Abstract
The yellow fever mosquito Aedes aegypti has been the subject of extensive genetic research due to its medical importance and the ease with which it can be manipulated in the laboratory. A molecular genetic linkage map was constructed using 148 amplified fragment length polymorphism (AFLP) and six single-strand conformation polymorphism (SSCP) markers. Eighteen AFLP primer combinations were used to genotype two reciprocal F2 segregating populations. Each primer combination generated an average of 8.2 AFLP markers eligible for linkage mapping. The length of the integrated map was 180.9 cM, giving an average marker resolution of 1.2 cM. Composite interval mapping revealed a total of six QTL significantly affecting Plasmodium susceptibility in the two reciprocal crosses of Ae. aegypti. Two common QTL on linkage group 2 were identified in both crosses that had similar effects on the phenotype, and four QTL were unique to each cross. In one cross, the four main QTL accounted for 64% of the total phenotypic variance, and digenic epistasis explained 11.8% of the variance. In the second cross, the four main QTL explained 66% of the variance, and digenic epistasis accounted for 16% of the variance. The actions of these QTL were either dominance or underdominance. Our results indicated that at least three new QTL were mapped on chromosomes 1 and 3. The polygenic nature of susceptibility to P. gallinaceum and epistasis are important factors for significant variation within or among mosquito strains. The new map provides additional information useful for further genetic investigation, such as identification of new genes and positional cloning.
MALARIA is one of the most fatal infectious diseases, causing significant human health problems in the subtropics and tropics. Nearly 500 million clinical malaria cases occur each year, resulting in 2.7 million deaths. These deaths are mainly among children under the age of 5 in sub-Saharan Africa. Malaria control strategies have been severely affected during the last two decades by the emergence of Plasmodium resistance to antimalaria drugs and mosquito vector resistance to insecticides.
The yellow fever mosquito Aedes aegypti is a natural vector of the avian malaria parasite, Plasmodium gallinaceum, and an important vector of dengue virus. It can be manipulated in the laboratory with ease and has been subject to extensive genetic research (e.g., Severson et al. 1995a,b; Yan et al. 1997; Yan and Severson 2003). For example, the genetic map of Ae. aegypti, which used 77 morphological, isozyme, and insecticide resistance markers covering 171 recombination map units, was the first linkage map for a mosquito species (Craig and Hickey 1967; Munstermann and Craig 1979). The first DNA marker-based genetic linkage map of Ae. aegypti was constructed in the early 1990s through a restriction fragment length polymorphism (RFLP) analysis of cDNA clones (Severson et al. 1993). This initial RFLP map for Ae. aegypti consisted of 50 DNA markers that covered 134 recombination units across the genome and included several morphological marker loci (Severson et al. 1993). Antolin et al. (1996) reported a linkage map of Ae. aegypti based on single-strand conformation polymorphism (SSCP) analysis of randomly amplified polymorphic DNA (RAPD) markers. A composite genetic linkage map for Ae. aegypti has been constructed based on the RFLP, SNP, and SSCP markers (Severson et al. 2002). The map consisted of 146 marker loci distributed across 205 cM and included several loci of morphological mutant markers. On the basis of the limited number of molecular markers and associated linkage map, quantitative trait loci (QTL) influencing Ae. aegypti susceptibility to the lymphatic filariasis nematode parasite and the avian malaria parasite were determined (Severson et al. 1994, 1995b; Beernsten et al. 1995). In particular, a major QTL on chromosome 2 and a minor QTL on chromosome 3 were identified in terms of vector competence to P. gallinaceum.
The statistical power of QTL mapping may be affected by factors such as the number of molecular markers used, the sample size of the segregating population, the number of genes controlling the traits, and the existence of gene interaction (Knapp and Bridges 1990; Melchinger et al. 1998; Coffman et al. 2003; Majumder and Ghosh 2005). Previous study of the identification of P. gallinaceum susceptibility QTL in Ae. aegypti used a limited number (16) of cDNA markers. Therefore, it is possible that other minor QTL could not be detected. In this study, we use significantly more molecular markers and a larger segregating population size to determine whether additional QTL conferring susceptibility to P. gallinaceum in Ae. aegypti may be detected. In addition, we aim to better define the genome regions of these QTL. High-resolution QTL mapping is critical for positional cloning and gene isolation. Here we report a high-resolution, amplified fragment length polymorphism (AFLP)-based genetic linkage map and the results of QTL mapping for malaria parasite susceptibility in the yellow fever mosquito Ae. aegypti. Our AFLP-based linkage map included 148 AFLP and six cDNA SSCP markers. The total map length was 180.9 cM, providing an average marker resolution of 1.2 cM. Using the AFLP markers and reciprocal F2 intercrosses between refractory and susceptible strains, we identified six QTL conferring Ae. aegypti susceptibility to P. gallinaceum.
MATERIALS AND METHODS
Mosquito populations, parasite infection, and phenotyping:
We developed two reciprocal F2 intercross segregating populations from crosses of the P. gallinaceum-susceptible Ae. aegypti RED strain and a P. gallinaceum-refractory Ae. aegypti strain selected from the Moyo-in-dry strain (MOYO-R). The selection procedure of the MOYO-R strain, its relative parasite susceptibility, and the RED strain were described by Thathy et al. (1994). In cross R5-5, an F1 generation was produced using pairwise mating between one RED female and one MOYO-R male, and F1 intercross progeny (n = 146) were produced through sibling mating. In cross M7-3, the F1 generation was produced using one RED male and one MOYO-R female; F1 intercross progeny (n = 122) were produced through sibling mating. Mosquito larvae were reared on a suspension of dried beef liver powder. Individual egg rafts were reared in separate rearing pans, and pupae were transferred to cages for adult exclusion and sibling mating. Parents and F1 generation pupae were separated by sex before adult emergence. Adults were kept in 20 × 20 × 30-cm cages and were provided cotton soaked with 2% sugar solution ad libitum. The mosquitoes were reared and maintained in an environmental chamber at 25°, 80% RH, and 16:8 hr (L:D) light cycle. A 30-min crepuscular period was set at the beginning and end of each light cycle. The F2 female mosquitoes were allowed to engorge on restrained White Leghorn chicks that were naturally infected with P. gallinaceum essentially as described by Kilama and Craig (1969), with some modifications by Thathy et al. (1994) and in accordance with animal testing guidelines. The engorged F2 females were dissected 6–7 days after being fed by the infectious blood, and the numbers of P. gallinaceum parasites that had successfully developed into oocysts in the midguts of individual mosquitoes were counted under a microscope to quantify the phenotype.
DNA extraction:
Genomic DNA was extracted individually from all the parents, F1, and F2 populations following the phenol/chloroform method (Severson 1997). The parents and F1 generation were used to establish the segregation pattern of the molecular markers.
AFLP analysis:
All individuals were subjected to genotyping with AFLP markers according to Vos et al. (1995) with some modifications (Zhong et al. 2003). Briefly, genomic DNA was double digested with EcoRI and MseI. The DNA fragments were ligated with EcoRI and MseI adaptors, generating template DNA for PCR amplification. Two primers used for PCR amplification were designed on the basis of adaptor sequences and restriction site sequences. Selective nucleotide sequences were added to the 3′-end of each primer. PCR amplification was conducted in two steps: a preselective amplification and a selective amplification. Polymorphism screening of AFLP products was conducted on a Li-Cor model 4200 automated DNA analyzer with 6.5% polyacrylamide gels. The gel electrophoresis was maintained under a constant temperature of 45°. Allele sizes were determined using the computer software, GENE IMAGIR, provided by the manufacturer (Li-Cor, Lincoln, NE). For the selective amplification, a total of 100 primer combinations were screened. Among them, 18 primer pairs that produced fragments with clear dominance inheritance patterns and reproducibility were used for linkage analysis. These included L1B1, L1A1, L1A8, L1A9, L1A10, L2B1, L2A8, L2A9, L2A10, L2A14, L3B1, L3A8, L3A9, L3A10, L4B1, L4A8, L4A9, and L4A10. These AFLP primer sequences and marker designations were reported by Zhong et al. (2003).
SSCP analysis:
A total of 20 SSCP markers were selected from the map of Fulton et al. (2001) for screening polymorphisms between the two parent strains, RED and MOYO-R. On the basis of marker polymorphism and location on three chromosomes, six markers (Hexam2, Peroxnc, Fax, Sin3, Gpd-1, and Apyr2) were selected for genotyping the mosquito populations. PCR was completed in thin-walled polycarbonate plates, each with 96 wells (Fisher Scientific, Pittsburgh). SSCP analyses were conducted on a dedicated height nucleic acid sequencing system (C.B.S. Scientific, Del Mar, CA) using 0.6× mutation detection enhancement (MDE) gels, following Bosio et al. (2000) and Martins-Lopes et al. (2001) with modifications. Briefly, 10 μl of PCR product was denatured by adding 4 μl of loading dye (95% formamide, 10 mm EDTA, 1.0% bromophenol blue), followed by heating at 95° for 5 min and quenching on ice. Six microliters of sample were loaded on standard sequencing gels (33 cm × 42 cm × 0.4 mm) containing 18 ml 2× MDE gel solution (BMA, Rockland ME), 3.6 ml 10× TBE buffer, and 38.4 ml water, which was polymerized by the addition of 0.6 ml of 10% w/v ammonium persulfate and 60 μl of TEMED. The gel electrophoresis was maintained at a constant power of 8 W for 5 hr at room temperature. Gels were stained with silver nitrate following the method of Bassam et al. (1991) and Zhong et al. (2004).
Linkage analysis and QTL mapping:
Linkage map construction and QTL analysis followed Zhong et al. (2003, 2004). Briefly, we analyzed the data using the Kosambi map function (Kosambi 1944) of Mapmaker/Exp v 3.0 (Lincoln et al. 1992) to develop a linkage map for each population. The computer software JoinMap (Stam 1993; Van Ooijen and Voorrips 2001) was used to produce the composite map. QTL analyses were conducted separately for crosses R5-5 and M7-3 to determine whether similar QTL could be identified in the two crosses. The computer software Mapmanager QTX (Manly et al. 2001) was used to determine the QTL positions, the expected additive and dominance effects, and the phenotypic variance explained by individual QTL. The LOD threshold value for declaring the presence of a QTL was determined by a permutation test (n = 1000) (Churchill and Doerge 1994). Average levels of dominance (h) were estimated using the ratio dominance (d)/additive effects (a) (Stuber et al. 1987). The individual QTL designation was made using the following format: pgs [n, y], where pgs is P. gallinaceum susceptibility, n is the linkage group number, and y is the AFLP marker closest to the QTL.
RESULTS
AFLP primer screening:
A total of 100 primer combinations were screened. Eighteen primer pairs that produced polymorphic fragments with clear dominance inheritance patterns and reproducibility were used for linkage analysis (18%). Among the 100 AFLP primer combinations, 48 primer combinations produced polymorphic fragments between the RED and MOYO-R strains. We selected 18 pairs of AFLP primer combinations for segregation analysis on the F2 intercross populations on the basis of reproducibility and the extent of polymorphism. Only the polymorphic fragments that showed dominant segregation and could be scored unambiguously were used for construction of a linkage map. A total of 146 and 138 qualified polymorphic fragments were selected in the crosses R5-5 and M7-3, respectively. In R5-5, a total of 41 (45.6%) dominant alleles were descended from the RED strain. Similarly, 50 (53.2%) dominant alleles were descended from the RED strain in cross M7-3. All markers with dominant alleles descended from the RED strain are underlined in Figure 1. There were 97 fragments common to the two crosses. On average, each primer combination generated 7.7–8.1 fragments, with sizes ranging from 45 to 487 bp, which could be used for linkage mapping. The L2/A9 primer combination produced the most qualifying polymorphic fragments in both crosses (15 for R5-5 and 14 for M7-3), whereas the L2/A8 primer combination produced the fewest (2 each for both crosses).
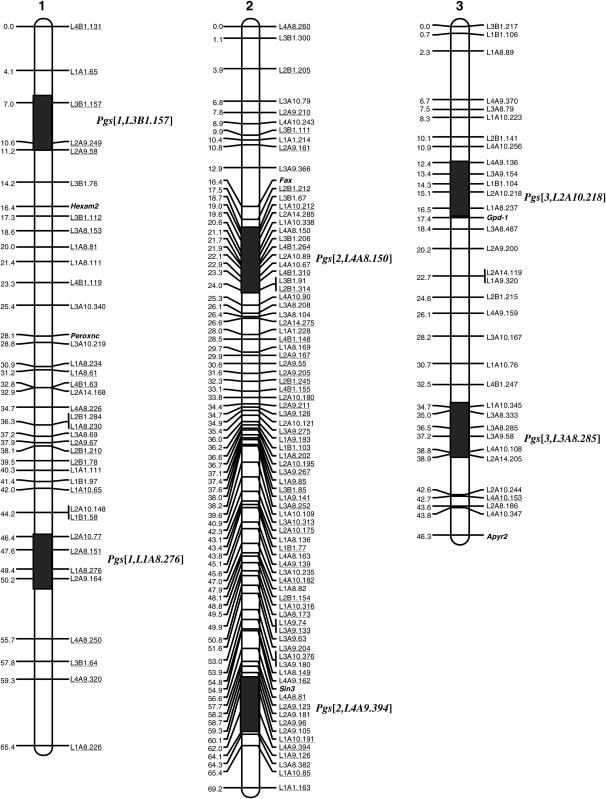
Figure 1.—
AFLP-based linkage map of Ae. aegypti and QTL locations for the susceptibility to the malaria parasite P. gallinaceum detected in two reciprocal F2 intercrosses. The numbers on the left side of each linkage group are genetic distances in Kosambi centimorgans. AFLP markers are designated following Zhong et al. (2003). The SSCP markers are indicated by italics. With underlined markers, the dominant allele was descended from the RED strain while with the markers without underlining, the dominant allele was descended from the MOYO-R strain.
Map construction:
The linkage map derived from the cross R5-5 contained 126 AFLP and six SSCP markers that were assigned to three linkage groups at the LOD threshold of 4.5. Twenty AFLP markers could not be assigned to any linkage group. The total map distance was 186 cM. A total of 30 markers (23.8%) showed significant distortion from the Mendelian segregation ratio, and most of these markers were on chromosome 1. For cross M7-3, 120 AFLP and six SSCP markers were mapped to three linkage groups with a total map length of 176 cM. Eighteen AFLP markers could not be assigned to any linkage group. Twenty-five markers (20.8%), found mostly in the middle of chromosome 1, exhibited significant distortion from the Mendelian segregation ratio.
The linkage maps generated from the two independent segregating populations were very similar in marker order and map distance in each linkage group. Alignment of the two maps revealed that the markers common to the two maps fell into the same linkage groups. Consequently, an integrated map comprising markers from both populations was constructed using a LOD threshold of 4.0. The integrated linkage map consisted of 148 AFLP markers and six SSCP markers (Figure 1). The six SSCP loci were used as anchor markers for assigning linkage groups to each mosquito chromosome, following the map of Fulton et al. (2001). The total recombination distance over the three chromosomes was 180.9 cM after correction for double crossovers using the Kosambi function; this was longer than the SSCP map (Fulton et al. 2001). The AFLP markers did not show any significant clustering near the centromeres or the distal region of the chromosomes, suggesting that they provide good coverage of the genome (Figure 1).
Phenotypic variability in Plasmodium susceptibility:
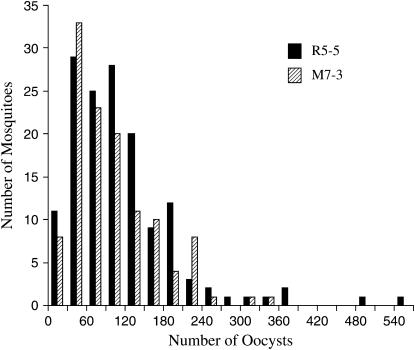
Significant genetic variation for Plasmodium susceptibility segregated in the two F2 populations. The number of oocysts in the F2 population ranged from 1 to 570. The oocyst frequency distribution was determined for two F2 segregating populations from a reciprocal intercross between the P. gallinaceum-susceptible RED strain and the P. gallinaceum-refractory MOYO-R strain (Figure 2). The mean oocyst counts and standard deviations (SD) within the F2 population were 90.1 ± 87.4 (n = 146) and 76.7 ± 66.2 (n =122) for R5-5 and M7-3, respectively.
Figure 2.—
Frequency distribution of the number of P. gallinaceum oocysts in two F2 segregating Ae. aegypti populations derived from pairwise mating between RED and MOYO-R strains. R5-5 represents an F2 segregating population from a cross between a RED female and a MOYO-R male, while M7-7 represents a cross between a MOYO-R female and a RED male.
QTL analysis:
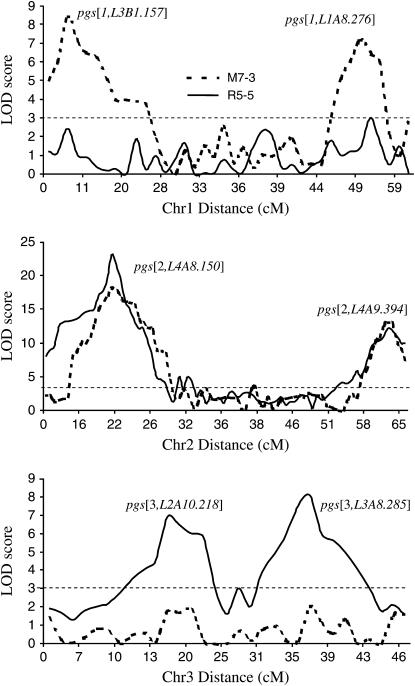
Using the composite interval mapping method, we detected four QTL on chromosomes 2 and 3 that significantly affect the parasite susceptibility in the R5-5 group (Figure 1). The LOD score plots for linkage groups with the identified QTL provide a basis for identifying the molecular markers most closely linked to the QTL (Figure 3). These four QTL are designated as pgs [2, L4A8.150], pgs [2, L4A9.394], pgs [3, L2A10.218], and pgs [3, L3A8.285]. The detected QTL explained 30, 18, 8, and 10%, respectively, of the phenotypic variations in parasite susceptibility (Table 1). The four QTL collectively explained ∼64% of the total phenotypic variation. The permutation tests indicated that all four QTL were statistically significant (P < 0.01), with additive effects ranging from 9.7 to 52.6 oocysts (Table 1). A positive additive regression coefficient suggested that the susceptible strain RED contributed the alleles for increased parasite susceptibility (Table 1). The two QTL (pgs [2, L4A8.150] and pgs [3, L2A10.218]) that had a positive ratio of additive regression coefficient to dominance regression coefficient (h) indicated that the gene action at these QTL was primarily dominance or partial dominance. In contrast, pgs [2, L4A9.394] and pgs [3, L3A8.285] exhibited positive additive and negative dominance regression coefficients with a negative h value, suggesting that the gene action at these two QTL was underdominance or recessive.
Figure 3.—
Composite interval mapping of the susceptibility to the malaria parasite P. gallinaceum in Ae. aegypti. Cross R5-5 represents an F2 segregating population from a cross between a RED female and a MOYO-R male, while M7-3 represents a cross between a RED male and a MOYO-R female. Significance thresholds are indicated by dashed horizontal lines, with LOD = 3.0 (genomewide P < 0.001) as determined by 1000 permutations of our mapping data (Churchill and Doerge 1994).
TABLE 1.
Genetic parameters as estimated by composite interval mapping of the QTL affecting the susceptibility to the malaria parasite P. gallinaceum in Ae. aegypti
| Cross | QTLa | Marker interval | LOD | R2 (%) | P | ab | dc | hd |
|---|---|---|---|---|---|---|---|---|
| R5-5 | pgs [2, L4A8.150] | L4A8.150–L3B1.206 | 23.1 | 30 | 0.000 | 52.6 | 32.7 | 0.62 |
| pgs [2, L4A9.394] | L4A9.394–L1A9.126 | 12.2 | 18 | 0.000 | 42.9 | −22.1 | −0.52 | |
| pgs [3, L2A10.218] | L2A10.218–L1A8.237 | 6.9 | 8 | 0.004 | 9.7 | 12.8 | 1.32 | |
| pgs [3, L3A8.285] | L3A8.333–L3A8.285 | 8.1 | 10 | 0.003 | 21.7 | −22.8 | −1.05 | |
| R7-3 | pgs [1, L3B1.157] | L3B1.157–L2A9.249 | 10.6 | 11 | 0.001 | −11.1 | 44.0 | −3.96 |
| pgs [1, L1A8.276] | L2A8.151–L1A8.276 | 7.2 | 9 | 0.003 | 8.9 | 11.2 | 1.23 | |
| pgs [2, L4A8.150] | L4A8.150–L3B1.206 | 18.3 | 26 | 0.000 | 48.5 | 40.9 | 0.84 | |
| pgs [2, L4A9.394] | L4A9.394–L1A9.126 | 13.0 | 20 | 0.000 | 40.7 | −20.3 | −0.50 |
Individual QTL designation has the following format: pgs [n, y], where pgs is P. gallinaceum susceptibility, n is the linkage group number, and y is the AFLP marker that is closer to the QTL region.
The additive regression coefficient for the association or the additive effect due to substitution of a RED allele by the corresponding MOYO-R allele. Positive value represents increasing mosquito susceptibility; negative represents decreasing mosquito susceptibility.
The dominance effect associated with the heterozygote.
The ratio dominance/additive effects. Underdominance or recessive if h < 0, additive if h = 0–0.20, partial dominance if h = 0.21–0.80, dominance if h = 0.81–1.20, and overdominance if h > 1.20 (Stuber et al. 1987).
For M7-3, four QTL (Figure 1), designated as pgs [1, L3B1.157], pgs [1, L1A8.276], pgs [2, L4A8.150], and pgs [2, L4A9.394], were identified. These four QTL were statistically significant at the level of P < 0.01 and accounted for 11, 9, 26, and 20% of variances in parasite susceptibility, respectively (Table 1). The QTL pgs [2, L4A8.150] and pgs [2, L4A9.394] identified in M7-3 exhibited the same gene actions as in R5-5. In addition to the two QTL detected on chromosome 1 in R5-5 (pgs [2, L4A8.150] and pgs [2, L4A9.394]), another two QTL (pgs [1, L3B1.157] and pgs [1, L1A8.276]) were found in M7-3 on chromosome 1 (Figure 3). The QTL pgs [1, L3B1.157] and pgs [1, L1A8.276] on chromosome 1 had additive effects of 11.1 and 8.9 parasite susceptibility, respectively (Table 1). The positive additive regression coefficients at pgs [1, L1A8.276] suggested that the susceptible RED strain contributed the alleles for increased parasite susceptibility. The gene action was underdominance or recessive at pgs [1, L3B1.157], but dominance at pgs [1, L1A8.276], as shown by the high h values (Table 1).
Digenic epistasis:
Two digenic epistatic QTL were detected for parasite susceptibility in each population (Table 2). The two digenic epistatic QTL in the R5-5 group involved three previously identified QTL markers (L4A8.150, L3A8.285, and L2A10.218), and they accounted for 11.8% of the total phenotypic variation. However, in M7-3, only one of the digenic epistatic QTL involved a previously identified QTL marker (L4A9.394), and the two digenic epistatic QTL accounted for 16.0% of the total phenotypic variation.
TABLE 2.
Digenic epistatic QTL affecting the susceptibility to the malaria parasite P. gallinaceum in Ae. aegypti
| LOD for:
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Cross | Chromosome | Marker i | Chromosome | Marker j | αi | αj | αiαj | R2 (%)a |
| R5-5 | 1 | L2A8.151 | 2 | L4A8.150 | 6.4 | 4.9 | 14.4 | 5.6 |
| 3 | L3A8.285 | 3 | L2A10.218 | 8.3 | 9.5 | 17.3 | 6.2 | |
| M7-3 | 1 | L4A8.226 | 2 | L2A14.275 | 7.8 | 10.8 | 18.3 | 8.3 |
| 3 | Apyr2 | 2 | L4A9.394 | 5.9 | 10.5 | 15.2 | 7.7 | |
Italics indicate that a marker is a main-effect QTL marker identified by composite interval mapping.
R2 is the proportion of trait variation (parasite susceptibility) explained by the digenic epistatic QTL.
DISCUSSION
In this study, we developed an AFLP-based linkage map for the yellow fever mosquito Ae. aegypti. The map consisted of 148 AFLP markers and six previously mapped SSCP markers. The length of the AFLP-based genetic map was 180.9 cM, giving an average marker resolution of 1.2 cM. Using this map, a total of six QTL that significantly affect the mosquito's susceptibility to P. gallinaceum were detected in the two reciprocal crosses in Ae. aegypti. The six QTL were located on all three of the mosquito's chromosomes. Two common QTL on linkage group 2 were identified in both crosses, with similar effects on the phenotype. Four QTL were unique to each cross. In one cross, the four main QTL accounted for 64% of the total phenotypic variance, and digenic epistasis explained 11.8% of the variance. In the second cross, the four main QTL explained 66% of the variance, and digenic epistasis accounted for only 16% of the variance. The actions of three QTL (one on each chromosome) were either dominance or partial dominance, while the actions of another three QTL (one on each chromosome) were underdominance or recessive.
Map analyses showed that the AFLP markers generated with the EcoRI/MseI restriction enzymes were not distributed among the mosquito's three chromosomes in proportion to the chromosomes' genetic length. Chromosome 2 contained more markers than expected, primarily due to an enrichment of markers around its centromere. However, the AFLP markers were found along nearly the entire length of the Ae. aegypti genome, and most genetic distances between the consecutive pairs of the AFLP markers were smaller than 5 cM. Therefore, the AFLP markers provided good coverage for the majority of the Ae. aegypti genome, showing that this technology efficiently generates dense molecular linkage maps in a relatively short period of time compared with RFLP or other PCR-based markers.
In the study, we found deviations from the expected 3:1 segregation ratio in the reciprocal F2 intercross population of Ae. aegypti. These deviations were 53.8% of AFLP loci on chromosome 1, 21.7% of the AFLP loci on chromosome 2, and 12.5% of AFLP loci on chromosome 3. This type of distorted segregation in the molecular markers used for linkage mapping has been repeatedly observed in plants, invertebrates, and vertebrates. Lu et al. (1998) reported that ∼15% of the AFLP markers deviated from the expected 3:1 or 1:2:1 segregation ratios in the K62-68 family of peach rootstocks. Likewise, Xu et al. (1997) observed that 6.8–31.8% of the mapped marker loci showed segregation distortions of AFLP in populations of rice (Oryza sativa L.). Also, Paillard et al. (1996) found that 12% of RFLP markers and 20% of RAPD markers in a linkage map of a doubled-haploid population of coffee (Coffea canephora) deviated from the expected segregation ratio of 1:1. Abnormal inheritance also occurred with ∼25% of the AFLP markers observed in soybean (Prabhu and Gresshoff 1994). Using AFLP markers, a distortion rate of 54% was reported for silkworm (Tan et al. 2001), 16% for channel catfish (Liu et al. 2003), and 14.5% for Tribolium castaneum (Zhong et al. 2004). In Ae. aegypti, Severson et al. (1995b) observed that significant deviations from the expected 1:2:1 ratio were evident for F2 progeny for all RFLP loci on chromosomes 1, 2, and 3. We found a distortion rate of 22% [(30+25)/(126+120)] in this study.
Several reasons may account for the observed marker ratio distortion, including a marker's location in relation to a chromosomal inversion polymorphism, as commonly found in Anopheles mosquitoes such as An. arabiensis (Temu and Yan 2005) and An. gambiae (Severson et al. 2004). Partially isolated subtaxa, M and S forms of An. gambiae, with extensive gene flow occurring between them, possess certain chromosomal regions referred to as “speciation islands” that harbor significant genetic differentiation with genes responsible for reproductive isolation (Turner et al. 2005). Segregation of molecular markers located in such a speciation island chromosomal region is likely to exhibit a ratio distortion. Another reason for such a distortion might be amplification of a single-sized fragment derived from several genomic regions (Faris et al. 1998). Or it may be simply because we sampled an infinite mapping population of selected strains with genetic characteristics arising from many years of colonization in the laboratory.
In our study, all the AFLP loci on chromosome 3 (except on marker L4A10.153) reflected a deficiency in the RED genotype. The deficiency of the RED strain genotype suggested that the segregation distortion might be caused by a partial lethal factor on chromosome 3 acting on the F2 generations (Severson et al. 1995b). The high proportion (53.8%) of AFLP loci on chromosome 1 deviated from expected Mendelian segregation ratios, probably due to the sex gene on chromosome 1 in Ae. aegypti. This single autosomal gene determines the sex, with maleness being the dominant allele (Gilchrist and Haldane 1947). Therefore, the observed segregation ratios for female F2 progeny would reflect a bias toward the maternal genotype (Severson et al. 1993).
Severson et al. (1995b) used RFLP markers to examine susceptibility to P. gallinaceum in Ae. aegypti and identified two QTL with significant effects on the phenotype. One QTL on chromosome 2, designated pgs [2, LF98] or pgs1, accounted for 49 and 65% of the phenotypic variance in two independently prepared populations and exhibited a partial dominance effect on susceptibility. A second QTL on chromosome 3, designated pgs [3, MalI] or pgs2, accounted for 10 and 14% of the observed phenotypic variance in the populations and exhibited an additive effect on susceptibility. The identification of multiple QTL regions illustrated the complexity of genetic mechanisms involved in P. gallinaceum susceptibility (Jimenez et al. 2004). Our results suggested that the locations and effects of the two QTL (pgs [2, L2A150] and pgs [3, L2A10]) were consistent with the QTL affecting mosquito susceptibility previously identified using the RFLP marker (pgs [2, LF98] and pgs [3, MalI]) because of the two SSCP markers (Fax and Gpd-1) that were very close to the two QTL regions. Furthermore, we identified at least three new QTL (two QTL on chromosome 1 and one QTL on chromosome 3) that had not been previously reported.
Coevolution has developed complex and often antagonistic interactions between Plasmodium species and mosquito species. Comparative studies of different genera and species of mosquitoes can provide new information for unraveling the complex processes of parasite–mosquito interaction. A broad-scale, genomewide chromosomal comparative analysis of Ae. aegypti and An. gambiae showed apparent extensive conservation of chromosome segments between the two species of different genera, with limited evidence for linear order conservation within these genome segments (Severson et al. 2004). However, different genomic organization (Knudson et al. 2002) and the different rearrangements of chromosomal inversions are prominent in the evolution of anopheline chromosomes (Coluzzi et al. 2002; Sharakhov et al. 2002). Therefore, for robust genome comparisons that allow determination of the genetic basis of interesting phenotypes among related dipterans, high-resolution gene maps and complete genome sequencing of representative dipteran species are required for more species than An. gambiae alone.
Understanding of parasite–mosquito interactions, especially of the genes and enzyme systems involved, is essential for developing strategies that will reduce malaria transmission through the mosquito vector. Although anopheline mosquitoes are responsible for transmission of human malaria (P. falciparum), experimental models have demonstrated that An. stephensi, An. gambiae, and Ae. aegypti have the ability to transmit the avian (chicken) malaria parasite P. gallinaceum. The rodent malaria parasite P. berghei can complete its life cycle in anophelines such as An. gambiae and An. stephensi, but it is not transmissible through Ae. aegypti (Alavi et al. 2003; Abraham and Jacobs-Lorena 2004). The mosquito midgut is one of the major impediments between the parasite and the mosquito. Failure of the parasite to negotiate this barrier deters its development and is likely the main cause of mosquito refractoriness. Parasite–vector combinations and ookinete development efficiency vary among different mosquito species due to species-specific incompatibilities at the midgut epithelium. This suggests that these parasite–mosquito combinations and mosquito susceptibility to malarial infection are regulated by multiple interventions of many genes and enzymes in the development of the parasites (Alavi et al. 2003; Abraham and Jacobs-Lorena 2004).
A mosquito-specific salivary gland surface protein (SGS) family is widespread among mosquito species. Certain anti-SGS antibodies inhibit sporozoite invasion into the salivary glands in vivo, confirming that this protein family encompasses candidate sporozoite receptors (Korochkina et al. 2006). Furthermore, microarray analyses for identification of Plasmodium refractoriness in Ae. aegypti have identified 28 EST of differentially expressed candidate genes. Among them, 10 showed no significant similarity to any known genes, 6 clones matched with unannotated genes of An. gambiae, and 12 clones exhibited significant similarity to known genes (Chen et al. 2004). Anophelines and culicines, including Ae. aegypti, have evolved fundamentally different biochemistry in cellular responses and mechanisms of epithelial repair after parasite invasion; the midgut responses to parasite invasion were not universally conserved in different vector–parasite pairs (Gupta et al. 2005). Such variations are mostly likely mediated by different genes and enzyme systems, a consequence resulting from the considerable evolutionary history separating the anopheline and culicine genera and the bird-, rodent-, and human-infecting Plasmodium species.
In summary, we developed a genetic linkage map based on AFLP markers. The reported map, composed of 148 AFLP loci across 180.9 cM with an average distance of 1.2 cM, will be useful in further genetic investigation such as gene identification and positional cloning. We identified six QTL that affected Ae. aegypti susceptibility to the parasite P. gallinaceum. These QTL exhibited small to medium effects on susceptibility, and the effects of these QTL were dominance or underdominance. Digenic epistasis was a major source of variation in the susceptibility seen, and its effects depended on the genetic background of the mosquitoes. Therefore, the polygenic nature of susceptibility to P. gallinaceum and epistasis were important mechanisms leading to significant variation in susceptibility within or among mosquito strains.
Acknowledgments
We thank Paul Zwiers and Monica Karsay-Klein for their assistance in this study. This research was supported by National Institutes of Health grants D43 TW01505 and R01 AI50243 and United Nations Development Programme/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases grant A10429.
References
- Abraham, E. G., and M. Jacobs-Lorena, 2004. Mosquito midgut barriers to malaria parasite development. Insect Biochem. Mol. Biol. 34: 667–671. [DOI] [PubMed] [Google Scholar]
- Alavi, Y., M. Arai, J. Mendoza, M. Tufet-Bayona, R. Sinha et al., 2003. The dynamics of interactions between Plasmodium and the mosquito: a study of the infectivity of Plasmodium berghei and Plasmodium gallinaceum, and their transmission by Anopheles stephensi, Anopheles gambiae and Aedes aegypti. Int. J. Parasitol. 33: 933–943. [DOI] [PubMed] [Google Scholar]
- Antolin, M. F., C. F. Bosio, J. Cotton, W. Sweeney, M. R. Strand et al., 1996. Intensive linkage mapping in a wasp (Bracon hebetor) and a mosquito (Aedes aegypti) with single-strand conformation polymorphism analysis of random amplified polymorphic DNA markers. Genetics 143: 1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassam, B. J., G. Caetano-Anolles and P. M. Gresshoff, 1991. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal. Biochem. 196: 80–83. [DOI] [PubMed] [Google Scholar]
- Beernsten, B. T., D. W. Severson, J. A. Klinkhammer, V. A. Kassner and B. M. Christensen, 1995. Aedes aegypti: a quantitative trait locus (QTL) influencing filarial worm intensity is linked to QTL for susceptibility to other mosquito-borne pathogens. Exp. Parasitol. 81: 355–362. [DOI] [PubMed] [Google Scholar]
- Bosio, C. F., R. E. Fulton, M. L. Salasek, B. J. Beaty and W. C. Black, Iv, 2000. Quantitative trait loci that control vector competence for dengue-2 virus in the mosquito Aedes aegypti. Genetics 156: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., J. Wang, P. Liang, M. Karsay-Klein, A. A. James et al., 2004. Microarray analysis for identification of Plasmodium-refractoriness candidate genes in mosquitoes. Genome 47: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Churchill, G. A., and R. W. Doerge, 1994. Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman, C., R. W. Doerge, M. Wayne and L. McIntyre, 2003. Intersection tests for single marker QTL analysis can be more powerful than two marker QTL analysis. BMC Genet. 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluzzi, M., A. Sabatini, A. Della Torre, M. A. Di Deco and V. Petrarca, 2002. A polytene chromosome analysis of the Anopheles gambiae species complex. Science 298: 1415–1418. [DOI] [PubMed] [Google Scholar]
- Craig, G. B. J., and W. A. Hickey, 1967. Genetics of Aedes aegypti, pp. 67–131 in Genetics of Insect Vectors of Disease, edited by J. W. Wright and R. Pal. Elsevier, Amsterdam.
- Gupta, L., S. Kumar, Y. S. Han, P. F. Pimenta and C. Barillas-Mury, 2005. Midgut epithelial responses of different mosquito-Plasmodium combinations: the actin cone zipper repair mechanism in Aedes aegypti. Proc. Natl. Acad. Sci. USA 102: 4010–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris, J. D., B. Laddomada and B. S. Gill, 1998. Molecular mapping of segregation distortion loci in Aegilops tauschii. Genetics 149: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton, R. E., M. L. Salasek, N. M. Duteau and W. C. Black, IV, 2001. SSCP analysis of cDNA markers provides a dense linkage map of the Aedes aegypti genome. Genetics 158: 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist, B. M., and J. B. S. Haldane, 1947. Sex linkage and sex determination in a mosquito, Culex molestus. Hereditas 33: 175–190. [Google Scholar]
- Jimenez, L. V., B.-K. Kang, B. Debruyn, D. D. Lovin and D. W. Severson, 2004. Characterization of an Aedes aegypti BAC library and chromosomal assignment of BAC clones for physical mapping QTL that influence Plasmodium susceptibility. Insect Mol. Biol. 13: 37–44. [DOI] [PubMed] [Google Scholar]
- Kilama, W. L., and J. G. B. Craig, 1969. Monofactorial inheritance of susceptibility to Plasmodium gallinaceum in Aedes aegypti. Ann. Trop. Med. Parasitol. 63: 419–432. [DOI] [PubMed] [Google Scholar]
- Knapp, S. J., and W. C. Bridges, 1990. Using molecular markers to estimate quantitative trait locus parameters: power and genetic variances for unreplicated and replicated progeny. Genetics 126: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson, D. L., S. E. Brown and D. W. Severson, 2002. Culicine genomics. Insect Biochem. Mol. Biol. 32: 1193–1197. [DOI] [PubMed] [Google Scholar]
- Korochkina, S., C. Barreau, G. Pradel, E. Jeffery, J. Li et al., 2006. A mosquito-specific protein family includes candidate receptors for malaria sporozoite invasion of salivary glands. Cell Microbiol. 8: 163–175. [DOI] [PubMed] [Google Scholar]
- Kosambi, D. D., 1944. The estimation of map distance from recombination values. Ann. Eugen. 12: 172–175. [Google Scholar]
- Lincoln, S., M. Daly and E. Lander, 1992. Constructing Genetics Maps With MAPMAKER/EXP 3.0. Whitehead Technical Report, Ed. 2. Whitehead Institute, Cambridge, MA.
- Liu, Z., A. Karsi, P. Li, D. Cao and R. Dunham, 2003. An AFLP-based genetic linkage map of channel catfish (Ictalurus punctatus) constructed by using an interspecific hybrid resource family. Genetics 165: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z. X., B. Sosinski, G. L. Reighard, W. V. Baird and A. G. Abbott, 1998. Construction of a genetic linkage map and identification of AFLP markers for resistance to root-knot nematodes in peach rootstocks. Genome 41: 199–207. [Google Scholar]
- Majumder, P. P., and S. Ghosh, 2005. Mapping quantitative trait loci in humans: achievements and limitations. J. Clin. Invest. 115: 1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly, K. F., J. R. Cudmore and J. M. Meer, 2001. Map Manager QTX: cross-platform software for genetic mapping. Mamm. Genome 12: 930–932. [DOI] [PubMed] [Google Scholar]
- Martin-Lopes, P., H. Zhang and R. Koebner, 2001. Detection of single nucleotide mutations in wheat using single strand conformation polymorphism gels. Plant Mol. Biol. Rep. 19: 159–162. [Google Scholar]
- Melchinger, A. E., H. F. Utz and C. C. Schon, 1998. Quantitative trait locus (QTL) mapping using different testers and independent population samples in maize reveals low power of QTL detection and large bias in estimates of QTL effects. Genetics 149: 383–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munstermann, L. E., and G. B. J. Craig, 1979. Genetics of Aedes aegypti: updating the linkage map. J. Hered. 70: 291–296. [Google Scholar]
- Paillard, M., P. Lashermes and V. Petiard, 1996. Construction of a molecular linkage map in coffee. Theor. Appl. Genet. 93: 41–47. [DOI] [PubMed] [Google Scholar]
- Prabhu, R. H., and P. M. Gresshoff, 1994. Inheritance of polymorphic markers generated by DNA amplification fingerprinting and their use as genetic markers in soybean. Plant Mol. Biol. 26: 105–116. [DOI] [PubMed] [Google Scholar]
- Severson, D. W., 1997. RFLP analysis of insect genomes, pp. 309–320 in The Molecular Biology of Insect Disease Vectors, edited by J. M. Crampton, C. B. Beard and C. Louis. Chapman & Hall, London.
- Severson, D. W., A. Mori, Y. Zhang and B. M. Christensen, 1993. Linkage map for Aedes aegypti using restriction fragment length polymorphisms. J. Hered. 84: 241–247. [DOI] [PubMed] [Google Scholar]
- Severson, D. W., A. Mori, Y. Zhang and B. M. Christensen, 1994. Chromosomal mapping of two loci affecting filarial worm susceptibility in Aedes aegypti. Insect Mol. Biol. 3: 67–72. [DOI] [PubMed] [Google Scholar]
- Severson, D. W., A. Mori, V. A. Kassner and B. M. Christensen, 1995. a Comparative linkage maps for the mosquitoes, Aedes albopictus and Aedes aegypti, based on common RFLP loci. Insect Mol. Biol. 4: 41–45. [DOI] [PubMed] [Google Scholar]
- Severson, D. W., V. Thathy, A. Mori, Y. Zhang and B. M. Christensen, 1995. b Restriction fragment length polymorphism mapping of quantitative trait loci for malaria parasite susceptibility in the mosquito Aedes aegypti. Genetics 139: 1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson, D. W., J. K. Meece, D. D. Lovin, G. Saha and I. Morlais, 2002. Linkage map organization of expressed sequence tags and sequence tagged sites in the mosquito, Aedes aegypti. Insect Mol. Biol. 11: 371–378. [DOI] [PubMed] [Google Scholar]
- Severson, D. W., B. Debruyn, D. D. Lovin, S. E. Brown, D. L. Knudson et al., 2004. Comparative genome analysis of the yellow fever mosquito Aedes aegypti with Drosophila melanogaster and the malaria vector mosquito Anopheles gambiae. J. Hered. 95: 103–113. [DOI] [PubMed] [Google Scholar]
- Sharakhov, I. V., A. C. Serazin, O. G. Grushko, A. Dana, N. Lobo et al., 2002. Inversions and gene order shuffling in Anopheles gambiae and Anopheles funestus. Science 298: 182–185. [DOI] [PubMed] [Google Scholar]
- Stam, P., 1993. Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant J. 5: 739–744. [Google Scholar]
- Stuber, C. W., M. D. Edwards and J. F. Wendel, 1987. Molecular markers facilitated investigations of quantitative trait loci in maize. II. Factors influencing yield and its component traits. Crop Sci. 27: 639–648. [Google Scholar]
- Tan, Y. D., C. Wan, Y. Zhu, C. Lu, Z. Xiang et al., 2001. An amplified fragment length polymorphism map of the silkworm. Genetics 157: 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temu, E., and G. Yan, 2005. Microsatellite and mitochondrial genetic differentiation of Anopheles arabiensis in Kenya. Am. J. Trop. Med. Hyg. 73: 726–733. [PubMed] [Google Scholar]
- Thathy, V., D. W. Severson and B. M. Christensen, 1994. Reinterpretation of the genetics of susceptibility of Aedes aegypti to Plasmodium gallinaceum. J. Parasitol. 80: 705–712. [PubMed] [Google Scholar]
- Turner, T. L., M. W. Hahn and S. V. Nuzhdin, 2005. Genomic islands of speciation in Anopheles gambiae. PLoS Biol. 3: e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen, J. W., and R. E. Voorrips, 2001. JoinMap Version 3.0: Software for the Calculation of Genetic Linkage Maps. Plant Research International, Wageningen, The Netherlands.
- Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van de Lee et al., 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23: 4407–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., L. Zhu, J. Xiao, N. Huang and S. R. Mccouch, 1997. Chromosomal regions associated with segregation distortion of molecular markers in F2, backcross, double haploids, and recombinant inbred populations in rice (Oryza sativa L.). Mol. Gen. Genet. 253: 535–545. [DOI] [PubMed] [Google Scholar]
- Yan, G., and D. W. Severson, 2003. Dynamics of molecular markers linked to the resistance loci in a mosquito-Plasmodium system. Genetics 164: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, G., B. Christensen and D. Severson, 1997. Comparisons of genetic variability and genome structure among mosquito strains selected for refractoriness to a malaria parasite. J. Hered. 88: 187–194. [DOI] [PubMed] [Google Scholar]
- Zhong, D., A. Pai and G. Yan, 2003. Quantitative trait loci for susceptibility to tapeworm infection in the red flour beetle. Genetics 165: 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, D., A. Pai and G. Yan, 2004. AFLP-based genetic linkage map for the red flour beetle, Tribolium castaneum. J. Hered. 95: 53–61. [DOI] [PubMed] [Google Scholar]