Abstract
The nature of epistatic interactions between genes encoding interacting proteins in hybrid organisms can have important implications for the evolution of postzygotic reproductive isolation and speciation. At this point very little is known about the fitness differences caused by specific closely interacting but evolutionarily divergent proteins in hybrids between populations or species. The intertidal copepod Tigriopus californicus provides an excellent model in which to study such interactions because the species range includes numerous genetically divergent populations that are still capable of being crossed in the laboratory. Here, the effect on fitness due to the interactions of three complex III proteins of the electron transport system in F2 hybrid copepods resulting from crosses of a pair of divergent populations is examined. Significant deviations from Mendelian inheritance are observed for each of the three genes in F2 hybrid adults but not in nauplii (larvae). The two-way interactions between these genes also have a significant impact upon the viability of these hybrid copepods. Dominance appears to play an important role in mediating the interactions between these loci as deviations are caused by heterozygote/homozygote deleterious interactions. These results suggest that the fitness consequences of the interactions of these three complex III-associated genes could influence reproductive isolation in this system.
EPISTASIS contributes to a wide range of evolutionary phenomena such as reproductive isolation, the potential for the evolution of sex, and the nature of adaptation (Whitlock and Phillips 1995). However, specific interactions between individual loci and the impact of these interactions on fitness are largely unknown for naturally occurring variation in most animal species. In the context of speciation, epistasis plays a primary role in postzygotic reproductive isolation, which is thought to be caused primarily by negative fitness interactions between loci. These deleterious interactions are known as Dobzhansky–Muller (D–M) incompatibilities (the model was originally proposed by Dobzhansky 1936 and Muller 1942). D–M incompatibilities are hypothesized to result from the breakup of positive epistasis that develops between interacting genes within distinct evolutionary lineages (coadapted gene complexes). These interactions are generally thought to be between two or more nuclear-encoded genes; however, rapidly evolving mitochondrial-encoded genes in many taxa provide another potential partner in deleterious interactions. Despite the crucial role of D–M incompatibilities in postzygotic reproductive isolation, there are very few examples of the types of specific molecular interactions that are involved. Several cases are known from Drosophila, where one gene involved in an interaction has been identified but the interacting partner(s) and the physiological process that are disrupted remain to be elucidated (Ting et al. 1998; Barbash et al. 2003, 2004; Presgraves et al. 2003). Here I look for evidence that nuclear and mitochondrial-encoded proteins in the electron transport system (ETS) in Tigriopus californicus could be involved in deleterious interactions and contribute to reproductive isolation in hybrids from population crosses of this species.
In taxa with partial reproductive isolation that can be crossed, patterns of segregation of molecular markers or chromosomal regions can be examined in hybrid offspring to determine candidate regions for D–M incompatibilities. Studies of crosses between species often reveal widespread detrimental interactions (where nonparental genotype combinations have a negative effect on fitness) and occasional positive interactions (conversely, when nonparental genotype combinations have increased fitness) (Palopoli and Wu 1994; Rieseberg et al. 1996; Burke et al. 1998; Coyne et al. 1998; Fishman et al. 2001; Presgraves 2003). Other studies have shown evidence for deleterious epistatic interactions in crosses between divergent populations of the same species (Dobzhansky 1950; Armbruster et al. 1997; Li et al. 1997; Galloway and Fenster 1999; Fenster and Galloway 2000a,b; Hall and Willis 2005). One caveat for many of these segregation studies is that the observed patterns of non-Mendelian inheritance could result from meiotic drive rather than from the breakdown of coadapted gene complexes; this has been shown to play a role for at least one well-characterized locus in an interspecific plant cross (Fishman and Willis 2005). Studies of segregation of genotypes in hybrids will sometimes allow the proportion of heterozygous/homozygous (where one locus is homozygous for the same allele and the second locus is heterozygous for two alleles) to homozygous/homozygous (both loci are homozygous in a two-locus comparison) incompatibilities to be estimated. The ratio of these types of incompatibilities can have significant effects on the results obtained from models of postzygotic isolation (Turelli and Orr 2000).
The intertidal copepod T. californicus has proven to be a useful system in which to examine the initial stages of allopatric speciation. Geographically distinct populations of this copepod from the Pacific coast of North America are dramatically divergent in mitochondrial DNA (mtDNA) even over relatively short distances (Burton and Lee 1994; Burton 1998; Burton et al. 1999; Edmands 2001; Willett and Burton 2004). The changes in mtDNA include a large number of replacement differences leading to substantial divergence in these mtDNA-encoded proteins. Crosses between these copepod populations typically show that F2 hybrids (but not F1 hybrids) have lower fitness by a variety of measures when each class is compared to the parental populations, suggesting that coadapted gene complexes are disrupted in these hybrids (Burton 1986, 1987, 1990; Edmands 1999; Edmands and Deimler 2004). Some genetic regions that contribute to this reduction in F2 hybrid fitness have been identified by segregation studies of molecular markers in crosses of divergent copepod populations (Burton 1987; Harrison and Edmands 2006).
The intimate interactions between mtDNA-encoded proteins and nuclear-encoded proteins in the physiologically indispensable ETS, coupled with the high rate of divergence in mtDNA in T. californicus, suggested this system as a candidate system in which to examine the evolution of epistasis and its potential role in F2 hybrid breakdown. Several studies have suggested that mitochondrial/nuclear coadaptation has diverged in these genetically isolated copepod populations: Edmands and Burton (1999) found evidence for mitochondrial/nuclear coadaptation in backcross hybrids between populations. Rawson and Burton (2002) uncovered functional coadaptation between nuclear-encoded cytochrome c (CYC) and complex IV (which contains three mtDNA-encoded proteins) in studies of enzymatic rates. Willett and Burton (2001, 2003) demonstrated that the fitness of CYC genotypes in hybrids is influenced by the cytoplasmic type of the cross and one specific cross yielded results consistent with coadaptation between CYC and mtDNA-encoded proteins in both F2 and advanced generation hybrids. In this cross [between populations from Santa Cruz (SC) and Abalone Cove (AB)] the selection against non-mtDNA type CYC genotypes occurred during the juvenile life stages (postzygote), thereby ruling out meiotic drive as an explanation for skewed ratios. Results obtained in the prior studies of coadaptation between mtDNA-encoded and nuclear-encoded proteins appear to be highly influenced by the particular pair of populations crossed (and the direction of the cross), suggesting that independent evolution has occurred in these coadaptation systems in each lineage.
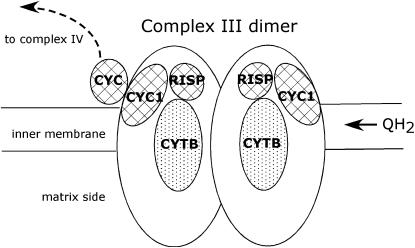
Complex III of the ETS (also known as ubiquinol/cytochrome c oxioreductase) includes both the mtDNA-encoded cytochrome b (CYTB) and a set of nuclear-encoded subunits (10 in mammals), 2 of which, cytochrome c1 (CYC1) and the rieske iron–sulfur protein (RISP), contain redox centers (Figure 1) and are found in the bacterial complex III homolog (Iwata et al. 1998). These three functionally important components of complex III have been characterized from several T. californicus populations and have been shown to have differences in amino acid sequence in comparisons across populations (Willett and Burton 2004). Much like the pattern seen for other mtDNA genes (Burton and Lee 1994; Burton 1998; Burton et al. 1999; Edmands 2001), CYTB was remarkably divergent between T. californicus populations with synonymous pairwise divergence exceeding 65% for comparisons between SC, AB, and San Diego (SD) copepod populations. In contrast, synonymous divergences for the CYC1 and RISP genes were ∼5% for comparisons of these same sets of populations (Willett and Burton 2004). Complex III interacts closely with CYC (a soluble protein that transfers electrons from complex III to complex IV; Saraste 1999); CYC has also diverged between copepod populations, divergence that has potentially been driven by positive selection (Rawson et al. 2000; Willett and Burton 2004).
Figure 1.—
Schematic of complex III and its position in the electron transport system. Complex III is known to function as a dimer in the inner membrane of the mitochondrion as depicted in the diagram. Within each half, the cytochrome b protein (CYTB) is mtDNA encoded, while the rieske iron–sulfur protein (RISP), cytochrome c1 (CYC1) proteins, and the soluble protein cytochrome c (CYC) are all encoded in the nucleus. Ubiquinol (QH2) is a lipid soluble compound that moves within the membrane and is oxidized by complex III. The diagram is derived from information in Saraste (1999) and Lange and Hunte (2002).
Given the importance of these ETS proteins for energy generation and the presence of changes in the amino acid sequences between copepod populations, an unanswered question is whether these differences in proteins will lead to detrimental interactions within the ETS that will negatively influence fitness in hybrids from genetically divergent populations. An examination of genotypic viabilities in F2 hybrids from T. californicus populations for these three interacting proteins (CYC, CYC1, and RISP) and their combined impact on viability affords the opportunity to uncover the nature of nuclear/nuclear interactions as well as nuclear/mtDNA interactions for this system. The degree to which heterozygote/homozygote vs. homozygote/homozygote types of interactions are occurring is also examined. Additional comparisons of levels of epistasis between these three proteins with two non-ETS proteins (malic enzyme homologs, ME1 and ME2) will help to illuminate the level of background interactions expected for arbitrarily chosen proteins. The results presented in this article suggest that for a cross between two Southern California copepod populations, Abalone Cove and San Diego, interactions between these three complex III nuclear proteins are having an impact upon F2 hybrid fitness. These interactions appear to be nuclear/nuclear and primarily heterozygote/homozygote in type and could be responsible for deleterious interactions that act as D–M incompatibilities in these hybrid copepods.
MATERIALS AND METHODS
Crosses and culturing conditions:
T. californicus were collected from intertidal rock pools at two sites in southern California, SD (32°44.74′ N, 117°15.30′ W, San Diego County) and AB (33°44.26′ N, 118°22.52′ W, Los Angeles County). The copepods were maintained in mass culture in artificial seawater (Instant Ocean, Aquarium Systems) in 400-ml beakers at 20° with a 12:12 L:D photoperiod. Cultures were maintained at a concentration of 35 parts per thousand seawater and fed with commercial flake fish food (O.S.I. Spirulina, Ocean Science International), but copepods were also allowed to consume natural algal growth.
To test for the existence of epistatic interactions between ETS proteins, crosses were set up using copepods from the AB and SD populations that had been maintained under the culturing conditions described above for at least 1 year (multiple generations are likely to have occurred under these laboratory conditions). Virgin females were obtained by separating clasped pairs (Burton 1985) and placing these females in dishes with males from the other population. Crosses were done in petri dishes with 15 males and 15 females, and two plates were set up for each of the reciprocal crosses (ABf × SDm and ABm × SDf). Female copepods were moved to new dishes when copepodids of the next generation were observed (if copepodids molt to the final adult stage, then generations will be indistinguishable). Males were removed and discarded because females mate only once in their lifetime and store sufficient sperm to produce multiple clutches. F2 progeny were obtained by allowing F1 male and female copepods to mate after replicate crosses were mixed (to minimize the chance of inbreeding). Nucleotide variability (which should be a proxy for functionally important genetic variation) is much lower within these populations than between them, reflecting the apparent long-term restrictions on gene flow between these populations. F1 females were then transferred to new petri dishes when F2 copepodids were observed. This crossing design has the potential disadvantage of not allowing cross-to-cross variability to be uncovered but it does permit a large number of F2 progeny to be collected, thereby increasing power to detect epistatic interactions.
F2 copepods were collected as both nauplii and adults: First-stage nauplii were obtained by incubating mature egg sacs (red in color) from F1 females in seawater at which point nauplii would hatch out and were immobilized on filter paper and then collected into 10 μl of proteinase-K cell-lysis buffer (Hoelzel and Green 1992). Adults were separated according to sex and then collected and placed into 20 μl of proteinase-K cell-lysis buffer. Both adults and nauplii were placed in 96-well polymerase chain reaction (PCR) plates and incubated at 65° for 1 hr and 95° for 15 min to prepare DNA for subsequent PCR reactions. Two 96-well plates each of nauplii, adult males, and adult females were collected and stored at −20°.
ETS gene marker scoring and data analysis:
PCR-based markers were developed for each of the three ETS proteins to allow the scoring of the genotypes of hybrids by length differences, i.e., allele-specific PCR products of different sizes. Genotyping assays for the gene CYC were described in Willett and Burton (2001). Primers for RISP and CYC1 took advantage of length differences in introns between AB and SD populations uncovered in these sequences in Willett and Burton (2004). The primers for RISP had the following sequences: RISPcon.f 5′-TGGCAGATCCAAGGCAAATACTAACAAT-3′ and RISPcon.r 5′-TGGTTAGATTTGAATACGGAACCTTCA-3′. The CYC1 primers were CYC1ex3con.f 5′-GACTCGTTTGACCATGCTTCCATTCG-3′ and CYC1ex4con.r 5′-CGGCATGGGTAGATAGTCGGTCAAT-3′. PCR reactions were run under standard conditions (with 55° annealing temperatures for both sets of reactions) and genotypes were determined by visualizing differences in the size of PCR products on 1.5% agarose gels stained with ethidium bromide.
Each of the complex III markers was tested for departures from homogeneity between sexes, using contingency tests. Deviations from Mendelian 1:2:1 ratios were then examined for the sexes separately or combined. This test can be used to test the significance of differences in genotypic viability that can then be compared across reciprocal crosses (with different mtDNA backgrounds) to determine whether mitochondrial/nuclear interactions are influencing F2 hybrid fitness. Relative viabilities of homozygous genotypes and their standard deviations were calculated according to Haldane's (1956) formulas. For the estimated viability value this is simply wAA = 2NAA/(NAa + 1), where NAA is the number of one homozygous class and NAa is the number of heterozygotes. The standard deviation of this value is
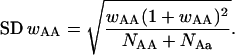 |
To test for epistatic interactions between nuclear-encoded gene products (nuclear/nuclear interactions), a multiplicative model was used in which the null hypothesis was that single-locus effects are mutually independent to generate expected numbers without epistasis. The expected number of each two-locus genotype class was calculated by using observed single-locus genotype frequencies to correct for deviations associated with each of the two single loci. This was done for each genotypic combination (nine total) by multiplying the two observed genotypic frequencies of the two loci and then multiplying these by the total number of copepods scored for both markers. This simplifies to
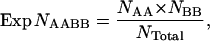 |
where Exp NAABB is the expected number of a two-locus genotypic class with corresponding numbers of NAA and NBB observed for each locus individually and Ntotal equal to the total number of copepods scored for both loci. Three-way epistatic interactions between RISP, CYC1, and CYC were detected using a procedure analogous to that described above for two-way interactions. The expected number for each of the 27 three-locus classes was derived from the average of each of the three two-locus classes multiplied by the excluded locus class. This simplifies to the formula
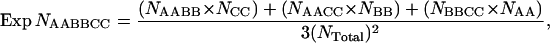 |
where Exp NAABBCC is the expected number of a three-locus genotypic class; the observed number in each two-locus class is NAABB, NAACC, or NBBCC; the observed number of each single-locus class is NAA, NBB, or NCC; and NTotal is the total sample size for which all three genotypes were obtained. The significance of deviations from independence in single-, two-, and three-locus comparisons was tested with a χ2-test. A sequential Bonferroni correction was used to determine a critical P-value for each table that adjusted for multiple tests. The significance of interactions with the functionally distantly related malic enzyme homologs (ME1 and ME2) was calculated using genotypes collected from the same F2 individuals scored herein (genotypes from J. Berkowitz and C. S. Willett, unpublished data). These markers (in addition to two-way interaction results for histone H1 from Willett and Burton 2001) will provide some insight into the background level of epistatic interactions in these crosses.
RESULTS
Deviations from Mendelian inheritance for ETS markers:
To test the potential contribution of nuclear-encoded ETS genes to hybrid breakdown in population crosses in T. californicus the genotypic-specific viabilities of the three ETS loci were examined individually. The genotypes for CYC, CYC1, and RISP were determined both from hybrid F2 adults and from first-stage nauplii from two reciprocal crosses between AB and SD populations (Table 1). There are significant deviations from Mendelian inheritance for each of these markers in both of the reciprocal crosses for F2 adults but no significant deviations are observed for F2 nauplii, suggesting that fitness differences between genotypic classes are leading to selection during development from nauplii to adult for each of these three markers in these hybrids. The ABf × SDm cross shows significant differences between the sexes for RISP and CYC1 genotypic ratios (only for CYC1 when significance values are corrected for multiple comparisons). For all three ETS markers there is a marked reduction in viability for both homozygote classes in adults when compared to the heterozygous class (Table 2).Willett and Burton (2001) performed an analogous cross under similar conditions and scored AB × SD F2 adults for CYC; these results are summarized in Table 2 for comparison.
TABLE 1.
Observed genotypic numbers for three ETS genes in F2 progeny from interpopulation crosses of Tigriopus californicus between Abalone Cove (AB) and San Diego (SD)
| No. of genotypic class
|
Male vs. female χ2 | |||||||
|---|---|---|---|---|---|---|---|---|
| ETS gene and reciprocal cross | Sex/stagea | SD/SD | AB/SD | AB/AB | Pbc | 1:2:1 χ2 | P | |
| CYC-ABm × SDf | Female | 35 | 121 | 32 | 4.29 | 0.11 | ||
| Male | 46 | 121 | 20 | |||||
| M + F | 81 | 242 | 52 | 36.2 | <0.0001* | |||
| Nauplii | 50 | 79 | 48 | 2.08 | 0.35 | |||
| CYC-ABf × SDm | Female | 32 | 123 | 29 | 1.27 | >0.5 | ||
| Male | 33 | 132 | 22 | |||||
| M + F | 65 | 255 | 51 | 53.1 | <0.0001* | |||
| Nauplii | 50 | 87 | 48 | 0.70 | >0.5 | |||
| CYC1-ABm × SDf | Female | 25 | 125 | 38 | 0.67 | >0.5 | ||
| Male | 31 | 120 | 36 | |||||
| M + F | 56 | 245 | 74 | 37.0 | <0.0001* | |||
| Nauplii | 38 | 104 | 36 | 5.1 | 0.078 | |||
| CYC1-ABf × SDm | Female | 13 | 124 | 49 | 17.6 | 0.0001* | 34.6 | <0.0001* |
| Male | 40 | 115 | 35 | 8.7 | 0.013 | |||
| M + F | 53 | 239 | 84 | 32.8 | <0.0001* | |||
| Nauplii | 50 | 100 | 33 | 4.7 | 0.094 | |||
| RISP-ABm × SDf | Female | 34 | 122 | 27 | 0.71 | >0.5 | ||
| Male | 29 | 112 | 41 | |||||
| M + F | 63 | 234 | 68 | 29.2 | <0.0001* | |||
| Nauplii | 45 | 88 | 44 | 0.014 | >0.5 | |||
| RISP-ABf × SDm | Female | 33 | 130 | 18 | 8.21 | 0.016 | 37.0 | <0.0001* |
| Male | 29 | 122 | 39 | 16.4 | 0.0003* | |||
| M + F | 62 | 252 | 57 | 47.8 | <0.0001* | |||
| Nauplii | 43 | 97 | 41 | 0.98 | >0.5 | |||
M, male; F, female.
Female and male are F2 adults, while nauplii are first-stage nauplii.
The significance of deviations from expected Mendelian 1:2:1 ratio and equal genotypic ratios between sexes was tested with contingency χ2-tests and P-values were computed with 2 d.f. P-values <0.05 are indicated in italics.
Application of the sequential Bonferroni method to the data yields a critical P-value of 0.004 (α = 0.05); an * denotes values exceeding this significance threshold.
TABLE 2.
Relative viabilities of ETS markers for F2 hybrids in AB × SD population crosses
| RV, current resultsa
|
RV, 2001 resultsb
|
|||||
|---|---|---|---|---|---|---|
| Locus | Cross | Sex | SD/SD | AB/AB | SD/SD | AB/AB |
| CYC | ABm × SDf | Combinedc | 0.67 (0.08)d | 0.43 (0.05) | 1.03 (0.14) | 0.87 (0.12) |
| ABf × SDm | Female | 0.52 (0.09) | 0.47 (0.08) | 0.77 (0.15) | 1.86 (0.33) | |
| Male | 0.50 (0.08) | 0.33 (0.06) | 1.31 (0.26) | 0.34 (0.09) | ||
| CYC1 | ABm × SDf | Combined | 0.46 (0.06) | 0.60 (0.07) | ||
| ABf × SDm | Female | 0.21 (0.05) | 0.78 (0.12) | |||
| Male | 0.69 (0.11) | 0.60 (0.10) | ||||
| RISP | ABm × SDf | Combined | 0.54 (0.07) | 0.58 (0.07) | ||
| ABf × SDm | Female | 0.50 (0.08) | 0.27 (0.05) | |||
| Male | 0.47 (0.08) | 0.63 (0.10) | ||||
RV, relative viabilities.
Underlined viabilities are cases when the higher viability of the homozygote classes matches the mtDNA background of the cross.
Previous results for CYC are from Willett and Burton (2001).
Combined sexes for the ABm × SDf cross for each marker because there was no significant difference between the sexes (either in Table 1 or in Willett and Burton 2001).
Relative viability is calculated assigning a heterozygote a fitness of 1. Standard deviations of relative viabilities are in parentheses.
The potential for CYC coadaptation with mtDNA-encoded proteins has been suggested by a number of studies (Edmands and Burton 1999; Willett and Burton 2001, 2003); however, these studies have focused largely on a different population cross (Abalone Cove and Santa Cruz in central California). Both RISP and CYC1 are found in the same protein complex as CYTB, which sets up the potential for the coadaptation of these nucDNA-encoded proteins with the mtDNA-encoded CYTB. To test for nuclear/mitochondrial coadaptation it is instructive to look at the representation of the alternate homozygous genotypic classes with the expectation that there will be a 1:1 ratio between them (or equivalent viabilities when compared to the heterozygous genotypic class). An examination of the viabilities in Table 2 does not lend support to a large role of mitochondrial/nuclear coadaptation in determining F2 hybrid fitness in the AB × SD crosses for these three markers. There is not widespread agreement with a simple model of ETS gene/mtDNA coadaptation when comparing bias in alternate homozygous classes with the maternal parent in each cross for each of the three genes. With maternal inheritance of mtDNA, nuclear/mtDNA coadaptation would predict that the viability of the homozygous class not matching the female parent should be reduced. For the current results, only three of nine possible comparisons between homozygous classes in Table 2 (underlined) show biases matching this prediction for the current results.
Epistatic interactions between ETS genes:
Given the close interactions of CYC, CYC1, and RISP proteins in complex III of the ETS, hybridization could result in the breakdown of coadaptation between these proteins in this complex. These nuclear/nuclear gene interactions within complex III could then be a potential source of hybrid inviability. To test for the presence of this coadaptation, I have examined the patterns of epistasis observed between the markers in the three complex III-associated genes in the F2 hybrid populations examined above. There are substantial deviations from independence for two-way comparisons of these three markers in adult F2 hybrids but no evidence of departures in F2 nauplii (Table 3). The departures from multiplicative independence were calculated by using expected values that correct for the fitness effects of each of the single loci. The two comparisons involving CYC1 display significant deviations for the adults summed across the reciprocal crosses and large deviations (approaching significance only when correcting for multiple tests) for at least one of each of the reciprocal crosses. For the RISP/CYC comparison, only the total female sample has a marginally significant deviation from independence (approaching the critical sequential Bonferroni-corrected P-value).
TABLE 3.
Two-way epistatic interactions between ETS loci in F2 hybrids
| Nauplii
|
Females
|
Males
|
Adultsa
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cross | Genes | χ2b | P | χ2 | Pc | χ2 | P | χ2 | P |
| ABf × SDm | CYC1/CYC | 1.82 | >0.95 | 14.7 | 0.066 | 12.54 | 0.13 | 24.08 | 0.0022 |
| ABm × SDf | 2.30 | >0.95 | 20.94 | 0.0073 | 7.61 | 0.47 | 24.87 | 0.0016 | |
| Total | 0.41 | >0.95 | 26.3 | 0.0009* | 16.0 | 0.04 | 39.21 | 0.00006* | |
| ABf × SDm | RISP/CYC | 5.03 | 0.75 | 19.8 | 0.011 | 3.01 | 0.93 | 11.83 | 0.16 |
| ABm × SDf | 2.81 | 0.95 | 6.69 | 0.57 | 2.25 | >0.95 | 4.96 | 0.76 | |
| Total | 2.64 | >0.95 | 20.8 | 0.0077 | 4.16 | 0.84 | 13.78 | 0.08 | |
| ABf × SDm | CYC1/RISP | 3.27 | 0.92 | 6.79 | 0.56 | 14.7 | 0.065 | 11.98 | 0.15 |
| ABm × SDf | 3.91 | 0.87 | 13.1 | 0.11 | 14.2 | 0.077 | 23.02 | 0.0033 | |
| Total | 4.98 | 0.75 | 14.6 | 0.67 | 22.6 | 0.0039 | 28.56 | 0.0004* | |
Adults are the total of male and female F2 hybrids for indicated cross(es).
χ2-value is measure of deviation from a model of independent gene action for an indicated pair of genes (discussed in materials and methods); P-values are calculated with 8 d.f. P-values <0.05 are indicated in italics.
Application of the sequential Bonferroni method to the data yields a critical P-value of 0.0015 (α = 0.05); an * denotes values exceeding this significance threshold.
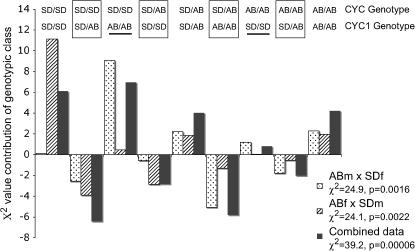
The nature of deviations from independence could yield insights into the types of epistasis occurring between these genes. The simplest pattern that could be generated by D–M incompatibilities would be an excess of parental-type double homozygotes and a deficit of non-parental-type double homozygotes. However, more complex patterns of homozygote/heterozygote incompatibilities are also possible (modeled by Turelli and Orr 2000). Figure 2 separates the components of the χ2-deviations into the nine different two-locus genotypic classes for CYC and CYC1. This provides a qualitative assessment of which interactions are overrepresented vs. underrepresented for these two genes in these hybrid copepods. For the CYC and CYC1 genotypic combinations, the homozygote/heterozygote classes are all underrepresented, suggesting their potential involvement in deleterious interactions. Doubly parental SD homozygotes (e.g., CYCSD/SD/CYC1SD/SD) show large positive deviations (as does the nonparental type, CYCSD/SD/CYC1AB/AB).
Figure 2.—
Relative contributions to χ2-deviations for genotype-by-genotype classes in F2 hybrids of a cross between SD and AB for T. californicus for CYC and CYC1. The magnitude of the deviation in χ2 is shown by the length of the bars on the y-axis while the direction indicates more (positive) or less (negative) in each category than expected. The genotypic combinations are indicated at the top while the reciprocal cross or combined result is indicated by shading as shown in the inset. Boxed classes are heterozygous/homozygous comparisons while the two underlined classes are nonparental homozygous/homozygous comparisons. The χ2-value for each cross and associated P-value are given below each cross name in the inset.
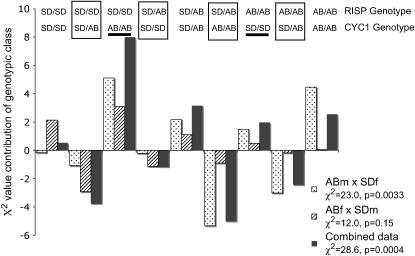
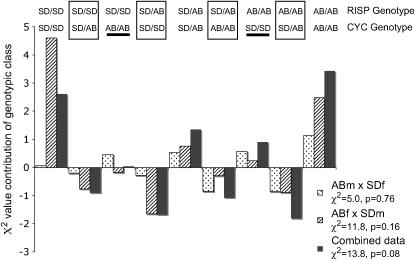
A similar pattern is found in the RISP/CYC1 comparison for deviations from multiplicative independence (Figure 3). Once again the heterozygous/homozygous classes are underrepresented. The large positive effect of the RISPSD/SD/CYC1AB/AB class and the similar result seen for a nonparental genotype with CYC and CYC1 are not consistent with completely negative interactions between genes from different populations. The deviations for RISP/CYC (for which the overall χ2-values were largely marginally significant or insignificant) are shown in Figure 4. For these two genes the largest contribution to the overall χ2-deviation comes from positive deviations for both parental-type double homozygous classes. The heterozygous/homozygous genotypic combinations are once again all underrepresented from RISP/CYC.
Figure 3.—
Relative contributions to χ2-deviations for genotype-by-genotype classes in F2 hybrids of a cross between SD and AB for T. californicus for CYC1 and RISP. The magnitude, direction, and cross type are all as indicated in Figure 1. Boxed classes are heterozygous/homozygous comparisons while the two underlined classes are nonparental homozygous/homozygous comparisons. The χ2-value for each cross and associated P-value are given below each cross name in the inset. Note the difference in scale of the y-axis from Figure 2.
Figure 4.—
Relative contributions to χ2-deviations for genotype-by-genotype classes in F2 hybrids of a cross between SD and AB for T. californicus for CYC and RISP. The magnitude, direction, and cross type are all as indicated in Figure 1. Boxed classes are heterozygous/homozygous comparisons while the two underlined classes are nonparental homozygous/homozygous comparisons. The χ2-value for each cross and associated P-value are given below each cross name in the inset. Note the difference in scale of the y-axis from Figures 2 and 3.
There is limited evidence for significant three-way interactions between RISP, CYC, and CYC1 when the effects of the two-way interactions are factored out (Table 4). When all adults in both reciprocal crosses are summed (total adults in Table 4), they deviate substantially from independence between the three ETS genes but do not reach the significance threshold with a sequential Bonferroni correction. The total adult genotypic class deviates from independence by having an observed excess of triple heterozygotes, and, in addition, several double-heterozygote/single-homozygote classes are underrepresented (results not shown). For the three-way interactions as well as the two-way interactions previously, the female classes show greater deviations from independence than do the male classes. Although the interactions between these three ETS genes appear to be complex, it appears that their interactions have significant effects upon hybrid fitness in this population cross.
TABLE 4.
Three-way interactions between RISP, CYC1, and CYC genes in F2 hybrids
| Nauplii
|
Females
|
Males
|
Adultsa
|
|||||
|---|---|---|---|---|---|---|---|---|
| Cross | χ2b | P | χ2 | Pc | χ2 | P | χ2 | P |
| ABf × SDm | 14.0 | >0.9 | 38.2 | 0.058 | 21.7 | 0.71 | 34.9 | 0.11 |
| ABm × SDf | 9.4 | >0.9 | 24.2 | 0.56 | 15.1 | >0.9 | 27.2 | 0.40 |
| Total | 6.9 | >0.9 | 38.2 | 0.058 | 28.3 | 0.34 | 46.8 | 0.007 |
Adults are the total of male and female F2 hybrids for indicated cross(es).
χ2 value is measure of deviation from a model of independent gene action for the three genes, RISP, CYC1, and CYC (discussed in methods); P-values are calculated with 26 d.f. P-values <0.05 are indicated in italics.
Application of the sequential Bonferroni method to the data in this table yields a critical P-value of 0.0042 (α=0.05); none of the values exceed this threshold.
Interactions of ETS proteins with ME homologs:
Markers have been developed for two different malic enzyme homologs from T. californicus, and these have been used to genotype the same F2 hybrids genotyped for the three nuclear-encoded ETS genes in this study (J. Berkowitz and C. S. Willett, unpublished results). There were significant departures from 1:2:1 Mendelian expectations for these two loci when examined singly that involved greatly reduced numbers of both homozygous classes (results not shown). We can gain some insight into the levels of background epistasis by looking at two-way interactions between the ME proteins and the three ETS proteins in the F2 hybrids. The same multiplicative model of independent gene action was tested for the comparisons of each of these two ME markers with the three ETS genes (Table 5). None of the deviations were significant when corrected for multiple tests using the sequential Bonferroni procedure. The ABf × SDm cross for ME/CYC had a large departure from independence that approached the sequential Bonferroni-adjusted P-value. This departure was driven by an excess of individuals in the CYCSD/SD/MEAB/AB class that led to a significant deviation for the F2 adult females; F2 males of the reciprocal cross had a nonsignificant trend for a deviation in the same genotypic class. In comparison with the results involving the three ETS proteins, these data suggest fewer fitness interactions in hybrids between these two ME homologs with the ETS proteins (and linked genes).
TABLE 5.
Two-way epistatic interactions between ETS loci and malic enzyme homologs in F2 hybrids
| Nauplii: | Males: | Females: | Adults:a | ||
|---|---|---|---|---|---|
| Cross | Genes | χ2b | χ2 | χ2cd | χ2 |
| ABf × SDm | ME/CYC | 6.99 | 2.96 | 21.7** | 20.4** |
| ABm × SDf | 2.32 | 10.4 | 1.68 | 6.69 | |
| Total | 1.50 | 19.2* | |||
| ABf × SDm | ME2/CYC | 3.27 | 3.30 | 1.57 | 0.59 |
| ABm × SDf | 5.07 | 2.20 | 5.03 | 2.78 | |
| Total | 7.33 | 3.74 | |||
| ABf × SDm | ME/CYC1 | 0.07 | 10.0 | 4.13 | 9.67 |
| ABm × SDf | 4.09 | 1.15 | 4.08 | 4.61 | |
| Total | 1.80 | 11.49 | |||
| ABf × SDm | ME2/CYC1 | 2.14 | 5.96 | 6.79 | 5.94 |
| ABm × SDf | 6.01 | 3.60 | 1.21 | 1.66 | |
| Total | 2.37 | 4.35 | |||
| ABf × SDm | ME/RISP | 2.12 | 7.02 | 5.65 | 9.82 |
| ABm × SDf | 3.15 | 3.38 | 12.3 | 3.14 | |
| Total | 0.63 | 10.4 | |||
| ABf × SDm | ME2/RISP | 3.00 | 6.80 | 0.49 | 1.80 |
| ABm × SDf | 0.78 | 3.72 | 5.90 | 4.84 | |
| Total | 2.80 | 3.26 |
Adults are the total of male and female F2 hybrids for indicated cross(es).
χ2-value is measure of deviation from a model of independent gene action for an indicated pair of genes (discussed in materials and methods).
χ2-values exceeding P = 0.05 with 8 d.f. are indicated in italics, with the magnitude indicated by *P < 0.05 and **P < 0.01.
Application of the sequential Bonferroni method to the data yields a critical P-value of 0.0008 (α = 0.05); none of the P-values in this table exceeded this significance threshold (for comparison, for the highest value, a χ2 of 21.7, P = 0.0055).
DISCUSSION
Significant epistatic interactions between ETS proteins:
The three genes, CYC, CYC1, and RISP, encode proteins crucial for the function of complex III and are involved in epistatic interactions that have deleterious impacts upon hybrid fitness in crosses between AB and SD populations of T. californicus. The deleterious interactions for these crosses appear to be largely nuclear/nuclear in nature despite the extensive interactions of these proteins with mtDNA-encoded proteins (the limited evidence for mitochondrial/nuclear coadaptation for these markers in this cross is discussed later). For each of these markers the heterozygote/homozygote genotypic classes are underrepresented in comparison to numbers expected with no epistasis (Figures 2–4). This congruence in deviations from independence across reciprocal crosses is a strong argument for the pattern's significance despite the lack of replication of each reciprocal cross individually in the experimental design (all F2 adults were pooled to maximize sample size). These interactions between the genes encoding CYC, CYC1, and RISP could then be an example of both partners of a D–M incompatibility leading to lowered hybrid fitness. Below, I explore other potential explanations for this pattern and argue that they are unlikely to be leading to the observed pattern.
If any of these three genes are linked to another, this could generate a significant pattern of nonindependence; however, other work examining patterns of segregation in backcrosses with these markers has demonstrated that they are on three separate chromosomes (H. B. Leisy and C. S. Willett, unpublished results). The lack of significant deviations from independence in two-way comparisons for first-stage nauplii also demonstrates that these genes are not linked to one another. Another possibility is that genes linked to these three markers are involved in the interactions rather than the three ETS genes. Given the close nature of interactions between each of these proteins in complex III they are clearly strong candidates for causing the observed epistatic interactions.
An examination of background levels of epistasis between genes that are not functionally interacting would provide a measure of the uniqueness of the observed interactions. Epistatic interactions approaching significance were much less common for other two-way comparisons using these markers and the two ME homologs or histone H1 (Willett and Burton 2001) or for the ME homologs to each other (J. Berkowitz and C. S. Willett, unpublished results). Although malic enzyme is not directly in the ETS, most animals have an isoform of this protein that functions in the mitochondrion (Kochan et al. 1995; Dolezal et al. 2004) and could lead to functional interactions with complex III. Harrison and Edmands (2006) have shown that a combination of microsatellite and RAPD markers on different chromosomes only rarely shows significant pairwise epistatic interactions in F2 backcrosses between SD and a population closely related to AB (Royal Palms). Only 6 of 132 possible pairwise comparisons were significant in their data set (only 2 after Bonferroni correction); however, measures across all chromosomes for this cross in their study do suggest the presence of more complex interactions. Combined, these results suggest that the three nuclear-encoded ETS proteins show many more significant pairwise epistatic interactions than other non-ETS genes and that high numbers of significant epistatic interactions are not ubiquitous across markers in crosses of these copepod populations.
Another argument for the epistatic interactions being caused by the deleterious interaction of the protein products of the CYC, CYC1, and RISP genes is that the magnitude of interactions is related to the degree of structural and functional interactions within complex III (and to CYC). The soluble CYC protein is bound to complex III at the CYC1 protein during the electron-transfer phase from complex III to CYC (Lange and Hunte 2002). In comparison with the 26-amino-acid differences between the AB and SD CYTB proteins, there is only a single (CYC1) or two (RISP, CYC) amino acid differences between these three nuclear-encoded proteins. Although some of the amino acid changes between T. californicus populations in these proteins fall near the interacting regions, none of them are the interacting residues themselves (Willett and Burton 2004). If, as this study suggests, these changes have important fitness consequences, this would imply that the basic interactions such as the residues responsible for binding have remained unchanged while the residues that have changed will cause smaller modifications to the complex III enzyme. The RISP protein interacts both in structure and in function with the CYC1 and CYTB proteins but has no direct interactions with CYC (Iwata et al. 1998). For two-way comparisons between these three genes, the comparisons between RISP and CYC had smaller deviations from independence as would be predicted by the more limited structural/functional interactions. If we use yeast or mammal complex III as a model there are either six or seven (respectively) other nuclear-encoded subunits in complex III. Of these subunits, QCR6p (corresponding to subunit 8 in mammals) and QCR9p (corresponding to subunit 10) are close in the structure to the active sites of CYC1 and RISP (Iwata et al. 1998; Zhang et al. 1998; Lange and Hunte 2002). Although complex III can function without these two subunits, it appears that they are important for complex assembly and may modify functional interactions as well (Kim et al. 1987; Schmitt and Trumpower 1991; Zara et al. 2004). To gain a complete understanding of the evolution of interactions in this complex for these hybrid copepods, these additional subunits should be characterized as well.
For the F2 adult hybrids of the AB and SD populations, dominance appears to play a large role in structuring the interactions between these genes. The two-way genotypes with homozygous/heterozygous combinations are all underrepresented for comparisons between the three ETS gene markers (Figures 2–4). This pattern suggests that deleterious interactions involving components of complex III may involve one heterozygous locus and one homozygous locus. In models of postzygotic isolation the ratio of heterozygous/homozygous to homozygous/homozygous incompatibilities influences the ability of reproductive isolation to evolve and can help explain the phenomenon known as the large X effect (Turelli and Orr 2000). They conclude that a small ratio of heterozygote/homozygote incompatibilities (h1 in their terminology) to homozygote/homozygote incompatibilities (h2) would make dominance a good explanation for the observation of a large X effect for differences between species. T. californicus does not have differentiated sex chromosomes so this large X effect is not applicable to this species, but the results for epistasis at complex III could indicate that heterozygous/homozygous deleterious incompatibilities can be uncoupled from homozygous/homozygous interaction effects. In results seen to date from Drosophila and wasp studies h1 is generally much lower than h2 (Breeuwer and Werren 1995; Hollocher and Wu 1996; True et al. 1996; Turelli and Orr 2000) although the results of Tao et al. (2003), which looked at hybrid male sterility in Drosophila simulans and D. mauritiana using introgressions, suggest that dominance/epistatic relationships are very complex for loci influencing this trait.
It is not clear what the proximate cause of high frequency of heterozygote/homozygote deleterious interactions would be for the genes in this study. Complex III is a stable dimer with each monomer containing one each of the CYC1, RISP, and CYTB proteins (Saraste 1999) (see Figure 1). There is the potential for both parental alleles to be present in the same complex III dimer, perhaps contributing to the observed interactions. It is also possible that the observed patterns of genotypic deviations derive from the influence of genes linked to the ETS markers combined with the interactions of the ETS proteins themselves. For this study F2 hybrids were examined, which allows for only one generation of recombination; therefore, there will be fairly large numbers of linked genes with the potential to influence the viability of the ETS markers scored in this study. Future studies are planned to isolate these three ETS markers from linked genes, which may help to clear up the nature of the interactions between these genes from the effects of background loci.
The homozygous–homozygous classes do not always deviate in a manner predicted by the D–M incompatibility model; in several cases the nonparental-type double-homozygote class (e.g., RISPSD/SD/CYC1AB/AB in Figure 3) is overrepresented. Clearly the nuclear/nuclear interactions between the three ETS proteins (or linked genes) have a complex relationship to fitness in the hybrid copepods of this population cross. If the observed epistasis is caused solely by the interactions of the ETS proteins, these results would suggest that both positive and negative fitness interactions can result even from single pairs of genes. Whether these loci with both negative and positive epistatic interactions will function as D–M incompatibilities overall will depend upon the relative strength of the effects and whether the negative outweighs the positive. The results of the epistatic interactions observed in this study suggest that D–M incompatibilities are very complex even when broken down to the level of individual loci.
Mitochondrial/nuclear coadaptation:
For the crosses between copepods from the AB and SD populations, coadaptation between the nuclear-encoded CYC1 and RISP genes and the mtDNA-encoded proteins does not appear to play a significant role in determining the fitness of F2 hybrids. RISP and CYC1 are part of the same complex with CYTB (Figure 1). CYTB is evolving more rapidly than the nuclear-encoded RISP and CYC1 proteins with 26-amino-acid differences between the AB and SD proteins (Willett and Burton 2004). For complex III the expectation from structural and functional considerations would be that RISP would have the closest interactions with CYTB (Iwata et al. 1998); however, the deviations seen in the F2 hybrids are not generally consistent with the deviations expected from mtDNA/nuclear coadaptation for this cross (Table 2). It is possible that such coadaptation could be obscured if the neighboring regions of the genome contain opposing interactions that are stronger in effect. These interactions may be detectable in advanced generation hybrids if they exist. Functional studies on complex III such as those performed by Rawson and Burton (2002) for CYC and complex IV could also help illuminate any potential coadaptation.
For the CYC gene, only the ABm × SDf cross for CYC has a lowered viability of the non-mtDNA type homozygous class for adults (SD mtDNA-type in this cross) and a nonsignificant difference between the sexes (Tables 1 and 2). The reciprocal cross does not lend additional support to the suggestion that CYC is involved in mitochondrial/nuclear coadaptation in hybrids of these two populations, however, as the same direction of bias toward SD homozygotes is observed in the two reciprocal crosses. The CYC protein interacts with complex IV (cytochrome c reductase), a complex primarily composed of three mtDNA-encoded proteins (Saraste 1999), in addition to the close interaction of CYC with complex III. In a cross between AB and a copepod population from SC, CYC did show fitness deviations consistent with mitochondrial/nuclear coadaptation in previous studies (Willett and Burton 2001, 2003). A physiological study has also suggested the possibility of coadaptation for CYC and complex IV enzymatic rates for the AB and SC populations (Edmands and Burton 1999). Combined, these studies suggest that if mitochondrial/nuclear coadaptation is contributing to hybrid breakdown in survival in these copepod hybrids it may involve interactions between CYC and complex IV. In other systems, few studies to date have looked at the contribution of individual ETS proteins to organismal fitness due to genomic coadaptation. A number of studies have used crosses between populations or species to examine the magnitude of mitochondrial/nuclear coadaptation on fitness (Clark and Lyckegaard 1988; Hutter and Rand 1995; Rand et al. 2001, 2006; James and Ballard 2003; Perrot-Minnot et al. 2004). Significant impacts of mitochondrial/nuclear coadaptation upon fitness have been found in these studies, demonstrating that such interactions might not be uncommon in diverging populations or species (along with significant effects of nuclear/nuclear interactions in some studies as well). The specific genetic interactions leading to these mitochondrial/nuclear interactions remain largely unknown at this point.
The results obtained in this study can be compared to results for CYC obtained from a previous study using the AB and SD populations (Willett and Burton 2001), and this comparison shows similar overall trends but a few larger differences as well (Table 2). The direction of bias between the AB/AB and SD/SD alternate homozygote classes in this 2001 experiment is qualitatively similar to the results obtained in the present study; the largest discrepancy for CYC is for adults in the ABf × SDm cross that show little difference between the viabilities of the alternate homozygotes in the current results but large differences in opposite directions between males and females in the 2001 results. This variation in results suggests that there can be differences from cross to cross in the magnitude and expression of hybrid incompatibilities even when conducted under similar conditions (the largest controlled difference was the use of artificial seawater for the current experiment vs. natural seawater for the previous experiment). It is conceivable that other experimental factors could influence the repeatability of these fitness effects; in particular, relative levels of competition (different numbers of nauplii will be in each of the F2 petri dishes) and algal growth in the cultures are factors that vary to some extent but not in a systematic way between experiments. It is also possible that the differences between studies result from genetic variation segregating within each of the populations. The copepods used in these two sets of crosses were from outbred lab populations sampled at different times from the field. It should be noted, however, that the magnitude of genetic variation within each population is much lower than the divergence seen between each of these three populations (typically around an order of magnitude lower for nuclear genes if variation at silent sites is compared within and between these two populations; Willett and Burton 2004). In addition, 30 females and 30 males were used to set up each cross, which should have the effect of averaging over any genetic variation present within each of the populations. Clearly, repeatability is an issue for interpreting the single-locus effects but could potentially reflect an underlying biological reality that many factors can influence the relative fitness of copepods, and at these relatively low levels of reproductive isolation, specific deleterious interactions might be somewhat transient in nature.
In support of the changeable nature of genetic interactions, the environment appeared to play a significant role in the expression of potential incompatibilities in a previous study of interpopulation hybrids of T. californicus. Willett and Burton (2003) found that temperature environment affected whether there was selection at CYC in a cross between AB and Santa Cruz. Temperature interactions would not be surprising given that these copepods are ectotherms and the ETS should have evolved to function best under the range of conditions that particular populations of copepods have experienced in their past. The stability of protein–protein interactions is often highly dependent upon temperature and may then lead to the widespread involvement of temperature in the expression of D–M incompatibilities. Replication of the current study under a different temperature environment could help reveal if nuclear/nuclear interactions detected in this study for ETS genes are influenced as dramatically by the environmental conditions.
Acknowledgments
I thank E. Hoddeson, Q. Qin, and J. Berkowitz for their help with the copepod crosses and sample preparation. Helpful suggestions on the manuscript were provided by R. Burton, M. Servedio, T. Vision, A. Bouck, and S. Hartman; by the University of North Carolina (UNC) evolgen reading group; and by two anonymous reviewers. I thank M. Servedio for help with analyses. C.W. was supported by money from UNC and National Science Foundation grant DEB-0516139.
References
- Armbruster, P., W. E. Bradshaw and C. M. Holzappel, 1997. Evolution of the genetic architecture underlying fitness in the pitcher-plant mosquito, Wyeomyia smithii. Evolution 51: 451–458. [DOI] [PubMed] [Google Scholar]
- Barbash, D. A., D. F. Siino, A. M. Tarone and J. Roote, 2003. A rapidly evolving MYB-related protein causes species isolation in Drosophila. Proc. Natl. Acad. Sci. USA 100: 5302–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbash, D. A., P. Awadalla and A. M. Tarone, 2004. Functional divergence caused by ancient positive selection of a Drosophila hybrid incompatibility locus. PloS Biol. 2: 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeuwer, J. A., and J. H. Werren, 1995. Hybrid breakdown between two haplodiploid species: the role of nuclear and cytoplasmic genes. Evolution 49: 705–717. [DOI] [PubMed] [Google Scholar]
- Burke, J. M., T. J. Voss and M. L. Arnold, 1998. Genetic interactions and natural selection in Louisiana iris hybrids. Evolution 52: 1304–1310. [DOI] [PubMed] [Google Scholar]
- Burton, R. S., 1985. Mating system of the intertidal copepod Tigriopus californicus. Mar. Biol. 86: 247–252. [Google Scholar]
- Burton, R. S., 1986. Evolutionary consequences of restricted gene flow in the intertidal copepod Tigriopus californicus. Bull. Mar. Sci. 39: 526–535. [Google Scholar]
- Burton, R. S., 1987. Differentiation and integration of the genome in populations of Tigriopus californicus. Evolution 41: 504–513. [DOI] [PubMed] [Google Scholar]
- Burton, R. S., 1990. Hybrid breakdown in developmental time in the copepod Tigriopus californicus. Evolution 44: 1814–1822. [DOI] [PubMed] [Google Scholar]
- Burton, R. S., 1998. Intraspecific phylogeography across the Point Conception biogeographic boundary. Evolution 52: 734–745. [DOI] [PubMed] [Google Scholar]
- Burton, R. S., and B.-N. Lee, 1994. Nuclear and mitochondrial gene genealogies and allozyme polymorphism across a major phylogeographic break in the copepod Tigriopus californicus. Proc. Natl. Acad. Sci. USA 91: 5197–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, R. S., P. D. Rawson and S. Edmands, 1999. Genetic architecture of physiological phenotypes: empirical evidence for coadapted gene complexes. Am. Zool. 39: 451–462. [Google Scholar]
- Clark, A. G., and E. M. S. Lyckegaard, 1988. Natural selection with nuclear and cytoplasmic transmission. III. Joint analysis of segregation and mtDNA in Drosophila melanogaster. Genetics 118: 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. A., S. Simeonidis and P. Rooney, 1998. Relative paucity of genes causing inviability in hybrids between Drosophila melanogaster and D. simulans. Genetics 150: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky, T., 1936. Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21: 113–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky, Th., 1950. Genetics of natural populations. XIX. Origin of heterosis through natural selection in populations of Drosophila pseudoobscura. Genetics 35: 288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal, P., S. Vanacova, J. Tachezy and I. Hrdy, 2004. Malic enzymes of Trichomonas vaginalis: two enzyme families, two distinct origins. Gene 329: 81–92. [DOI] [PubMed] [Google Scholar]
- Edmands, S., 1999. Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evolution 53: 1757–1768. [DOI] [PubMed] [Google Scholar]
- Edmands, S., 2001. Phylogeography of the intertidal copepod Tigriopus californicus reveals substantially reduced population differentiation at northern latitudes. Mol. Ecol. 10: 1743–1750. [DOI] [PubMed] [Google Scholar]
- Edmands, S., and R. S. Burton, 1999. Cytochrome c oxidase activity in interpopulation hybrids of a marine copepod: a test for nuclear-nuclear or nuclear-cytoplasmic coadaptation. Evolution 53: 1972–1978. [DOI] [PubMed] [Google Scholar]
- Edmands, S., and J. K. Deimler, 2004. Local adaptation, intrinsic coadaptation and the effects of environmental stress on interpopulation hybrids in the copepod Tigriopus californicus. J. Exp. Mar. Biol. Ecol. 303: 183–196. [Google Scholar]
- Fenster, C. B., and L. F. Galloway, 2000. a Inbreeding and outbreeding depression in natural populations of Chamaecrista fasciculata (Fabaceae). Conserv. Biol. 14: 1406–1412. [Google Scholar]
- Fenster, C. B., and L. F. Galloway, 2000. b Population differentiation in an annual legume: genetic architecture. Evolution 54: 1157–1172. [DOI] [PubMed] [Google Scholar]
- Fishman, L., and J. H. Willis, 2005. A novel meiotic drive locus completely distorts segregation in Mimulus (monkeyflower) hybrids. Genetics 169: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman, L., A. Kelly, E. Morgan and J. H. Willis, 2001. A genetic map in the Mimulus guttatus species complex reveals transmission ratio distortion due to heterospecific interactions. Genetics 159: 1701–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway, L. F., and C. B. Fenster, 1999. The role of nuclear and cytoplasmic factors in the adaptive evolution of populations of Chamaecrista fasciculata (Fabaceae). Evolution 53: 1734–1743. [DOI] [PubMed] [Google Scholar]
- Haldane, J. B. S., 1956. The estimation of viabilities. J. Genet. 59: 29–36. [Google Scholar]
- Hall, M. C., and J. H. Willis, 2005. Transmission ratio distortion in intraspecific hybrids of Mimulus guttatus: implications for genomic divergence. Genetics 170: 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, J. S., and S. Edmands, 2006. Chromosomal basis of viability differences in Tigriopus californicus interpopulation hybrids. J. Evol. Biol. (in press). [DOI] [PubMed]
- Hoelzel, A. R., and A. Green, 1992. Analysis of population-level variation by sequencing PCR-amplified DNA, pp. 159–187 in Practical Approach Series: Molecular Genetic Analysis of Populations, edited by A. R. Hoelzel. Oxford University Press, New York.
- Hollocher, H., and C.-I. Wu, 1996. The genetics of reproductive isolation in the Drosophila simulans clade: X vs. autosomal effects and male vs. female effects. Genetics 143: 1243–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter, C. M., and D. M. Rand, 1995. Competition between mitochondrial haplotypes in distinct nuclear genetic environments: Drosophila pseudoobscura vs. D. persimilis. Genetics 140: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata, S., J. L. Lee, K. Okada, J. K. Lee, M. Iwata et al., 1998. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281: 64–71. [DOI] [PubMed] [Google Scholar]
- James, A. C., and J. W. O. Ballard, 2003. Mitochondrial genotype affects fitness in Drosophila simulans. Genetics 164: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. H., C. Balny and T. E. King, 1987. Role of the hinge protein in the electron transfer between cardiac cytochrome c1 and c. J. Biol. Chem. 262: 8103–8108. [PubMed] [Google Scholar]
- Kochan, Z., J. Karbowska, F. Bukato and J. Swierczynski, 1995. Comparative studies on NADP+-linked malic enzyme in the central nervous system of ectothermic and endothermic animals. Comp. Biochem. Physiol. 110B: 309–314. [DOI] [PubMed] [Google Scholar]
- Lange, C., and C. Hunte, 2002. Crystal structure of the yeast cytochrome bc1 complex with its bound substrate cytochrome c. Proc. Natl. Acad. Sci. USA 99: 2800–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., S. R. M. Pinson, A. H. Paterson, W. D. Park and J. W. Stansel, 1997. Genetics of hybrid sterility and hybrid breakdown in an intrasubspecific rice (Oryza sativa L.) population. Genetics 145: 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, H. J., 1942. Isolating mechanisms, evolution, and temperature. Biol. Symp. 6: 71–125. [Google Scholar]
- Palopoli, M. F., and C.-I Wu, 1994. Genetics of hybrid male sterility between Drosophila sibling species: a complex web of epistasis is revealed in interspecific studies. Genetics 138: 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Minnot, M.-J., A. Migeon and M. Navajas, 2004. Intergenomic interactions affect female reproduction: evidence from introgression and inbreeding depression in a haplodiploid mite. Heredity 93: 551–558. [DOI] [PubMed] [Google Scholar]
- Presgraves, D. C., 2003. A fine-scale genetic analysis of hybrid incompatibilities in Drosophila. Genetics 163: 955–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves, D. C., L. Balagopalan, S. M. Abmayr and H. A. Orr, 2003. Adaptive evolution drives divergence of a hybrid inviability gene between two species of Drosophila. Nature 423: 715–719. [DOI] [PubMed] [Google Scholar]
- Rand, D. M., A. G. Clark and L. M. Kann, 2001. Sexually antagonistic cytonuclear fitness interactions in Drosophila melanogaster. Genetics 159: 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand, D. M., A. Fry and L. Sheldahl, 2006. Nuclear-mitochondrial epistasis and Drosophila aging: introgression of Drosophila simulans mtDNA modifies longevity in D. melanogaster nuclear backgrounds. Genetics 172: 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson, P. D., and R. S. Burton, 2002. Functional coadaptation between cytochrome c and cytochrome c oxidase within allopatric populations of a marine copepod. Proc. Natl. Acad. Sci. USA 99: 12955–12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson, P. D., D. A. Brazeau and R. S. Burton, 2000. Isolation and characterization of cytochrome c from the marine copepod Tigriopus californicus. Gene 248: 15–22. [DOI] [PubMed] [Google Scholar]
- Rieseberg, L. H., B. Sinervo, C. R. Linder, M. C. Ungerer and D. M. Arias, 1996. Role of gene interactions in hybrid speciation: evidence from ancient and experimental hybrids. Science 272: 741–745. [DOI] [PubMed] [Google Scholar]
- Saraste, M., 1999. Oxidative phosphorylation at the fin de siecle. Science 283: 1488–1493. [DOI] [PubMed] [Google Scholar]
- Schmitt, M. E., and B. L. Trumpower, 1991. The petite phenotype resulting from a truncated copy of subunit 6 results from loss of assembly of the cytochrome bc1 complex and can be suppressed by overexpression of subunit 9. J. Biol. Chem. 266: 14958–14963. [PubMed] [Google Scholar]
- Tao, Y., Z-B. Zeng, J. Li, D. L. Hartl and C. C. Laurie, 2003. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. II. Mapping hybrid male sterility loci on the third chromosome. Genetics 164: 1399–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting, C.-T., S.-C. Tsaur, M.-L. Wu and C.-I Wu, 1998. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science 282: 1501–1504. [DOI] [PubMed] [Google Scholar]
- True, J. R., B. S. Weir and C. C. Laurie, 1996. A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritiana chromosomes into D. simulans. Genetics 142: 819–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M., and H. A. Orr, 2000. Dominance, epistasis, and the genetics of postzygotic isolation. Genetics 154: 1663–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock, M. C., and P. C. Phillips, 1995. Multiple fitness peaks and epistasis. Annu. Rev. Ecol. Syst. 26: 601–629. [Google Scholar]
- Willett, C. S., and R. S. Burton, 2001. Viability of cytochrome c depends on cytoplasmic background in Tigriopus californicus. Evolution 55: 1592–1599. [DOI] [PubMed] [Google Scholar]
- Willett, C. S., and R. S. Burton, 2003. Environmental influences on epistatic interactions: viabilities of cytochrome c genotypes in interpopulation crosses. Evolution 57: 2286–2292. [DOI] [PubMed] [Google Scholar]
- Willett, C. S., and R. S. Burton, 2004. Evolution of interacting proteins in the mitochondrial electron transport system in a marine copepod. Mol. Biol. Evol. 21: 443–453. [DOI] [PubMed] [Google Scholar]
- Zara, V., I. Palmisano, L. Conte and B. L. Trumpower, 2004. Further insights into the assembly of the yeast cytochrome bc1 complex based on analysis of single and double mutants lacking supernumerary subunits and cytochrome b. Eur. J. Biochem. 271: 1209–1218. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., J. Huang, V. M. Sculmeister, Y.-I. Chi, K. K. Kim et al., 1998. Electron transfer by domain movement in cytochrome bc1. Nature 392: 677–684. [DOI] [PubMed] [Google Scholar]