Abstract
Normal aging is associated with progressive functional losses in perception, cognition, and memory. Although the root causes of age-related cognitive decline are incompletely understood, psychophysical and neuropsychological evidence suggests that a significant contribution stems from poorer signal-to-noise conditions and down-regulated neuromodulatory system function in older brains. Because the brain retains a lifelong capacity for plasticity and adaptive reorganization, dimensions of negative reorganization should be at least partially reversible through the use of an appropriately designed training program. We report here results from such a training program targeting age-related cognitive decline. Data from a randomized, controlled trial using standardized measures of neuropsychological function as outcomes are presented. Significant improvements in assessments directly related to the training tasks and significant generalization of improvements to nonrelated standardized neuropsychological measures of memory (effect size of 0.25) were documented in the group using the training program. Memory enhancement appeared to be sustained after a 3-month no-contact follow-up period. Matched active control and no-contact control groups showed no significant change in memory function after training or at the 3-month follow-up. This study demonstrates that intensive, plasticity-engaging training can result in an enhancement of cognitive function in normal mature adults.
Keywords: age-related cognitive decline, cognitive aging, cognitive rehabilitation, computer-based training
Cognitive decline is a nearly universal aspect of the aging process. Memory problems, for example, may begin as early as age 30 and, on the average, worsen slowly but steadily thereafter (1). Most older adults experience nonpathological losses in cognitive function, frequently called “age-related cognitive decline” (ARCD) (2–4); however, there is considerable interindividual variability in the age of onset and the course of memory or other cognitive loss (5, 6). Although ARCD does not profoundly affect real-world function, even modest reductions in cognitive function negatively impact the quality of life, independence, frequency and quality of social interaction, and engagement in cognitively stimulating activities in older adults. The potential for an eventual progression from ARCD to Alzheimer’s disease or other forms of senile dementia is of substantial concern to virtually every older individual.
Brain plasticity refers to the brain’s lifelong capacity for physical and functional change; it is this capacity that enables experience to induce learning throughout life (7, 8). Research in this field has demonstrated that the adult brain continuously adapts to disproportionately represent relevant sensory stimuli and behavioral outputs with well coordinated populations of neurons. This adaptation is achieved by engaging competitive processes in brain networks that refine the selective representations of sensory inputs or motor actions, typically resulting in increased strengths of cortical resources devoted to, and enhanced representational fidelity of, the learned stimulus or behavior (9). For example, in monkeys trained to detect a specific pattern of stimulation to the fingers the somatosensory cortex reorganizes to represent that specific input pattern with large, well organized, and spatiotemporally coherent responses (10–12), whereas violin players have been shown to have stronger and more distinct representations of the fingers in the right hemisphere, corresponding to the individuated finger movements required by their left hands (13).
Because plasticity processes are inherently competitive, there always will be a competitive “winner” and “loser”; thus, plastic changes with negative consequences are just as common as those with positive outcomes. One example of plasticity with negative consequences is seen in monkeys trained under conditions in which heavy synchronous input is delivered across fingers (12, 14, 15) or the entire hand (16). In response to this type of sensory stimulation, the somatosensory cortex reorganizes to adaptively represent the undifferentiated spatiotemporal characteristics of the trained input. This results in an undifferentiated map with abnormally large, overlapping receptive fields and degraded spatial and temporal response characteristics.
We have argued (17) that ARCD is caused by a combination of physical changes in the aging brain and brain plasticity with negative consequences. This brain plasticity is likely to result in a self-reinforcing downward spiral of degraded brain function involving disuse, noisy processing, weakened neuromodulatory system function, and negative learning. This downward spiral hypothetically results from progressively reduced schedules of active learning and attentionally demanding behaviors, from the slow deterioration of the physical brain that partially stems from these age-dependent changes in brain engagement, and from progressively deteriorating sensory system machinery. The aggregate effect of these interrelated processes is a degradation of the fidelity and power of brain representations of sensory, cognitive, and motor events, manifested by a gradual, slowly growing loss of function that manifests as ARCD. One striking manifestation of this decline in representational fidelity is progressive changes in processing speed (18), which may be driven by the degraded signal-to-noise level conditions and longer time constants observed in older-aged brains for most dimensions of perceptual, cognitive, and motor responses (17).
These slowly embedded changes should be at least partially reversible. Brain plasticity research has shown that the adult brain is adaptive at any age, and it has a lifelong capacity to refine the spatial or temporal features of sensory inputs or of movements. This has been particularly well documented in rat models of aging, in which enriched environments and training have been shown to re-refine cortical maps of sensation and movement and progressively re-establish paw and limb movements (19, 20).
Previous work has also demonstrated that a brain plasticity-based training program focused on improving the brain’s ability to represent the spatiotemporal details of aural speech inputs is effective for enhancing language and reading abilities in school-age children. Improvements in language-related cognitive abilities, verbal memory, and processing efficiency (21–25) have been repeatedly recorded in a wide variety of child subgroups within the >650,000 children and young adults that have now undergone this brain plasticity-based training. Importantly, the same, positive receptive, cognitive/memory, and language-usage impacts of this training have repeatedly been shown to be independent of the subjects’ ages (26, 27). Functional MRI studies have revealed that the abnormal response patterns recorded while these trained subjects performed key language and reading behaviors could be consistently restored to more normal forms after training, in adults as well as children (28). Magnetoencephalography studies have also demonstrated that the neuronal representations of aural speech inputs are substantially more salient and reliable after training (29).
After this successful application of a brain plasticity-based intervention, we hypothesized that a training program designed on broadly similar principles and constructed specifically for adults could be expected to partially reverse normal age-related losses in memory. Accordingly, we applied similar scientific principles to the design of a training program for use by adults. This training program intensively exercises aural language reception accuracy and engages down-regulated neuromodulatory structures in the brain by engaging participants in sensory and cognitively demanding exercises where, to make progress in tasks, the participant must perform increasingly more difficult stimulus recognition, discrimination, sequencing, and memory tasks under conditions of close attentional control, high reward, and novelty.
Here we report results from a randomized, controlled study designed to assess the feasibility and benefits of this approach in adults aged 60 and over. Goals were to evaluate compliance and progressions through the training exercises in the target population, the appropriateness of a carefully matched active control (AC) activity, and the magnitudes of the effects resulting from intervention, as compared with AC and no-contact control (NCC) groups.
Results
Study Participants.
A total of 182 participants consented and were randomized into the study, with 62 in the experimental training (ET) group, 61 in the AC group, and 59 in the NCC group. During the training period, there were nine withdrawals from the ET group, eight from the AC group, and three from the NCC group; an additional three participants withdrew from the ET group during the 3-month follow-up period, two withdrew from the AC group, and two withdrew from the NCC group. The age range of the fully evaluated (at posttraining) study participants was 60 to 87 (mean of 70.9 years), the education range was 10 to 22 years (mean of 16.3); 50% were female. The groups had equivalent education levels (P = 0.43, one-way ANOVA) before the training period began; however, despite randomization there was a slight trend toward a difference in ages across the groups (P = 0.054, one-way ANOVA) with a small difference in mean age between the ET (mean 69.3) and the AC groups (mean 72.8) (the NCC was not statistically different from either group, mean 70.7).
Usability and Acceptability.
All ET participants were able to use the program independently and successfully after initial instruction. Dropouts from the ET group were generally caused by the time required (1 h per day) to comply with the training protocol; no participants dropped out because of difficulty using the training program or the computer.
Changes in Directly Trained Functions.
In any training study it is important to demonstrate changes in the directly trained functions before going on to examine questions of generalization. Changes in the ET group on the trained tasks were directly examined by reconstructing psychophysical functions for each task based on the stimuli presented over the first 25% of training (beginning phase) and the last 25% of training (ending phase). Each phase typically consisted of 10 training sessions for most participants completing 40 total sessions.
Psychophysical functions were plotted by counting the correct responses as a percentage of total responses for each difficulty level during the training phase, then fit with standard sigmoid functions. Eighty percent correct thresholds were estimated for each participant in each phase. Summary data are shown in Table 1. Most participants showed improvement in all tasks. Although it is difficult to compare improvement across tasks because of the different underlying nature of the task progressions in each task, substantial improvements were seen in all tasks, including speed of processing task (93% of participants showing improvement, mean improvement of 43%) and forward recognition memory span task (91% of participants showing improvement, mean improvement of 18%).
Table 1.
Training improves thresholds as measured in training tasks
| Improvement | Exercise |
||||
|---|---|---|---|---|---|
| Speed of processing | Spatial syllable match memory | Forward word recognition span | Working memory | Narrative memory | |
| Participants showing improvement | 93% | 77% | 91% | 80% | 91% |
| Average improvement | 41% | 10% | 18% | 13% | 18% |
Data for five of six training exercises are shown; data are not available for exercise 2 (syllable identification). The ET group was able to learn to perform the tasks and showed task-specific improvements after training.
A limitation of this approach is that the beginning phase and the ending phase each averaged over ≈10 training sessions to collect adequate data to fit psychophysical functions, yet training-driven changes may be occurring over that time period. To further this analysis, pretraining and posttraining thresholds were measured on the two exercises described above (speed of processing and forward word recognition span) by using assessments that were specifically designed to measure thresholds (as opposed to improve performance). Data from these before vs. after training assessments are shown in Table 2. Both measures showed significant improvement (P < 0.001 for speed of processing, P = 0.022 for forward word recognition span, two-tailed t test with repeated measures), with speed of processing performance improving by ≈90% and forward recognition memory span performance improving by ≈10%.
Table 2.
Training improves thresholds in directly trained measures
| Trained task | Pretraining | Posttraining | Difference P value |
|---|---|---|---|
| Speed of processing, ms | 194 ± 32 | 23 ± 8 | 176 ± 32P < 0.001 |
| Forward word recognition span, words | 3.0 ± 0.1 | 3.3 ± 0.1 | 0.3 ± 0.1P = 0.022 |
Mean and SEM shown for the ET group in that pretraining and posttraining period. Mean and SEM for change scores and P value for posttraining to pretraining comparison with two-tailed t test are shown. Speed of processing threshold in the ET group pretraining and posttraining is shown (n = 49). Word span threshold in a forward word recognition span task in the ET group pretraining and posttraining is shown (n = 50). ET participants showed improvements on directly trained tasks that are relevant to important areas of ARCD, including speed of processing and memory for word span.
These analyses (Tables 1 and 2) demonstrated that mature adults are capable of learning these exercises and that their performances on these tasks improve.
Generalization of Changes to Standardized Neuropsychological Measures of Memory Function.
Generalization of training-induced improvements to broader measures of memory performance is imperative if a training program is to have value beyond the specifics of its own training exercises (30). For this initial ET version, improvement on standardized tests of neuropsychological function would represent a generalization of improvement because the training exercises bear no resemblance to these standardized neuropsychological assessments.
The changes in global auditory memory score found in each group are shown in Table 3. Participants in the ET training group showed a statistically significant gain of 2.3 summed standard score points (P = 0.019, two-tailed t test with repeated measures). Given that the standard deviation of this measure in the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) normative population is 9.0, this finding represents an effect size (mean change in the ET group divided by SD in the normative population) of 0.25. In contrast, neither the AC nor the NCC group showed significant improvement (mean change of 1.0, P = 0.29; mean change of 1.1, P = 0.22, respectively).
Table 3.
Training improves global auditory memory score
| Group | Pretraining | Posttraining | Difference P value |
|---|---|---|---|
| ET | 48.9 ± 1.3 | 51.2 ± 1.2 | 2.3 ± 0.9P = 0.019 |
| AC | 50.2 ± 1.2 | 51.2 ± 1.2 | 1.0 ± 0.9P = 0.29 |
| NCC | 51.2 ± 1.0 | 52.3 ± 1.1 | 1.1 ± 0.9P = 0.22 |
Pretraining and posttraining data are shown for the ET (n = 53), AC (n = 53), and NCC (n = 56) groups. Normative population mean is 50 on this scale, standard deviation is 9.0. Mean and SEM for change scores are shown. P value for posttraining to pretraining comparison with two-tailed t test is shown. The change in the ET group is significant; changes within the two control groups are not significant. Participants in the ET group show improvements in nontrained, standardized assessments of memory.
Ceiling Effects in RBANS in This Population.
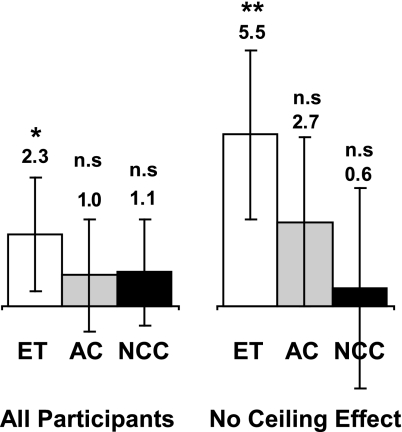
Inspection of the data suggested that many of the participants in this population showed high raw scores on their pretraining neuropsychological testing, which if close to the ceiling allowed by the test, may have impeded a measurement of a positive effect. Quantitative analysis of the pretraining data showed that 95 of the 161 participants (59%) had a raw score of 85% or higher than the maximum score possible on at least one of the five auditory measures in the RBANS at the pretesting measurement (excluding list recognition as virtually all neurologically normal older adults show nearly ceiling performance on this task). To get an estimate of the effect size uncontaminated by ceiling effects, the data analysis was rerun on the subset of participants that did not exhibit any pretraining ceiling effect (as defined as having no pretraining auditory RBANS subtest score >85% of the possible raw score). This analysis (conducted with 22 ET participants, 23 AC participants, and 22 NCC participants) showed a much larger effect size (Fig. 1) in the ET group (5.5 points, P < 0.001), a slightly larger effect size in the AC group (2.7 points, P = 0.069), and a smaller effect in the NCC group (0.6 points, P = 0.72), which represents an effect size of 0.61. These results suggest that the ET effect size was limited by ceiling effects in the measurement used to evaluate cognitive gains in this healthy population.
Fig. 1.
Impact of pretraining assessment ceiling effects on change in global auditory memory score. Change in global auditory memory score in all participants (Left) (ET, n = 53; AC, n = 53; and NCC, n = 56) and participants that had no pretraining RBANS subtest raw score >85% of the maximum possible (Right) (ET, n = 22; AC, n = 23; and NCC, n = 22) is shown. Error bars are 95% confidence limits. Significant effects are seen only in the ET groups. Including only participants without ceiling effects shows a larger effect in the ET group, suggesting that effect size in the entire group was limited by ceiling effects in the measure. ∗, P < 0.025; ∗∗, P < 0.001; n.s., not significant.
Longevity of Changes After the Training Period.
Longevity of training-induced improvements after the discontinuation of training is generally considered a key measure of the value of a training program (30). To address this question, the 3-month follow-up data were compared with the pretraining data. It is inappropriate to use list learning and story memory data in such analysis, because the RBANS has only two test versions, and participants could be expected to retain familiarity with the contents of earlier-heard test materials. However, the digit span forward data, which is intrinsically less learnable than the lists or stories, remains an appropriate measure of longitudinal retention. The ET group showed an enhancement of digit span score that was maintained at the 3-month follow-up point (Table 4, P = 0.044 immediately after training; P = 0.034 3 months later, paired t test); both control groups showed no significant change 3 months after training (AC, P = 0.20; NCC, P = 0.36).
Table 4.
Training improvements on digit span persist after 3-month no-contact follow-up period
| Group | Pretraining | Posttraining | 3-Month follow-up | Pretraining to 3-month follow-up difference | P value (paired t test, two-tailed) |
|---|---|---|---|---|---|
| ET | 11.1 ± 0.4 | 11.8 ± 0.3 | 11.8 ± 0.4 | 0.7 ± 0.3 | 0.034 |
| AC | 12.0 ± 0.3 | 11.7 ± 0.3 | 11.6 ± 0.3 | −0.4 ± 0.3 | 0.20 |
| NCC | 11.6 ± 0.3 | 11.6 ± 0.3 | 11.8 ± 0.4 | 0.2 ± 0.3 | 0.36 |
Pretraining, posttraining, and 3-month no-contact follow-up data are shown for ET (n = 50), AC (n = 51), and NCC (n = 54) groups. Mean and SEM for change scores and P values for posttraining to pretraining comparison with two-tailed t test are shown. The change in the ET group from pretraining to 3-month-follow-up is significant; changes within the two control groups are not significant. Improvements seen in digit span endure after the offset of training for at least 3 months.
Discussion
Goals of the Study.
The goal of this study was to evaluate the effect size of the ET program intervention and determine appropriate control activities in a randomized controlled double-blind study design. These data are relevant for creating a more advanced version of the ET program and designing and powering a larger trial to more fully evaluate the effects of the ET program.
Results of ET.
Use of the ET program for 8–10 weeks resulted in a significant increase in task-specific performance, indicating that participants were able to learn the exercises and intensive practice on these exercises yielded improvements on them. These improvements showed evidence for meeting the main criteria for the value of behavioral training programs: generalization (to nontrained assessments) and maintenance (of the gains after the completion of training). Generalization was demonstrated in the improvements seen in nontrained, standardized neuropsychological measures of memory functions. The effect size (mean change in the ET group divided by SD in the normative population) of the improvement on measures was 0.25 for the entire ET population and 0.61 for the ET population that showed no pretraining ceiling effects in the outcome measure. Maintenance was demonstrated in the digit-span forward assessment, showing that this dimension of improvement was maintained over a 3-month period after the completion of training during which no further training activities occurred.
Results of the Control Groups.
Individuals in the AC and NCC conditions showed no statistically significant changes in global auditory memory scores over the training period. Absolute differences in global auditory memory scores were small and nearly identical, suggesting that both groups showed a limited test–retest learning effect.
The lack of difference between the two control groups suggests that there is no meaningful placebo effect from study inclusion in this population with these outcome measures. AC participants were blinded as to their condition and received identical interaction with and coaching from research assistants. This result suggests that future studies may not need to include both types of control groups.
Comparison to Other Training Approaches.
To date, behavioral strategies have focused almost exclusively on compensatory strategies (e.g., mnemonics) to enhance memory function in mature adults (31–34), individuals with mild cognitive impairment (35), and those with Alzheimer’s disease (36–38). These studies demonstrated that significant short-term improvements in certain cognitive functions are achievable in both healthy and impaired populations
Although these compensatory strategies demonstrate the promise of training-based approaches, we expect that they will be incomplete because they do not address the fact that age-related decline in sensation, memory, cognition, and guided motor control has more fundamental roots in degraded brain processing. A further limitation of compensatory strategies is that people do not seem to transfer the use of these strategies into their daily lives (31) or retain the limited benefits of training (30). In contrast, a brain plasticity-based training program targeting degraded sensory processing and the down-regulation of neuromodulatory control nuclei may produce stronger, more durable, and more generalized improvements in cognitive functions. It is most likely, in our opinion, that these approaches will be complementary and may even show synergistic effects.
Conclusions and Future Directions.
These data support the hypothesis that brain plasticity-based training has improved the flow of information through auditory and language systems in the brain (as shown by the directly trained task data), such improvement generalizes to nontrained tasks (as shown by the neuropsychological assessment data), and the improvement may endure after the completion of training (as shown by digit span data).
In sum, the findings from this study demonstrate that a brain plasticity-based training program can significantly improve cognitive function, and memory in particular, in mature adults with normal ARCD.
Methods
Participant Recruitment.
Participants were recruited by direct mail and word-of-mouth referrals from the San Francisco Bay area and provided informed consent under the authority of an institutional review board before study participation. Participants typically lived independently in their own homes or apartments (87% of participants) with the remainder living in residential communities.
Inclusion and Exclusion Criteria.
Participants were required to be 60 years of age or over, to have a Mini-Mental Status Examination (39) score of 24 or higher, be a native English speaker, be available and willing to commit to the time requirements of the study, and be able to see, hear, and use their hands well enough (in the opinion of the clinician obtaining informed consent) to successfully participate in the ET or AC training groups. Patients who were active in another memory or cognitive-related clinical trial at the time of screening were excluded. Patients were also excluded if they had had head trauma with loss of consciousness or a stroke that had occurred within the previous year, any stroke that left them with expressive or receptive language problems, or any central nervous system disorder that could contribute to cognitive impairment. In addition, participants planning on beginning or changing dosing of cholinesterase inhibitors were excluded. There were no other exclusion criteria for concurrent medication use.
Group Assignment.
Participants were randomly assigned to one of three groups: (i) experimental computer-based training (ET), (ii) active computer-based control (AC), and (iii) NCC. Using balanced scripts, participants in both computer use groups (ET, AC) were informed that they would be receiving investigational cognitive training over 8–10 weeks.
Participants in the ET group worked on the training exercises for 60 min per day, 5 days per week, for 8–10 weeks using a computer in their own home that was provided to them for the duration of the study. Individuals were allowed to take breaks during the training as necessary and could skip training days if necessary, although frequent breaks were discouraged.
A true placebo control is impossible for a behavioral training program because a single active ingredient cannot generally be removed from the experimental condition. The AC was designed to control for the positive effects that could be attributed to study inclusion, computer use, and the duration and intensity of training. The AC group watched and listened to DVD-based educational lectures and other programs by using the DVD player capability of their computers. The AC group engaged in this control activity on exactly the same schedule as did the ET group (60 min per day, 5 days per week, 8–10 weeks).
Research assistants visited participants in both computer use groups to set up computers in their homes and explain how to use the training activity. Participants were visited once per week thereafter to ensure that they were using the activity correctly. Telephone support was provided to participants throughout their involvement.
Participants received a brief introduction to the software (ET or AC) and instructions about how to use the computer mouse. With a carefully balanced script, the trainer explained the rationale behind the training program (ET or AC), recorded field notes describing all usability problems encountered by participants, and regularly encouraged all participants to do their best. Both groups were kept blind to the hypothesis that ET would prove to be superior to AC.
ET group participants were trained until they met one of three exit criteria: completion of all exercise content, completion of 75% of the speed-of-processing exercise content (see below) by training session 40, or completion of 50 training sessions. To ensure that the average time in training was closely matched across groups, participants started the study in triads of ET/AC/NCC participants. Triads began in the same week; all exited in the same week as governed by the experimental program participant’s exit.
There were no study activities for the NCC group beyond consent and pre, post, and follow-up neuropsychological assessments.
ET Program Design.
The ET program is based on the hypothesis that a substantial contributor to ARCD is negative plasticity, learning driven changes in brain function in response to decreased brain use conditions and increased noise conditions that lead to reduced accuracy and speed of processing in brain representations of sensation, cognition, memory, and motor control and decreased function of neuromodulatory systems that control learning and memory (17). In this initial form, the program consisted of six exercises designed in aggregate to reverse these dysfunctions by improving representational fidelity in auditory/language systems and very intensively stimulating neuromodulatory structures throughout hour-long training sessions. The exercises adaptively progressed in difficulty, beginning with relatively easy task forms and progressively advancing in the listening accuracy challenges they presented as a user’s abilities improved.
The first of the six exercises required users to reconstruct the identity (upward or downward) and sequence of frequency-modulated sweeps. The task progressed by changing the duration and interstimulus interval between the sweeps. The second exercise required users to identify a synthetically generated syllable (e.g.,/ba/) from a confusable pair (e.g.,/ba/vs./da/). The task progressed by changing the duration and intensity of the sweep component of the initial consonant. The third exercise required users to match short spoken confusable consonant–vowel–consonant words (e.g., bad, dad) from a spatial grid. The task progressed by changing the number of potential matches and processing the spoken speech in a manner similar to Nagarajan et al. (40) to stretch and emphasize rapid transitions. The fourth exercise required users to reconstruct a sequence of short spoken words (identical to the third exercise stimuli). The task progressed by changing the number of words in the sequence and the level of speech processing. The fifth task required users to reconstruct a spoken series of instructions by using the computer mouse to click and drag icons on the computer screen. The task progressed by changing the number and complexity of the instructions and the level of speech processing. The sixth task required users to answer questions regarding short narratives. The task progressed by changing the length of the narratives and the level of speech processing.
Stimuli across the exercises were chosen such that they spanned the acoustic and organizational structure of speech, from very simple acoustic stimuli and tasks (e.g., time order judgments of rapidly successive frequency modulated sweeps) to the complex manipulations of continuous speech (e.g., narrative memory). In each case the progression of the exercise was governed by the user’s individual performance during a training session. As a user performed trials correctly, the difficulty level was increased, and as a user performed trials incorrectly, the difficulty level was decreased.
This training approach is described as brain plasticity-based training because the exercises are designed to induce brain plasticity by engaging mechanisms that have been directly shown in other work to lead to and promote brain plasticity (e.g., refs. 10 and 41–43).
Characterization Measures.
At study entry, basic demographic data were collected, including sex, age, years of education, and native language. In addition, the Mini-Mental Status Examination, Wechsler Test of Adult Reading (44), and Geriatric Depression Scale (15-item scale) were performed.
Outcome Measures.
The primary outcome measure was based on a standardized battery of neuropsychological assessments including the RBANS (45). The RBANS consists of a group of 12 neuropsychological assessments that included six tests of auditory cognition (list learning, story memory, digit span forward, delayed free list recall, delayed list recognition, and delayed free story recall). Because the training program focused on improving the operations of the aural speech system, the primary outcome measure used was a global auditory memory score based on the six tests of auditory cognition in the RBANS. The global auditory memory score was calculated by using the normative RBANS population data to construct age-normed (by decade) look-up tables allowing the conversion of raw score data on each test (which generally showed a strong skew) to scaled score data (optimally normally distributed with a population mean of 10 and a SD of 3). Delayed list recall and delayed list recognition were summed before scaling to allow the inclusion of the significantly skewed and otherwise unscalable delayed list recognition data. These look-up tables were then used to calculate scaled scores for each participant in this study for pretraining and posttraining assessments. The five scaled scores were then summed for each participant at each time point to yield a global auditory memory score.
In addition, participants in the ET group performed psychophysical assessments modeled closely on two exercises (speed of processing and forward aural speech recognition span). These assessments were performed before training began and after training ended. The speed of processing assessment required participants to correctly identify and order two frequency modulated sweeps (duration of 80 ms, sweep rate of 16 octaves per s, base frequency of 1 kHz). Interstimulus interval threshold was estimated by using an adaptive three-up one-down rule. The forward recognition memory word span exercise required participants to reconstruct an aurally presented sequence of one-syllable words. Word span threshold was also estimated by using an adaptive three-up one-down rule. Use of these assessments in the ET group represents a difference in the assessment experience between the groups; however, it is unlikely to have unblended participants because these assessments were performed during the setup and takedown of the computer in the participants’ homes and were perceived as part of the overall training experience, which was by design different between the groups.
Study Design and Participant Flow.
This study was randomized, double-blinded, and controlled. Participants were kept blind as to the hypothesis that one training program was considered the intervention and the other an AC through the use of balanced scripts and training material that described both activities as plausible cognitive training strategies. All neuropsychological assessments were performed by trained clinicians who were kept blind to the group membership of participants.
After recruitment, each participant completed an initial visit in which informed consent and relevant inclusion/exclusion measures were performed. Each participant then completed a pretraining assessment visit, after which they were randomized into the ET, AC, or NCC groups. After the training period (or no-contact period for the NCC group), each participant then completed a posttraining assessment visit and a 3-month follow-up assessment visit. Participants performed no study-related activities during the 3-month follow-up period. Identical neuropsychological evaluations were performed at each assessment visit. The RBANS has parallel forms with established equivalence and test–retest reliability; participants were randomly assigned to either the A or B form of the RBANS in the pretraining visit and used the other form in the posttraining visit and the original version for the 3-month follow-up.
Data Analysis.
The goal of this study was to evaluate the effect size of the ET program intervention and determine appropriate control activities; for this reason data analysis focused on comparing pretraining and posttraining and pre scores and follow-up scores within groups comprised of the participants who completed the assessment battery in the pretraining and posttraining/follow-up visits.
Acknowledgments
We thank Anne Bruce, Bradley Brummett, Jill Damon, Danielle Doan, Lisa Faille, Jason Minow, and Amy Walthall for performing assessments for this study; Chuck Armstrong, Audrey Chen, Teresa Freeman, Katie Holmes, Prashant Idury, Sarah Kim, John Motsinger, Kim Schilling, Dan Tinker, Rudy Walter, and Lauren Wholey for supporting the participants during the study; Dylan Bird, Ron Ruff, and Cate Stasio for helpful comments on the manuscript; Christoph Schreiner for a careful review of the manuscript; and Christopher Randolph for providing data and working on developing the RBANS scaled score analyses. This work was funded by Posit Science Corporation.
Glossary
Abbreviations
- ET
experimental training
- AC
active control
- NCC
no-contact control
- ARCD
age-related cognitive decline
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status.
Footnotes
Conflict of interest statement: M.M.M. is a founder and a minor stockholder of the company (Posit Science) that developed this brain science-based training strategy. All authors hold stock or stock options in Posit Science Corporation. All authors except M.M.M. are employees of Posit Science Corporation.
References
- 1.Christensen H. Aust. N. Z. J. Psychiatry. 2001;35:768–775. doi: 10.1046/j.1440-1614.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 2.Fillit H. M., Butler R. N., O’Connell A. W., Albert M. S., Birren J. E., Cotman C. W., Greenough W. T., Gold P. E., Kramer A. F., Kuller L. H., et al. Mayo Clin. Proc.; 2002. pp. 681–696. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie K., Artero S., Touchon J. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 4.Bischkopf J., Busse A., Angermeyer M. C. Acta Psychiatr. Scand. 2002;106:403–414. doi: 10.1034/j.1600-0447.2002.01417.x. [DOI] [PubMed] [Google Scholar]
- 5.Ylikoski R., Ylikoski A., Keskivaara P., Tilvis R., Sulkava R., Erkinjuntti T. Eur. J. Neurol. 1999;6:645–652. doi: 10.1046/j.1468-1331.1999.660645.x. [DOI] [PubMed] [Google Scholar]
- 6.Park H. L., O’Connell J. E., Thomson R. G. Int. J. Geriatr. Psychiatry. 2003;18:1121–1134. doi: 10.1002/gps.1023. [DOI] [PubMed] [Google Scholar]
- 7.Merzenich M. M., Jenkins W. M. In: The Adaptable Brain. Levy-Reiner S., editor. Vol. II. Washington, DC: Library of Congress; 1999. pp. 37–50. [Google Scholar]
- 8.Merzenich M. M., Jenkins W. M. In: Memory Concepts. Andersen P., editor. Amsterdam: Elsevier; 1993. pp. 437–453. [Google Scholar]
- 9.Buomomano D. V., Merzenich M. M. Annu. Rev. Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 10.Recanzone G. H., Merzenich M. M., Schreiner C. E. J. Neurophysiol. 1992;67:1071–1091. doi: 10.1152/jn.1992.67.5.1071. [DOI] [PubMed] [Google Scholar]
- 11.Recanzone G. H., Merzenich M. M., Jenkins W. M., Grajski K. A., Dinse H. R. J. Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 12.Wang X., Merzenich M., Sameshima K., Jenkins W. Nature. 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- 13.Elbert T., Pantev C., Wienbruch C., Rockstroh B., Taub E. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 14.Allard T., Clark S. A., Jenkins W. M., Merzenich M. M. J. Neurophysiol. 1991;66:1048–1058. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins W., Merzenich M., Ochs M., Allard T., Guic-Robles E. J. Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 16.Byl N., Merzenich M., Jenkins W. Neurology. 1996;47:508–520. doi: 10.1212/wnl.47.2.508. [DOI] [PubMed] [Google Scholar]
- 17.Mahncke H. W., Bronstone A., Merzenich M. M. In: Reprogramming the Brain. Møller A. R., Chapman S. B., Lomber S. G., editors. Amsterdam: Elsevier; 2006. pp. 81–110. [Google Scholar]
- 18.Salthouse T. A. Psychol. Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 19.Dinse H. R. Acta Neurochir. Suppl. 2005;93:79–84. doi: 10.1007/3-211-27577-0_12. [DOI] [PubMed] [Google Scholar]
- 20.Coq J. O., Xerri C. Neuroscience. 2001;104:705–715. doi: 10.1016/s0306-4522(01)00123-3. [DOI] [PubMed] [Google Scholar]
- 21.Merzenich M. M., Jenkins W. M., Johnston P., Schreiner C., Miller S. L., Tallal P. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- 22.Tallal P., Miller S., Jenkins W., Merzenich M. In: Foundations of Reading. Blachman B., editor. Cambridge, MA: MIT Press; 1996. pp. 71–88. [Google Scholar]
- 23.Tallal P., Miller S. L., Bedi G., Byma G., Wang X., Nagarajan S. S., Schreiner C., Jenkins W. M., Merzenich M. M. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- 24.Merzenich M. M., Tallal P., Peterson B., Miller S. L., Jenkins W. M. In: Neuroplasticity: Building a Bridge from the Laboratory to the Clinic. Grafman J., Cristen Y., editors. New York: Springer; 1998. pp. 169–187. [Google Scholar]
- 25.Merzenich M. M., Miller S., Jenkins W., Saunders G., Protopapas A., Peterson B., Tallal P. In: Basic Neural Mechanisms in Cognition and Language. Euler C., editor. Amsterdam: Elsevier; 1998. pp. 143–172. [Google Scholar]
- 26.Scientific Learning . Improved Reading Skills by Students in the Dallas Independent School District Who Used Fast ForWard(r) Products. Oakland, CA: Scientific Learning; 2005. MAPS for Learning: Educator Reports. [Google Scholar]
- 27.Scientific Learning . Improved Reading Skills by Students in the United Independent School District Who Used Fast ForWard(r) Products. Oakland, CA: Scientific Learning; 2005. MAPS for Learning: Educator Reports. [Google Scholar]
- 28.Temple E., Deutsch G. K., Poldrack R. A., Miller S. L., Tallal P., Merzenich M. M., Gabrieli J. D. Proc. Natl. Acad. Sci. USA. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes E. A., Warrier C. M., Nicol T. G., Zecker S. G., Kraus N. Clin. Neurophysiol. 2003;114:673–684. doi: 10.1016/s1388-2457(02)00414-5. [DOI] [PubMed] [Google Scholar]
- 30.Gatz M. PLoS Med. 2005;2:e7. doi: 10.1371/journal.pmed.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDougall G. J., Jr Annu. Rev. Nurs. Res. 1999;17:219–240. [PMC free article] [PubMed] [Google Scholar]
- 32.Ball K., Berch D. B., Helmers K. F., Jobe J. B., Leveck M. D., Marsiske M., Morris J. N., Rebok G. W., Smith D. M., Tennstedt S. L., et al. J. Am. Med. Assoc. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards J. D., Wadley V. G., Myers R. S., Roenker D. L., Cissell G. M., Ball K. K. Gerontology. 2002;48:329–340. doi: 10.1159/000065259. [DOI] [PubMed] [Google Scholar]
- 34.Yesavage J. A. Arch. Gerontol. Geriatr. Suppl. 1989;1:185–190. [PubMed] [Google Scholar]
- 35.Rapp S., Brenes G., Marsh A. P. Aging Mental Health. 2002;6:5–11. doi: 10.1080/13607860120101077. [DOI] [PubMed] [Google Scholar]
- 36.Loewenstein D. A., Acevedo A., Czaja S. J., Duara R. Am. J. Geriatr. Psychiatry. 2004;12:395–402. doi: 10.1176/appi.ajgp.12.4.395. [DOI] [PubMed] [Google Scholar]
- 37.Clare L., Wilson B. A., Carter G., Roth I., Hodges J. R. Neuropsychology. 2002;16:538–547. doi: 10.1037//0894-4105.16.4.538. [DOI] [PubMed] [Google Scholar]
- 38.Davis R. N., Massman P. J., Doody R. S. Alzheimer Dis. Assoc. Disord. 2001;15:1–9. doi: 10.1097/00002093-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Folstein M. F., Folstein S. E., McHugh P. R. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 40.Nagarajan S. S., Wang X., Merzenich M. M., Schreiner C. E., Johnston P., Jenkins W. M., Miller S., Tallal P. IEEE Trans. Rehabil. Eng. 1998;6:257–268. doi: 10.1109/86.712220. [DOI] [PubMed] [Google Scholar]
- 41.Recanzone G. H., Schreiner C. E., Merzenich M. M. J. Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polley D. B., Steinberg E. E., Merzenich M. M. J. Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xerri C., Merzenich M. M., Jenkins W., Santucci S. Cereb. Cortex. 1999;9:264–276. doi: 10.1093/cercor/9.3.264. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D. Wechsler Text of Adult Reading. San Antonio, TX: Harcourt Assessment; 2001. [Google Scholar]
- 45.Randolph C., Tierney M. C., Mohr E., Chase T. N. J. Clin. Exp. Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]