I, Barozzi L, Favaro L, Pierro A, De Matteis M, Borghi C, Padula A, Ferri S, Pavlica P. Dactylitis in patients with seronegative spondyloarthropathy: assessment by ultrasonography and magnetic resonance imaging. Arthritis Rheum. 1996;39:1524–1528. doi: 10.1002/art.1780390912. [DOI] [PubMed] [Google Scholar]
- Olivieri I, Barozzi L, Pierro A, De Matteis M, Padula A, Pavlica P. Toe dactylitis in patients with spondyloarthropathy: assessment by magnetic resonance imaging. J Rheum. 1997;24:926–930. [PubMed] [Google Scholar]
- Olivieri I, Salvarani C, Cantini F, Scarano E, Padula A, Niccoli L, Ciancio G, Barozzi L. Fast spin echo-T2-weighted sequences with fat saturation in dactylitis of spondyloarthritis. Arthritis Rheum. 2002;46:2964–2967. doi: 10.1002/art.10633. [DOI] [PubMed] [Google Scholar]
- Olivieri I, Scarano E, Padula A, Giasi V. Dactylitis involving most of the fingers. Clin Exp Rheumatol. 2003;21:406. [PubMed] [Google Scholar]
- Padula A, Barozzi L, Cantini F, Olivieri I. Dactylitis also involving the synovial sheaths in the palm of the hand: two more cases studied by magnetic resonance imaging. Ann Rheum Dis. 1998;57:61–62. doi: 10.1136/ard.57.1.61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula A, Belsito F, Barozzi L, Cantini F, Salvarani C, Pavlica P, Olivieri I. Isolated tenosynovitis associated with psoriasis triggered by physical injury. Clin Exp Rheumatol. 1999;17:104–104. [PubMed] [Google Scholar]
- Salvarani C, Cantini F, Olivieri I, Niccoli L, Senesi C, Macchioni L, Boiardi L, Padula A. Distal extremity swelling with pitting edema in psoriatic arthritis: Evidence of 2 pathological mechanisms. J Rheumatol. 1999;26:1831–1834. [PubMed] [Google Scholar]
- Savnik A, Malmskov H, Thomsen HS, Bretlau T, Graff LB, Nielsen H, Danneskiold-Samsoe B, Boesen J, Bliddal H. MRI of the arthritis small joints: comparison of extremity MRI (0.2T) vs high-field MRI (1.5T) Eur Radiol. 2001;11:1030–1038. doi: 10.1007/s003300000709. [DOI] [PubMed] [Google Scholar]
- Savnik A, Malmskov H, Thomsen HS, Graff LB, Nielsen H, Danneskiold-Samsoe B, Boesen J, Bliddal H. Magnetic resonance imaging of the wrist and finger joints in patients with inflammatory joint diseases. J Rheumatol. 2001;28:2193–2200. [PubMed] [Google Scholar]
- Savnik A, Malmskov H, Thomsen HS, Graff LB, Nielsen H, Danneskiold-Samsoe B, Boesen J, Bliddal H. MRI of the wrist and finger joints in inflammatory joint diseases at 1-year interval: MR features to predict bone erosions. Eur Radiol. 2002;12:1203–1210. doi: 10.1007/s003300101114. [DOI] [PubMed] [Google Scholar]
- Taylor PW, Stoecker W. Enthesitis of the elbow in psoriatic arthritis. J Rheumatol. 1997;24:11. [PubMed] [Google Scholar]
- Tehranzadeh J, Ashikyan O, Dascalso J, Dennehey C. MRI of large intraosseous lesions in patients with inflammatory arthritis. AJR Am J Roentgenol. 2004;183:1453–1463. doi: 10.2214/ajr.183.5.1831453. [DOI] [PubMed] [Google Scholar]
- Tuzun C, Peker O, Kucuktas F, Gulbahar S, Kovalikaya I, Fuzun S. An atypical psoriatic spondylitis case, successfully treated with methotrexate. Clin Rheum. 1996;15:403–409. doi: 10.1007/BF02230367. [DOI] [PubMed] [Google Scholar]
- Williamson L, Dockerty JL, Dalbeth N, McNally E, Ostlere S, Wordsworth BP. Clinical assessment of sacroiliitis and HLA-B27 are poor predictors of sacroiliitis diagnosed by MRI in psoriatic arthritis. Rheumatol. 2004;43:85–88. doi: 10.1093/rheumatology/keg475. [DOI] [PubMed] [Google Scholar]
- Reece RJ, Canete JD, Parsons WJ, Emery P, Veale DJ. Distinct vascular patterns of early synovitis in psoriatic, reactive and rheumatoid arthritis. Arthritis Rheum. 1999;42:1481–1484. doi: 10.1002/1529-0131(199907)42:7<1481::AID-ANR23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Goedkoop AY, Kraan MC, Teunissen MB, Picavet DI, de Rie MA, Bos JD, Tak PP. Early effects of tumour necrosis factor alpha blockade on skin and synovial tissue in patients with active psoriasis and psoriatic arthritis. Ann Rheum Dis. 2004;63:769–773. doi: 10.1136/ard.2003.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D, Conaghan PG, Emery P. Psoriatic arthritis. A unified concept twenty years on. Arthritis Rheum. 1999;42:1080–1086. doi: 10.1002/1529-0131(199906)42:6<1080::AID-ANR2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwartz EM. Mechanisms of TNFα and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–831. doi: 10.1172/JCI200316069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladman DD. Psoriatic arthritis. In: Khan MA, editor. Ankylosing Spondylitis and Related Spondyloarthropathies Spine: State of the art review. Philadelphia: Hanley and Belfus Inc; 1990. pp. 637–656. [Google Scholar]
- Østergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, Shnier R, O'Connor P, Klarlund M, Emery P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–1388. [PubMed] [Google Scholar]
- McQueen FM, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S, McLean L, Stewart N. Bone oedema scored on magnetic resonance scans of the dominant carpus at presentation predicts radiographic joint damage at the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:1814–1827. doi: 10.1002/art.11162. [DOI] [PubMed] [Google Scholar]
- Vasey FB, Seleznck MJ, Fenske NA, Espinosa LR. New signposts on the road to understanding psoriatic arthritis. J Rheumatol. 1989;6:1405–1406. [PubMed] [Google Scholar]
- Barozzi L, Olivieri I, De Matteis M, Padula A, Pavlic P. Seronegative spondyloarthropathies: imaging of spondylitis, enthesitis and dactylitis. Eur J Radiol. 1988;27:S12–S17. doi: 10.1016/S0720-048X(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Braun J, Baraliakos X, Golder W, Brandt J, Rudwaleit M, Listing J, Bollow M, Sieper J, van der Heijde D. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab. Arthritis Rheum. 2003;48:1126–1136. doi: 10.1002/art.10883. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Ohashi K, El-Khoury GY. Spondyloarthropathies: ankylosing spondylitis and psoriatic spondylitis. Radiol Clin N Am. 2004;42:121–134. doi: 10.1016/S0033-8389(03)00156-8. [DOI] [PubMed] [Google Scholar]
- Namey T, Rosenthall L. Periarticular uptake of 99mtechnetium diphosphonate in psoriatics: correlation with cutaneous activity. Arthritis Rheum. 1976;19:607–612. doi: 10.1002/art.1780190313. [DOI] [PubMed] [Google Scholar]
- Leonard DG, O'Duffy JD, Rogers RS. Prospective analysis of psoriatic arthritis in patients hospitalised for psoriasis. Mayo Clin Proc. 1978;53:511–518. [PubMed] [Google Scholar]


