Abstract
Pro has been shown to play an important role in ameliorating environmental stress in plants and microorganisms, including heavy metal stress. Here, we describe the effects of the expression of a mothbean Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene in the green microalga Chlamydomonas reinhardtii. We show that transgenic algae expressing the mothbean P5CS gene have 80% higher free-Pro levels than wild-type cells, grow more rapidly in toxic Cd concentrations (100 μM), and bind fourfold more Cd than wild-type cells. In addition, Cd-K edge extended x-ray absorption fine structure studies indicated that Cd does not bind to free Pro in transgenic algae with increased Pro levels but is coordinated tetrahedrally by sulfur of phytochelatin. In contrast to P5CS-expressing cells, Cd is coordinated tetrahedrally by two oxygen and two sulfur atoms in wild-type cells. Measurements of reduced/oxidized GSH ratios and analyses of levels of malondialdehyde, a product of the free radical damage of lipids, indicate that free Pro levels are correlated with the GSH redox state and malondialdehyde levels in heavy metal–treated algae. These results suggest that the free Pro likely acts as an antioxidant in Cd-stressed cells. The resulting increased GSH levels facilitate increased phytochelatin synthesis and sequestration of Cd, because GSH–heavy metal adducts are the substrates for phytochelatin synthase.
INTRODUCTION
Cd is widely used in a variety of industrial processes, including plastic manufacturing, electroplating, and Ni-Cd battery production, as well as in pigments (Alloway, 1995; Dudka and Adriano, 1997). These industries, as well as mining and smelting industries, release substantial amounts of Cd into the environment each year (Nriagu and Pacyna, 1988). In animals and humans, chronic or toxic exposure to Cd can induce a variety of health disorders, including kidney damage (Buchet et al., 1980; Hellstrom et al., 2001), osteomalacia (Nogawa and Kido, 1993; Tsuritani et al., 1996), developmental defects, and prostate cancer (Desi et al., 1998).
Plants readily take up Cd from the soil. However, exposure to high levels of Cd results in reduced rates of photosynthesis, chlorosis, growth inhibition, browning of root tips (Kahle, 1993), decreases in water and nutrient uptake, and finally death (di Toppi and Gabbrielli, 1999). At the molecular level, Cd toxicity is associated with the formation and disruption of sulfhydryl and metal thiolate bonds, alterations in protein secondary structure, changes in the redox status of the cell, and interference with essential metal uptake, transport, and metabolism (Brennan and Schiestl, 1996; Chaoui et al., 1997; Ouariti et al., 1997; Nies, 1999; Sandalio et al., 2001). In addition, Cd poisoning of metalloproteins involved in redox or electron transfer processes may result in increased free radical production, leading to nonspecific damage to proteins, lipids, and other biomolecules (Stohs et al., 2000; Schützendübel et al., 2001).
There are a variety of mechanisms by which organisms reduce heavy metal toxicity, including production of heavy metal binding factors and proteins (metallothionein, GSH, and phytochelatin conjugates), exclusion of toxic heavy metals from cells by ion-selective metal transporters, and excretion or compartmentalization (Howe and Merchant, 1992; Kaplan et al., 1995; Lee et al., 1996; Cohen et al., 1998; Guerinot, 2000; Hu et al., 2001). One mechanism by which many plants and algae respond to and apparently detoxify toxic heavy metals is the production of Pro (Delauney and Verma, 1993; Schat et al., 1997; Shah and Dubey, 1998; Mehta and Gaur, 1999; Verma, 1999). The accumulation of Pro in stressed plants is associated with reduced damage to membranes and proteins (Alia et al., 1997; Shah and Dubey, 1998; Verma, 1999). Pro synthesis has been implicated in the alleviation of cytoplasmic acidosis and may maintain NADP+/NADPH ratios at values compatible with metabolism (Hare and Cress, 1997). Rapid catabolism of Pro upon relief of stress also may provide reducing equivalents that support mitochondrial oxidative phosphorylation and the generation of ATP for recovery from stress-induced damage (Hare and Cress, 1997). However, there has been much disagreement regarding the mechanism(s) by which Pro reduces heavy metal stress. Free Pro has been proposed to act as an osmoprotectant (Paleg et al., 1984; Delauney and Verma, 1993; Taylor, 1996), a protein stabilizer (Kuznetsov and Shevyakova, 1997; Shah and Dubey, 1998), a metal chelator (Farago and Mullen, 1979), an inhibitor of lipid peroxidation (Mehta and Gaur, 1999), a hydroxyl radical scavenger (Smirnoff and Cumbes, 1989), and a singlet oxygen scavenger (Alia et al., 2001). It is evident that there is no clear consensus regarding the mechanism(s) by which Pro reduces heavy metal stress.
We have investigated the role of Pro in facilitating Cd detoxification and ameliorating salt stress in microalgae by comparing the responses of wild-type and transgenic algae, which have nearly twofold higher free Pro levels, with toxic levels of Cd and treatment with seawater. We demonstrate that increased free Pro levels provide enhanced protection from Cd- and salt-induced stress. Furthermore, we show that Pro reduces Cd stress not by sequestering Cd but by reducing Cd-induced free radical damage and by maintaining a more reducing environment (higher GSH levels) in the cell. The Pro-dependent increase of cytoplasmic GSH levels in Cd-treated cells facilitates Cd sequestration and its detoxification as phytochelatin conjugates.
RESULTS
Overexpression of the Mothbean Δ1-Pyrroline-5-Carboxylate Synthetase Gene in Chlamydomonas reinhardtii
To enhance Pro synthesis in transgenic algae, we introduced a mothbean Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene into the nuclear genome of Chlamydomonas. P5CS catalyzes the first dedicated step in Pro synthesis from glutamate (Hu et al., 1992). The mothbean P5CS cDNA was cloned into plasmid pSSCR7 and cotransformed with plasmid p389, encoding an Arg succinyl lyase (arg7-8), into the nuclear genome of Chlamydomonas strain CC-425 (cell-wall-less, arg7-8) by electroporation (Shimogawara et al., 1998). Transformed cells were selected for their ability to grow in the absence of Arg and for the presence of the P5CS gene by PCR amplification using P5CS gene-specific primers. The identity of the P5CS PCR product was confirmed by DNA sequence analysis. Genomic DNA gel blot analysis of three transgenic lines indicated that one to nine copies of the mothbean P5CS gene were inserted into the genomes of the various transgenic lines (data not shown).
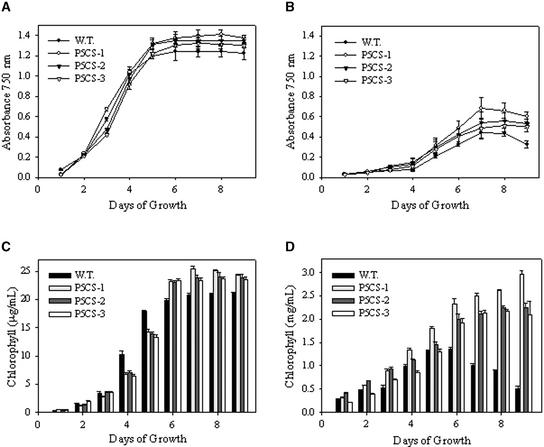
In the absence of Cd, all three P5CS transgenic algal strains grew to slightly higher stationary-phase cell densities than wild-type cells. These results suggest that enhanced Pro production does not impair and may slightly enhance the growth of C. reinhardtii under nonstressed growth conditions (Figure 1A). However, in the presence of toxic concentrations of Cd (100 μM), mothbean P5CS transgenic cells (P5CS-1) had as much as a 1.5-fold increased growth rate relative to wild-type cells (Figure 1B). In addition, algal cultures expressing the P5CS gene had significantly higher levels of chlorophyll per cell than wild-type cells when grown in the presence of toxic concentrations of Cd (100 μM) (Figures 1A to 1D). Among the three P5CS transformants screened, the chlorophyll content per cell of the P5CS-1 strain was highest when grown in Cd (100 μM). Surprisingly, algae expressing the mothbean P5CS gene (P5CS-1, P5CS-2, and P5CS-3) also had substantially higher Cd binding capacities (4.2-, 3.0-, and 2.5-fold, respectively) than wild-type cells (Table 1).
Figure 1.
Growth Rates and Chlorophyll Contents of Wild-Type and P5CS-Expressing Transgenic Algae Grown in the Presence and Absence of Cd.
(A) Growth rate in the absence of Cd.
(B) Growth rate in the presence of 100 μM Cd.
(C) Chlorophyll content in the absence of Cd.
(D) Chlorophyll content in the presence of 100 μM Cd.
Data shown are average absorbance units at 750 nm (cell concentration) or chlorophyll concentration per milliliter of culture ± sd from three separate experiments. W.T., wild type.
Table 1.
Cd Binding Capacity of Wild-Type (CC-425) and P5CS-expressing Transgenic Algae Grown in the Presence of 50 μM Cd
| Chlamydomonas | Cd Bound to Cell (ng Cd/absorbance at 750 nm) |
|---|---|
| Wild type (CC-425) | 189 ± 11 |
| P5CS-1 | 786 ± 112 |
| P5CS-2 | 564 ± 109 |
| P5CS-3 | 478 ± 69 |
Data shown are means ± SD of three separate experiments.
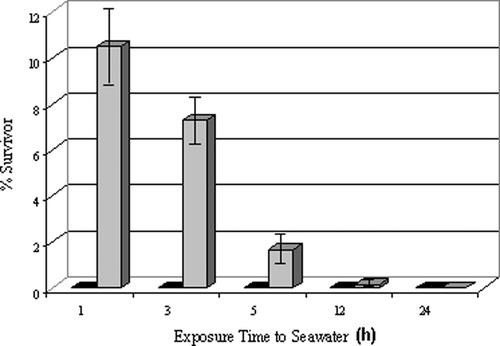
The P5CS-1 strain, which exhibited the fastest growth, highest chlorophyll content, and greatest Cd binding capacity, was selected for further physiological and molecular analyses. To determine whether expression of the P5CS gene conferred resistance to other types of stress, we compared the survivability of wild-type and P5CS-1 algae to different periods of seawater exposure. As shown in Figure 2, the P5CS-1 strain survived up to 12 h of exposure to seawater, whereas no survivors were recovered from wild-type cells after a 1-h incubation in seawater.
Figure 2.
Survival of Wild-Type and P5CS-1 Transgenic Algae in Seawater.
Cells were incubated with artificial seawater for 1, 3, 5, 12, or 24 h before growing in Tris-acetate-phosphate medium supplemented with Arg. Black bars represent wild-type cells, and gray bars represent P5CS-1 cells. Data shown are average percent survival after seawater incubation ± sd from three separate experiments.
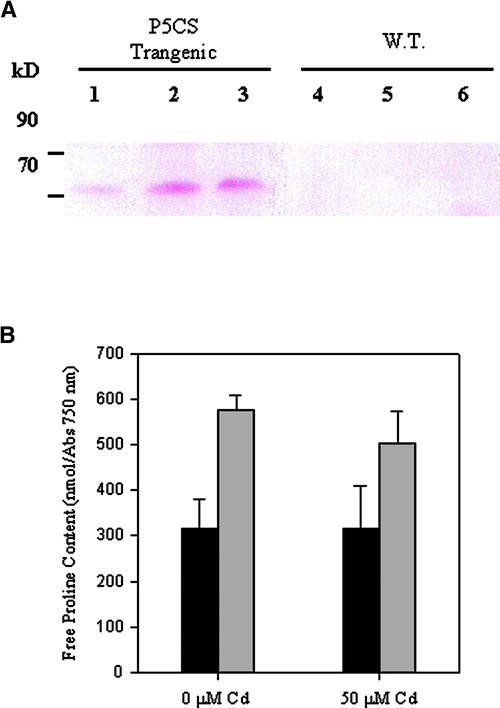
To determine whether the enhanced stress tolerance of the P5CS-1 strain was associated with expression of the P5CS protein, we performed protein gel blot analyses for expression of the P5CS protein in P5CS-1 and wild-type cells using mothbean P5CS-specific polyclonal antibodies (Figure 3A, lanes 1 to 3). As shown in Figure 3A, P5CS-1 transgenic cells expressed the mothbean P5CS protein. Significantly, the mothbean P5CS antibodies did not detect any Chlamydomonas proteins in wild-type cells, suggesting that Chlamydomonas may not express an antigenically similar enzyme. The expression of the P5CS protein also was associated with increased cytoplasmic free Pro levels. As shown in Figure 3B, transgenic algae expressing the mothbean P5CS gene had 80% higher free Pro levels than wild-type cells. Previous studies have reported enhanced accumulation of free Pro in some algal species after exposure to toxic concentrations of Cu or Cd (Wu et al., 1995). However, we observed no Cd-induced (50 μM) increases in free Pro content in wild-type or P5CS-expressing cells (Figure 3B).
Figure 3.
Expression of the Mothbean P5CS Protein and Pro Production in Transgenic Algae.
(A) Protein gel blot of total soluble proteins from P5CS transgenic algae (lanes 1 to 3) and wild-type (W.T.) algae (lanes 4 to 6). The samples were loaded at 10 μg (lanes 1 and 4), 15 μg (lanes 2 and 5), and 20 μg (lanes 3 and 6) of soluble protein. The membrane was immunodecorated with antibodies raised against purified mothbean P5CS protein. Molecular masses (kD) of protein markers (Gibco BRL) are indicated.
(B) Free Pro content of wild-type algae (black bars) and P5CS-1–expressing transgenic algae (gray bars) grown in the presence and absence of 50 μM Cd. Free Pro is expressed per absorbance unit at 750 nm ± sd (cell concentration) from three separate experiments.
Cd-K Edge Extended X-Ray Absorption Fine Structure Spectra
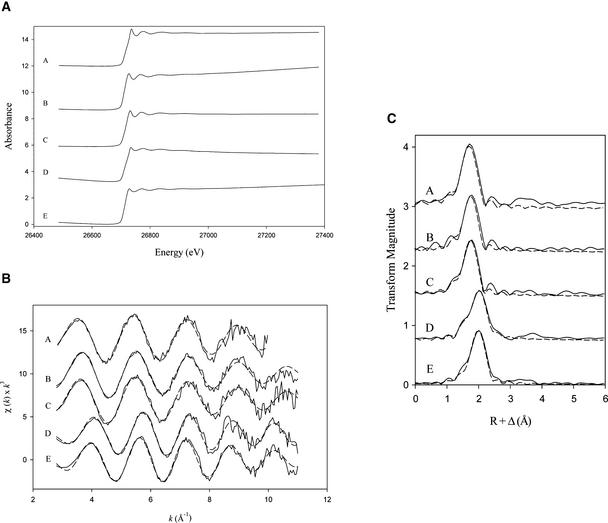
To determine whether cytoplasmic free Pro directly sequestered Cd, we determined the chemical identity of the atoms binding Cd and their associated bond lengths by extended x-ray absorption fine structure (EXAFS) spectroscopy. The Cd-K edge x-ray absorption spectra of the model solutions (Cd-GSH, Cd-Pro, and Cd-GSH-Pro) and of Chlamydomonas cells grown in the presence of 50 μM Cd are shown in Figure 4A. The corresponding EXAFS spectra and Fourier transforms are shown in Figures 4B and 4C, respectively. The absorption energy (26.72 keV) for Cd-electron ejection is consistent with the expected values for Cd. The spectra then were modeled and fit using the FEFF 8 (Ankudinov et al., 1998) and EXAFSPAK (George and Pickering, 1995) software packages. The raw and fitted data are shown in Figures 4B and 4C and listed in Table 2.
Figure 4.
Cd-K Edge X-Ray Absorption Spectra (A), EXAFS Spectra (B), and EXAFS Fourier Transform (C) of Cd-GSH, Cd-Pro, and Cd-Chlamydomonas Complexes.
Waves are as follows: A, GSH-Cd(NO3)2 solution; B, Pro-Cd(NO3)2 solution; C, GSH-Pro-Cd(NO3)2 solution; D, Chlamydomonas wild type (CC-425) grown in 50 μM Cd; E, Chlamydomonas expressing P5CS grown in 50 μM Cd. Solid lines represent experimental data, and dashed lines represent results of the least-squared fit to the EXAFS.
Table 2.
Cd-K Edge EXAFS Curve-Fitting Results for Chlamydomonas Cells Grown in the Presence of Cd (50 μM) and for Model Solutions
| Sample | Atom Types | N | R (Å) | σ2 (Å2) | ΔE0 (eV) |
|---|---|---|---|---|---|
| GSH + Cd(NO3)2 (5 mM each) |
Cd-O | 5.12 | 2.27 | 0.0096 | −4.08 |
| Pro + Cd(NO3)2 (5 mM each) |
Cd-O | 4.95 | 2.27 | 0.0077 | −0.01 |
| GSH (2.5 mM) + Pro (2.5 mM) + Cd(NO3)2 (5 mM) |
Cd-O | 4.90 | 2.27 | 0.0072 | −1.91 |
| Wild-type algae | Cd-O | 1.15 | 2.21 | 0.0039 | 4.95 |
| Cd-S | 1.40 | 2.52 | 0.0021 | 4.95 | |
| Transgenic algae | Cd-S | 3.24 | 2.50 | 0.0086 | −0.57 |
N, coordination number; R, interatomic distance; σ 2, Debye-Waller factor; ΔE0, difference between the resulting absorption energy and the constant input in the FEFF8 calculation.
The results of the EXAFS fit to all three model solutions (Cd-GSH, Cd-Pro, and Cd-GSH-Pro) are in agreement with the crystallographic data for CdCO3 (otavite) (Graf, 1961). For CdCO3, the coordination number is 6 and the interatomic distance is 2.2892 Å. The Cd-O interatomic distance between all three model samples and CdCO3 differs by <0.02 Å. In addition, the results of the EXAFS fit to the first shell of the Cd-GSH, Cd-Pro, and Cd-GSH-Pro solutions (Table 2) show strong similarities between the model compounds and CdCO3. Finally, the coordination numbers for the model compounds are close to the ideal value of 6 for pure O coordination. As expected, there is an ∼20% error in calculating the coordination in the first shell. These results suggest that the Cd-GSH model solutions were oxidized and that the sulfhydryl group was unavailable for Cd coordination.
In the wild-type microalgal samples, the Cd EXAFS spectra could be fit only by including both O and S in the first coordination shell, at bond lengths of 2.2 and 2.5 Å, respectively (Figure 4). As shown in Figures 4B and 4C and Table 2, the results of the EXAFS fit to Chlamydomonas wild-type cells are in excellent agreement with a combination of crystallographic data for CdCO3 (otavite) (Graf, 1961) and Cd-S (hawleyite) (Traill and Boyle, 1955). For Cd-S (hawleyite) and CdCO3 (otavite), the coordination numbers are 4 and 6 and the interatomic distances are 2.53 and 2.29 Å, respectively. The Cd-O and Cd-S interatomic distances of wild-type cells differ from those of CdCO3 and Cd-S by <0.02 and 0.01 Å, respectively. The presence of O ligands for Cd in wild-type cells is consistent with that in previous chemical titration and Fourier transform infrared studies of Cd binding to Chlamydomonas (Adhiya et al., 2002). By contrast, in P5CS-1 transgenic algae, the EXAFS spectrum is best fit by Cd coordination to four sulfur atoms at a bond distance of ∼2.5 Å. In addition, the results of the EXAFS fit to the transgenic algae are in agreement with the crystallographic data for Cd-S (Traill and Boyle, 1955) and for Cd-phytochelatin complexes (Pickering et al., 1999), including the ligand type, coordination number, and the Cd-ligand interatomic distance. Although one might anticipate that S ligation to Cd would produce a less “peaky” edge than is observed for Cd-O moieties (Salt et al., 1995), careful examination of Figure 4 shows that the EXAFS oscillations of the wild-type and P5CS-1 strains are somewhat out of phase. Overall, the EXAFS data strongly suggest that Cd is sequestered by phytochelatins and not Pro in P5CS-1 transgenic algae. This interpretation is further supported by comparative analyses of the model compounds and the algal samples. As indicated in Table 2, the Debye-Waller factors (0.0072 to 0.0096 Å2 for Cd-O) for the model solutions were increased relative to that for wild-type algae (0.0039 Å2 for Cd-O). The increased Debye-Waller factors for the model samples presumably arise from static disorder within the Cd-O populations. This disorder also is observed in transgenic algae, suggesting that Cd is not complexed by the same conjugates as those observed in wild-type algae.
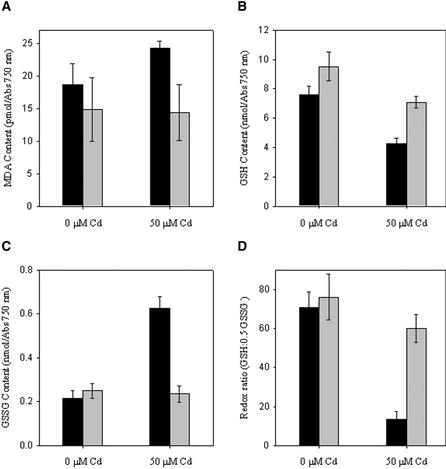
To further define the role of sulfhydryls in Cd binding in P5CS transgenic cells, we measured GSH levels in cells grown in the presence and absence of sufficient Cd (50 μM) to induce phytochelatin synthesis (Howe and Merchant, 1992; Wu et al., 1995; Cai et al., 1999). Under these conditions, GSH can bind Cd directly, and the heavy metal adduct serves as the substrate for phytochelatin synthase (Vatamaniuk et al., 2000). As discussed previously, conjugation of heavy metals to phytochelatin is a primary means of detoxifying heavy metals in plants and algae. As shown in Figure 5C, there was a nearly threefold increase in GSSG levels in wild-type cells when grown in the presence of Cd. By contrast, there was no increase in GSSG levels in P5CS transgenic cells. When the reduced and oxidized forms of GSH were expressed as a molar ratio (GSH:0.5 GSSG), it was apparent that wild-type cells had a fourfold reduction in their GSH:0.5 GSSG ratio relative to P5CS-1–expressing cells when grown in the presence of Cd. These results suggest that in the presence of Cd, the redox state of the cytoplasm of P5CS-expressing cells remains more reducing than that of wild-type cells.
Figure 5.
MDA Content and GSH:0.5 GSSG Redox State of Wild-Type (black bars) and P5CS-1–Expressing Transgenic Algae (gray bars).
(A) MDA content per cell.
(B) Redox state of cells as measured by GSH content.
(C) GSSG content per cell.
(D) GSH:0.5 GSSG redox ratio.
Data are means ± sd of three separate experiments.
The production of free radicals has obvious implications for control of the redox state of the cell. Free radicals often are quenched by reductants, including GSH. Cd has been demonstrated to cause increased levels of free radicals in cells in part by impairment of normal electron transfer processes (Hussain et al., 1987; Brennan and Schiestl, 1996; Chaoui et al., 1997; Ouariti et al., 1997; Stohs et al., 2000; Sandalio et al., 2001; Schützendübel et al., 2001). To quantify the relative Cd-dependent free radical damage in P5CS-1 transgenic cells and wild-type cells, we compared the extent of free radical–mediated damage of cells grown in the presence and absence of Cd. As shown in Figure 5A, the levels of malondialdehyde (MDA), a product of lipid peroxidation (Heath and Packer, 1968), were 70% higher in wild-type algae than in P5CS-1 algae when grown in the presence of Cd (50 μM Cd). Significantly, transgenic algae expressing the P5CS gene exhibited no increase in MDA levels when grown in the presence of 50 μM Cd, unlike wild-type cells. Importantly, the relative reduction (70%) in MDA levels in Cd-treated P5CS-1 cells compared with wild-type cells was inversely proportional to the increase in free Pro content of the P5CS-1 cells (80%). These results strongly suggest that Pro acts directly as an antioxidant to protect the cell from free radical damage and maintain a more reducing environment that is favorable for phytochelatin synthesis and Cd sequestration.
DISCUSSION
It was shown previously that exposure of Chlamydomonas to toxic levels of Cd induces phytochelatin synthesis (Howe and Merchant, 1992; Kaplan et al., 1995; Hu et al., 2001; Rubinelli et al., 2002). The induction of phytochelatin synthesis by Cd results in a severalfold increase in cellular Cd levels (Howe and Merchant, 1992; Kaplan et al., 1995; Cai et al., 1999; Hu et al., 2001). In addition to its effects on phytochelatin synthesis, Cd has been shown to alter patterns of gene expression, most notably that of genes whose products are involved in chlorophyll synthesis and Fe uptake. We have observed that the steady state transcript levels of the chlL gene, which encodes the regulatory Fe-S subunit of the light-independent protochlorophyllide synthase, and the CRD1 gene, which encodes an enzyme involved in chlorophyll synthesis (Rubinelli et al., 2002), are increased after short-term exposure to Cd. In addition, the expression of the H43 gene, which encodes a periplasmic Fe chaperonin, is upregulated substantially (×40) by Cd exposure (25 μM for 2 h) (Rubinelli et al., 2002). Presumably, the function of increased H43 expression is to facilitate Fe2+ uptake to competitively reduce Cd uptake through a common metal ion transporter (Cohen et al., 1998; Guerinot, 2000).
Here, we show that exposure of Chlamydomonas to minimally toxic levels (50 to 100 μM) of Cd results in reduced growth rates and chlorophyll content per cell (70% less chlorophyll per cell) and increased lipid peroxidation and GSH oxidation. Although many plants and algae respond to heavy metal stress with increased steady state levels of Pro, it is apparent that Chlamydomonas does not accumulate more Pro in response to Cd exposure. We found that transgenic strains expressing the mothbean P5CS were only slightly more tolerant to toxic levels of Cd (50 μM) than wild-type strains (Figure 5D), but P5CS-1 transgenic algae had fourfold higher Cd levels per cell than wild-type strains (Table 1). This increased Cd content per cell seems counterintuitive given the enhanced Cd tolerance of P5CS-expressing strains. The chemical form in which this excess Cd is sequestered in P5CS-expressing strains differs from the Cd conjugates observed in wild-type strains. In P5CS-expressing strains, most Cd is bound by sulfur ligands that have physical properties consistent with coordination by phytochelatin (Pickering et al., 1999). In wild-type Chlamydomonas, we observed that a substantial portion of the Cd ligands are provided by oxygen. These results are consistent with earlier Fourier transform infrared and titration studies for wild-type strains, which indicated that carboxylate groups accounted for nearly half of all Cd binding sites (Adhiya et al., 2002).
Notably, Chlamydomonas does not appear to make class I or II metallothioneins (Cai et al., 1999). Therefore, there are two possible mechanisms to account for the increased Cd-thiolate or Cd-phytochelatin complexes present in P5CS transgenic algae. These mechanisms include enhanced phytochelatin synthesis or reduced degradation and/or export of phytochelatins (Cd) from the cell (Lee et al., 1996). We favor the first hypothesis, because it is evident that there were substantially increased GSH levels (80% higher), relative to GSSG levels, in P5CS-expressing strains (Figures 5B and 5C). As indicated previously, Cd-GSH adduct and not GSSG is the substrate for phytochelatin synthesis (Vatamaniuk et al., 2000). Thus, increased GSH-Cd levels would lead to enhanced phytochelatin-Cd synthesis and accumulation. Although phytochelatin-Cd export has been demonstrated in microalgae, we cannot account for any mechanism by which Pro could alter or reduce the rate of phytochelatin (Cd) export from algal cells. In fact, we observed increased Cd export from P5CS-expressing cells relative to wild-type cells, consistent with greater flux (export) of Cd from P5CS transgenic cells as phytochelatin-Cd adducts (our unpublished observation).
To account for the increased GSH levels in P5CS transgenic cells, we turn to indirect evidence that indicates the mothbean P5CS-expressing cells have lower levels of products derived from free radical damage than wild-type cells (when grown in the presence of Cd). Analyses of the levels of MDA, a product of lipid peroxidation, in wild-type and P5CS-1 strains grown in the presence of Cd indicate that there is no increase in free radical–dependent peroxidation of lipids in high-Pro strains (Figure 5A). By contrast, exposure of wild-type strains to Cd resulted in nearly a twofold increase in MDA levels and a fourfold reduction in the GSH/0.5 GSSG ratio (relative to P5CS cells in the presence of Cd) (Figure 5D). The increase in GSSG content relative to GSH content presumably is attributable to oxidation by free radicals (Figures 5B and 5C) (Misra, 1974; Albro et al., 1986). Similar increases in Cd-dependent free radical damage have been observed in other biological systems, including plants and chloroplasts (Alia et al., 1997). Significantly, the increase in GSH/0.5 GSSG ratios (fourfold) in P5CS cells relative to wild-type cells was identical to the relative increase in bound Cd per cell (fourfold).
The mechanisms by which Pro reduces free radical damage include physical quenching of oxygen singlets and chemical reaction with hydroxyl radicals (Rustgi et al., 1977; Floyd and Nagy, 1984; Smirnoff and Cumbes, 1989; Lissi et al., 1993; Alia et al., 2001). Floyd and Nagy (1984) demonstrated that Pro readily forms stable nitroxyl radicals (in vitro) in the presence of hydroxyl radicals (·OH), whereas Rustgi et al. (1977) have shown that C5 of Pro can react with hydroxyl radicals. In addition, Pro has been shown to react directly with singlet oxygen (1O2), forming reversible charge-transfer complexes, which effectively quench free radicals. The propensity to form charge-transfer complexes via singlet oxygen is dependent in part on the ionization potential of the amine (Alia et al., 2001). Significantly, Pro has a low ionization potential and thus can readily form reversible charge-transfer complexes with 1O2, effectively quenching this reactive oxygen species (Alia et al., 2001). Pro also has been shown to quench free radicals in vivo. Alia et al. (1997) demonstrated that Pro can reduce free radical–mediated damage in isolated chloroplasts exposed to high light intensities.
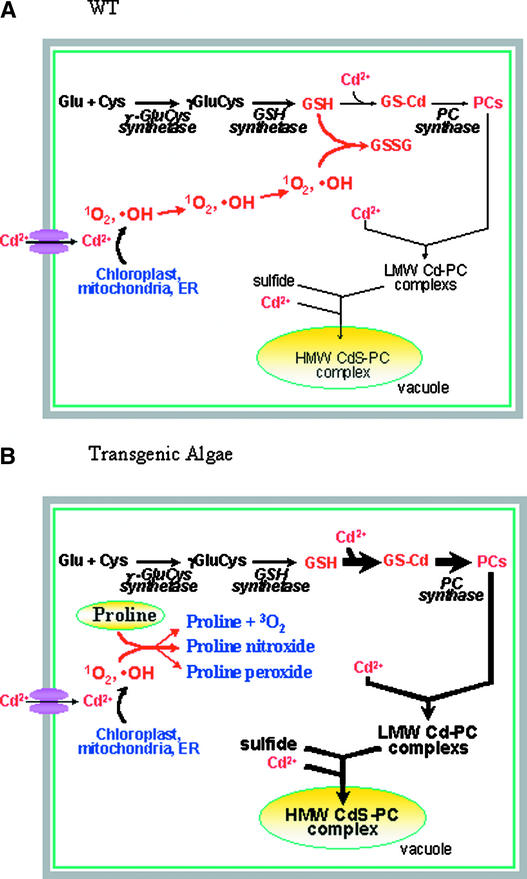
A model summarizing the role of free Pro in reducing free radical damage and enhancing Cd tolerance is presented in Figure 6. We propose that Pro reduces heavy metal stress by detoxification of free radicals produced as a result of Cd poisoning (Figure 6A). Pro may physically quench oxygen singlets or react directly with hydroxyl radicals. These reactions result in reduced free radical damage (lower MDA levels) and a more reducing cellular environment (higher GSH levels). The high GSH levels in turn facilitate phytochelatin synthesis and sequestration of heavy metal phytochelatin conjugates in the vacuole. This enhanced sequestration of Cd-phytochelatin complexes in the vacuole accounts for the transiently increased Cd content of P5CS-expressing cells (Figure 6B).
Figure 6.
Role of Free Pro in Free Radical Quenching.
Phytochelatin synthesis requires the synthesis of GSH by γ-GluCys synthetase and GSH synthetase, followed by binding of Cd and synthesis of Cd-phytochelatin by phytochelatin synthase. ER, endoplasmic reticulum; LMW, low molecular mass.
(A) In wild-type algae, Cd2+ induces the production of reactive oxygen species that rapidly oxidize GSH to GSSG. The resulting decreased GSH levels reduce phytochelatin synthesis.
(B) In transgenic algae, reactive oxygen species are reduced in quantity by reacting with free Pro. The resulting increased GSH levels facilitate increased phytochelatin production to sequester Cd.
METHODS
Strains and Media
The rec A− Escherichia coli strain XLI-Blue (Stratagene) was used in all recombinant DNA work. The Chlamydomonas reinhardtii strain used was the cell wall–deficient, Arg-requiring mutant CC-425 (arg7-8 cw15 mt+ sr-u-2-60), which was obtained from the Chlamydomonas Culture Collection at Duke University (Durham, NC). Chlamydomonas was grown in Tris-acetate-phosphate (TAP) medium supplemented with 50 μg Arg/mL for liquid medium or 100 μg Arg/mL for solid medium, when required, at 22 to 27°C. Illumination was continuous at 10 μmol·m−2·s−1 from fluorescent tubes.
Transformation of Chlamydomonas
A KpnI-ApaI fragment (∼2.45 kb) carrying the Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene (Hu et al., 1992) was subcloned into plasmid pSSCR7 and designated pCRP5CS. The plasmids pCRP5CS and p389 (encoding the arg7-8 gene used for complementation of the Arg auxotrophic mutation in CC-425) were cotransformed into the nucleus of the cell-wall-less Chlamydomonas strain CC-425 by electroporation (Shimogawara et al., 1998). Five micrograms of total plasmid DNA containing a 1:3 (mol/mol) ratio of p389 (arg7-8) and pCRP5CS was used for cotransformation. To increase the efficiency of insertion of the transforming DNA, the plasmids were linearized. Transformed colonies appeared 7 to 8 days after DNA transformation.
Cell Growth and Chlorophyll Content
Cell growth was determined by measuring the optical density at 750 nm (Sager and Granick, 1953). Chlorophyll determinations were performed according to Arnon (1949).
Protein Gel Blot Analysis
Cells from late-log-phase cultures of transgenic or wild-type lines were harvested, frozen in liquid nitrogen, and ground with a mortar and pestle in 50 mM Na-Tricine buffer, pH 8.0, containing 2 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, 20 μM leupeptin, and 2 mM l-1-chloro-3-(4-tosylannido)-4-phenyl-2-butanone. After centrifugation at 100,000g for 60 min, total soluble proteins were resolved on SDS-PAGE, transferred to polyvinylidene fluoride membranes, and immunoblotted using antibody against the purified mothbean (Vigna aconitifolia) P5CS protein (Cai et al., 1999).
Pro Determination
Free Pro was determined according to the method of Bates et al. (1973) One liter of cells from late-log-phase cultures of transgenic or wild-type cells was suspended in 10 mL of 3% salicylic acid, broken by French press at 5000 pounds, and centrifuged at 4000g for 10 min to remove cell debris. To 2 mL of the supernatant, 2 mL of acid ninhydrin was added, followed by the addition of 2 mL of glacial acetic acid and boiling for 60 min. The mixture was extracted with toluene, and the free Pro was quantified spectrophotometrically at 520 nm from the organic phase.
Cell Growth in Response to Salt Stress
A 1% (v/v) inoculum (∼1 to 4 × 104 cells/mL) for wild-type or P5CS-1 cells was used to inoculate 100 mL of artificial seawater medium. After 1, 3, 5, 12, and 24 h of incubation in artificial seawater medium, the viable cell number of each culture was determined by spreading cells on TAP plates containing Arg and counting the colonies.
Cd-K Edge Extended X-Ray Absorption Fine Structure Spectroscopy
Sample Preparation
Both wild-type and P5CS transgenic algae were grown in 4 L of TAP medium containing 50 μM Cd for 5 days. Cells were collected by centrifugation at 800g for 10 min. Cells were washed twice with deionized water and packed into sample holders for the Cd-K edge extended x-ray absorption fine structure (EXAFS) spectroscopic measurements.
Data Collection and Analysis
Cd-K EXAFS spectra were collected at room temperature on beam line 4-3 of the Stanford Synchrotron Radiation Laboratory, which is equipped with Ar-filled ionization chambers. Fluorescence spectra were collected with a 13-element Ge solid-state detector. Counting times were adjusted to provide at least 106 counts per energy step above the Cd-K edge. A total of six scans were collected for the nonbiological samples: 1 mM Cd(NO3)2, 5 mM each GSH and Cd(NO3)2, and 2.5 mM Pro, 2.5 mM GSH, and 5 mM Cd(NO3)2. For the wild-type and transgenic algae, a total of 45 and 24 scans were collected, respectively. Data analysis was performed using the EXAFSPAK programs (George and Pickering, 1995) according to standard methods. EXAFS spectra were extracted from the raw absorption data with a two-range spline concomitant with normalizing the amplitude to the edge jump. The EXAFS oscillations [χ(k)] were analyzed by a curve-fitting procedure to the equation
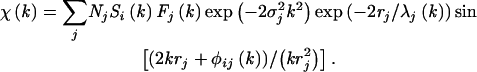 |
where Fj(k) is the back-scattering amplitude from each Nj neighboring atom of the jth type with a Debye-Waller factor of σj (to account for thermal vibration and static disorder); rj is the distance away from the central atom; and φij(k) is the total phase shift experienced by the photoelectron. The term exp(−2rj/λj) is caused by inelastic losses in the scattering process with λj. λj is the electron mean free path. Si(k) is the amplitude reduction factor attributable to a many-body effect, such as shake up/off at the central atom denoted by i. EXAFS phase, amplitude, and mean free path functions for the Cd-O or Cd-S shell were calculated for CdCO3 (otavite) or Cd-S (hawleyite) using the program FEFF 8 (Traill and Boyle, 1955; Graf, 1961; Konningsberger and Prins, 1988; Ankudinov et al., 1998).
Malondialdehyde Determination
Malondialdehyde (MDA) was determined in both transgenic and wild-type cells grown with or without Cd (50 μM) according to the method of Hodges et al. (1999). Cells were collected from a 1-L culture by centrifugation at 800g for 5 min and extracted with 25 mL of 80:20 (v/v) ethanol:water. One milliliter of the algal extract was added to a test tube with 1 mL of either 20% (w/v) trichloroacetic acid and 0.01% butyrated hydroxytoluene (−TBA solution) or 20% (w/v) trichloroacetic acid, 0.01% butyrated hydroxytoluene, and 0.65% thiobarbituric acid (+TBA solution). The solutions then were mixed vigorously, heated at 95°C for 25 min, cooled, and centrifuged at 3000g for 10 min, and the absorbance was measured at 440, 532, and 600 nm. The absorbance of the −TBA solution was used to correct the MDA concentration from the interfering compounds, which can absorb at 532 nm. MDA equivalents were calculated as described by Hodges et al. (1999).
GSH and GSSG Determination
GSH and GSSG were determined in the cell extracts via the GSH reductase–dependent enzymatic cycle (Anderson, 1985). Cells from a 1-L culture were collected by centrifugation at 800g for 5 min and frozen in liquid nitrogen. Five volumes per gram fresh weight of 5% 5-sulfosalicylic acid was added, and the freeze-thaw process was repeated three times to disrupt the cells. After centrifugation at 20,000g for 10 min to remove debris, nonprotein thiol levels were determined spectrophotometrically using Ellman's reagent with GSH as a standard. GSSG levels were determined after derivatization with 2 μM 2-vinylpyridine for 60 min. GSH levels were obtained by subtracting the GSSG levels from the total GSH level in the sample.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
This research was supported by a grant from the National Oceanographic and Atmospheric Administration to R.T.S. and S.T. and by a grant to D.P.S.V.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.004853.
References
- Adhiya, J., Cai, X.-H., Sayre, R.T. , and Traina, S. (2002). Binding of aqueous cadmium by the lyophilized biomass of Chlamydomonas reinhardtii. Colloids Surf. A Physiochem. Eng. Aspects 210, 1–11. [Google Scholar]
- Albro, P.W., Corbett, J.T., and Schroeder, J.L. (1986). Generation of hydrogen-peroxide by incidental metal ion-catalyzed autooxidation of glutathione. J. Inorg. Biochem. 27, 191–203. [DOI] [PubMed] [Google Scholar]
- Alia, Mohanty, P., and Matysik, J. (2001). Effect of proline on the production of singlet oxygen. Amino Acids 21, 195–200. [DOI] [PubMed] [Google Scholar]
- Alia, Saradhi, P.P., and Mohanty, P. (1997). Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J. Photochem. Photobiol. B 38, 253–257. [Google Scholar]
- Alloway, B.J. (1995). Cadmium Heavy Metals in Soils, 2nd ed, B.J. Alloway, ed (London: Chapman and Hall), pp. 122–151.
- Anderson, M.E. (1985). Tissue glutathione. In Handbook of Methods for Oxygen Radical Research, R.A. Greenwald, ed (Boca Raton, FL: CRC Press), pp. 317–323.
- Ankudinov, A.L., Ravel, B., Rehr, J.J., and Conradson, S.D. (1998). Real-space multiple-scattering calculation and interpretation of x-ray-absorption near-edge structure. Physiol. Rev. B 58, 7565–7576. [Google Scholar]
- Arnon, D.I. (1949). Copper enzymes in isolated chloroplast: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, L.S., Waldren, R.P., and Teare, I.D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. [Google Scholar]
- Brennan, R.J., and Schiestl, R.H. (1996). Cadmium is an inducer of oxidative stress in yeast. Mutat. Res. 356, 171–178. [DOI] [PubMed] [Google Scholar]
- Buchet, J.P., Roels, H., Bernard, A., and Lauwerys, R. (1980). Assessment of renal-function of workers exposed to inorganic lead, cadmium, or mercury-vapor. J. Occup. Environ. Med. 22, 741–750. [PubMed] [Google Scholar]
- Cai, X.-H., Brown, C., Adhiya, J., Traina, S.J., and Sayre, R.T. (1999). Growth and heavy metal binding properties of transgenic Chlamydomonas expressing a foreign metallothionein gene. Int. J. Phytoremediation 1, 53–65. [Google Scholar]
- Chaoui, A., Mazhoudi, S., Ghorbal, M.H., and Ferjani, E.E. (1997). Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 127, 139–147. [Google Scholar]
- Cohen, C.K., Fox, T.C., Garvin, D.F., and Kochian, L.V. (1998). The role of iron-deficiency stress responses in stimulating heavy metal transport in plants. Plant Physiol. 116, 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delauney, A.J., and Verma, D.P.S. (1993). Proline biosynthesis and osmoregulation in plants. Plant J. 4, 215–223. [Google Scholar]
- Desi, I., Nagymajtenyi, L., and Schulz, H. (1998). Behavioural and neurotoxicological changes caused by cadmium treatment of rats during development. J. Appl. Toxicol. 18, 63–70. [DOI] [PubMed] [Google Scholar]
- di Toppi, L.S., and Gabbrielli, R. (1999). Response to cadmium in higher plants. Environ. Exp. Bot. 41, 105–130. [Google Scholar]
- Dudka, S., and Adriano, D.C. (1997). Environmental impacts of metal ore mining and processing: A review. J. Environ. Qual. 26, 590–602. [Google Scholar]
- Farago, M.E., and Mullen, W.A. (1979). Plants which accumulate metals. Part IV. A possible copper-proline complex from the roots of Armeria maritima. Inorg. Chim. Acta 32, L93–L94. [Google Scholar]
- Floyd, R.A., and Nagy, I. (1984). Formation of long-lived hydroxyl free-radical adducts of proline and hydroxyproline in a Fenton reaction. Biochim. Biophys. Acta. 790, 94–97. [DOI] [PubMed] [Google Scholar]
- George, G.N., and Pickering, I.F. (1995). EXAFSPAK. (Stanford, CA: Stanford Synchrotron Radiation Laboratory), http://www-ssrl.slac.stanford.edu/exafspak.html.
- Graf, D.L. (1961). Crystallographic tables for the rhombohedral carbonates. Am. Mineral. 46, 1283–1316. [Google Scholar]
- Guerinot, M.L. (2000). The ZIP family of metal transporters. Biochim. Biophys. Acta 1465, 190–198. [DOI] [PubMed] [Google Scholar]
- Hare, P.D., and Cress, W.A. (1997). Metabolism implications of stress-induced proline accumulation in plants. Plant Growth Regul. 21, 79–102. [Google Scholar]
- Heath, R.L., and Packer, L. (1968). Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198. [DOI] [PubMed] [Google Scholar]
- Hellstrom, L., Elinder, C.G., Dahlberg, B., Lundberg, M., Jarup, L., Persson, B., and Axelson, O. (2001). Cadmium exposure and end-stage renal disease. Am. J. Kidney Dis. 38, 1001–1008. [DOI] [PubMed] [Google Scholar]
- Hodges, D.M., DeLong, J.M., Forney, C.F., and Prange, R.K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611. [DOI] [PubMed] [Google Scholar]
- Howe, G., and Merchant, S. (1992). Heavy metal-activated synthesis of peptides in Chlamydomonas reinhardtii. Plant Physiol. 98, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C.-C.A., Delauney, A.J., and Verma, D.P.S. (1992). A bifunctional enzyme (Δ1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc. Natl. Acad. Sci. USA 89, 9354–9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S., Lau, K.W.K., and Wu, M. (2001). Cadmium sequestration in Chlamydomonas reinhardtii. Plant Sci. 161, 987–996. [Google Scholar]
- Hussain, T., Shukla, G.S., and Chandra, S.V. (1987). Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: In vivo and in vitro studies. Pharmacol. Toxicol. 60, 355–358. [DOI] [PubMed] [Google Scholar]
- Kahle, H. (1993). Response of roots of trees to heavy metals. Environ. Exp. Bot. 33, 99–119. [Google Scholar]
- Kaplan, D., Heimer, Y.M., Abeliovich, A., and Goldsbrough, P.B. (1995). Cadmium toxicity and resistance in Chlorella sp. Plant Sci. 109, 129–137. [Google Scholar]
- Konningsberger, D.C., and Prins, R. (1988). X-Ray Absorption: Principles, Applications, Techniques of EXAFS, SEXAFS and XANES, D.C. Konningsberger and R. Prins, eds (New York: John Wiley & Sons).
- Kuznetsov, V.V., and Shevyakova, N.I. (1997). Stress responses of tobacco cells to high temperature and salinity: Proline accumulation and phosphorylation of polypeptides. Physiol. Plant. 100, 320–326. [Google Scholar]
- Lee, J.G., Ahner, B.A., and Morel, F.M.M. (1996). Export of cadmium and phytochelatin by the marine diatom Thalassiosira weissflogii. Environ. Sci. Technol. 30, 1814–1821. [Google Scholar]
- Lissi, E.A., Encinas, M.V., Lemp, E., and Rubio, M.A. (1993). Singlet O2 bimolecular processes: Solvent and compartmentalization effects. Chem. Rev. 93, 699–723. [Google Scholar]
- Mehta, S.K., and Gaur, J.P. (1999). Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol. 143, 253–259. [Google Scholar]
- Misra, H.P. (1974). Generation of superoxide free radical during the autooxidation of thiols. J. Biol. Chem. 249, 2151–2155. [PubMed] [Google Scholar]
- Nies, D.H. (1999). Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 51, 730–750. [DOI] [PubMed] [Google Scholar]
- Nogawa, K., and Kido, T. (1993). Biological monitoring of cadmium exposure in itai-itai disease epidemiology. Int. Arch. Occup. Environ. Health 65, S43–S46. [DOI] [PubMed] [Google Scholar]
- Nriagu, J.O., and Pacyna, J.M. (1988). Quantitative assessment of worldwide contamination of air, water and soils by trace-metals. Nature 333, 134–139. [DOI] [PubMed] [Google Scholar]
- Ouariti, O., Boussama, N., Zarrouk, M., Cherif, A., and Ghorbal, M.H. (1997). Cadmium- and copper-induced changes in tomato membrane lipids. Phytochemistry 45, 1343–1350. [DOI] [PubMed] [Google Scholar]
- Paleg, L.G., Stewart, G.R., and Bradbeer, J.W. (1984). Proline and glycine betaine influence protein solvation. Plant Physiol. 75, 974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering, I.J., Prince, R.C., George, G.N., Rauser, W.E., Wickramasinghe, W.A., Watson, A.A., Dameron, C.T., Dance, I.G., Fairlie, D.P., and Salt, D.E. (1999). X-ray absorption spectroscopy of cadmium phytochelatin and model systems. Biochim. Biophys. Acta 1429, 351–364. [DOI] [PubMed] [Google Scholar]
- Rubinelli, P., Siripornadulsil, S., Gao-Rubinelli, F., and Sayre, R.T. (2002). Cadmium- and iron-stress-inducible gene expression in the green alga Chlamydomonas reinhardtii: Evidence for H43 protein function in iron assimilation. Planta 215, 1–13. [DOI] [PubMed] [Google Scholar]
- Rustgi, S., Joshi, A., Moss, H., and Riesz, P. (1977). ESR of spin-trapped radicals in aqueous solutions of amino acids: Reactions of the hydroxyl radical. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 31, 415–440. [DOI] [PubMed] [Google Scholar]
- Sager, R., and Granick, S. (1953). Nutritional studies with Chlamydomonas reinhardtii. Ann. N.Y. Acad. Sci. 56, 831–838. [DOI] [PubMed] [Google Scholar]
- Salt, D.E., Prince, R.C., Pickering, I.J., and Raskin, I. (1995). Mechanisms of cadmium mobility and accumulation in Indian Mustard. Plant Physiol. 109, 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalio, L.M., Dalurzo, H.C., Gómez, M., Romero-Puertas, M.C., and del Río, L.A. (2001). Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 52, 2115–2126. [DOI] [PubMed] [Google Scholar]
- Schat, H., Sharma, S.S., and Vooijs, R. (1997). Heavy metal-induced accumulation of free proline in a metal-tolerant and a nontolerant ecotype of Silene vulgaris. Physiol. Plant. 101, 477–482. [Google Scholar]
- Schützendübel, A., Schwanz, P., Teichmann, T., Gross, K., Langenfeld-Heyser, R., Godbold, D.L., and Polle, A. (2001). Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol. 127, 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, K., and Dubey, R.S. (1998). Effect of cadmium on proline accumulation and ribonuclease activity in rice seedlings: Role of proline as a possible enzyme protectant. Biol. Plant. 40, 121–130. [Google Scholar]
- Shimogawara, K., Fujiwara, S., Grossman, A., and Usada, H. (1998). High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 148, 1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff, N., and Cumbes, Q.J. (1989). Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28, 1057–1060. [Google Scholar]
- Stohs, S.J., Bagchi, D., Hassoun, E., and Bagchi, M. (2000). Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 19, 201–213. [PubMed] [Google Scholar]
- Taylor, C.B. (1996). Proline and water deficit: Ups, downs, ins and outs. Plant Cell 8, 1221–1224. [Google Scholar]
- Traill, R.J., and Boyle, R.W. (1955). Hawleyite, isometric cadmium sulphide, a new mineral. Am. Mineral. 40, 555–559. [Google Scholar]
- Tsuritani, I., Honda, R., Ishizaki, M., Yamada, Y., and Nishijo, M. (1996). Ultrasonic assessment of calcaneus in inhabitants in a cadmium-polluted area. J. Toxicol. Environ. Health 48, 131–140. [DOI] [PubMed] [Google Scholar]
- Vatamaniuk, O.K., Mari, S., Lu, Y.P., and Rea, P.A. (2000). Mechanism of heavy metal ion activation of phytochelatin (PC) synthase: Blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. J. Biol. Chem. 275, 31451–31459. [DOI] [PubMed] [Google Scholar]
- Verma, D.P.S. (1999). Osmotic stress tolerance in plants: Role of proline and sulfur metabolisms. In Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants, K. Shinozaki and K. Yamaguchi-Shinozaki, eds (Austin, TX: R.G. Landers), pp. 153–168.
- Wu, J.-T., Chang, S.J., and Chou, T.L. (1995). Intracellular proline accumulation in some algae exposed to copper and cadmium. Bot. Bull. Acad. Sin. 36, 89–93. [Google Scholar]