The potential demand for genetic tests has many firms seeking to discover and patent information about genetic markers that are correlated (however tenuously) with disease.1,2,3,4,5,6 Policy-makers worldwide are considering the potential impact — both good and bad — of new predictive genetic tests on the cost, quality and equity of health care systems.7,8 Policy discussion has tended to focus on the per-unit costs of genetic tests, and particularly on how patent law influences this. However, because the expanded use of health care products and services sold on the basis of test results may be as important in determining the health system impact of predictive genetic testing as the tests themselves, policy-makers should pay close attention to all aspects of complete testing services and their various effects on health system costs.1
The ultimate cost impact of a genetic test will depend on how the availability of the test changes health care behaviours.1,9 The immediate cost impact is the cost of screening itself. Genetic testing can change screening costs by changing the unit cost per case screened, the number of cases screened, or both. Genetic tests per se are currently very expensive; moreover, appropriate counselling represents a potentially major, and always necessary, component of genetic testing.10,11 However, even if the per-unit cost of genetic screening were lower than that of conventional screening, the total cost of the screening program could increase if a genetic test were applied more widely than conventional screening. This might be anticipated if the genetic test were more convenient, or if it were heavily promoted by those who held patents on the tests themselves or on the products sold on the basis of test results.2,3
The long-term financial consequences of predictive genetic testing include changes in the uptake of diagnostic and preventive services.12,13,14 Surveillance activities, undertaken to detect the development of disease early in its progression for those identified as being at high risk, may vary widely. In the case of BRCA1 and BRCA2 testing, or other tests for hereditary forms of cancer, the resulting surveillance could range from inexpensive self-examination to high-cost diagnostic imaging or surgical biopsy.15,16,17,18 When added to a program cost on a year-after-year basis, increased diagnostic testing or molecular imaging, or both, may be particularly important determinants of the health system impact of a genetic testing service. Preventive activities that are undertaken to reduce the likelihood or severity of disease onset can have similar effects on health system costs. They may range from simple behaviour change in order to avoid known environmental risk factors, to costly prophylactic surgical removal of healthy tissue, to lifelong pharmacologic management.1,18,19
Effective surveillance and preventive activities that are a consequence of positive genetic test results will ideally reduce or avert the treatment costs for the associated disease. In many cases, however, the effectiveness of interventions targeted at individuals with a specific genetic makeup will be difficult to prove in the short term. Moreover, although outcomes should improve when cost-effective surveillance and preventive activities are taken up by appropriate patients, population health outcomes can worsen if false- negative genetic test results reduce the uptake of otherwise cost-effective surveillance or prevention,20 if genetic testing increases the uptake of unnecessary and ineffective surveillance and prevention activities, or if testing diverts health care resources away from more cost-effective means of promoting health.21 Attention must therefore be paid to the relative risks and benefits of interventions and behaviour changes for patients who are tested versus those who are not, and for patients with positive test results versus those with negative test results.
Two important determinants of the ultimate balance of these cost considerations are the timing of the respective costs and the degree to which the test service is appropriately targeted at candidate populations.1,12,22,23 The cost of testing is always incurred in the present, and surveillance and prevention costs can begin almost immediately and continue for years, yet saved treatment costs often do not become evident until far into the future. The longer the lag between current spending and future savings, the greater the savings must be to justify current spending from a purely financial perspective. This is because of both the year-after-year nature of surveillance or prevention and the fact that a given level of future savings or costs is worth more today than it is in the future — the further into the future savings arise, the less they are worth investing in today. This “discounting” of future returns to investment reflects interest costs and other economic considerations.24 If, for example, the interest rate is 5%, the treatment savings required to offset $1 per year of ongoing prevention or surveillance, or both, would need to be about $5 if treatment costs are expected to occur in 5 years, $12 if treatment costs occur within 10 years, $34 for 20 years and $128 for 40 years. When prevention is inexpensive and effective this is not a problem, but the costs of long-term risk management can become substantial for daily pharmaceutical consumption or the routine use of high-tech imaging and surveillance equipment.
The extent to which a test is targeted at high-risk populations is a major determinant of the overall cost of screening. In addition, the precision of targeting affects the nature of the information produced by the test and how that information affects the behaviour of tested individuals and their health care providers. Although several factors influence the positive predictive value of a test in practice, one of the most important is the prevalence of the genetic risk factor in the target population.22 Targeting a screening program toward populations at risk reduces in particular the proportion of “false positives” among positive test results. This improvement in the positive predictive value of the test reduces the unnecessary uptake of prevention and surveillance services.
The impact on health systems will vary considerably across different genetic tests. Policy-makers should be most concerned about the adverse health and economic effects of tests to identify elevated risk for common, multifactorial conditions such as heart disease and cancer. Although only a few of these tests are now in clinical use, several (such as apolipoprotein E [ApoE] testing for sporadic Alzheimer's disease) are being pursued strongly by those with a commercial interest in the tests themselves or in the products sold based on test results.2,3,4 On the other hand, genetic tests for strongly hereditary conditions, such as Huntington's disease, are unlikely to generate a large impact because such conditions are rare, and the test can be appropriately targeted to family members. Such tests can even save health care costs by obviating the need for surveillance for those family members confirmed not to have the genetic anomaly.1,17
Genetic testing services are plotted in Fig. 1 along the dimensions of the scope of testing offered (the horizontal axis) and the cost impact per test (the vertical axis). The total health system cost impact can range from significant cost savings to significant cost increases. Quadrant I represents a best case scenario for health care funders, but unfortunately is perhaps the least likely to occur in reality because highly predictive tests generally apply only to rare conditions. Quadrant IV represents a worst case scenario associated with risk factor tests — tests which, despite a relatively low predictive value, have a broad “market” potential for those promoting the tests or the goods or services associated with test results.21 The shaded slope illustrates where economic assessment would place most current genetic tests: those that have a favourable cost impact per person tested tend to be focused screening programs.1 As genetic testing services extend beyond rare familial disorders to broader populations, the cost impact will tend to grow and become less favourable due to increased screening costs and reduced predictive power. Even good tests, when applied too broadly or without adequate information and support, can generate large, unwarranted cost impacts.
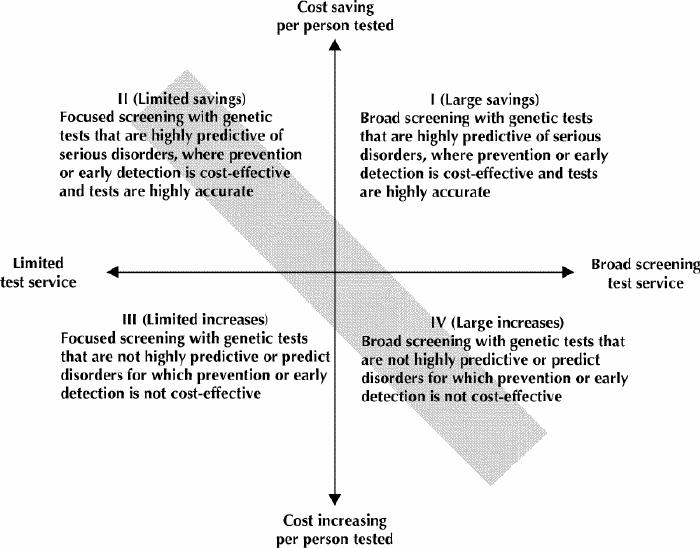
Fig. 1: The cost impact of predictive genetic tests on health systems. Most current genetic tests fall within the shaded slopes.
Because the cost of providing a test may often be relatively small compared with surveillance, prevention and treatment costs, policy-makers have a clear interest in evaluating all aspects of a genetic testing service. Public funders of health care services may have a financial interest in controlling the use and targeting of new predictive genetic tests regardless of who pays for the tests themselves. In many cases, the best way to guide use may be to design well-targeted, publicly funded screening programs rather than allowing access to be determined by private market forces. Policy-makers should focus attention on tests that could have the broadest health system impact. They should also ensure appropriate population targeting, which is at least as important as the technical accuracy of a test in influencing impact on a health system. Wise policy choices can ensure that savings are realized where possible and that use and related costs are justified by associated improvements in health.
Acknowledgments
We thank Patricia Collins and Gioia Buckley for research assistance, and we thank Phil Jackson and Barbara Slater of the Ontario Ministry of Health and Long-term Care for helpful comments. None of the above is responsible in any way for the ideas and conclusions expressed in this work.
Footnotes
This article has been peer reviewed.
Contributors: All authors contributed to the project inception, conceptual framework, data collection, and manuscript preparation and revision. Drs. Morgan and Hurley drafted the initial manuscript. Drs. Miller and Giacomini developed the conceptual framework for assessing genetic tests. All authors approved the final version to be published.
This research was supported by funding from the Ontario Ministry of Health and Long-term Care. Dr. Morgan is supported in part by a Canadian Institutes of Health Research Post-Doctoral Fellowship. Dr. Giacomini is supported by a National Health Research Scholar Award from the Canadian Institutes of Health Research/National Health Research and Development Program of Health Canada. The work also benefited from the resources of the Centre for Health Economics and Policy Analysis, McMaster University, which is funded in part by the Ontario Ministry of Health and Long-term Care.
Competing interests: None declared.
Correspondence to: Dr. Steve Morgan, Centre for Health Services and Policy Research, University of British Columbia, 429–2194 Health Sciences Mall, Vancouver BC V6T 1Z3; fax 604 822-7012; morgan@chspr.ubc.ca
References
- 1.Miller F, Hurley J, Morgan S, Goereee R, Collins P, Blackhouse G, et al. Predictive genetic tests and health care costs: final report prepared for the Ontario Ministry of Health and Long Term Care. Hamilton (ON): Centre for Health Economics and Policy Analysis, McMaster University; 2002. Available: www.gov.on.ca/health/english/pub/ministry/geneticsrep02/chepa.html (accessed 2003 Jan 14).
- 2.Herper M. Gene tests: medicine's new gold mine. Forbes 2001 Dec 14. Available: www.forbes.com/2001/12/14/1214roche.html (accessed 2003 Feb 26).
- 3.Herper M. Pharmacia's next trick: gene tests. Forbes 2002 Feb 5. Available: www.forbes.com/2002/02/05/0205pharmacia.html (accessed 2003 Feb 26).
- 4.Savill J. Science, medicine, and the future. Prospecting for gold in the human genome.BMJ 1997;314(7073):43-5. [DOI] [PMC free article] [PubMed]
- 5.Mathew C. Science, medicine, and the future. Postgenomic technologies: hunting the genes for common disorders. BMJ 2001;322(7293):1031-4. [DOI] [PMC free article] [PubMed]
- 6.Willison DJ, MacLeod SM. Patenting of genetic material: Are the benefits to society being realized? CMAJ 2002;167(2):262-4. [PMC free article] [PubMed]
- 7.Baird PA. Current challenges to appropriate clinical use of new genetic knowledge in different countries. Community Genet 2001;4(1):12-7. [DOI] [PubMed]
- 8.Organisation for Economic Co-operation and Development. Genetic testing. Policy issues for the new millennium. OECD Proceedings. Paris: OECD; 2000.
- 9.Miller FA, Giacomini M. Defining the characteristics of predictive genetic tests: a framework for evaluation and decision-making. Background Paper to Final Report. Hamilton (ON): Subcommittee on Evaluation. Ontario Advisory Committee on New Predictive Genetic Technologies; 2001.
- 10.Caulfield T. Gene testing in the biotech century: Are physicians ready? CMAJ 1999;161(9):1122-4. [PMC free article] [PubMed]
- 11.Petersen GM, Brensinger JD, Johnson KA, Giardiello FM. Genetic testing and counseling for hereditary forms of colorectal cancer.Cancer 1999; 86 (Suppl 11):2540-50. [DOI] [PubMed]
- 12.Cairns J, Shackley P. Sometimes sensitive, seldom specific: a review of the economics of screening. Health Econ 1993;2(1):43-53. [DOI] [PubMed]
- 13.Mushlin AI, Ruchlin HS, Callahan MA. Cost effectiveness of diagnostic tests. Lancet 2001;358(9290):1353-5. [DOI] [PubMed]
- 14.Foucar E. Predictive genetics and predictive morphology have certain similarities. BMJ 2001;323(7311):514. [PMC free article] [PubMed]
- 15.Tempany CM, McNeil BJ. Advances in biomedical imaging. JAMA 2001; 285 (5): 562-7. [DOI] [PubMed]
- 16.Martin SP, Ulrich CD. Pancreatic cancer surveillance in a high-risk cohort. Is it worth the cost? Med Clin North Am 2000;84(3):739-47. [DOI] [PubMed]
- 17.Bapat B, Noorani H, Cohen Z, Berk T, Mitri A, Gallie B, et al. Cost comparison of predictive genetic testing versus conventional clinical screening for familial adenomatous polyposis. Gut 1999;44(5):698-703. [DOI] [PMC free article] [PubMed]
- 18.Matloff ET, Shappell H, Brierley K, Bernhardt BA, McKinnon W, Peshkin BN. What would you do? Specialists' perspectives on cancer genetic testing, prophylactic surgery, and insurance discrimination. J Clin Oncol 2000; 18 (12): 2484-92. [DOI] [PubMed]
- 19.Fasouliotis S, Schenker J. BRCA1 and BRCA2 gene mutations: decision-making dilemmas concerning testing and management. Obstet Gynecol Surv 2000; 55 (6): 373-84. [DOI] [PubMed]
- 20.Marteau TM, Lerman C. Genetic risk and behavioural change. BMJ 2001; 322 (7293):1056-9. [DOI] [PMC free article] [PubMed]
- 21.Baird PA. Genetic technologies and achieving health for populations. Int J Health Serv 2000;30(2):407-24. [DOI] [PubMed]
- 22.Grimes DA, Schulz KF. Uses and abuses of screening tests. Lancet 2002;359 (9309): 881-4. [DOI] [PubMed]
- 23.Stewart-Brown S, Farmer A. Screening could seriously damage your health [editorial]. BMJ 1997;314:533. [DOI] [PMC free article] [PubMed]
- 24.Drummond M, Stoddart G, Torrance G. Methods for the economic evaluation of health care programmes. 2nd ed. Oxford: Oxford University Press; 1997.


