Abstract
Fibromyalgia (FM) pain is frequent in the general population but its pathogenesis is only poorly understood. Many recent studies have emphasized the role of central nervous system pain processing abnormalities in FM, including central sensitization and inadequate pain inhibition. However, increasing evidence points towards peripheral tissues as relevant contributors of painful impulse input that might either initiate or maintain central sensitization, or both. It is well known that persistent or intense nociception can lead to neuroplastic changes in the spinal cord and brain, resulting in central sensitization and pain. This mechanism represents a hallmark of FM and many other chronic pain syndromes, including irritable bowel syndrome, temporomandibular disorder, migraine, and low back pain. Importantly, after central sensitization has been established only minimal nociceptive input is required for the maintenance of the chronic pain state. Additional factors, including pain related negative affect and poor sleep have been shown to significantly contribute to clinical FM pain. Better understanding of these mechanisms and their relationship to central sensitization and clinical pain will provide new approaches for the prevention and treatment of FM and other chronic pain syndromes.
Introduction
Fibromyalgia syndrome (FM) is a chronic pain syndrome that has been defined by widespread pain for more than 3 months and the presence of ≥11 out of 18 tender points [1]. In addition, most FM patients complain of disturbed sleep, emotional distress, and pronounced fatigue. FM represents the extreme end of the spectrum of musculoskeletal pain in the general population and is a chronic illness that disproportionably affects women (9:1 ratio of women to men affected). Like many other clinical syndromes, FM has no single specific feature but represents a symptom complex of self reported or elicited findings.
Pain in FM is consistently felt in the musculature and is related to sensitization of central nervous system (CNS) pain pathways. Although not specific for FM, abnormal concentration of CNS neuropeptides, biogenic amines, and alterations of the hypothalamic-pituitary-adrenal axis have been described [2-5]. There is a large body of evidence for a generalized lowering of pressure pain thresholds in FM patients [6-10], but the mechanical pain hypersensitivity (allodynia) of FM patients is not limited to tender points and appears to be widespread [10]. In addition, almost all studies of FM patients have shown abnormalities of pain sensitivity while using different methods of sensory testing.
Although relevant for many clinical pain syndromes like FM, nociception alone cannot explain the human pain experience because it always undergoes modulation in the CNS by conscious and unconscious mental activity [11]. In addition, socio-cultural influences, beliefs or biases can strongly influence pain, particularly those related to cause, control, duration, outcome, and blame. These beliefs are frequently linked to negative emotions, like anger, fear, and depression [12]. Generally, pain has two emotional components, including the unpleasantness of the sensation (primary pain affect) as well as negative feelings like depression, anger and fear (secondary pain affect). This relationship of emotions with pain is bidirectional because modulation of negative feelings can powerfully alter the pain experience [13]. Due to the fact that pain is a personal (first person) experience it can only be partially captured by definitions. The International Association for the Study of Pain has defined pain as an "unpleasant sensory and emotional experience associated with actual and potential tissue damage or described in terms of such damage" [14]. This definition of pain, however, has significant shortcomings because it does not encompass all aspects of pain.
Thus, abnormalities of pain processing appear to play an important role for FM pain, particularly those related to deep tissue impulse input, central sensitization, and mood abnormalities. Some of the important contributions of abnormal central pain mechanisms to clinical FM pain include temporal summation of pain (or windup) and central sensitization.
Pathogenesis of fibromyalgia pain
FM is a pain amplification syndrome of patients who are highly sensitive to painful and non-painful stimuli, including touch, heat, cold, chemicals, light, sound, and smell. The cause for the heightened sensitivity of FM patients is unknown, but is likely to involve abnormalities in CNS sensory processing as well as peripheral tissue abnormalities. Central abnormalities appear to be related to blunting of the hypothalamic-pituitary axis responses to stressors [15,16], increased levels of substance P [2,17], excitatory amino acids [18] and neurotrophins [19] in the cerebro-spinal fluid of FM patients.
Although previous FM studies did not show consistent peripheral tissue abnormalities [20], more recent evidence points to possibly relevant alterations in skin and muscles. These abnormalities include increased substance P in muscle tissue [21], DNA fragmentation of muscle fibers [22], increased IL-1 in cutaneous tissues [23], and muscle perfusion deficits [24,25]. These peripheral changes may contribute to increased tonic nociceptive input into the spinal cord that results in augmented windup and central sensitization. In addition, there is compelling evidence for the contribution of peripheral pain to overall clinical pain in FM [26]. In a large study of FM patients, ratings of peripheral pain areas accounted for 27% of the variance of overall clinical pain [26], thus emphasizing the important role of peripheral impulse input for FM pain. These findings represent a possible link between peripheral input and FM pain. Importantly, nociceptive activity in peripheral tissues of FM patients does not necessarily have to be extensive, because central sensitization requires little sustained input for the maintenance of the sensitized state and chronic pain [26].
Despite increasing evidence emphasizing the role of sensory abnormalities in chronic widespread pain in FM, the contribution of psychological factors to FM pain must also be recognized. Several psychological risk factors for FM are common in Western populations, including somatic symptoms, negative life events [27], psychological distress [28], increased focus on bodily symptoms [29], and passive pain-coping mechanisms [30]. Both community and clinic patients with FM are also more likely than the general population to have a diagnosis of psychiatric disorders, particularly depression and anxiety [31,32]. In a prospective study of 214 women with self-reported pain, 39 (18%) were diagnosed with FM at study entry, and 33% satisfied FM criteria after 5.5 years of follow up [33]. Self-reported depression at baseline was associated with a more than six-fold increased likelihood of reporting FM symptoms at follow up and was found to be the strongest independent predictor. In addition, psychosocial factors, including high levels of distress, fatigue, and frequent health care seeking behavior, are strong predictors for chronic widespread pain and FM [34].
In this context, several studies have reported FM to be co-morbid with major depressive disorder [35,36]. A recent large family study of FM subjects showed that FM and major depressive disorder are characterized by shared, familial risk factors [37], thus emphasizing the strong relationship between negative affect and FM pain.
Peripheral and central sensitization
Although heightened pain sensitivity is a hallmark of FM, little is known about the genetic and other factors that contribute to this abnormality. Tissue sensitization after injury has long been recognized as making an important contribution to pain. This form of sensitization is related to changes in the properties of primary nociceptive afferents (peripheral sensitization), whereas central sensitization requires functional changes in the CNS (neuroplasticity). Such CNS changes can result in central sensitization, which manifests itself in several ways, including increased excitability of spinal cord neurons after an injury, enlargement of the receptive fields of these neurons, reduction in pain threshold, or recruitment of novel afferent inputs. Behaviorally, centrally sensitized patients like FM sufferers report abnormal or heightened pain sensitivity with spreading of hypersensitivity to uninjured sites and the generation of pain by low threshold mechano-receptors that are normally silent in pain processing. Thus, tissue injury might not only cause pain but also an expansion of dorsal horn receptive fields and central sensitization.
Central sensitization can occur as an immediate or delayed phenomenon [38], resulting in increased sensitivity of wide dynamic range and nociception specific neurons of the spinal cord. Whereas delayed central sensitization depends mostly on transcriptional and translational neuronal changes during afferent barrage, immediate central sensitization relies mainly on dorsal horn receptor mechanisms, including the N-methyl-D-aspartate (NMDA) and neurokinin-1 receptors [39].
Peripheral and central pain amplification
Peripheral nociceptors can become increasingly sensitive after tissue trauma and/or after up-regulation of nociceptor expression in peripheral nerve endings. Subsequent activation of these receptors will lead to increased firing rates and pain. This mechanism (peripheral sensitization) seems to play an important role in FM pain, although only indirect evidence is available at this time to support this assumption [26]. Impulses from peripheral nociceptors are transmitted to the CNS by myelinated A-δ (first pain) and unmyelinated C-fibers (second pain). A-δ mediated pain signals are rapidly conducted to the CNS (at about 10 m/s), whereas C-fiber impulses travel relatively slowly (at about 1.6 m/s). When the distance of C-fiber transmission is sufficiently long (like the length of the arm or leg) this delay of C-fiber compared to A-δ fiber impulses can be easily detected by study subjects. An important test of central pain amplification relies on summation of second pain or windup [40]. This technique reveals sensitivity to input from unmyelinated (C) afferents and the status of the NMDA receptor system [41], which is implicated in a variety of chronic pain conditions. Thermal, mechanical, or electrical windup stimuli can be applied to the skin or musculature of patients and commercial neurosensory stimulators are readily available for windup testing.
Temporal summation of second pain or windup
In 1965, Mendell and Wall described for the first time that repetitive C-fiber stimulation can result in a progressive increase of electrical discharges from second order neurons in the spinal cord [42]. This important mechanism of pain amplification in the dorsal horn neurons of the spinal cord is related to temporal summation of second pain or windup. First pain, which is conducted by myelinated A-δ pain fibers, is often described as sharp or lancinating and can be readily distinguished from second pain by most study subjects. In contrast, second pain (transmitted by unmyelinated C-fibers), which is strongly related to chronic pain states, is most frequently reported as dull, aching, or burning. Second pain increases in intensity when painful stimuli are applied more often than once every three seconds (Figure 1). This progressive increase represents temporal summation or windup and has been demonstrated to result from central rather than a peripheral nervous system mechanism (Figure 1). Animal studies have demonstrated similar windup of C afferent-mediated responses of dorsal horn nociceptive neurons and this summation has been found to involve NMDA receptor mechanisms. Importantly, windup and second pain can be inhibited by NMDA receptor antagonists, including dextromethorphan and ketamine [43-45].
Figure 1.
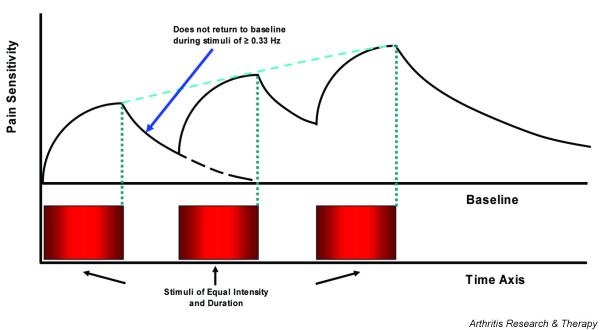
Temporal summation of second pain (windup). When identical stimuli are applied to normal subjects at frequencies of ≥0.33 Hz, pain sensations will not return to baseline during the interstimulatory interval. Windup is strongly dependent on stimulus frequency and is inversely correlated with interstimulatory interval [75]. In contrast to normal subjects, FM patients windup at frequencies of < 0.33 Hz and require lower stimulus intensities [40].
Abnormal windup of fibromyalgia patients
Recent investigations in FM patients have focused on windup and central sensitization because this chronic pain syndrome is associated with extensive secondary hyperalgesia and allodynia [46]. Several studies provided psychophysical evidence that input to central nociceptive pathways is abnormal in FM patients [40,47-51]. When windup pain is evoked both in FM patients and in normal controls, the perceived pain increase by experimental stimuli (mechanical, heat, cold, or electricity) is greater for FM patients compared with control subjects, as is the amount of temporal summation or windup within a series of stimuli (Figure 2). Following a series of stimuli, windup after-sensations are greater in magnitude, last longer and are more frequently painful in FM subjects. These results indicate both augmentation and prolonged decay of nociceptive input in FM patients and provide convincing evidence for a role for central sensitization in the pathogenesis of this syndrome.
Figure 2.
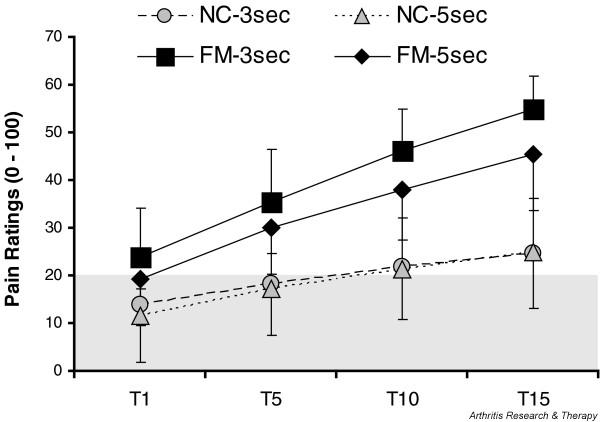
Windup pain ratings of normal control (NC) and fibromyalgia syndrome (FM) patients. All subjects received 15 mechanical stimuli (taps (T)) to the adductor pollicis muscles of the hands at interstimulatory intervals of 3 s and 5 s. FM patients showed mechanical hyperalgesia during the first tap and greater temporal summation than NCs at both interstimulatory intervals. A numerical pain scale was used (0 to 100). The shaded area represents pain threshold.
Several important points appear relevant for clinical practice. As previously mentioned, when central sensitization has occurred in chronic pain patients, including FM patients, little additional nociceptive input is required to maintain the sensitized state. Thus, seemingly innocuous daily activities might contribute to the maintenance of the chronic pain state. In addition, the decay of painful sensations is very prolonged in FM and patients do not seem to experience drastic changes in their pain levels during brief therapeutic interventions. Many frequently used analgesic medications do not improve central sensitization, and some medications, including opioids, have been shown to maintain or even worsen this CNS phenomenon. Sustained administration of opioids in rodents over one week can not only elicit hyperalgesia but also induce neurochemical CNS changes commonly seen with inflammatory pain [52]. Thus, long-term analgesic therapy may sometimes result in unintended worsening of the targeted pain processing abnormalities.
Windup measures as predictors of fibromyalgia pain intensity
The important role of central pain mechanisms for clinical pain is also supported by their usefulness as predictors of clinical pain intensity in FM patients. Thermal windup ratings correlate well with clinical pain intensity (Peason's r = 0.53), thus emphasizing the important role of this pain mechanism for FM. In addition, hierarchical regression models that include tender point count, pain related negative affect, and windup ratings have been shown to account for 50% of the variance in FM clinical pain intensity [53].
Mechanisms underlying abnormal pain sensitivity
The mechanisms underlying the central sensitization that occurs in patients with FM relies on hyperexcitability of spinal dorsal horn neurons that transmit nociceptive input to the brain. As a consequence, low intensity stimuli delivered to the skin or deep muscle tissue generate high levels of nociceptive input to the brain as well as the perception of pain. Specifically, intense or prolonged impulse input from A-δ and C afferents sufficiently depolarizes the dorsal horn neurons and results in the removal of the Mg2+ block of NMDA-gated ion channels. This is followed by the influx of extracellular Ca2+ and production of nitric oxide, which diffuses out of the dorsal horn neurons. Nitric oxide, in turn, promotes the exaggerated release of excitatory amino acids and substance P from presynaptic afferent terminals and causes the dorsal horn neurons to become hyperexcitable. Subsequently, low intensity stimuli evoked by minor physical activity may be amplified in the spinal cord resulting in painful sensations.
Role of glia in central sensitization
Accumulating evidence suggests that dorsal horn glia cells might have an important role in producing and maintaining abnormal pain sensitivity [54,55]. Synapses within the CNS are encapsulated by glia that do not normally respond to nociceptive input from local sites. Following the initiation of central sensitization, however, spinal glia cells are activated by a wide array of factors that contribute to hyperalgesia, such as immune activation within the spinal cord, substance P, excitatory amino acids, nitric oxide, and prostaglandins. Precipitating events known to induce glial activation include viral infections, including HIV, hepatitis C, and influenza [56]. Once activated, glia cells release proinflammatory cytokines, including tumor necrosis factor, IL-6 and IL-1, substance P, nitric oxide, prostaglandins, excitatory amino acids, ATP, and fractalkine [57], that, in turn, further increase the discharge of excitatory amino acids and substance P from the A-δ and C afferents that synapse in the dorsal horn and also enhance the hyper-excitability of the dorsal horn neurons [54,58]. Recent evidence also points towards a possible role for NMDA receptors in glial activation and pain [59].
Possible causes of central sensitization
As a normal response to tissue trauma, injury is followed by repair and healing. Inflammation occurs, which results in a cascade of electrophysiological and chemical events that resolve over time and the patient becomes pain free. In persistent pain, however, the local, spinal, and even supraspinal responses are considerably different from those that occur during acute pain. While defining the relationship between tissue events and pain is necessary for understanding the clinical context of these pathologies, defining the relationship between injury and specific and relevant nociceptive responses is crucial for understanding the central mechanisms of persistent pain in FM. It must be emphasized, however, that specific abnormalities in persons with FM have not been identified that might produce the prolonged impulse input that is necessary to initiate the events underlying the development of central sensitization and/or spinal glia cell activation. After central sensitization has occurred, low threshold A-β afferents, which normally do not serve to transmit a pain response, are recruited to transmit spontaneous and movement-induced pain. This central hyperexcitability is characterized by a 'windup' response of repetitive C fiber stimulation, expanding receptive field areas, and spinal neurons taking on properties of wide dynamic range neurons [60]. Ultimately, A-β fibers stimulate postsynaptic neurons to transmit pain, where these A-β fibers previously had no role in pain transmission, all leading to central sensitization. Nociceptive information is transmitted from the spinal cord to supraspinal sites, such as the thalamus and cerebral cortex, by ascending pathways.
Muscle tissue as a source of nociceptive input
A potential source of nociceptive input that might account for FM pain is muscle tissue [61]. Several types of muscle abnormalities have been reported in FM patients, including the appearance of ragged red fibers, inflammatory infiltrates, and moth-eaten fibers [62-64]. Possible mechanisms for such muscle changes might include repetitive muscle microtrauma, which could contribute to the postexertional pain and other painful symptoms experienced by these patients. In addition, prolonged muscle tension and ischemia was found in muscles of FM patients [25,65,66]. Changes in muscle pH related to ischemia [67] might provide a powerful mechanism for the sensitization of spinal and supraspinal pain pathways [68]. Investigations using 31P nuclear magnetic resonance spectroscopy have shown that FM patients display significantly lower phosphorylation potential and total oxidative capacity in the quadriceps muscle during rest and exercise [69]. FM patients also exhibit significantly lower levels of muscle phosphocreatine and ATP, as well as a lower phosphocreatine/inorganic phosphate ratio [62,63]. Furthermore, nuclear magnetic resonance testing of muscles in FM patients showed an increased prevalence of phosphodiester peaks, which have been associated with sarcolemmal membrane damage [69,70].
Focal muscle abnormalities, including trigger points, are frequently detectable in FM patients and may play an important role as pain generators. Using sensitive microdialysis techniques, concentrations of protons, bradykinin, calcitonin gene-related peptide, substance P, tumor necrosis factor-α, IL-1b, serotonin, and norepinephrine have been found to be significantly higher in trigger points than normal muscle tissue [71,72]. Recent studies have shown that advanced glycation end products may also be relevant for FM pain. These can trigger the synthesis of cytokines, particularly IL-1b and tumor necrosis factor-α, and elevated advanced glycation end product levels have been detected in interstitial connective tissue of muscles and in serum of FM patients [73]. All these biochemical mediators can sensitize muscle nociceptors and thus indirectly contribute to central sensitization and chronic pain. Because nociceptive input from muscles is very powerful in inducing and maintaining central sensitization [74], FM muscle abnormalities may strongly contribute to pain through important mechanisms of pain amplification.
Conclusion
FM is a chronic pain syndrome that is characterized by widespread pain in peripheral tissues, psychological distress, and central sensitization. Whereas the role of psychological factors in FM patients' pain has been well established, little is known about the origin of the sensory abnormalities for pain. Deep tissue impulse input is most likely relevant for the initiation and/or maintenance of abnormal central pain processing and represents an important opportunity for new treatments and prevention of this chronic pain syndrome. Three important strategies for FM therapy appear useful at this time: reduction of peripheral nociceptive input, particularly from muscles; improvement or prevention of central sensitization; and treatment of negative affect, particularly depression. The first strategy is most likely relevant for acute FM pain exacerbations and includes physical therapy, muscle relaxants, muscle injections, and anti-inflammatory analgesics. Central sensitization can be successfully ameliorated by cognitive behavioral therapy, sleep improvement, NMDA receptor antagonists, and anti-seizure medications. The pharmacological and behavioral treatment of secondary pain affect (anxiety, anger, depression) is equally important and may currently be one of the most powerful interventions for FM pain. Whether narcotics are useful for the treatment of FM pain is currently unknown because of insufficient trial experience.
Abbreviations
CNS = central nervous system; FM = fibromyalgia; IL = interleukin; NMDA = N-methyl-D-aspartate.
Competing interests
The authors declare that they have no competing interests.
Note
This review is part of a series on "Biology and therapy of fibromyalgia" edited by Leslie Crofford. Other articles in this series can be found at http://arthritis-research.com/articles/review-series.asp?=ar_fibromyalgia.
Acknowledgments
Acknowledgements
Supported by NIH grant NS-38767 and the American Fibromyalgia Syndrome Association.
References
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Orr MD, Littman B, Vipraio GA, Alboukrek D, Michalek JE, Lopez Y, MacKillip F. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37:1593–1601. doi: 10.1002/art.1780371106. [DOI] [PubMed] [Google Scholar]
- Bradley LA, Alarcon GS, Sotolongo A, Weigent DA, Alberts KR, Blalock JE, Kersh BC, Domino ML, De Waal D. Cerebrospinal fluid (CSF) levels of substance P (SP) are abnormal in patients with fibromyalgia (FM) regardless of traumatic or insidious pain onset. Arthritis Rheum. 1998;41:S256. doi: 10.1002/1529-0131(199802)41:2<256::AID-ART9>3.0.CO;2-E. [DOI] [Google Scholar]
- Vaeroy H, Helle R, Forre O, Kass E, Terenius L. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain. 1988;32:21–26. doi: 10.1016/0304-3959(88)90019-X. [DOI] [PubMed] [Google Scholar]
- Neeck G. Neuroendocrine and hormonal perturbations and relations to the serotonergic system in fibromyalgia patients. Scand J Rheumatol. 2000;29:8–12. [PubMed] [Google Scholar]
- Lautenschlager J, Bruckle W, Schnorrenberger CC, Muller W. Measuring pressure pain of tendons and muscles in healthy probands and patients with generalized tendomyopathy (fibromyalgia syndrome) Z Rheumatol. 1988;47:397–404. [PubMed] [Google Scholar]
- Quimby LG, Block SR, Gratwick GM. Fibromyalgia: generalized pain intolerance and manifold symptom reporting. J Rheumatol. 1988;15:1264–1270. [PubMed] [Google Scholar]
- Tunks E, Crook J, Norman G, Kalaher S. Tender points in fibromyalgia. Pain. 1988;34:11–19. doi: 10.1016/0304-3959(88)90176-5. [DOI] [PubMed] [Google Scholar]
- Mikkelsson M, Latikka P, Kautiainen H, Isomeri R, Isomaki H. Muscle and bone pressure pain threshold and pain tolerance in fibromyalgia patients and controls. Arch Phys Med Rehabil. 1992;73:814–818. [PubMed] [Google Scholar]
- Kosek E, Ekholm J, Hansson P. Increased pressure pain sensibility in fibromyalgia patients is located deep to the skin but not restricted to muscle tissue. Pain. 1995;63:335–339. doi: 10.1016/0304-3959(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Loeser JD. What is chronic pain? Theor Med. 1991;12:213–225. doi: 10.1007/BF00489607. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological Mechanisms of Pain and Analgesia: Progress in Pain Research and Management. 15. Seattle: IASP Press; 1999. [Google Scholar]
- Rainville P, Bao QV, Chretien P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain. 2005;118:306–318. doi: 10.1016/j.pain.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Merskey H, Bogduk N. Classification of Chronic Pain: Description of Chronic Pain Syndromes and Definition of Pain Terms. 2. Seattle: IASP Press; 1994. [Google Scholar]
- Crofford LJ, Young EA, Engleberg NC, Korszun A, Brucksch CB, McClure LA, Brown MB, Demitrack MA. Basal circadian and pul-satile ACTH and cortisol secretion in patients with fibromyal-gia and/or chronic fatigue syndrome. Brain Behav Immun. 2004;18:314–325. doi: 10.1016/j.bbi.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Crofford LJ. The hypothalamic-pituitary-adrenal axis in fibromyalgia: Where are we in 2001? J Musculoskelet Pain. 2002;10:215–220. doi: 10.1300/J094v10n01_18. [DOI] [Google Scholar]
- Bradley LA, Sotolongo A, Alberts KR, Alarcon GS, Mountz JM, Liu HG, Kersh BC, Domino ML, DeWaal D, Weigent DA, et al. Abnormal regional cerebral blood flow in the caudate nucleus among fibromyalgia patients and non-patients is associated with insidious symptom onset. J Musculoskelet Pain. 1999;7:285–292. doi: 10.1300/J094v07n01_29. [DOI] [Google Scholar]
- Larson AA, Giovengo SL, Russell IJ, Michalek JE. Changes in the concentrations of amino acids in the cerebrospinal fluid that correlate with pain in patients with fibromyalgia: implications for nitric oxide pathways. Pain. 2000;87:201–211. doi: 10.1016/S0304-3959(00)00284-0. [DOI] [PubMed] [Google Scholar]
- Giovengo SL, Russell IJ, Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999;26:1564–1569. [PubMed] [Google Scholar]
- Simms RW. Fibromyalgia is not a muscle disorder. Am J Med Sci. 1998;315:346–350. doi: 10.1097/00000441-199806000-00002. [DOI] [PubMed] [Google Scholar]
- Sprott H, Bradley LA, Oh SJ, Wintersberger W, Alarcon GS, Mussell HG, Tseng A, Gay RE, Gay S. Immunohistochemical and molecular studies of serotonin, substance P, galanin, pituitary adenylyl cyclase-activating polypeptide, and secre-toneurin in fibromyalgic muscle tissue. Arthritis Rheum. 1998;41:1689–1694. doi: 10.1002/1529-0131(199809)41:9<1689::AID-ART21>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Sprott H, Salemi S, Gay RE, Bradley LA, Alarcon GS, Oh SJ, Michel BA, Gay S. Increased DNA fragmentation and ultra-structural changes in fibromyalgic muscle fibres. Ann Rheum Dis. 2004;63:245–251. doi: 10.1136/ard.2002.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemi S, Rethage J, Wollina U, Michel BA, Gay RE, Gay S, Sprott H. Detection of interleukin 1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyal-gia. J Rheumatol. 2003;30:146–150. [PubMed] [Google Scholar]
- Graven-Nielsen T, Arendt-Nielsen L. Is there a relation between intramuscular hypoperfusion and chronic muscle pain? J Pain. 2002;3:261–263. doi: 10.1054/jpai.2002.125927. [DOI] [PubMed] [Google Scholar]
- Elvin A, Siosteen AK, Nilsson A, Kosek E. Decreased muscle blood flow in fibromyalgia patients during standardised muscle exercise: A contrast media enhanced colour doppler study. Eur J Pain. 2006;10:137–144. doi: 10.1016/j.ejpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Robinson ME, Price DD. Overall fibromyal-gia pain is predicted by ratings of local pain and pain related negative affect: possible role of peripheral tissues. Rheumatology. 2006 doi: 10.1093/rheumatology/kel121. [DOI] [PubMed] [Google Scholar]
- Wigers SH. Fibromyalgia outcome: the predictive values of symptom duration, physical activity, disability pension, and critical life events–a 4.5 year prospective study. J Psychosom Res. 1996;41:235–243. doi: 10.1016/0022-3999(96)00070-0. [DOI] [PubMed] [Google Scholar]
- Macfarlane GJ, Thomas E, Papageorgiou AC, Schollum J, Croft PR, Silman AJ. The natural history of chronic pain in the community: a better prognosis than in the clinic? J Rheumatol. 1996;23:1617–1620. [PubMed] [Google Scholar]
- Winfield JB. FMS as functional somatic syndrome. Arthritis Rheum. 2001;44:751–753. doi: 10.1002/1529-0131(200104)44:4<751::AID-ANR130>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Hunt IM, Silman AJ, Benjamin S, McBeth J, Macfarlane GJ. The prevalence and associated features of chronic widespread pain in the community using the 'Manchester' definition of chronic widespread pain. Rheumatology. 1999;38:275–279. doi: 10.1093/rheumatology/38.3.275. [DOI] [PubMed] [Google Scholar]
- Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol. 1993;20:710–713. [PubMed] [Google Scholar]
- Benjamin S, Morris S, McBeth J, Macfarlane GJ, Silman AJ. The association between chronic widespread pain and mental disorder – A population-based study. Arthritis Rheum. 2000;43:561–567. doi: 10.1002/1529-0131(200003)43:3<561::AID-ANR12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Forseth KO, Forre O, Gran JT. A 5.5 year prospective study of self-reported musculoskeletal pain and of fibromyalgia in a female population: Significance and natural history. Clin Rheumatol. 1999;18:114–121. doi: 10.1007/s100670050067. [DOI] [PubMed] [Google Scholar]
- McBeth J, Macfarlane GJ, Benjamin S, Silman AJ. Features of somatization predict the onset of chronic widespread pain: results of a large population-based study. Arthritis Rheum. 2001;44:940–946. doi: 10.1002/1529-0131(200104)44:4<940::AID-ANR151>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Arnold LM, Hudson JI, Hess EV, Ware AE, Fritz DA, Auchenbach MB, Starck LO, Keck PE. Family study of fibromyalgia. Arthritis Rheum. 2004;50:944–952. doi: 10.1002/art.20042. [DOI] [PubMed] [Google Scholar]
- Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med. 2004;66:837–844. doi: 10.1097/01.psy.0000146329.63158.40. [DOI] [PubMed] [Google Scholar]
- Raphael KG, Janal MN, Nayak S, Schwartz JE, Gallagher RM. Familial aggregation of depression in fibromyalgia: a community-based test of alternate hypotheses. Pain. 2004;110:449–460. doi: 10.1016/j.pain.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–108. doi: 10.1016/0304-3959(96)03114-4. [DOI] [PubMed] [Google Scholar]
- Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/S0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Price DD, Mao J, Frenk H, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59:165–174. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Robinson ME, Price DD. Effects of the NDMA receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal controls. J Pain. 2005;6:323–332. doi: 10.1016/j.jpain.2005.01.357. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated ther-mode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci USA. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Domingo M. Evidence for abnormal pain processing in fibromyalgia syndrome. Pain. 2001;2:208–215. doi: 10.1046/j.1526-4637.2001.01030.x. [DOI] [PubMed] [Google Scholar]
- Staud R, Domingo M. New Insights into the pathogenesis of fibromyalgia syndrome. Med Aspects Hum Sex. 2001;1:51–57. [Google Scholar]
- Vierck CJ, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002;4:299–305. doi: 10.1007/s11926-002-0038-5. [DOI] [PubMed] [Google Scholar]
- Price DD, Staud R, Robinson ME, Mauderli AP, Cannon RL, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/S0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- King T, Gardell LR, Wang RZ, Vardanyan A, Ossipov MH, Malan TP, Vanderah TW, Hunt SP, Hruby VJ, Lai J, et al. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–288. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R, Robinson ME, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–222. doi: 10.1016/S0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/S0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Holguin A, O'Connor KA, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan ED, Hansen MK, Spataro L, Maksimova E, Brav-mann C, et al. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-1 (nNOS) Pain. 2004;110:517–530. doi: 10.1016/j.pain.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Wieseler-Frank J, Maier SF, Watkins LR. Central proinflamma-tory cytokines and pain enhancement. Neurosignals. 2005;14:166–174. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. When good pain turns bad. Curr Directions Psych Sci. 2003;12:232–236. doi: 10.1046/j.0963-7214.2003.01268.x. [DOI] [Google Scholar]
- Salter MW. Cellular signalling pathways of spinal pain neuro-plasticity as targets for analgesic development. Curr Topics Med Chem. 2005;5:557–567. doi: 10.2174/1568026054367638. [DOI] [PubMed] [Google Scholar]
- Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- Henriksson KG. Is fibromyalgia a distinct clinical entity? Pain mechanisms in fibromyalgia syndrome. A myologist's view. Best Pract Res Clin Rheumatol. 1999;13:455–461. doi: 10.1053/berh.1999.0035. [DOI] [PubMed] [Google Scholar]
- Bengtsson A, Henriksson KG, Larsson J. Muscle biopsy in primary fibromyalgia. Light-microscopical and histochemical findings. Scand J Rheumatol. 1986;15:1–6. doi: 10.3109/03009748609092661. [DOI] [PubMed] [Google Scholar]
- Bengtsson A, Henriksson KG, Jorfeldt L, Kagedal B, Lennmarken C, Lindstrom F. Primary fibromyalgia. A clinical and laboratory study of 55 patients. Scand J Rheumatol. 1986;15:340–347. doi: 10.3109/03009748609092601. [DOI] [PubMed] [Google Scholar]
- Pongratz DE, Spath M. Morphologic aspects of fibromyalgia. Z Rheumatol. 1998;57:47–51. doi: 10.1007/s003930050234. [DOI] [PubMed] [Google Scholar]
- Bennett RM, Clark SR, Goldberg L, Nelson D, Bonafede RP, Porter J, Specht D. Aerobic fitness in patients with fibrositis. A controlled study of respiratory gas exchange and 133xenon clearance from exercising muscle. Arthritis Rheum. 1989;32:454–460. doi: 10.1002/anr.1780320415. [DOI] [PubMed] [Google Scholar]
- Lund N, Bengtsson A, Thorborg P. Muscle tissue oxygen pressure in primary fibromyalgia. J Rheumatol. 1986;15:165–173. doi: 10.3109/03009748609102084. [DOI] [PubMed] [Google Scholar]
- de Kerviler E, Leroy-Willig A, Jehenson P, Duboc D, Eymard B, Syrota A. Exercise-induced muscle modifications: study of healthy subjects and patients with metabolic myopathies with MR imaging and P-31 spectroscopy. Radiology. 1991;181:259–264. doi: 10.1148/radiology.181.1.1887044. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperal-gesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::AID-MUS4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Park JH, Phothimat P, Oates CT, Hernanz-Schulman M, Olsen NJ. Use of P-31 magnetic resonance spectroscopy to detect metabolic abnormalities in muscles of patients with fibromyalgia. Arthritis Rheum. 1998;41:406–413. doi: 10.1002/1529-0131(199803)41:3<406::AID-ART5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Jubrias SA, Bennett RM, Klug GA. Increased incidence of a resonance in the phosphodiester region of 31P nuclear magnetic resonance spectra in the skeletal muscle of fibromyalgia patients. Arthritis Rheum. 1994;37:801–807. doi: 10.1002/art.1780370604. [DOI] [PubMed] [Google Scholar]
- Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microana-lytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- Rosendal L, Kristiansen J, Gerdle B, Sogaard K, Peolsson M, Kjaer M, Sorensen J, Larsson B. Increased levels of interstitial potassium but normal levels of muscle IL-6 and LDH in patients with trapezius myalgia. Pain. 2005;119:201–209. doi: 10.1016/j.pain.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Ruster M, Franke S, Spath M, Pongratz DE, Stein G, Hein GE. Detection of elevated N-epsilon-carboxymethyllysine levels in muscular tissue and in serum of patients with fibromyalgia. Scand J Rheumatol. 2005;34:460–463. doi: 10.1080/03009740510026715. [DOI] [PubMed] [Google Scholar]
- Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol Lond. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]


