Abstract
Plants with mutations in one of three maize genes, mop1, rmr1, and rmr2, are defective in paramutation, an allele-specific interaction that leads to meiotically heritable chromatin changes. Experiments reported here demonstrate that these genes are required to maintain the transcriptional silencing of two different transgenes, suggesting that paramutation and transcriptional silencing of transgenes share mechanisms. We hypothesize that the transgenes are silenced through an RNA-directed chromatin mechanism, because mop1 encodes an RNA-dependent RNA polymerase. In all the mutants, DNA methylation was reduced in the active transgenes relative to the silent transgenes at all of the CNG sites monitored within the transgene promoter. However, asymmetrical methylation persisted at one site within the reactivated transgene in the rmr1-1 mutant. With that one mutant, rmr1-1, the transgene was efficiently resilenced upon outcrossing to reintroduce the wild-type protein. In contrast, with the mop1-1 and rmr2-1 mutants, the transgene remained active in a subset of progeny even after the wild-type proteins were reintroduced by outcrossing. Interestingly, this immunity to silencing increased as the generations progressed, consistent with a heritable chromatin state being formed at the transgene in plants carrying the mop1-1 and rmr2-1 mutations that becomes more resistant to silencing in subsequent generations.
DURING the past 20 years extensive studies on transgene expression in plants have revealed the potential for transcriptional or post-transcriptional silencing (Flavell 1994; Baulcombe 1996; Finnegan et al. 2001; Vaucheret and Fagard 2001; Matzke et al. 2002). Transcriptional gene silencing (TGS) is mediated through repression of transcription, while post-transcriptional gene silencing (PTGS) is mediated through mRNA degradation. TGS is a mitotically and often meiotically heritable phenomenon, while PTGS is usually meiotically reversible. Silencing can happen in cis or in trans, affecting unlinked sequences (including transgenes, endogenous genes, or viruses) that share high sequence similarity.
TGS at either endogenous or transgenic loci has been associated with a number of different epigenetic hallmarks. Examples of correlations with TGS include changes in DNA methylation, histone methylation, histone acetylation, and chromatin structure (reviewed in Meyer 2000). TGS is often associated with DNA methylation in the promoter regions and can be induced by production of a double-stranded RNA homologous to a targeted promoter (Mette et al. 2000).
In this report, mutants defective in paramutation were used to investigate whether paramutation is mechanistically related to transgene silencing. Paramutation involves transcommunication between homologous sequences, resulting in heritable changes in chromatin (Chandler and Stam 2004) associated with transcriptional gene silencing (Patterson et al. 1993; Hollick et al. 2000).
The three mutations that prevent paramutation were originally isolated in genetic screens using two paramutation systems, b1 and pl1, both genes that control the production of purple anthocyanin pigment in seedlings and mature plant tissues. The recessive mop1-1 mutation (mediator of paramutation1) prevents paramutation at three genes that undergo paramutation (Dorweiler et al. 2000) and increases the transcription rate of the two silenced paramutant alleles tested. Two mutations, rmr1-1 and rmr2-1 (required to maintain repression), were isolated on the basis of their ability to disrupt the silencing associated with paramutation at pl1 (Hollick and Chandler 2001), but their effects on paramutation at other genes have not been reported. The mop1-1 (Dorweiler et al. 2000), rmr1-1, and rmr2-1 (V. Chandler and Y. Lin, unpublished data) mutations do not affect global DNA methylation, as none of the mutants show altered DNA methylation levels within repeated sequences such as centromeres and ribosomal DNA genes, but Mutator transposable elements are hypomethylated in all three mutants (J. Hollick and D. Lisch, personal communication; Dorweiler et al. 2000). The mop1-1 mutation also reactivates Mutator transposable elements, but the reactivation takes multiple generations in the mop1-1 background (Lisch et al. 2002; Woodhouse et al. 2006). The experiments reported herein demonstrate that each of the mutants could reactivate the previously silent trangenes, but the mutants differed with respect to the associated DNA methylation changes and whether the transgene could remain active in subsequent generations.
MATERIALS AND METHODS
Constructs and transgenic lines:
The silent transgenic lines used for this work include the genomic region of the B-I allele, which corresponds to nucleotides 22,136–27,972 of the b1 gene contained in GenBank AF466203 (Selinger et al. 1998). This portion of the gene includes the genomic sequence spanning the complete coding region and both the 5′ and 3′ UTRs of the b1 gene. The 35SBTG transgene also includes the 35S CaMV promoter, corresponding to nucleotides 7012–7435 of GenBank V00140, and the first intron of maize alcohol dehydrogenase1 (included as an enhancer of expression), corresponding to nucleotides 1222–1774 of GenBank X04049. In the BBBS transgene, the B-Bolivia promoter was used to drive expression of the same genomic region of the b1 gene as in the 35SBTG construct. B-Bolivia is an allele that does not undergo paramutation, and its promoter was previously characterized (Selinger and Chandler 2001). The BBBS construct (Selinger and Chandler 2001) and the protocol used to generate transgenic maize plants with biolistic particle bombardment have been described previously (Selinger et al. 1998).
Both constructs were co-bombarded with a selectable marker for identifying transgenic plants—the bar gene driven by the 35S CaMV promoter, as described previously (Selinger and Chandler 2001). The bar gene confers resistance to glufosinate–ammonium herbicides (Dennehey et al. 1994).
Genetic stocks and genotyping:
Following transformation of the maize inbred line CG00526, the regenerated T0 plants were outcrossed for two generations with b r-g stocks and transgenic lines were selected that were homozygous for b and r-g (Selinger et al. 1998). The b1 gene is highly homologous to r1 and both encode functionally equivalent bHLH proteins (Chandler et al. 1989). The r-g allele is recessive and produces no pigment in either the plant or the seed. The b allele is also recessive and produces no pigment. The stocks used for the mop1-1 and rmr1-1 experiments were either r-r/r-g or r-g; these genotypes result in colorless seeds and purple or yellow anthers, respectively. The recessive r allele in the seeds of these stocks enabled unambiguous identification of individuals carrying the transgenes, as b1 expression in the aleurone layer results in purple kernels. The rmr1-1, rmr2-1, and some of the mop1-1 stocks also carried the Pl′ allele. The Pl′ allele allowed for scoring of heterozygous vs. homozygous mutants in some families, as all the mutants upregulate the Pl′ allele (Dorweiler et al. 2000; Hollick and Chandler 2001). Anthers of plants that are −r and Pl′ are lightly pigmented and sectored when heterozygous and solid purple when homozygous for each of the mutations.
For the experiments with rmr2-1, a dominant r1 allele (R-r) segregated, which also conferred purple aleurone pigment. As R-r segregates independently of the transgene and rmr2, one-third of the plants derived from purple seed would be expected to receive the transgene and be homozygous for rmr2-1. Segregation analysis, testcrosses, PCR with transgene-specific primers, and Southern blots with transgene-specific probes were used to determine the genotype of progeny plants.
Northern blot analysis:
Total RNA extractions, blotting, and hybridizations were performed as described previously (Chandler et al. 1989). The probe for b1 corresponds to coordinates 1–1970 in Genbank accession X57276, and the bar probe corresponds to coordinates 317–767 in Genbank accession AY572837. Both probes were generated by PCR. All plasmid and primer sequences utilized in this work are available upon request. Northern blots were analyzed with a STORM PhosphorImager and ImageQuant software (GE Healthcare). Northern blot analysis on small RNA-enriched samples was performed according to an unpublished protocol (N. Doetsch and R. Jorgensen, personal communication) derived from published work in other laboratories (Hutvagner et al. 2000; Mette et al. 2000).
Nuclear run-on assays:
Transcription rates were determined using transcription assays on isolated nuclei as described (Dorweiler et al. 2000). Briefly, nuclei from 10 to 15 g of sheath or husk tissue from plants at anthesis were prepared using a modified chromatin isolation protocol. Denatured PCR product or linearized plasmid (100 ng of DNA) of each gene listed below was slot blotted onto nitrocellulose filter membranes. With the exception of the linearized backbone of the transgene (SK+), all probes were generated by PCR. The coordinates and relevant accession numbers are:
35S CaMV promoter: 3577–4096 in AY553053;
b1 coding region: 1–1970 in X57276;
bar gene: 317–767 in AY572837;
c2 coding region: 156–791 in AY109395;
ubi gene: 975 bp Ubi-2 specific probe (Christensen et al. 1992);
a1 coding region: 1–1436 in AY105150.
Data normalization was performed as previously described (Dorweiler et al. 2000).
Analysis of transgene structure:
The structures of the transgene were determined using Southern blot analysis. DNA isolation, blotting, and hybridization protocols have been described previously (Dorweiler et al. 2000). The b1 coding region and 35S CaMV promoter probes utilized for Southern blots are the same sequences described in the nuclear run-on assay methods.
Analysis of DNA methylation:
Total genomic DNA was digested by a combination of methylation-sensitive and -insensitive restriction endonucleases. The methylation-sensitive enzymes DdeI, PstI, and BstBI were each used together with BstNI, a methylation-insensitive enzyme, to test for DNA methylation within the transgene. BstNI sites occur 5′ of the 35S promoter in the 35SBTG construct and at a position 280 bp from the 5′-end of the adh1 intron; the other restriction sites utilized lie between these two BstNI sites. Following complete digestion according to the manufacturer's instructions for each enzyme, Southern blot analysis was performed. The 35S CaMV promoter probe is the same as that described in the nuclear run-on assay methods. A probe specific for the adh1 intron was used to identify regions of the 35S CaMV promoter that were adjacent to the adh1 intron and b1 transgene. The probe was amplified by PCR from pMCG161 (Genbank AY572837) with the probe sequence corresponding to coordinates 4837–5402 of that accession.
RESULTS
Two silent transgenes can be reactivated by three paramutation mutants:
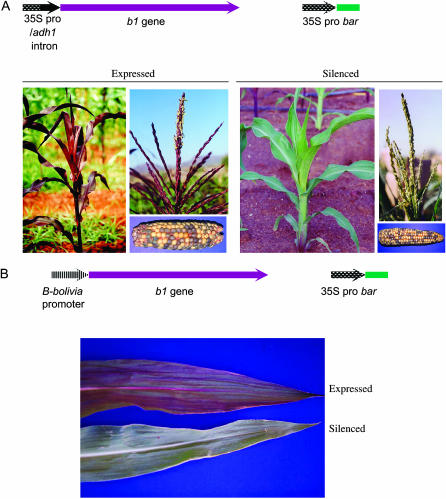
Previous work had generated two transgenic lines, each containing distinct b1 transgenes originally produced to study anthocyanin gene regulation. The b1 gene encodes a transcription factor that activates the anthocyanin biosynthetic pathway (Chandler et al. 1989). Expression of the b1 gene in a particular tissue will give rise to purple pigment in that tissue, if the other regulatory and biosynthetic proteins are present. The 35SBTG transgenic line carries the 35S CaMV promoter driving the B-I genomic sequences spanning the transcribed region, including all introns, exons, and 5′ and 3′ UTRs (Figure 1A). Active 35SBTG lines had darkly pigmented seeds and pigment throughout the vegetative and many of the floral parts of the plant. The only obvious above-ground epidermal tissue that was not pigmented was the anthers (Figure 1A). The BBBS-derived transgenic line carries the promoter from the B-Bolivia allele fused to the same B-I genomic sequence as the 35SBTG construct (Figure 1B). Active BBBS lines showed medium-to-dark pigment in the seeds and had medium pigment in a variety of vegetative tissues (Figure 1B; see Selinger and Chandler 2001 for details on the pigment phenotype of plants with active BBBS transgenes).
Figure 1.—
The (A) 35SBTG and (B) BBBS constructs contain the same b1 genomic sequences but expression is driven by different promoters, which control expression in different tissues. Each construct was co-bombarded with a 35S CaMV promoter:bar construct as a selectable marker. For unknown reasons, both transgenes were expressed in the aleurone even in individuals displaying a silenced phenotype in vegetative tissue. The ears shown in the pictures were a result of crossing an individual hemizygous for the transgene with a wild type, nontransgenic individual, producing the 1:1 segregation of purple:colorless kernels.
To test whether the paramutation mutants could affect transgene silencing, lines were identified in which transgenes were silenced. To help ensure that the transgenes had the potential to be reactivated, lines were used in which the transgene was silenced in vegetative and reproductive plant tissues, yet remained active in the aleurone layer of the seed. The fact that the transgenes were expressed in the seed indicated that there was an intact coding region present within the transgene array capable of activating anthocyanin biosynthesis. It is not known why these transgenic lines were completely silent in vegetative and floral tissue while expression was observed in the aleurone layer of the seed. A similar pattern of expression has been reported for other transgenes (Cocciolone et al. 2000), and one speculation is that the chromatin structure is distinct in endosperm and related aleurone tissues as compared to the embryo and resulting plant.
The 35SBTG line used for this work contained ∼2 copies of the introduced b1 construct and ∼3 copies of the selectable marker gene, bar, all segregating as a single locus (data not shown). In this transgenic line, expression of the 35SBTG construct had never been detected in the plant. The BBBS transgenic locus was more complex; it contained ∼15 copies of the introduced b1 construct, which segregated as a single locus (Selinger and Chandler 2001). This transgenic line originally produced vegetative and seed pigment (Selinger and Chandler 2001). Individual progeny plants that were green because the transgene had become silenced in plant tissues were observed at a frequency of ∼1%.
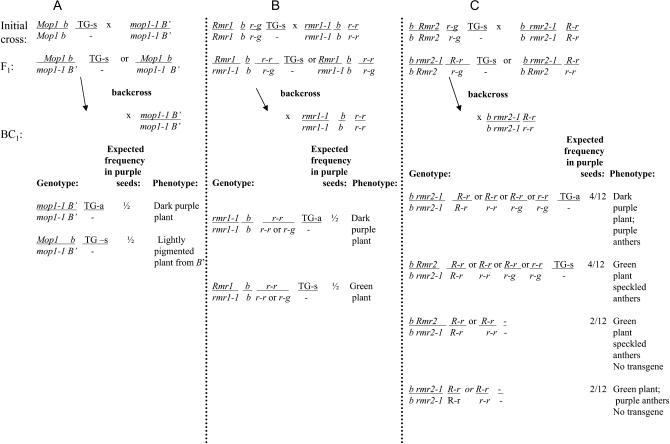
Three mutations defective in paramutation (mop1-1, rmr1-1, and rmr2-1) were each separately combined with the two transgenic lines by genetic crosses (Figure 2). Resulting F1 plants heterozygous for each mutant and containing the transgene had the same low level of pigment as their nontransgenic siblings, indicating that the transgene remained silent. The F1 plants were lightly pigmented in the mop1-1 experiments, because the weakly expressed B′ allele was present, or green in the rmr experiments, in turn because a null b1 allele was present. Transgenic F1 plants were backcrossed (BC1) to plants homozygous for the particular mutation, yielding families segregating for the transgene and heterozygous or homozygous for the mutation being tested. In the rmr2-1 families, R-r segregated, producing purple or mottled seed color, depending on the direction of the cross (Kermicle and Alleman 1990). Thus, in the rmr2-1 families, not all purple seed is expected to result from the transgene (Figure 2C).
Figure 2.—
Crossing strategy to test the ability of three paramutation mutants to reactivate the transgenes. Plants with silent transgenes (Tg-s) were crossed individually with plants homozygous for each of the three mutants. F1 progeny were crossed again with plants homozygous for the appropriate mutant to generate a backcross population segregating homozygous and heterozygous mutations, and hemizygous for or lacking the transgene. The resulting genotypes and the phenotypes expected for activation of the transgene by each homozygous mutant are listed for each experiment; data are in Table 1. Both mop1 and rmr2 are linked to b1; rmr1 segregates independently. The original transgene lines were Pl, which becomes paramutated to Pl′ when crossed with the Pl′ carried in the rmr1-1 and rmr2-1 stocks. Thus, because Pl′ is in all plants, it is not shown.
For all three mutants, reactivated transgenes (Tg-a) segregated in the BC1 generation (Figure 3, A–C), and the frequencies observed were consistent with activation in all plants homozygous for the mutants (Table 1). The dark pigment phenotype conferred by the reactivated transgene was equivalent in all three mutants. When the backcross progeny were Pl′ and −r, the anthocyanin color in the anthers (where the transgenes are not expressed) could be used to assess whether the plant was heterozygous or homozygous for a particular mutant (Figure 3D). Each of the homozygous mutants upregulate expression of Pl′, resulting in darkly pigmented anthers (Dorweiler et al. 2000; Hollick and Chandler 2001). Independent of whether anther color could be used to assess whether the mutation was heterozygous or homozygous, every plant was crossed with appropriate mutant testers to determine or confirm the mutant genotype. Not all crosses were successful, but in all cases when a plant was confirmed homozygous for the mutations, the transgene was activated and no activation was seen in plants heterozygous for the mutations.
Figure 3.—
Reactivation of the silent transgenes resulting in dark-purple pigmentation throughout all the vegetative tissue of the plant, while the transgene remained silent in plants heterozygous for the mutations. These photographs illustrate rmr1-1/rmr1-1 plants and are representative of the phenotype observed with all three mutants. (A) The 35SBTG transgene was reactivated in plants homozygous for each of the mutations. (B and C) The BBBS transgene was also reactivated in the plants homozygous for the mutations. Plants heterozygous for the mutations have a silent transgene and are green (B), while plants homozygous for the mutations have a reactivated transgene with an intermediate level of pigmentation in the leaf and culm (C). In plants homozygous for each mutation, Pl′ is upregulated, producing darkly pigmented anthers (D); neither transgene is expressed in the anthers.
TABLE 1.
Activation of two silent transgenes
| 35SBTG
|
BBBS
|
|||||
|---|---|---|---|---|---|---|
| Mutation | No. active | No. silent | χ2a | No. active | No. silent | χ2a |
| mop1-1 (1:1) | 213 | 192 | 1.1 | 27 | 26 | 0.02 |
| rmr1-1 (1:1) | 55 | 52b | 0.09 | 15 | 13 | 0.14 |
| rmr2-1 (1:3) | 26 | 95 | 0.79 | 28 | 96 | 0.39 |
Each of the three mutants was introduced into the silent transgene lines as indicated in Figure 2. The resulting BC1 progeny segregated active and silent transgenes. The observed ratios were consistent with the plants carrying active transgenes homozygous for each mutant; the expected ratio for this hypothesis is summarized for each mutant in Figure 2.
Chi square tests (χ2) were performed to estimate the degree of confidence for each hypothesis. The hypothesis tested in each case is indicated in parentheses next to the indicated mutation.
Forty-four of these individuals had anther color scores (ACS) between 1 and 4, consistent with a genotype of Rmr1/rmr1-1. In contrast, 8 individuals had ACS of 6–7. These 8 individuals could be due to reversion of Pl′ to Pl-Rh (Hollick et al. 2000), with these individuals being Rmr1/rmr1-1 and not activating the transgene. Alternatively, the plants with ACS6-7 could have been rmr1-1 homozygotes, but the transgene was not activated. Unfortunately, testcrosses of these 8 individuals were not obtained so the rmr1 genotype could not be assessed.
Reactivation of the 35SBTG transgene occurs at the transcriptional level:
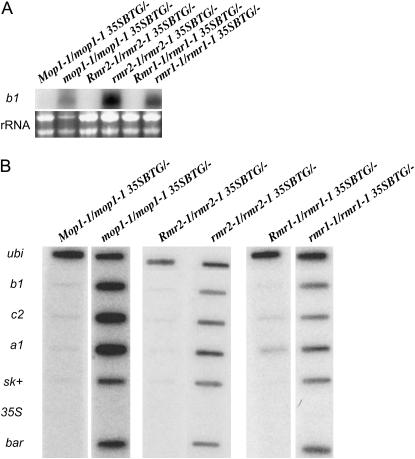
Given that all three mutants increase transcription of the B′ and Pl′ alleles (Dorweiler et al. 2000; Hollick and Chandler 2001), a reasonable hypothesis was that the mutations also increase transcription from the transgene. Because of the stronger pigment phenotype, the experiments examining RNA levels and transcription were performed with the 35SBTG lines. RNA was isolated from sheath tissue of transgenic plants heterozygous or homozygous for each mutation and examined on Northern blots. The darkly pigmented plants (homozygous for mop1-1, rmr1-1, or rmr2-1) had dramatic increases in transgene-encoded b1 RNA (Figure 4A). No b1 RNA was detectable in the plants heterozygous for the mutations. RNA from the bar gene, originally used to select for transformants, was also increased in plants homozygous for the mutants (data not shown).
Figure 4.—
Steady-state transcript abundance and the transcription rate of several elements of the 35SBTG transgene were increased in the homozygous mutant plants relative to heterozygous siblings. (A) Northern blot of total RNA from transgenic siblings segregating homozygous and heterozygous mutants hybridized with the b1 probe. The ethidium-bromide-stained gel shows the bands corresponding to rRNA, a loading control. (B) Nuclear run-on analysis reveals that the transgene is transcriptionally silenced in wild type and that transcription increases in the presence of each homozygous mutant. Slot blots containing the indicated gene sequences were hybridized with RNA isolated from transcription assays with nuclei of the indicated genotypes. The ubiquitin gene (ubi) is the positive control. The b1, sk+, 35S, and bar sequences were all in the 35SBTG locus. The c2 and a1 genes are transcriptionally activated by B (Coe et al. 1988). Additional biological replicates revealed results comparable to those shown.
To investigate whether the increased b1 RNA levels were caused by increases in transcription or increases in RNA stability, nuclear run-on assays were performed (Figure 4B). Transcription of the 35SBTG transgene is dramatically increased in plants homozygous for the mutants. The transgene activation results in an active B protein, as two genes that are controlled by B, c2 and a1 (Coe et al. 1988), are also transcribed. There was also an increase in transcription of the bar gene and the vector backbone (+SK). This transgene locus contains several rearranged copies of the introduced constructs (data not shown), which may have fortuitously placed the vector sequences adjacent to a promoter.
Expression of promoter sequences can trigger RNA-directed DNA methylation and subsequent transcriptional silencing (Mette et al. 2000). Thus, a probe for the CaMV 35S promoter was included in the nuclear run-on experiments to determine if transcription from the CaMV 35S promoter could be detected, but no promoter transcripts were detected (Figure 4B). Northern blot analysis was also performed on small RNA-enriched samples to determine if small interfering RNAs (siRNAs) homologous to the transgene were present. Small RNAs were not detected with probes specific for any part of the 35SBTG construct (data not shown), although this protocol has been successfully used in the laboratory to detect small RNAs for other experiments (data not shown).
Heritability of the transgene reactivation:
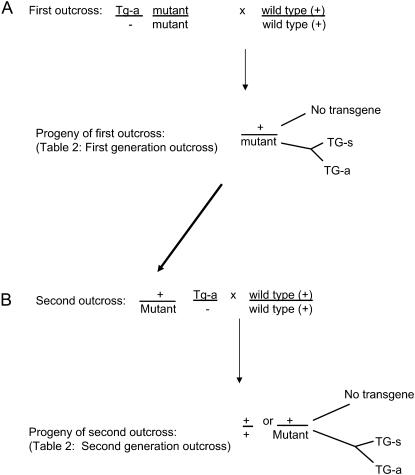
Plants containing the activated 35SBTG transgene were used to test whether the transgene is efficiently resilenced or if it remained active in progeny plants after outcrosses to wild-type individuals. Multiple individual plants with the active 35SBTG transgene and homozygous for mop1-1, rmr1-1, or rmr2-1 were outcrossed with b r-g Pl stocks, which contained dominant functional Mop1, Rmr1, and Rmr2 alleles (Figure 5A). Purple seeds were planted and the resulting plants were scored for pigment level (Table 2). With the rmr1-1 experiment, all of the resulting plants were green, indicating that the transgene was efficiently resilenced in the presence of a functional Rmr1 allele. For mop1-1 and rmr2-1, the transgene was resilenced in many of the progeny, but there were multiple families in which the transgene remained active in some of the progeny (Table 2, Figure 6A), all of which had functional alleles of Mop1 and Rmr2. Southern blot analysis indicated no obvious rearrangements of the transgene associated with resilencing or continued activation (data not shown).
Figure 5.—
To test for resilencing upon introduction of wild-type proteins, plants with an active transgene (Tg-a) and homozygous for each mutation were crossed with nontransgenic, wild-type plants. Phenotypic data for the resulting progeny are reported in the first-generation outcross columns of Table 2 and in Figure 6A. A subsequent outcross to wild-type nontransgenic plants was done with Tg-a plants heterozygous for the mutations. Phenotypic data for the resulting progeny data are reported in the second-generation outcross columns of Table 2 and in Figure 6B.
TABLE 2.
Immunity of activated transgenes to silencing in subsequent generations
| First-generation outcross
|
Second-generation outcross
|
|||
|---|---|---|---|---|
| Inducing mutation | Homozygotes yielding Tg-a progeny/ total outcrossed | Tg-a progeny/ total progeny scored | Heterozygotes yielding Tg-a progeny/ total outcrossed | Tg-a progeny/ total progeny scored |
| mop1-1 | 7/10 | 87/198 | 9/9 | 1491/1566 |
| rmr2-1 | 5/8 | 21/127 | 11/12 | 370/620 |
| rmr1-1 | 0/44 | 0/877 | NA | NA |
Homozygous mutants were outcrossed with wild type as described in Figure 5A. Some individuals produced progeny that maintained an active transgene (Tg-a) in the presence of wild-type proteins (first-generation outcross). The Tg-a heterozygous mutant plants were outcrossed again to wild type as described in Figure 5B and the results are summarized in the columns under second-generation outcross. NA, not applicable.
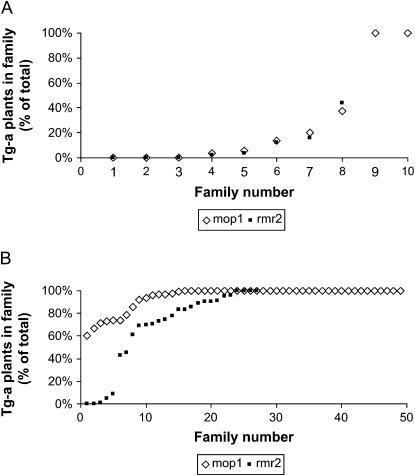
Figure 6.—
Activation of the previously silent transgene can be maintained after outcrossing to a wild-type individual. After the initial reactivation in the homozygous mutants and subsequent outcrossing to wild type, some progeny retained an active transgene in mop1-1 and rmr2-1 heterozygotes. Seed from each ear resulting from an outcross becomes one family. For each family, the percentage of plants with active transgenes (Tg-a) is indicated. (A) Data from first-generation outcross diagrammed in Figure 5A. (B) Data from second-generation outcross diagrammed in Figure 5B.
A second outcross was done with the Tg-a, mop1-1, or rmr2-1 heterozygotes (Figure 5B). In these experiments, one-half the progeny would be expected to be wild type and one-half would be heterozygous for each mutation. For both sets of outcrosses, almost all of the plants gave rise to large numbers of dark progeny (Table 2), and this continued for a third generation of outcrosses (data not shown). In the second generation of outcrossing after reactivation, the number of plants expressing the transgene in each family was usually much higher than in the previous generation (Figure 6B), with the most dramatic results observed in the mop1-derived families.
DNA methylation correlates with silencing of the transgene:
Mutants that show reduction in DNA methylation can exhibit loss of gene silencing (Mittelsten Scheid and Paszkowski 2000; Bender 2004). To test whether DNA methylation changes within the transgene correlated with its activation, methylation patterns of the transgene in plants heterozygous and homozygous for each mutation were examined.
Methylation of DNA within the 35S promoter and the adjacent adh1 intron sequence was assayed using Southern blots. When possible, the adh1 intron sequence was used as a probe so that the 35S promoter driving the b1 transgene could be distinguished from the 35S promoter driving the bar transgene, but in some cases a 35S promoter probe was used. Multiple restriction sites with different methylation sensitivities are distributed across the promoter/intron region (Figure 7A). Restriction sites were chosen such that both symmetrical (CNG) and asymmetrical (CNN) cytosine methylation could be evaluated. Symmetrical CG methylation was not evaluated because in this region there were no CG methylation sites within the recognition sites for methylation-sensitive enzymes that could be distinguished with the probes utilized for this study.
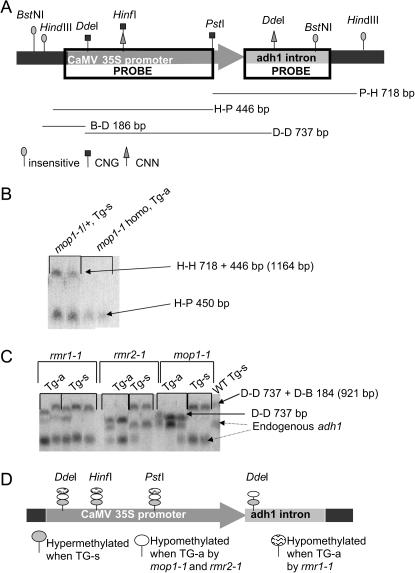
Figure 7.—
DNA methylation is different in the promoter/enhancer regions of silent (Tg-s) and active (Tg-a) transgenes. (A) Restriction sites for enzymes with differing methylation sensitivities are present within the promoter (CaMV 35S) and enhancer (adh1 intron) sequences of 35SBTG. Restriction site locations and methylation sensitivities are indicated and the predicted band sizes for digestions used in B and C are described below the promoter/enhancer map. (B) DNA samples from leaf tissue of individuals with the indicated genotype were digested with HindIII (methylation insensitive) and PstI (methylation sensitive), Southern blots were prepared and hybridized with the CaMV probe indicated in A. Each lane contains DNA from a different plant. The product corresponding to complete digestion is 418 bp, and the 1164-bp fragment corresponds to no digestion of the PstI site within the 35S CaMV promoter. Represenative data are shown for mop1-1 mutants, and similar results were observed for all mutants. (C) DNA was extracted from leaves of individual plants and subjected to electrophoresis and Southern blotting. The resulting blot was hybridized with the adh1 intron probe illustrated in A. The 921-bp fragment results when the site is methylated and consequently not cut; when the site is hypomethylated and fully digested, a 737-bp fragment is observed. The two lower bands indicated with dashed arrows are the endogenous adh1 gene; two distinct alleles are segregating. (D) Summary of methylation across the promoter/enhancer region of the 35SBTG.
Methylation was compared in the heterozygous and homozygous mutants in the first generation in which reactivation was observed (BC1). Differential transgene methylation was observed, with a decrease in CNG methylation correlating with reactivation (Figure 7, B and D). One exception was that CNN methylation persisted at one site upon reactivation, but only in the transgene upregulated in homozygous rmr1-1 plants (Figure 7C). Transgenes reactivated in mop1-1 and rmr2-1 homozygous plants were hypomethylated at this site (Figure 7C).
DISCUSSION
Mutations in three different genes required for paramutation (mop1, rmr1, and rmr2) have been shown to reactivate two silenced transgenes. Nuclear run-on experiments demonstrated that the transgenes were silenced at the transcriptional level and that transcription increases in the presence of the mutants. The fact that the mutants were able to prevent paramutation and to reactivate transcriptionally silent transgenes suggests that there are shared mechanisms between paramutation and transcriptional silencing of transgenes. Importantly, with two of the mutations, the transgene can remain active after the wild-type protein is reintroduced, suggesting that the absence of the wild-type proteins establishes an epigenetic state that can be immune to silencing in the presence of the wild-type proteins. Decreases in DNA methylation correlated with increased transgene expression. In the rmr1-1 mutant that retained asymmetrical methylation of one site in the transgene, the transgene was efficiently resilenced upon introduction of wild-type proteins by outcrossing. In contrast, in the mop1-1 and rmr2-1 lineages that lost all tested DNA methylation, the transgene could escape resilencing.
All of the mutants were originally isolated on the basis of their ability to prevent paramutation. The transgenes that they reactivated contain the b1 gene, which is subject to paramutation. It could be argued that the transgenes were reactivated simply because they contained b1 sequences; this is unlikely because none of the transgenes contain the sequences required for b1 paramutation, which are located ∼100 kb upstream of the b1 gene (Stam et al. 2002). Additional experiments with other transcriptionally silenced transgenes will reveal the generality of transgene reactivation by the mutants.
In a subset of individuals, the transgenes reactivated by mop1-1 and rmr2-1 were able to escape resilencing when outcrossed with wild-type plants, suggesting that the absence of the MOP1 and RMR2 proteins can induce a heritable chromatin state immune to silencing upon reintroduction of the wild-type proteins. The number of individuals with active transgenes increased over multiple generations of outcrossing with wild-type plants, demonstrating that the immunity to silencing was highly heritable and actually increased over multiple generations of outcrossing. In Arabidopsis, failure to resilence has been described as delayed resilencing for several mutants shown to impact TGS (Mittelsten Scheid et al. 1998). However, it is difficult to directly compare our results with those previously reported, because heritability was not a focus of the previous studies nor was the activity of the transgene followed over multiple generations. It has been speculated (Mittelsten Scheid et al. 1998; Brzeski and Jerzmanowski 2004) that delayed resilencing could be due to an epigenetic condition that takes multiple generations to reestablish in a wild-type or heterozygous plant. The data reported here are not consistent with that idea, as the number of plants maintaining an active state increases rather than decreases over multiple generations.
The ability of an expressed locus to remain active even when the mutation causing activation is segregated away is reminiscent of the ddm1 mutant in Arabidopsis. Plants carrying the ddm1 mutation accumulate developmental abnormalities that are associated with activation of previously silent loci (Kakutani et al. 1996, 1999; Kakutani 1997). The unlinked loci can remain active in subsequent outcrosses to wild type (Stokes and Richards 2002).
In contrast to the results observed for rmr2-1- and mop1-1-derived lines, transgenes reactivated by rmr1-1 are effectively resilenced upon outcrossing with wild-type plants. This is similar to sil1 (hda6; Probst et al. 2004) and drd1 mutants, which are capable of reactivating silent loci, but not in a manner that is heritable when the mutation is segregated away (Furner et al. 1998; Kanno et al. 2004).
Another interesting interpretation of the heritability data involves acquired characters and genetic assimilation, first described by C. H. Waddington in the 1950s. Alternative phenotypes, or polyphenisms, in Drosophila melanogaster were induced by subjecting individuals to multiple types of environmental stressors (Waddington 1953, 1956, 1959). After selectively breeding the individuals that exhibited polyphenisms under the influence of the environmental stressor, the acquired trait was eventually assimilated, such that the trait would be displayed in some progeny without requiring exposure to the inducing stressor. Waddington demonstrated that the assimilated traits were heritable and polygenic and speculated that the exposure of the organism to environmental stressors uncovered hidden genetic variability that could be incorporated via selective breeding into the heritable information passed on to the progeny of the flies exhibiting the phenotypes.
With genetic assimilation, the frequency of the phenotype increases over multiple generations of selective breeding, just as the number of Tg-a-containing plants increased over multiple generations of outcrossing in this study. Since Waddington's pioneering work, there have been many interesting reports of polyphenisms, and it is clear that epigenetic modifications can alter gene expression and cause divergent, heritable phenotypes. One intriguing speculation is that the underlying mechanism of genetic assimilation is alterations in chromatin structure or other epigenetic marks, resulting in a concomitant shift in gene expression.
Methylation of cytosine in a symmetrical CG context is a highly conserved DNA modification found across the plant, animal, and fungal kingdoms (Antequera and Bird 1993; Finnegan et al. 1996). Plants contain additional types of DNA methylation such as methylation of symmetrical CNG sequences and of asymmetrical CNN sequences (reviewed in Chan et al. 2005). Decreases in symmetrical DNA methylation in the promoter correlated with 35SBTG transgene activation by the mutations. However, these mutations do not globally demethylate the genome as centromeric and rDNA repeats remain methylated in the mutants (Dorweiler et al. 2000; Y. Lin and V. Chandler, unpublished data). Many of the mutants in other organisms that have been able to reactivate silent transgenes also affect DNA methylation. Initially, TGS was highly correlated with cytosine methylation at silenced loci, leading to the speculation that methylation was a major contributor to TGS (Kilby et al. 1992). However, further investigations revealed that maintenance of DNA methylation is not required for TGS at all loci (Dieguez et al. 1998; Mittelsten Scheid et al. 1998; Amedeo et al. 2000; reviewed in Mittelsten Scheid and Paszkowski 2000). Extensive genetic studies in Arabidopsis have indicated that control of DNA methylation and transcriptional silencing may be locus and sequence specific (Cao and Jacobsen 2002; Lippman et al. 2003), suggesting that a number of different mechanisms, both methylation dependent and independent, could potentially affect a given locus.
When 35SBTG was reactivated by rmr1-1, hypomethylation was observed at CNG sequences within the 35S CaMV promoter. However, asymmetrical (CNN) cytosine methylation persisted upon reactivation by rmr1-1 in at least one site within the promoter/enhancer region of the transgene. In contrast, transgenes reactivated by mop1-1 and rmr2-1 showed reduced methylation at all symmetric and asymmetric sequences tested within the promoter/enhancer region. This distinction is intriguing because the persistence of asymmetrical methylation upon reactivation coincides with an immediate resilencing of the transgene upon introduction of the wild-type protein by outcrossing. Possibly, the persistence of asymmetric methylation in the transgene activated by the absence of RMR1 facilitates resilencing in the next generation. Conversely, the loss of CNN methylation in the promoter region of the reactivated transgenes in the mop1-1 and rmr2-1 lineages may reflect a heritable chromatin state that hinders resilencing by wild-type proteins.
Although the underlying mechanism of silencing of the 35SBTG is not known, it seems likely that this silencing phenomenon involves RNA-directed chromatin changes. Recently, mop1 was cloned and shown to be the maize ortholog of RDR2 (Alleman et al. 2006), an Arabidopsis protein involved in an RNA-interference-mediated transcriptional silencing pathway correlating with DNA methylation of the silenced locus (Chan et al. 2004). The proposed function of MOP1 in paramutation is to amplify tandem repeat specific RNAs to a level sufficient to trigger chromatin changes (Alleman et al. 2006). This suggests that an RNA signal (“small” or otherwise) is necessary for mediating both paramutation at b1 and transcriptional silencing of the 35SBTG. Perhaps the RNA signal for transcriptional silencing of the transgene used in this study was not observed because it exists below the sensitivity range of the techniques used. Consistent with an involvement in RNA-mediated silencing, mop1-1 does reactivate silenced Mutator transposons (Lisch et al. 2002), whose regulation correlates with siRNAs (Slotkin et al. 2005).
The correlation of asymmetric methylation with transcriptional silencing has been previously demonstrated (Dieguez et al. 1998). It has been proposed that asymmetric methylation is established and maintained in response to small RNA signals that direct methylation at loci sharing sequence identity with small RNAs (Chan et al. 2005). Symmetric methylation can be established and maintained by this mechanism, but can also be maintained by an RNA-independent mechanism (Jones et al. 2001; Aufsatz et al. 2002).
The results herein, together with extensive studies from Arabidopsis, suggest a model for transcriptional silencing at this locus where an RNA signal is generated and amplified by maize DCL3, RDR2 (Xie et al. 2004), AGO4 (Zilberman et al. 2003), and RNA polymerase IV (Herr et al. 2005) orthologs and interpreted by other proteins in this pathway, including the de novo methyltransferase DRM1/2, the maintenance methyltransferases CMT3 (Cao et al. 2003) and MET1 (Aufsatz et al. 2004), and the putative SNF2 chromatin-remodeling protein DRD1 (Kanno et al. 2004). It is possible that, similar to mop1 (which encodes the maize RDR2 ortholog), rmr1 and rmr2 encode other proteins involved in establishing or interpreting RNA-directed chromatin formation.
To summarize, maize proteins encoded by mop1, rmr1, and rmr2 are required for transcriptional silencing of some transgenes, in addition to their previously reported roles in paramutation and transposon silencing. Intriguingly, the loss of the MOP1 and RMR2 proteins results in a chromatin structure that enables the transgene to be maintained in an active state after reintroducing the wild-type protein through crosses. In the case of mop1, this means that restoration of RNA-dependent RNA polymerase activity does not always result in resilencing, suggesting that amplified RNAs (siRNA or others) are not sufficient for silencing. It is likely that further investigation into the unique properties of paramutation, transposon silencing, and transgene silencing will continue to yield novel insights.
Acknowledgments
The authors gratefully acknowledge Bruce Walsh for a valuable discussion regarding genetic assimilation; Matthew Scholz for proofreading the manuscript; Damon Lisch, Jay Hollick, Natalie Doetsch, and Rich Jorgensen for providing access to unpublished data and protocols; Robert Sandoval for technical assistance with methylation analyses; and the National Science Foundation for funding (DBI-0421619 to K.M. and V.C.; MCB-0235329 and MCB-9982447 to V.C.).
References
- Alleman, M., L. M. Sidorenko, K. McGinnis, V. Seshadri, J. Dorweiler et al., 2006. An RNA-dependent RNA polymerase is required for paramutation, an allelic interaction that establishes a heritable chromatin state. Nature (in press). [DOI] [PubMed]
- Amedeo, P., Y. Habu, K. Afsar, O. Mittelsten Scheid and J. Paszkowski, 2000. Disruption of the plant gene MOM releases transcriptional silencing of methylated genes. Nature 405: 203–206. [DOI] [PubMed] [Google Scholar]
- Antequera, F., and A. Bird, 1993. Number of CpG islands and genes in human and mouse. Proc. Natl. Acad. Sci. USA 90: 11995–11999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufsatz, W., M. F. Mette, J. Van Der Winden, A. J. Matzke and M. Matzke, 2002. RNA-directed DNA methylation in Arabidopsis. Proc. Natl. Acad. Sci. USA 99 (Suppl. 4): 16499–16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufsatz, W., M. F. Mette, A. J. Matzke and M. Matzke, 2004. The role of MET1 in RNA-directed de novo and maintenance methylation of CG dinucleotides. Plant Mol. Biol. 54: 793–804. [DOI] [PubMed] [Google Scholar]
- Baulcombe, D. C., 1996. RNA as a target and an initiator of post-transcriptional gene silencing in transgenic plants. Plant Mol. Biol. 32: 79–88. [DOI] [PubMed] [Google Scholar]
- Bender, J., 2004. DNA methylation and epigenetics. Annu. Rev. Plant Biol. 55: 41–68. [DOI] [PubMed] [Google Scholar]
- Brzeski, J., and A. Jerzmanowski, 2004. Plant chromatin–epigenetics linked to ATP-dependent remodeling and architectural proteins. FEBS Lett. 567: 15–19. [DOI] [PubMed] [Google Scholar]
- Cao, X., and S. E. Jacobsen, 2002. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proc. Natl. Acad. Sci. USA 99 (Suppl. 4): 16491–16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., W. Aufsatz, D. Zilberman, M. F. Mette, M. S. Huang et al., 2003. Role of the DRM and CMT3 methyltransferases in RNA-directed DNA methylation. Curr. Biol. 13: 2212–2217. [DOI] [PubMed] [Google Scholar]
- Chan, S. W., D. Zilberman, Z. Xie, L. K. Johansen, J. C. Carrington et al., 2004. RNA silencing genes control de novo DNA methylation. Science 303: 1336. [DOI] [PubMed] [Google Scholar]
- Chan, S. W., I. R. Henderson and S. E. Jacobsen, 2005. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat. Rev. Genet. 6: 351–360. [DOI] [PubMed] [Google Scholar]
- Chandler, V. L., and M. Stam, 2004. Chromatin conversations: mechanisms and implications of paramutation. Nat. Rev. Genet. 5: 532–544. [DOI] [PubMed] [Google Scholar]
- Chandler, V. L., J. P. Radicella, T. P. Robbins, J. Chen and D. Turks, 1989. Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. Plant Cell 1: 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, A. H., R. A. Sharrock and P. H. Quail, 1992. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 18: 675–689. [DOI] [PubMed] [Google Scholar]
- Cocciolone, S. M., L. V. Sidorenko, S. Chopra, P. M. Dixon and T. Peterson, 2000. Hierarchical patterns of transgene expression indicate involvement of developmental mechanisms in the regulation of the maize P1-rr promoter. Genetics 156: 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe, E. H., Jr., D. A. Hoisington and M. G. Neuffer, 1988. The genetics of corn, pp. 81–258 in Corn and Corn Improvement, edited by G. Sprague and J. Dudley. American Society of Agronomy, Madison, WI.
- Dennehey, B. K., W. L. Petersen, C. Fordsantino, M. Pajeau and C. L. Armstrong, 1994. Comparison of selective agents for use with the selectable marker gene Bar in maize transformation. Plant Cell Tissue Organ Cult. 36: 1–7. [Google Scholar]
- Dieguez, M. J., H. Vaucheret, J. Paszkowski and O. Mittelsten Scheid, 1998. Cytosine methylation at CG and CNG sites is not a prerequisite for the initiation of transcriptional gene silencing in plants, but it is required for its maintenance. Mol. Gen. Genet. 259: 207–215. [DOI] [PubMed] [Google Scholar]
- Dorweiler, J. E., C. C. Carey, K. M. Kubo, J. B. Hollick, J. L. Kermicle et al., 2000. Mediator of paramutation1 is required for establishment and maintenance of paramutation at multiple maize loci. Plant Cell 12: 2101–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan, E. J., W. J. Peacock and E. S. Dennis, 1996. Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc. Natl. Acad. Sci. USA 93: 8449–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan, E. J., M. Wang and P. Waterhouse, 2001. Gene silencing: fleshing out the bones. Curr. Biol. 11: R99–R102. [DOI] [PubMed] [Google Scholar]
- Flavell, R. B., 1994. Inactivation of gene expression in plants as a consequence of specific sequence duplication. Proc. Natl. Acad. Sci. USA 91: 3490–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furner, I. J., M. A. Sheikh and C. E. Collett, 1998. Gene silencing and homology-dependent gene silencing in Arabidopsis: genetic modifiers and DNA methylation. Genetics 149: 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr, A. J., M. B. Jensen, T. Dalmay and D. C. Baulcombe, 2005. RNA polymerase IV directs silencing of endogenous DNA. Science 308: 118–120. [DOI] [PubMed] [Google Scholar]
- Hollick, J. B., and V. L. Chandler, 2001. Genetic factors required to maintain repression of a paramutagenic maize pl1 allele. Genetics 157: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollick, J. B., G. I. Patterson, I. M. Asmundsson and V. L. Chandler, 2000. Paramutation alters regulatory control of the maize pl locus. Genetics 154: 1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner, G., L. Mlynarova and J. P. Nap, 2000. Detailed characterization of the posttranscriptional gene-silencing-related small RNA in a GUS gene-silenced tobacco. RNA 6: 1445–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, L., F. Ratcliff and D. C. Baulcombe, 2001. RNA-directed transcriptional gene silencing in plants can be inherited independently of the RNA trigger and requires Met1 for maintenance. Curr. Biol. 11: 747–757. [DOI] [PubMed] [Google Scholar]
- Kakutani, T., 1997. Genetic characterization of late-flowering traits induced by DNA hypomethylation mutation in Arabidopsis thaliana. Plant J. 12: 1447–1451. [DOI] [PubMed] [Google Scholar]
- Kakutani, T., J. A. Jeddeloh, S. K. Flowers, K. Munakata and E. J. Richards, 1996. Developmental abnormalities and epimutations associated with DNA hypomethylation mutations. Proc. Natl. Acad. Sci. USA 93: 12406–12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakutani, T., K. Munakata, E. J. Richards and H. Hirochika, 1999. Meiotically and mitotically stable inheritance of DNA hypomethylation induced by ddm1 mutation of Arabidopsis thaliana. Genetics 151: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno, T., M. F. Mette, D. P. Kreil, W. Aufsatz, M. Matzke et al., 2004. Involvement of putative SNF2 chromatin remodeling protein DRD1 in RNA-directed DNA methylation. Curr. Biol. 14: 801–805. [DOI] [PubMed] [Google Scholar]
- Kermicle, J. L., and M. Alleman, 1990. Gametic imprinting in maize in relation to the angiosperm life cycle. Dev. Suppl., pp. 9–14. [PubMed]
- Kilby, N. J., H. M. O. Leyser and I. J. Furner, 1992. Promoter methylation and progressive transgene inactivation in Arabidopsis. Plant Mol. Biol. 20: 103–112. [DOI] [PubMed] [Google Scholar]
- Lippman, Z., B. May, C. Yordan, T. Singer and R. Martienssen, 2003. Distinct mechanisms determine transposon inheritance and methylation via small interfering RNA and histone modification. PLoS Biol. 1: E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisch, D., C. C. Carey, J. E. Dorweiler and V. L. Chandler, 2002. A mutation that prevents paramutation in maize also reverses Mutator transposon methylation and silencing. Proc. Natl. Acad. Sci. USA 99: 6130–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke, M. A., W. Aufsatz, T. Kanno, M. F. Mette and A. J. Matzke, 2002. Homology-dependent gene silencing and host defense in plants. Adv. Genet. 46: 235–275. [DOI] [PubMed] [Google Scholar]
- Mette, M. F., W. Aufsatz, J. van Der Winden, M. A. Matzke and A. J. Matzke, 2000. Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J. 19: 5194–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, P., 2000. Transcriptional transgene silencing and chromatin components. Plant Mol. Biol. 43: 221–234. [DOI] [PubMed] [Google Scholar]
- Mittelsten Scheid, O., and J. Paszkowski, 2000. Transcriptional gene silencing mutants. Plant Mol. Biol. 43: 235–241. [DOI] [PubMed] [Google Scholar]
- Mittelsten Scheid, O., K. Afsar and J. Paszkowski, 1998. Release of epigenetic gene silencing by trans-acting mutations in Arabidopsis. Proc. Natl. Acad. Sci. USA 95: 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, G. I., C. J. Thorpe and V. L. Chandler, 1993. Paramutation, an allelic interaction, is associated with a stable and heritable reduction of transcription of the maize b regulatory gene. Genetics 135: 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst, A. V., M. Fagard, F. Proux, P. Mourrain, S. Boutet et al., 2004. Arabidopsis histone deacetylase HDA6 is required for maintenance of transcriptional gene silencing and determines nuclear organization of rDNA repeats. Plant Cell 16: 1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger, D. A., and V. L. Chandler, 2001. B-Bolivia, an allele of the maize b1 gene with variable expression, contains a high copy retrotransposon-related sequence immediately upstream. Plant Physiol. 125: 1363–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger, D. A., D. Lisch and V. L. Chandler, 1998. The maize regulatory gene B-Peru contains a DNA rearrangement that specifies tissue-specific expression through both positive and negative promoter elements. Genetics 149: 1125–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin, R. K., M. Freeling and D. Lisch, 2005. Heritable transposon silencing initiated by a naturally occurring transposon inverted duplication. Nat. Genet. 37: 641–644. [DOI] [PubMed] [Google Scholar]
- Stam, M., C. Belele, W. Ramakrishna, J. E. Dorweiler, J. L. Bennetzen et al., 2002. The regulatory regions required for B′ paramutation and expression are located far upstream of the maize b1 transcribed sequences. Genetics 162: 917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes, T. L., and E. J. Richards, 2002. Induced instability of two Arabidopsis constitutive pathogen-response alleles. Proc. Natl. Acad. Sci. USA 99: 7792–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret, H., and M. Fagard, 2001. Transcriptional gene silencing in plants: targets, inducers and regulators. Trends Genet. 17: 29–35. [DOI] [PubMed] [Google Scholar]
- Waddington, C. H., 1953. Genetic assimilation of an acquired character. Evolution 7: 118–126. [Google Scholar]
- Waddington, C. H., 1956. Genetic assimilation of the bithorax phenotype. Int. J. Org. Evol. 10: 1–13. [Google Scholar]
- Waddington, C. H., 1959. Canalization of development and genetic assimilation of acquired characters. Nature 183: 1654–1655. [DOI] [PubMed] [Google Scholar]
- Woodhouse, M. R., M. Freeling and D. Lisch, 2006. The mop1 (mediator of paramutation1) mutant progressively reactivates one of the two genes encoded by the MuDR transposon in maize. Genetics 172: 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z., L. K. Johansen, A. M. Gustafson, K. D. Kasschau, A. D. Lellis et al., 2004. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2: E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman, D., X. Cao and S. E. Jacobsen, 2003. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 299: 716–719. [DOI] [PubMed] [Google Scholar]