Abstract
The aim of the present work is to compare drug survival and safety of infliximab, etanercept, and adalimumab (tumor necrosis factor [TNF] antagonists) in spondylarthritis (SpA) with those of rheumatoid arthritis (RA). To this purpose, we analysed the data in BIOBADASER (2000–2005), a drug registry launched in 2000 for long-term follow-up of the safety of these biologics in rheumatic diseases. The rates of drug discontinuation and adverse events (AEs) in SpA (n = 1,524) were estimated and compared with those of RA (n = 4,006). Cox regression analyses were used to adjust for independent factors. Total exposure to TNF antagonists for SpA was 2,430 patient-years and 7,865 for RA. Drug survival in SpA was significantly greater than in RA at 1, 2, and 3 years. The hazard ratio (HR) for discontinuation in SpA compared with RA was 0.66 (95% confidence interval [CI], 0.57–0.76) after adjustment for age, gender, and use of infliximab. The difference remained after controlling for the individual medication and its place in the sequence of treatment. There were fewer SpA patients with AEs (17%) than RA patients (26%; p < 0.001). The HR for AEs in SpA was 0.80 (95% CI, 0.70–0.91) compared with RA after adjustment for age, disease duration, and use of infliximab. In conclusion, due in part to a better safety profile, survival of TNF antagonists in SpA is better than in RA. TNF antagonists are at present a safe and effective therapeutic option for long-term treatment of patients with SpA failing to respond to traditional drugs. Because chronic therapy is necessary, continual review of this issue is necessary.
Introduction
The term spondylarthritis (SpA) refers to a group of conditions with inflammation at the entheses, axial skeleton, peripheral joints, and non-articular structures [1-3]. It includes ankylosing spondylitis (AS), reactive arthritis, undifferentiated SpA, juvenile spondylitis, and the arthritis associated with psoriasis or inflammatory bowel diseases. These conditions occur in approximately 1% of the general population [3]. Because of overlapping clinical features, diagnosis of any single one from among the several within the group is sometimes difficult. Nevertheless, treatment does not differ very much among the different conditions. Non-steroidal anti-inflammatory drugs (NSAIDs) have a role in symptom modification and disease control in patients with AS [4,5] as do methotrexate and sulfasalazine with psoriatic arthritis (PsA) and AS [6-17]. In both conditions, these drugs have demonstrated some benefit in peripheral arthritis. In axial disease, evidence is lacking.
Recently, tumor necrosis factor (TNF) inhibitors have been found to be safe and effective in the short-term management of AS, PsA, enteropathic arthritis, and juvenile SpA in patients failing to respond to traditional therapies [17-34]. Unlike in rheumatoid arthritis (RA), however, their long-term efficacy and safety in such conditions are largely unknown.
In February 2000, the Spanish Society of Rheumatology (SER) launched a drug registry (BIOBADASER) of patients with any rheumatic condition treated with biologic disease modifiers. In the past 5 years, more than 5,000 patients from 100 centres have been included in the registry and followed up with [35]. Although the emphasis of BIOBADASER is in drug safety, information on drug discontinuation for any cause is gathered as well. For prescription of any biological disease modifier in a context of universal health coverage in Spain, the physician commits himself to assess effectiveness and safety regularly and discontinue medication when appropriate to meet our current guidelines. Thus, drug survival in this particular clinical setting may be considered a surrogate for effectiveness. Consistency of the data in our registry, which have been externally assessed as described in Materials and methods, and comparison of drug survival in different conditions offer a unique opportunity for the detection of relevant differences in safety and effectiveness. In the present work, we describe the differences in the survival and safety of TNF antagonist in SpA compared with the well-known profile in RA.
Materials and methods
A description of BIOBADASER has been published elsewhere [28], and its protocol and periodical reports are available on its Web page [36]. In brief, BIOBADASER is a drug registry established in February 2000 for active long-term follow-up of rheumatic patients being treated with biological response modifiers. Patients treated with infliximab before the start of the registry were also included if complete history of treatment and information on adverse events (AEs) were available. The registry, which is supported by the SER and funded in part by the Spanish Agency for Medicines and Health-Service Products (Agencia Española de Medicamentos y Productos Sanitarios), notes relevant AEs (RAEs) occurring during treatment. All hospital and community-based Rheumatology Units in Spain were invited to participate in setting up the project. Participation is voluntary, covering approximately 60% of the patients treated with these therapies for rheumatic diseases in Spain. The large number of participating units (100) ensures a true mix of hospital and community-based practices.
A random code is assigned to every patient entered. This code will be kept throughout the follow-up, until death, or until the study closure date. The registry protocol and methods were approved by the Spanish Medicines Agency (Ministerio de Sanidad y Consumo), and the information regarding patients was gathered in the registry and handled according to current official regulations on data protection.
Data collected systematically include gender, date of birth, diagnosis, date of diagnosis, treatment type, date of start, and date of discontinuation. Should a patient discontinue treatment, the main reason for end is also recorded (for example, inefficacy, AE, or other causes). When a patient has an RAE, additional data are registered; these data include the date of occurrence, type, and classification of event according to the World Health Organization Adverse Reaction Dictionary.
Completeness and agreement of data with patient charts were assessed in situ by audits of 15% of patients' records between December 2003 and March 2004 and by telephone of 10% of patients' records between December 2004 and January 2005. Non-communication of the TNF antagonist discontinuation was detected in 6% of cases and non-communication of RAE in 16%; non-communication of either discontinuation or RAE was present in 18% of the sample. All errors were corrected accordingly and yielded an expected error rate, in the whole BIOBADASER sample, of approximately 11%.
Guidelines for the use of TNF antagonists in patients with SpA were available through the SER at the beginning of 2005. Criteria for eligibility of patients with axial disease comprise failure to respond to two NSAIDs, bath AS disease activity index (BASDAI) greater than 4, and additional clinical criteria. For peripheral joint disease, TNF antagonists are recommended for patients who failed to respond to two NSAIDs and sulphasalazine and who have a self-assessment of the disease in a visual analog scale greater than 4 cm and an elevation of acute-phase reactants. These guidelines do not contain recommendations for discontinuation. Prior to this date, biologics were used based on the judgement of the rheumatologist, most often following the criteria settled previously for their application as compassionate medication. These criteria were more stringent and include a BASDAI greater than 6.5 units for spinal disease, more than five inflamed joints, and radiographic erosive changes for peripheral arthritis in any case in which a patient failed to respond to two NSAIDs and two disease-modifying antirheumatic drugs (DMARDs). In Spain, infliximab was available for clinical use in August 1999, etanercept in April 2003, and adalimumab in February 2004. For AS, infliximab was approved in May 2003 and etanercept in January 2004. For PsA, infliximab was available in September 2004 and etanercept in December 2002. For the other types of SpA, biologics are used as compassionate medication. All three biologics (infliximab, etanercept, and adalimumab) are provided free of charge to patients and without restriction at the hospital level by the National Health Service.
Data in the present study correspond to those gathered from the start of the registry to February 2005. Data that are incomplete are censored at the time the last reliable information was recorded. The censoring was done to avoid having long drug survival that reflected under-reporting rather than true drug retention.
Statistical methods
The cumulative rate of discontinuation was calculated using the actuarial method, accounting for multiple failures per patient and for the calendar year. The log-rank test was used to compare the different types of SpA and to compare the survival curves of SpA and RA. Cox regression models were used to measure the differences in discontinuation rate between the two diagnostic groups as well as to measure association between risk factors and discontinuation. Besides estimation of drug survival and retention rate, the incidence rates of AEs were compared, and Cox regression analyses were used to detect and adjust for independent factors other than the diagnostic group. All analyses were performed using the Stata 9.1 program (StataCorp LP, College Station, TX, USA).
Results
A majority of the patients registered in BIOBADASER were diagnosed with RA (n = 4,006; 68.46%). SpA accounts for 26.04% (n = 1,524), AS 43% (n = 657), and PsA 37% (n = 570). The characteristics of patients are summarised in Table 1.
Table 1.
Characteristics of patients with spondylarthritis and rheumatoid arthritis in BIOBADASER and medications used by diagnosis
| Total treatments with biologics, n (percentage) by diagnosis | |||||||
| n (percentage) by diagnosis in BIOBADASER | Age* | n (percentage), women | Disease duration* | Infliximab | Etanercept | Adalimumab | |
| Rheumatoid arthritis | 4,006 (68.5) | 54 ± 13 | 3,166 (79.0) | 10 ± 7 | 2,789 (60.6) | 1,170 (25.4) | 577 (12.5) |
| All spondylarthritis | 1,524 (26.0) | 45 ± 12† | 537 (35.2)† | 10 ± 8 | 1,180 (70.7) | 472 (28.3) | 15 (0.9)† |
| Ankylosing spondylitis | 657 (11.2) | 45 ± 12 | 152 (23.1) | 12 ± 8 | 570 (80.5) | 136 (19.2) | 2 (0.3) |
| Psoriatic arthritis | 570 (9.7) | 47 ± 12 | 270 (47.4) | 9 ± 7 | 347 (54.5) | 281 (44.1) | 9 (1.4) |
| Undifferentiated spondylarthritis | 187 (3.2) | 43 ± 12 | 70 (37.4) | 8 ± 7 | 162 (79.0) | 41 (20.0) | 2 (1.0) |
| Crohn's disease arthritis | 68 (1.2) | 38 ± 13 | 32 (47.1) | 7 ± 8 | 68 (94.4) | 2 (2.8) | 1 (1.4) |
| Juvenile spondylarthritis | 19 (0.3) | 30 ± 12 | 7 (36.8) | 17 ± 14 | 17 (89.5) | 2 (10.5) | - |
| Reactive arthritis | 12 (0.2) | 49 ± 9 | 2 (16.7) | 8 ± 7 | 10 (83.3) | 2 (16.7) | - |
| Chronic seronegative oligoarthritis | 11 (0.2) | 35 ± 9 | 4 (36.4) | 4 ± 3 | 6 (40.0) | 8 (53.3) | 1 (6.7) |
*In years, mean ± standard deviation
†p < 0.001 of the difference between rheumatoid arthritis and spondylarthritis as a group.
Compared with patients with RA, patients with SpA were younger (p < 0.001) and more frequently male (p < 0.001). Adalimumab was rarely used in SpA (15 treatments [0.90%] versus 577 [12.72%] in RA; p < 0.001).
The total exposure to TNF antagonists was 2,430 patient-years for SpA and 7,865 for RA. A detailed exposure rate by biologic and diagnosis is shown in Table 2. As expected, patients with RA not only had larger exposure in terms of patient-years, but also had used different biologic treatments more frequently. Adalimumab contributed little to the exposure to biologics in our population.
Table 2.
Exposure to the different biologic response modifiers depending on diagnosis in BIOBADASER
| Diagnosis | Infliximab | Etanercept | Adalimumab |
| Rheumatoid arthritis | 5,521 (840) | 1,724 (188) | 526 (67) |
| All spondylarthritis | 1,915 (211) | 507 (35) | 5.7 (3) |
| Ankylosing spondylitis | 873 (87) | 134 (13) | 0.8 (0) |
| Psoriatic arthritis | 636 (84) | 325 (16) | 2.6 (2) |
| Undifferentiated spondylarthritis | 257 (28) | 35 (4) | 1.4 (0) |
| Crohn's related spondylarthritis | 124 (9) | 2.8 (0) | 0.7 (0) |
| Other spondylarthritis | 22 (3) | 9.5 (2) | 0.3 (1) |
Numbers indicate total patient-years. In parentheses are the numbers of treatments discontinued.
Survival of TNF antagonists in SpA is significantly greater than in RA at 1, 2, and 3 years (Table 3), and the difference seems even larger with prolonged exposures (Figure 1). As shown in Table 3 and Figure 1, there are no significant differences in drug survival in the different types of SpA, although the group of "other SpA," which includes reactive arthritis, juvenile SpA, and chronic seronegative oligoarthritis, has lower drug survival (yet due to the small size of the group did not reach statistical significance [n = 42]).
Table 3.
Drug survival at one, two, and three years by diagnosis
| Drug survival [95% confidence interval] | |||
| Diagnosis | One year | Two years | Three years |
| Rheumatoid arthritis | 0.83 [0.81–0.84] | 0.72 [0.71–0.74] | 0.65 [0.63–0.67] |
| All spondylarthritis | 0.88 [0.86–0.90]* | 0.82 [0.79–0.84]* | 0.74 [0.71–0.78]* |
| Ankylosing spondylitis | 0.88 [0.85–0.91] | 0.82 [0.78–0.86] | 0.76 [0.70–0.80] |
| Psoriatic arthritis | 0.88 [0.84–0.90] | 0.81 [0.77–0.84] | 0.73 [0.67–0.78] |
| Undifferentiated spondylarthritis | 0.88 [0.81–0.91] | 0.83 [0.76–0.88] | 0.72 [0.60–0.80] |
| Crohn's related spondylarthritis | 0.90 [0.79–0.95] | 0.86 [0.73–0.93] | 0.82 [0.69–0.91] |
| Other spondylarthritis | 0.79 [0.53–0.92] | 0.61 [0.31–0.81] | 0.61 [0.31–0.81] |
*p of the difference versus survival at the same time in rheumatoid arthritis < 0.001 (by log-rank test).
Figure 1.
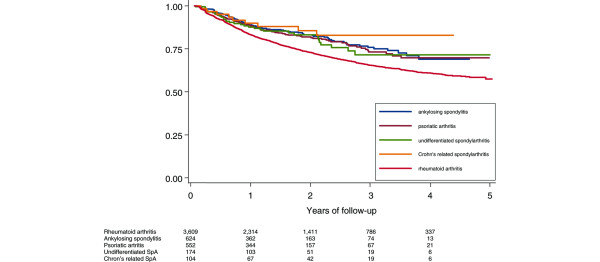
Survival of tumor necrosis factor antagonists in patients with spondylarthritis (SpA) and rheumatoid arthritis
The better survival in SpA is expressed by a hazard ratio (HR) for discontinuation of 0.66 (95% confidence interval [CI], 0.58–0.76) compared with RA. There are unevenly distributed factors in SpA and RA (Table 1) that had an impact on discontinuation: being older than 60 (HR = 1.21 [95% CI, 1.08–1.36]), being female (HR = 1.27 [95% CI, 1.13–1.43]), and using infliximab (HR = 1.53 [95% CI, 1.31–1.78]). After adjustment for these three factors, the HR for discontinuation in SpA was 0.66 [95% CI, 0.57–0.76] compared with RA. Survival of infliximab as first medication in SpA was significantly better than in RA (p = 0.0065). Similarly, survival of etanercept as first medication in SpA was significantly better than in RA (p = 0.0439). There were no differences however, when survival of infliximab or etanercept as second medication in RA was compared with their survival as second drug in SpA.
The distribution and the causes for discontinuation do not differ significantly in SpA and RA (pchi-square = 0.131). They are AE (45.4% versus 48.7%), lack of efficacy (34.6% versus 36.0%), and others (19.9% versus 15.3%). The type of infection was also distributed equally in SpA and RA. Herpes zoster virus and Mycobacterium tuberculosis were the leading identified microorganisms causing infection. Recommendations for screening and treatment of latent tuberculosis were put in place in March 2002 [35], and their impact has been reported elsewhere [37].
The HR for discontinuation due to AEs does not differ significantly among the types of SpA. On the contrary, the HR for discontinuation due to lack of efficacy is statistically greater for chronic seronegative arthritis than for the other types of SpA (HR = 6.7 [95% CI, 1.6–28.2]). Compared with patients with RA, patients with SpA treated with TNF antagonists have fewer AEs (17% versus 26%, respectively, had one or more AEs) that occur at a lower rate (13 events [95% CI, 11–14] in SpA versus 17 events per 1,000 patient-years in RA [95% CI, 16–18]). The incidence risk ratioSpA versus RA is 0.78 [95% CI, 0.68–0.89]). The HR of presenting an AE in SpA compared with RA is 0.69 [95% CI, 0.61–0.78]. However, as confirmed by the confidence intervals in Table 4, there were no differences in the rate of any particular AE. For the purpose of this study, discontinuation for AEs was considered when infusion or injection treatment after the event was not received within fourfold of the expected therapeutic interval. Under this definition, treatment was discontinued in 553 of 1,388 (39.8%) AEs in RA and in 123 of 329 (37.4%) in SpA (p = 0.412). Furthermore, the proportion of AEs that ended in hospitalisation or a prolonged hospital stay was 26.4% in RA and 21.3% in SpA (p = 0.056). Rate of AEs associated with death was 3.9% in RA and 1.5% in SpA (p = 0.034).
Table 4.
Types of adverse events occurring in patients with spondylarthritis and rheumatoid arthritis treated with biologics
| Spondylarthritis | Rheumatoid arthritis | |||||||||||
| Biologic and total exposure | Infliximab 1,915 | Etanercept 507 | Adalimumab 5.7 | Infliximab 5,521 | Etanercept 1,724 | Adalimumab 526 | ||||||
| Type of adverse event | Cases | IR [95% CI] | Cases | IR [95% CI] | Cases | IR [95% CI] | Cases | IR [95% CI] | Cases | IR [95% CI] | Cases | IR [95% CI] |
| Infusion reaction | 72 | 4.14 [3.29–5.22] | 1 | 0.20 [0.03–1.4] | 0 | - | 252 | 5.09 [4.50–5.76] | 7 | 0.43 [0.21–0.91] | 6 | 1.36 [0.61–3.03] |
| Infection | 102 | 5.87 [4.83–7.13] | 5 | 1.01 [0.42–2.43] | 1 | 18.90 [2.66–134.14] | 350 | 7.07 [6.37–7.85] | 28 | 1.74 [1.20–2.52] | 56 | 12.71 [9.78–16.52] |
| Cutaneous | 22 | 1.27 [0.83–1.92] | 2 | 0.40 [0.10–1.61] | 1 | 18.90 [2.66–134.14] | 50 | 1.01 [0.77–1.33] | 21 | 1.30 [0.85–2.00] | 40 | 9.08 [6.66–12.38] |
| Cytopenia | 2 | 0.12 [0.03–0.46] | 0 | - | 0 | - | 27 | 0.54 [0.37–0.80] | 3 | 0.19 [0.06–0.58] | 3 | 0.68 [0.22–2.11] |
| Neoplastic | 6 | 0.35 [0.16–0.77] | 1 | 0.20 [0.03–1.4] | 0 | - | 20 | 0.40 [0.26–0.63] | 8 | 0.50 [0.25–0.99] | 1 | 0.23 [0.03–1.61] |
| Pulmonary | 4 | 0.23 [0.09–0.61] | 0 | - | 0 | - | 21 | 0.42 [0.28–0.65] | 4 | 0.25 [0.09–0.66] | 3 | 0.68 [0.22–2.11] |
| Cardiovascular | 13 | 0.75 [0.43–1.29] | 3 | 0.61 [0.20–1.88] | 0 | - | 64 | 1.29 [1.01–1.65] | 8 | 0.50 [0.25–0.99] | 13 | 2.95 [1.71–5.08] |
| Endocrine | 1 | 0.06 [0.01–0.41] | 0 | - | 0 | - | 5 | 0.10 [0.04–0.24] | 0 | - | 0 | - |
| Gastrointestinal | 25 | 1.44 [0.97–2.13] | 3 | 0.61 [0.20–1.88] | 0 | - | 32 | 0.64 [0.46–0.91] | 5 | 0.31 [0.13–0.75] | 19 | 4.31 [2.75–6.76] |
| Ophthalmologic | 4 | 0.23 [0.09–0.61] | 0 | - | 0 | - | 2 | 0.04 [0.01–0.16] | 3 | 0.19 [0.06–0.58] | 2 | 0.45 [0.11–1.82] |
| Psychiatric | 3 | 0.17 [0.06–0.54] | 0 | - | 0 | - | 2 | 0.04 [0.01–0.16] | 0 | - | 6 | 1.36 [0.61–3.03] |
| Neurological | 5 | 0.29 [0.12–0.69] | 1 | 0.20 [0.03–1.43] | 0 | - | 15 | 0.30 [0.18–0.50] | 1 | 0.06 [0.01–0.44] | 8 | 1.82 [0.91–3.63] |
| Gynecological | 0 | - | 0 | - | 0 | - | 3 | 0.06 [0.02–0.19] | 0 | - | 1 | 0.23 [0.03–1.61] |
| Urological | 4 | 0.23 [0.09–0.61] | 0 | - | 0 | - | 4 | 0.08 [0.03–0.22] | 1 | 0.06 [0.01–0.44] | 3 | 0.68 [0.22–2.11] |
| Others | 17 | Not estimated | 2 | Not estimated | 0 | Not estimated | 83 | Not estimated | 10 | Not estimated | 23 | Not estimated |
Data are presented as cases documented and as incidence rate (IR) and 95% confidence interval (CI) per 100 patient-years of exposure.
After adjustment for being older than 60 years (HR = 1.51 [95% CI, 1.37–1.67]), having a disease duration longer than three years prior to therapy with TNF antagonist (HR = 1.29 [95% CI, 1.12–1.48]), and using infliximab (HR = 2.52 [95% CI, 2.11–2.99]), the HR for an AE in SpA versus RA was 0.80 [95% CI, 0.70–0.91]. Adjustment for age as a continuous variable did not produce different results than adjustment for age as a dichotomized variable (older than 60 years).
Discussion
In the present study, we have analysed the survival of TNF antagonists in patients with SpA in clinical practice in comparison with RA patients in a registry. We have found that, due in part to the occurrence of fewer AEs, patients with SpA have a 33% lower probability than patients with RA to discontinue TNF antagonists.
Measuring effectiveness of drugs in registries such as BIOBADASER is exposed to limitations. The voluntary medical registries need to be of high quality to be reliable [38]. The quality of our database was ensured by repeated external audits of the participating centres, as reported in Materials and methods. Following these quality assessment interventions, we expect an error of approximately 11% in the information considered relevant to the analysis. This is within the 0%-17% range reported by others [39].
Survival of a drug could be taken as an indication of drug effectiveness in the clinical setting. Furthermore, studies with extended follow-up and large numbers of patients are common in rheumatology in assessing long-term effectiveness of treatments [40]. However, these studies face limitations such as confounding by indication, patient selection, and the absence of a washout period [41].
Until recently, AS and PsA were difficult to evaluate in therapeutic trials because of the lack of standardised, widely accepted, or validated instruments for assessing disease and clinical response. This has changed in the past few years [42,43]. Although the pathogeneses of SpA have not been fully recognised, there appears to be a significant expression of TNF in the entheses and peripheral joints in all the conditions [44-47]. This has led to the use of TNF antagonists in the treatment of SpA resistant to NSAIDs and the traditional DMARDs. However, long-term studies in patients with SpA treated with TNF antagonists are not yet available, and optimal duration of therapy has not been established. In routine clinical practice, physicians tend to treat patients with SpA over protracted periods (as in RA) and, as such, these insights from our BIOBADASER database can provide valuable information about physicians' prescribing patterns. Of note is the better survival of TNF antagonists in patients with SpA than in patients with RA.
A limitation of our study is that measures of joint inflammation and damage were not collected, and therefore we can neither adjust for baseline severity nor measure improvement in each group. However, had we measured these parameters, it would be difficult to compare activity in RA with activity in PsA. Several parameters other than efficacy and safety (such as co-morbidity, costs, availability of other therapies, patient and physician expectations, and medication compliance) have an impact on the rate of discontinuation of a drug [48]. Absence of alternative medications in the treatment of SpA may have contributed to the favourable retention rate compared with RA. Another explanation could be the lower rate of AEs in SpA. This is explained, in part, by younger age and fewer co-medications in SpA than in RA, because age and co-medications are associated with a greater number of AEs [49]. Nevertheless, the difference in risk of AEs cannot be fully explained by these factors, because after the proper adjustments there is still a 20% lower risk in SpA compared with RA. Adjustment for potential confounders did not change the overall HR for discontinuation of SpA. Because all confounders were more likely to be present in RA, this suggests an interaction of one of the variables and the type of disease. We cannot prove that patients with SpA have fewer co-morbidities and use fewer concomitant medications than do patients with RA, because these data were collected only in patients who had AEs. However, comparison of both co-morbidities and concomitant medication in SpA and in RA patients who had had an AE reveals a significant difference (Mann-Whitney U test, p < 0.001). Also, RA itself could increase the rate of AEs in patients treated with TNF antagonists. Interestingly, the higher doses of infliximab recommended for SpA did not increase the rate of AEs. Although the dose was not actually noted, the recommended dose is 5 mg/kg for SpA and 3 mg/kg for RA (Summary of Product Characteristics of infliximab). Neither was it recorded whether the interval between infusions was shortened in patients not responding. This might have been the case in the early phases, when only infliximab was available, but was less probable thereafter.
Of note is that the HR of discontinuation of PsA versus RA was 0.81 (95% CI, 0.66–0.99). After adjustment for age, it lost its statistical significance. It may be that the symptoms of patients with PsA who predominantly have small-joint peripheral disease (that is, who appear to have RA) behave more like those of RA. This cannot be fully substantiated, because clinical data to assess the type of PsA were not included in the registry.
In patients with SpA, the rate of AEs with infliximab was greater than with etanercept. This could merely reflect an over-representation of patients treated with infliximab and with a longer follow-up. Furthermore, a bias toward the use of a "newer better" drug in the most severe cases or in non-responder patients)[50] may have distorted the observed efficacy; the availability of etanercept may have led to early discontinuation of infliximab, which had been cleared for use 40 months before etanercept. Head-to-head comparison will be needed to answer this question.
Conclusion
Survival of TNF antagonists in SpA is better than in RA. At present, TNF antagonists are an optimal, safe, and effective therapeutic option for patients with severe SpA who fail to respond to traditional drugs. Because chronic therapy is necessary, continual review of this issue is necessary.
Abbreviations
AE = adverse event; AS = ankylosing spondylitis; BASDAI = bath ankylosing spondylitis disease activity index; CI = confidence interval; DMARD = disease-modifying antirheumatic drug; HR = hazard ratio; NSAID = non-steroidal anti-inflammatory drug; PsA = psoriatic arthritis; RA = rheumatoid arthritis; RAE = relevant adverse event; SER = Spanish Society of Rheumatology; SpA = spondylarthritis; TNF = tumor necrosis factor.
Competing interests
JJGR is on Advisory Boards of Schering-Plough, Wyeth, and Roche and has received lecture fees from Abbott Laboratories, Wyeth, and Schering-Plough.
Authors' contributions
Both LC and JJGR were the designers of the study. JJGR prepared the manuscript, which was reviewed and modified by LC. LC planned and ran the analyses. The BIOBADASER Study group collected and checked the data without receiving any economic reward. Both authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We are indebted to Raquel Ruiz for her careful monitoring of the registry and to Rocío Gonzalez for her help in the statistical analysis and data management. BIOBADASER is supported by the Spanish Society of Rheumatology. This study was funded, in part, by the Spanish Agency for Medicines and Medical Devices (Ministry of Health, Spain).
The following is a list of contributors (and centers) in BIOBADASER, with the steering committee members identified with an asterisk:
D Montero* (División de Farmacoepidemiología y Farmacovigilancia, Agencia Española de Medicamentos y Productos Sanitarios); A Erra, S Marsal (CS Vall D'hebron); M Fernández Castro, J Mulero*, J L Andréu, (Clínica Puerta de Hierro); M Rodríguez Gómez (CH de Ourense); M Larrosa Pardo, E Casado, (CH del Parc Taulí); E Leonor Sirvent, D Reina, C García Gómez, (H de Bellvitge); B Joven, P Carreira, (H 12 de Octubre); MV Hernández (H Clinic i Provincial); E Loza (H Clínico Univ. San C); A Alonso, E Uriarte (H de Cruces); L Pantoja, M Valvanera (H del Bierzo); T Mariné (H de L'Esperit Sant); R García de Vicuña, A M Ortiz, I González Álvaro, A Laffón*, J M Álvaro-Gracia* (H Univ. de La Princesa); C Díaz López, A Rodríguez de la Serna (H de La Santa Creu i Sant Pau); E Loza (H de Navarra); M V Irigoyen, I Ureña, V Coret (H Gral. C Haya); P Vela, E Pascual* (H Gral. Univ. de Alicante); MA Belmonte, J Beltrán, JJ Lerma (H Gral. de Castellón); M Liz (H Clínico Univ. de Santiago); SM Gelman (H Gral. de Manresa); E Ciruelo, E Tomero, O Amengual (H Gral. de Segovia); JC Cobeta (H Gral. de Teruel Obispo Polanco); E Saiz, J Gálvez (H Gral. Morales Meseguer); G Iglesias de la Torre (H Gral. Río Carrión); R Roselló, C Vázquez (H Gral. San Jorge); JP Valdazo (H Gral. Virgen de La Concha); X Tena*, V Ortiz (H Univ. Germans Trias i Pujol); M Fernández Prada, JA Piqueras, J Tornero* (H Gral. Univ. de Guadalajara); L Cebrián, L Carreño* (H Gregorio Marañón); J J García Borras (H La Fe); F J Manero (H Univ. Miguel Servet); M Pujol, J Granados (H Mutua Terrassa); JL Cuadra, FJ Paulino, M Paulino (H Ntra. Sra. del C); O Maiz, E Barastay, M Figueroa* (H de Donosti); C Torres, M Corteguera (H Ntra. Sra. de Sonsoles); C Rodríguez Lozano, F Francisco, I Rua (H de Gran Canaria Dr. Negrín); O Illera, AC Zea, P García de la Peña, M Valero (H Ramón y Cajal); E Aznar, R Gutiérrez (H Reina Sofía); A Cruz, M Crespo, F Cabero (H Severo Ochoa); MT Ruiz Jimeno (H Comarcal Sierrallana); J Fiter, L Espadaler (H Son Dureta); JC Vesga, E Cuende (H Txagorritxu); S Sánchez Andrada, V Rodríguez Valverde* (H Univ. Marques de Valdecilla); I Ferraz, T González (H Univ. de Canarias); JL Marenco*, E Rejón (H Univ. de Valme); E Collantes, MC Castro (H Univ. Reina Sofía); B Hernández, JV Montes de Oca, F Navarro, FJ Toyos (H Univ. Virgen Macarena); C Marras, LF Linares, J Moreno (H Virgen de La Arrixaca); C González-Montagut (H Virgen de La Luz); A García Aparicio (H Virgen de la Salud); R Cáliz*, C Idalgo (H Virgen de las Nieves); A Sánchez-Andrade (H Xeral-Calde); E Martín Mola*; T Cobo, A Hernández (H La Paz); X Arasa (H de Tortosa); J R Noguera, FJ Navarro Blasco, JV Tovar (H Gral. Univ. de Elche); JC Rosas, G Santos (H del S.V.S. de Villajoyosa); I Ibero, V Jovani, R Martín, (H Gral. de Elda); J del Blanco Barnusell (H Sant Jaume de Calella); MA Abad, M Torresano (H Virgen del Puerto); G Pérez Lidon, M Tenorio (H del Insalud Ceuta); I Bañegil (H de Mendaro); J Carbonell*; J Maymo, C Pérez García (IMAS. H de L'Esperança y del Mar); VE Quevedo (H Comarcal de Monforte); J Rivera, T González (Instituto Provincial de Rehabilitación); J M Rodríguez Heredia, A Gallegos Cid, J García Arroba, M Cantalejo (H Univ. de Getafe); R Almodóvar, J Quirós, P Zarco, R Mazzucchelli (H Fundación Alcorcón); A Corrales (H Comarcal de Laredo); D Boquet (H Arnau de Vilanova); F Pérez Torres (H Gral. de Requena); J Ivorra (H Gral de Onteniente); X Suris (H Gral. de Granollers); T Pérez Sandoval (H Virgen B); J Calvo Catalá, C Campos (H Gral. Univ. de Valencia); MF Pina (H Rafael Méndez); C Hidalgo (H de La Santísima Trinidad); J García Consuegra, R Merino (H Infantil La Paz); M Sala Gómez (H de Figueres); M Centellas (H de Mataró); JM Ruiz Martín (H de Viladecans); A Juan, I Ros (Fundación H Son Llàtzer); J Fernández Campillo, R González Molina (H del S.V.S. Vega Baja); M Mínguez Vega, Gaspar Panadero (H San J de Alicante); J Ibáñez (Policlínico Vigo, S.A. (Povisa)); A Martínez Cristóbal, P Trenor (H de La Ribera); J Graña Gil (H Santa T); MT Bosque Peralta (H Clínico Univ. Lozano Blesa); A Urruticoechea (H Can Misses de Ibiza); J Román Ivorra, I Chalmeta (H Univ. Dr. Peset); J Alegre, B Álvarez Lario, J L Alonso Valdivielso, J Fernández Melón (H Gral. Yagüe); MA Belmonte (Clínica A. Belmonte).
Contributor Information
Loreto Carmona, Email: lcarmona@ser.es.
Juan J Gómez-Reino, Email: juan.gomez-reino.carnota@sergas.es.
References
- Khan MA. Update on spondyloarthropathies. Ann Intern Med. 2002;136:896–907. doi: 10.7326/0003-4819-136-12-200206180-00011. [DOI] [PubMed] [Google Scholar]
- Wright V, Moll JMH. Seronegative Polyarthritis. Amsterdam: North Holland Publishing Co; 1976. [Google Scholar]
- Gladman DD. Spondyloarthropathies. Curr Opin Rheumatol. 2004;16:329–330. doi: 10.1097/01.bor.0000130163.21746.82. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cortinovis D, Stone MA. Recent advances in the treatment of the spondyloarthropathies. Curr Opin Rheumatol. 2004;16:357–365. doi: 10.1097/01.bor.0000129719.21563.35. [DOI] [PubMed] [Google Scholar]
- Wanders A, Heijde D, Landewe R, Behier JM, Calin A, Olivieri I, Zeidler H, Dougados M. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52:1756–1765. doi: 10.1002/art.21054. [DOI] [PubMed] [Google Scholar]
- Sampaio-Barros PD, Costallat LT, Bertolo MB, Neto JF, Samara AM. Methotrexate in the treatment of ankylosing spondylitis. Scand J Rheumatol. 2000;29:160–162. doi: 10.1080/030097400750002021. [DOI] [PubMed] [Google Scholar]
- Clegg DO, Reda DJ, Mejias E, Cannon GW, Weisman MH, Taylor T, Budiman-Mak E, Blackburn WD, Vasey FB, Mahowald ML, et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheum. 1996;39:2013–2020. doi: 10.1002/art.1780391210. [DOI] [PubMed] [Google Scholar]
- Clegg DO, Reda DJ, Abdellatif M. Comparison of sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondylarthropathies: a Department of Veterans Affairs cooperative study. Arthritis Rheum. 1999;42:2325–2329. doi: 10.1002/1529-0131(199911)42:11<2325::AID-ANR10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ferraz MB, Tugwell P, Goldsmith CH, Atra E. Meta-analysis of sulfasalazine in ankylosing spondylitis. J Rheumatol. 1990;17:1482–1486. [PubMed] [Google Scholar]
- Jones G, Crotty M, Brooks P. Psoriatic arthritis: a quantitative overview of therapeutic options. The Psoriatic Arthritis Meta-Analysis Study Group. Br J Rheumatol. 1997;36:95–99. doi: 10.1093/rheumatology/36.1.95. [DOI] [PubMed] [Google Scholar]
- van der Linden S, van der Heijde D. Clinical aspects, outcome assessment, and management of ankylosing spondylitis and postenteric reactive arthritis. Curr Opin Rheumatol. 2000;12:263–268. doi: 10.1097/00002281-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Black RL, O'Brien WM, Vanscott EJ, Auerbach R, Eisen AZ, Bunim JJ. Methotrexate therapy in psoriatic arthritis: double-blind study on 21 patients. JAMA. 1964;189:743–747. [PubMed] [Google Scholar]
- Willkens RF, Williams HJ, Ward JR, Egger MJ, Reading JC, Clements PJ, Cathcart ES, Samuelson CO, Jr, Solsky MA, Kaplan SB, et al. Randomized, double-blind, placebo controlled trial of low-dose pulse methotrexate in psoriatic arthritis. Arthritis Rheum. 1984;27:376–381. doi: 10.1002/art.1780270403. [DOI] [PubMed] [Google Scholar]
- Fraser SM, Hopkins R, Hunter JA, Neumann V, Capell HA, Bird HA. Sulphasalazine in the management of psoriatic arthritis. Br J Rheumatol. 1993;32:923–925. doi: 10.1093/rheumatology/32.10.923. [DOI] [PubMed] [Google Scholar]
- Combe B, Goupille P, Kuntz JL, Tebib J, Liote F, Bregeon C. Sulphasalazine in psoriatic arthritis: a randomized, multicentre, placebo-controlled study. Br J Rheumatol. 1996;35:664–668. doi: 10.1093/rheumatology/35.7.664. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Grober JS, Hamilton TA, Ellis CN, Siegel MT, Voorhees JJ, McCune WJ. Sulfasalazine therapy for psoriatic arthritis: a double blind, placebo controlled trial. J Rheumatol. 1995;22:894–898. [PubMed] [Google Scholar]
- Dougados M, vam der Linden S, Leirisalo-Repo M, Huitfeldt B, Juhlin R, Veys E, Zeidler H, Kvien TK, Olivieri I, Dijkmans B, et al. Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum. 1995;38:618–627. doi: 10.1002/art.1780380507. [DOI] [PubMed] [Google Scholar]
- Boeger CA, Wittwer H, Schattenkirchner M, Kellner H, Kellner W. Treatment of ankylosing spondylitis with infliximab. Ann Rheum Dis. 2001;60:1159–1160. doi: 10.1136/ard.60.12.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Haibel H, Cornely D, Golder W, Gonzalez J, Reddig J, Thriene W, Sieper J, Braun J. Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis Rheum. 2000;43:1346–1352. doi: 10.1002/1529-0131(200006)43:6<1346::AID-ANR18>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Brandt J, Haibel H, Reddig J, Sieper J, Braun J. Successful short term treatment of severe undifferentiated spondyloarthropathy with the anti-tumor necrosis factor-alpha monoclonal antibody infliximab. J Rheumatol. 2002;29:118–122. [PubMed] [Google Scholar]
- Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, Gromnica-Ihle E, Kellner H, Krause A, Schneider M, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002;359:1187–1193. doi: 10.1016/S0140-6736(02)08215-6. [DOI] [PubMed] [Google Scholar]
- Brandt J, Khariouzov A, Listing J, Haibel H, Sorensen H, Grassnickel L, Rudwaleit M, Sieper J, Braun J. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum. 2003;48:1667–1675. doi: 10.1002/art.11017. [DOI] [PubMed] [Google Scholar]
- Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, Gromnica-Ihle E, Kellner H, Schneider M, Sorensen H, et al. Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis. 2005;64:229–234. doi: 10.1136/ard.2004.025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bosch F, Kruithof E, Baeten D, Herssens A, de Keyser F, Mielants H, Veys EM. Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum. 2002;46:755–765. doi: 10.1002/art.511. [DOI] [PubMed] [Google Scholar]
- Maksymowych WP, Jhangri GS, Lambert RG, Mallon C, Buenviaje H, Pedrycz E, Luongo R, Russell AS. Infliximab in ankylosing spondylitis: a prospective observational inception cohort analysis of efficacy and safety. J Rheumatol. 2002;29:959–965. [PubMed] [Google Scholar]
- Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356:385–390. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- Marzo-Ortega H, McGonagle D, O'Connor P, Emery P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum. 2001;44:2112–2117. doi: 10.1002/1529-0131(200109)44:9<2112::AID-ART363>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Gorman JD, Sack KE, Davis JC., Jr Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349–1356. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- Davis JC, Jr, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, Kivitz A, Fleischmann R, Inman R, Tsuji W, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48:3230–3236. doi: 10.1002/art.11325. [DOI] [PubMed] [Google Scholar]
- Salvarani C, Cantini F, Olivieri I, Macchioni P, Padula A, Niccoli L, Catanoso MG, Scocco GL, Boiardi L. Efficacy of infliximab in resistant psoriatic arthritis. Arthritis Rheum. 2003;49:541–545. doi: 10.1002/art.11201. [DOI] [PubMed] [Google Scholar]
- Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, Furst DE, Molitor J, Keystone E, Gladman D, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52:1227–1236. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Pinho P, Walco G, Higgins G, Hummell D, Szer I, Henrickson M, Watcher S, Reiff A. Etanercept treatment in patients with refractory systemic onset juvenile rheumatoid arthritis. J Rheumatol. 2005;32:935–942. [PubMed] [Google Scholar]
- Henrickson M, Reiff A. Prolonged efficacy of etanercept in refractory enthesitis-related arthritis. J Rheumatol. 2004;31:2055–2061. [PubMed] [Google Scholar]
- Homeff G, Burgos-Vargas R. TNF-alpha antagonists for the treatment of juvenile-onset spondyloarthritides. Clin Exp Rheumatol. 2002;20:S137–142. [PubMed] [Google Scholar]
- Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- BIOBADASER http://biobadaser.ser.es
- Carmona L, Gómez-Reino JJ, Rodriguez-Valverde V, Montero D, Pascual-Gómez E, la EM, Carreno L, Figueroa M, BIOBADASER Group Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766–1772. doi: 10.1002/art.21043. [DOI] [PubMed] [Google Scholar]
- Arts DG, De Keizer NF, Scheffer GJ. Defining and improving data quality in medical registries: a literature review, case study, and generic framework. J Am Med Inform Assoc. 2002;9:600–611. doi: 10.1197/jamia.M1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen JH, Jacob M, Copley L. Assessing the quality of the data in a transplant registry: the European Liver Transplant Registry. Transplantation. 2003;75:2164–2167. doi: 10.1097/01.TP.0000080272.48725.8D. [DOI] [PubMed] [Google Scholar]
- Maetzel A, Wong A, Strand V, Tugwell P, Wells G, Bombardier C. Meta-analysis of treatment termination rates among rheumatoid arthritis patients receiving disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2000;39:975–981. doi: 10.1093/rheumatology/39.9.975. [DOI] [PubMed] [Google Scholar]
- Krishnan E, Fries JF. Measuring effectiveness of drugs in observational databanks: promises and perils. Arthritis Res Ther. 2004;6:41–44. doi: 10.1186/ar1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladman DD, Helliwell P, Mease PJ, Nash P, Ritchlin C, Taylor W. Assessment of patients with psoriatic arthritis: a review of currently available measures. Arthritis Rheum. 2004;50:24–35. doi: 10.1002/art.11417. [DOI] [PubMed] [Google Scholar]
- Calin A, Mackay K, Santos H, Brophy S. A new dimension to outcome: application of the Bath Ankylosing Spondylitis Radiology Index. J Rheumatol. 1999;26:988–992. [PubMed] [Google Scholar]
- Grom AA, Murray KJ, Luyrink L, Emery H, Passo MH, Glass DN, Bowlin T, Edwards C., 3rd Patterns of expression of tumor necrosis factor alpha, tumor necrosis factor beta, and their receptors in synovia of patients with juvenile rheumatoid arthritis and juvenile spondylarthropathy. Arthritis Rheum. 1996;39:1703–1710. doi: 10.1002/art.1780391013. [DOI] [PubMed] [Google Scholar]
- Baeten D, Kruithof E, Van den Bosch F, Demetter P, Van Damme N, Cuvelier C, De Vos M, Mielants H, Veys EM, De Keyser F. Immunomodulatory effects of anti-tumor necrosis factor alpha therapy on synovium in spondylarthropathy: histologic findings in eight patients from an open-label pilot study. Arthritis Rheum. 2001;44:186–195. doi: 10.1002/1529-0131(200101)44:1<186::AID-ANR25>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Danning CL, Illei GG, Hitchon C, Greer MR, Boumpas DT, McInnes IB. Macrophage-derived cytokine and nuclear factor kappaB p65 expression in synovial membrane and skin of patients with psoriatic arthritis. Arthritis Rheum. 2000;43:1244–1256. doi: 10.1002/1529-0131(200006)43:6<1244::AID-ANR7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ritchlin C, Haas-Smith SA, Hicks D, Cappuccio J, Osterland CK, Looney RJ. Patterns of cytokine production in psoriatic synovium. J Rheumatol. 1998;25:1544–1552. [PubMed] [Google Scholar]
- Wolfe F. The epidemiology of drug treatment failure in rheumatoid arthritis. Baillieres Clin Rheumatol. 1995;9:619–632. doi: 10.1016/s0950-3579(05)80305-x. [DOI] [PubMed] [Google Scholar]
- Shelton PS, Fritsch MA, Scott MA. Assessing medication appropriateness in the elderly: a review of available measures. Drugs Aging. 2000;16:437–450. doi: 10.2165/00002512-200016060-00004. [DOI] [PubMed] [Google Scholar]
- Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med. 1991;10:577–581. doi: 10.1002/sim.4780100409. [DOI] [PubMed] [Google Scholar]


