Abstract
In growing Arabidopsis root hairs, the nucleus locates at a fixed distance from the apex, migrates to a random position during growth arrest, and moves from branch to branch in a mutant with branched hairs. Consistently, an artificial increase of the distance between the nucleus and the apex, achieved by entrapment of the nucleus in a laser beam, stops cell growth. Drug studies show that microtubules are not involved in the positioning of the nucleus but that subapical fine F-actin between the nucleus and the hair apex is required to maintain the nuclear position with respect to the growing apex. Injection of an antibody against plant villin, an actin filament-bundling protein, leads to actin filament unbundling and movement of the nucleus closer to the apex. Thus, the bundled actin at the tip side of the nucleus prevents the nucleus from approaching the apex. In addition, we show that the basipetal movement of the nucleus at root hair growth arrest requires protein synthesis and a functional actin cytoskeleton in the root hair tube.
INTRODUCTION
Root hairs are tubular structures that emerge from certain root epidermal cells (Haberlandt, 1883). They expand by localized exocytosis of cell wall matrix contained in Golgi-derived vesicles at the cell apex into a plastic cell wall, a phenomenon referred to as tip growth (reviewed by Derksen and Emons, 1990). During the process of tip growth, a distinct organization of the cell can be observed. The apical area of the root hair is cytoplasm dense with endoplasmic reticulum (ER), Golgi cisternae, and mitochondria, whereas in the extreme apex, a high density of vesicles is present (Emons, 1987; Ridge, 1988; Galway et al., 1997; Miller et al., 2000). Net-axially aligned fine bundles of actin filaments (fine F-actin), which are involved in the delivery of Golgi-derived vesicles to the apex, are present in the subapical area (Miller et al., 1999). Another typical observation in root hairs involves the position of the nucleus. In growing root hairs, the nucleus is located at a distinct distance from the apex (Haberlandt, 1887; reviewed by Miller et al., 1997). This precise positioning of the nucleus during the growth process suggests that the nucleus is part of the growth machinery involved in root hair elongation. How the nucleus maintains a fixed position from the growing tip and how it is involved in the tip growth machinery is not clear.
Mutants can be helpful tools to gain insight into various processes. In this study, we used the cow1-2 mutant (Grierson et al., 1997), which produces branches in ∼20% of its root hairs. The length of the branches in a branched cow1-2 root hair can be greater than the distinct distance between the nucleus and the tip; therefore, the behavior of the nucleus in a branched root hair could provide valuable insight into the role of the nucleus in tip growth.
Here, we demonstrate that the presence of the nucleus at a fixed distance from growing root hair tips is essential for Arabidopsis root hair growth and that the positioning is dependent on the actin cytoskeleton, but not the tubulin cytoskeleton, during hair growth and growth arrest. Furthermore, we show that actin filament bundling is involved in the positioning of the nucleus in root hairs and that the movement of the nucleus away from the hair tip during growth arrest is both actin based and dependent on new protein synthesis.
RESULTS
Position of the Nucleus during Root Hair Growth and Growth Arrest
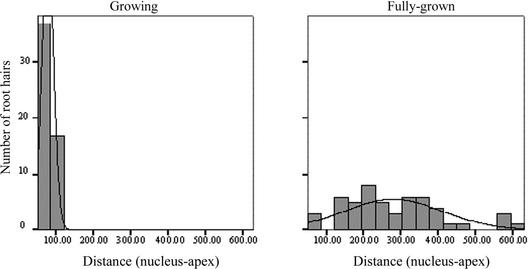
To determine the movement of nuclei in root hairs, time-lapse recordings were made of developing hairs (Figure 1). The distance between the nucleus and the apex of growing Arabidopsis ecotype Columbia root hairs was 77 ± 15 μm (19%; n = 54), whereas in fully grown root hairs, the nucleus was located at a random position in the hair (247 ± 134 μm [54%]; n = 57). In Figure 2, the position distribution of nuclei in growing versus fully grown root hairs is displayed. The nucleus remains at a fixed distance from the apex of growing hairs and moves back when growth terminates at a speed between 0 and 60 μm/min (12 ± 23 μm/min; n = 25), so that eventually it obtains a random position in the hair. Chytilova et al. (2000) reported occasional division of nuclei into subnuclear structures in root hairs of Arabidopsis, and these remain connected by thread-like structures. We did not observe such divisions using Hoffman modulation contrast or differential interference contrast microscopy of living hairs. However, they used a green fluorescent protein (GFP)–β-glucuronidase fusion construct with a nuclear localization sequence that accumulates in the nucleoplasm. The loading of the nucleoplasm with the GFP–β-glucuronidase protein may be the reason for the creation of subnuclear structures. The fixed distance between the nucleus and the apex in a growing root hair can be observed clearly in the supplemental data online of Chytilova et al. (2000).
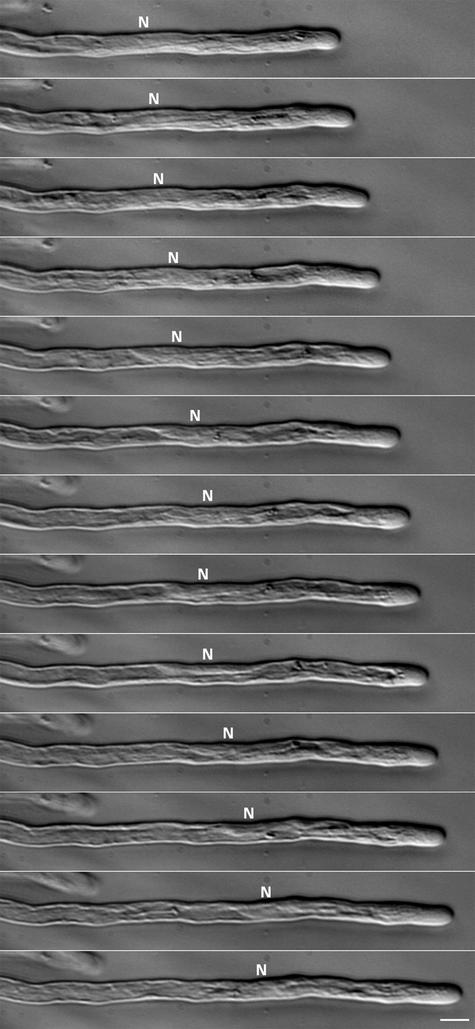
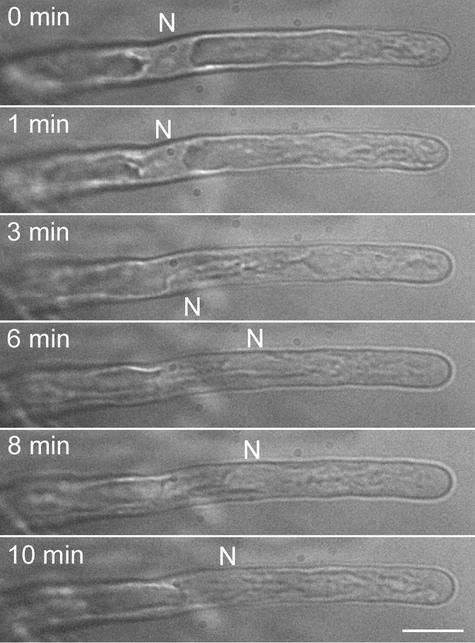
Figure 1.
Hoffman Modulation Contrast Images from a Time-Lapse Recording of a Growing Arabidopsis Root Hair.
Photographs were taken every 4 min. The nucleus (N) is indicated. The sequential images demonstrate that the distance between the nucleus and the tip in growing root hairs is more-or-less fixed. Bar = 10 μm.
Figure 2.
Distribution of the Distance between the Apex and the Nucleus in Growing and Fully Grown Root Hairs of Arabidopsis.
The bars represent the frequency distribution, and the lines represent a fitted normal distribution curve.
Behavior of the Nucleus in a Mutant with Branching Root Hairs
The cow1-2 mutant (Grierson et al., 1997) has shorter and wider root hairs than those of wild-type plants and reduced growth speed. It produces a branch in ∼20% of its root hairs. We studied the position of the nucleus during branch formation by time-lapse video microscopy. In nonbranching root hairs of the cow1-2 mutant, the positional behavior of the nucleus was comparable to that of wild-type root hairs in that the distance was smaller and more constant in growing root hairs than in fully grown root hairs (the distance from nucleus to apex in growing root hairs was 31 ± 6 μm [19%; n = 57], and the distance in fully grown root hairs was 106 ± 70 μm [66%; n = 61]). The shorter distance between the nucleus and the apex in the mutant correlated with the reduced length and increased width of cow1-2 root hairs.
Branches develop from the sides of growing root hairs (Figure 3), a process preceded by the movement of the nucleus to that site. Subsequently, the nucleus moves from one hair tip to the other and is located within 31 μm from the growing tip between 30 and 70% of the time (n = 20). When the nucleus moves back from a tip, that tip continues to elongate until the smooth area at the tip, containing the Golgi-derived vesicles, has disappeared. The tip to which the nucleus moves starts to elongate when such a smooth area has been built up. We conclude that in the cow1-2 mutant, the presence of the nucleus at a fixed distance from a growing root hair tip correlates with the sustained growth of that tip.
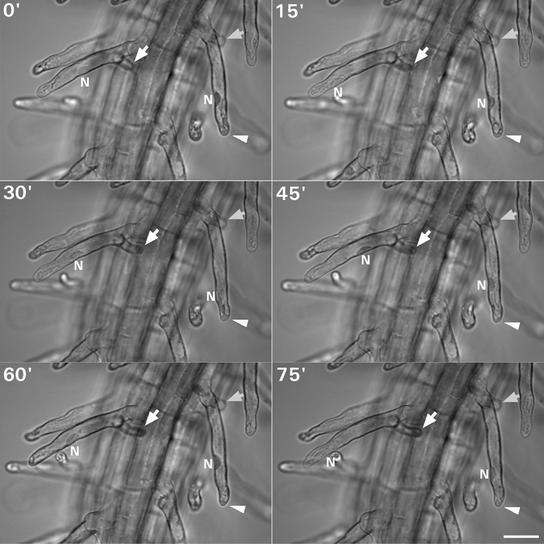
Figure 3.
Sequential Images from a Time-Lapse Recording of Growing cow1-2 Root Hairs That Develop Branches.
One photograph was taken every 15 min. The nuclei (N) are indicated. During this time lapse, both tips of the left root hair were growing, and the nucleus was moving between the two branches. In the right root hair, only one tip was growing. The nucleus was at a fixed distance from this tip. The branches of the root hairs are marked with arrows, and in the root hair in which only one tip was growing, the growing tip is marked with an arrowhead. Bar = 25 μm.
Prolonged Mechanical Inhibition of the Movement of the Nucleus by Optical Trapping Leads to Growth Inhibition
Because the presence of the nucleus at a fixed distance from a growing tip is correlated with growth and the nucleus alternates between the growing tips of the cow1-2 mutant, we studied the effect of direct manipulation of the position of the nucleus in growing wild-type root hairs. To achieve noninvasive manipulation of the nucleus, an optical trap was used. This technique allows the trapping of a micrometer-sized object with an index of refraction greater than the surrounding medium in a focused laser beam. In practice, we trapped the nucleolus, which has a higher density and hence a higher index of refraction than the nucleus and also is smaller (specific conditions of the optical trap are given in Methods). During the experiments, we caught the nucleolus in the laser beam and were able to keep it at a fixed position, so that the movement of the nucleolus, and thus the nucleus, was inhibited. It is not feasible to trap the nucleus and observe the root hair tip simultaneously because of the large distance between the nucleus and the hair tip at the high magnification required. Therefore, it was not possible to check for root hair growth before the optical trap was released, so root hair growth was checked before and after optical trapping of the nucleus.
When the nucleolus was held at a fixed position, in the first minutes of the experiments, the position of the nucleolus in the nucleus changed, because the nucleus moved forward at the speed of growth, maintaining the fixed distance to the growing tip. After 1 to 3 min, when the nucleolus was located at the rear end of the nucleus, nuclear movement was inhibited and the distance between the nucleus and the tip increased at the speed of cell growth.
When the trap was released after <10 min, root hair growth continued. After 10 to 15 min of continued trapping, the nucleus suddenly struggled to move toward the root hair tip for ∼0.5 min. When the trap was released after this time, root hair growth was found to have stopped. Because a root hair grows 10 to 15 μm in 10 to 15 min, the nucleus struggles to move out of the trap when the distance between the nucleus and the tip has increased by 10 to 15 μm. This increased distance correlates very well with a value slightly greater than the largest known distance between the nucleus and the tip in growing root hairs. In addition, inspection of the cytoarchitecture of the root hair at this time revealed that the subapical cytoplasm-dense region found in growing root hairs was disappearing and that the cytoarchitecture of the hair was becoming more similar to that of a fully grown hair. On a few occasions, the generated force on the nucleus in the basipetal direction was so great that the nucleolus escaped from the trap. However, it never escaped toward the tip, but only backward. After this basipetal movement of the nucleus, its position in the root hair did not alter for at least 30 min (n = 5).
To determine whether optical damage of the nucleolus, or its movement inside the nucleus, could have caused the root hairs to terminate growth, we trapped the nucleolus and moved the trap in the direction of growth every 30 s. The nucleolus relocated within the nucleus during the trapping time, but it recovered its original position once the trap was released. During this experiment, the nucleus followed the growing tip, and this treatment did not lead to the inhibition of growth. This experiment demonstrates that there is no relevant damage caused by the optical adsorption and relocation of the nucleolus inside the nucleus. In addition, pointing the optical trap to a fixed position in the subapical cytoplasm did not lead to changes in growth speed and nuclear positioning, showing that the trapping procedure does not disturb the tip-growth cytoarchitecture. In Table 1, the results of the different treatments are listed. We conclude that the location of the nucleus plays a role in the growth of root hairs and that the subapical cytoplasm-dense area plays a role in the localization of the nucleus in wild-type growing root hairs.
Table 1.
Mechanical Increase of the Distance between the Nucleus and the Tip by Trapping the Nucleolus in a Laser Beam in Growing Root Hairs Leads to Inhibition of Growth after 10 to 15 Min
| Growth before Trapping | Trapping Time (min) | Treatment | Growth after Trapping (μm/min) |
|---|---|---|---|
| + | 5:00 | Nucleolus | −0.90 |
| + | 5:00 | Nucleolus | −0.86 |
| + | 6:00 | Nucleolus | −0.96 |
| + | 10:00 | Nucleolus | −0.03 |
| + | 12:00 | Nucleolus | −0.05 |
| + | 13:00 | Nucleolus | −0.01 |
| + | 17:00 | Nucleolus | −0.04 |
| + | 26:00 | Nucleolus | −0.00 |
| + | 10:00 | Follow | −0.87 |
| + | 10:00 | Follow | −0.93 |
| + | 15:30 | Follow | −0.93 |
The treatments were trapping of the nucleolus at a fixed position (nucleolus) and the control treatment as described in the text (follow). Pointing the trap for at least 15 min to a location in the cytoplasm did not lead to growth inhibition (n = 5 traps of cytoplasm).
Role of the Cytoskeleton in Maintaining a Fixed Distance between the Nucleus and the Hair Tip
Microtubules form a shallow helix in the cortical cytoplasm of Arabidopsis root hairs (Figure 4A). The addition to the medium of 1 μM oryzalin, which binds specifically to tubulin dimers and prevents their incorporation into microtubules (Anthony et al., 1998), led to complete depolymerization of all microtubules in root hairs within 5 min (Figure 4B). Time-lapse imaging shows that during and after microtubule depolymerization, root hair growth continued at the same speed of ∼1 μm/min (0.96 ± 0.15 μm compared with 0.93 ± 0.17 μm in untreated root hairs). The cytoarchitecture of the cells did not change dramatically, but the root hairs became slightly wavy, as has been reported previously with oryzalin treatment (Bibikova et al., 1999) and the mutant mor1 (Whittington et al., 2001). Consistently, the position of the nucleus was not altered significantly by the microtubule-depolymerizing drug (67 ± 14 μm [21%] at 10 min after oryzalin application compared with 77 ± 15 μm [19%] in untreated growing root hairs by Kolmogorov-Smirnov test). We conclude that microtubules are not essential for nuclear positioning in growing Arabidopsis root hairs.
Figure 4.
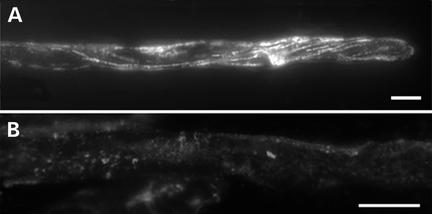
The Microtubule Cytoskeleton in Arabidopsis Root Hairs.
(A) Microtubules formed a shallow helix in Arabidopsis root hairs and were located cortically.
(B) Application of 1 μM oryzalin led to the depolymerization of all microtubules within 5 min. Bars = 10 μm.
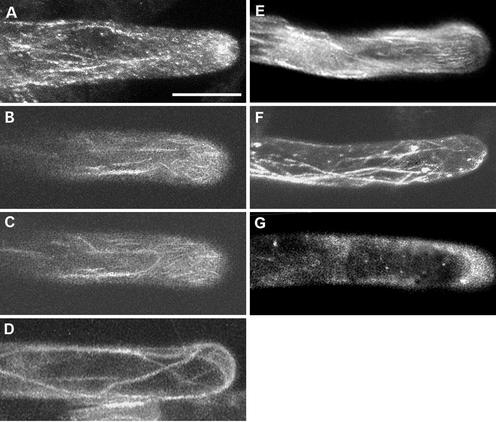
Using GFP fused to the actin binding domain of mouse talin (Kost et al., 1998), Baluška et al. (2000) reported the occurrence of filamentous actin in living root hairs of Arabidopsis. In their work, the labeling of filamentous actin in the subapex was not very distinct, possibly as a result of the dynamics of the actin cytoskeleton or the inability of the GFP–actin binding domain of mouse talin fusion to bind to the subapical fine F-actin. We reported previously that actin filament staining with fluorescent phalloidin of root hairs of Vicia sativa after an optimized fixation procedure (ester-aldehyde-choline [EAC]) (Emons and De Ruijter, 2000) gives the same results as immunocytochemistry after rapid freeze fixation and freeze substitution, although with more detail (Miller et al., 1999). We have now used both techniques with Arabidopsis root hairs. The immunocytochemistry technique after freeze fixation suffered from the fact that the fine bundles of actin filaments in the subapex were obscured (Figure 5A), probably by antibody attached to actin monomers, as reported by Miller et al. (1999). The EAC method gave the most crisp images of the actin cytoskeleton. In growing hairs, an area at the extreme tip was free of bundles of actin filaments, followed by a subapical area with fine F-actin (Figures 5B [cortex] and 5C [median plane of the same cell]), typical of growing root hairs (Miller et al., 1999). Fine F-actin was not present in fully grown root hairs (Figure 5D). In addition, we injected fluorescently labeled phalloidin into Arabidopsis root hairs, with similar results (Figure 5E). The organization of the actin cytoskeleton in the injected living hairs was similar to that of cytoskeleton fixed with the EAC procedure. Although hairs stayed alive, they mostly stopped growing, making microinjection of labeled phalloidin an unsuitable laboratory procedure for the determination of the actin cytoskeleton configuration in growing root hairs.
Figure 5.
The Actin Cytoskeleton in Arabidopsis Root Hairs.
(A) Full projection of a freeze-fixed root hair labeled with anti-actin antibody. A flat projection of a z-series of confocal images shows net-axially oriented, thick bundles of actin filaments in the root hair base that branch into a subapical network of thinner bundles of actin filaments. In the extreme apex of growing root hairs, an area was present in which actin filament bundles could not be detected. Bar = 10 μm.
(B) and (C) Partial projections (depth of 2 μm) of confocal images of an EAC-fixed and fluorescently labeled phallacidin-stained growing root hair show thin bundles of actin filaments in the cortical plane (B) and the median plane (C) of the subapical cytoplasm.
(D) A fully grown root hair stained with fluorescently labeled phallacidin shows thick bundles that loop through the tip. Fine subapical actin filament bundles were absent.
(E) A living root hair injected with fluorescently labeled phalloidin at 5 min after injection.
(F) Five minutes after the application of 5 μM CD, the bundles of actin filaments in the root hair tube were intact, but the subapical fine F-actin had disappeared. The photograph shows a freeze-fixed root hair labeled with anti-actin antibody.
(G) The application of 1 μM LA led to the depolymerization of all of the filamentous actin within 1 min. The photograph shows a freeze-fixed root hair labeled with anti-actin antibody.
To answer the question of whether the subapical fine F-actin is involved specifically in keeping the nucleus following the growing hair tip, we used cytochalasin D (CD). At a concentration of 1 to 5 μM, CD depolymerized fine F-actin in growing Arabidopsis root hairs and kept the thicker bundles in the root hair tube (Figure 5F), and thus cytoplasmic streaming, intact, as demonstrated by Miller et al. (1999) for V. sativa root hairs. The CD-induced fine F-actin depolymerization gave immature root hairs a cytoarchitecture similar to that of fully grown root hairs (Figure 5D) and arrested their growth. In this experiment, the nucleus behaved similar to normal growth-terminating root hairs and migrated away from its fixed distance from the apex back toward the root hair base (Figure 6). We conclude that in the absence of subapical fine F-actin, the nucleus does not maintain its position in relation to the growing root hair tip.
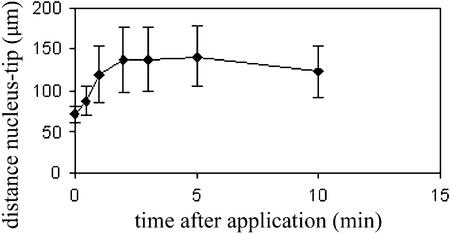
Figure 6.
Positioning of the Nucleus in Arabidopsis Root Hairs after the Application of 5 μM CD to the Medium.
After CD application, the distance between the nucleus and the apex increased, whereas root hairs terminated growth. The bars represent standard deviations. n ≥ 15 per time point.
Chytilova et al. (2000) demonstrated that CD-induced actin depolymerization inhibited the random movement of GFP-tagged nuclei in Arabidopsis intercalary growing root cells. However, these experiments were performed with high concentrations of this actin drug (50 and 100 μM cytochalasin B and CD), which in our hands led to a complete arrest of cytoplasmic streaming. Results obtained with such high concentrations of cytochalasin should be compared with results obtained with 1 μM latrunculin A (LA), in which case all actin filaments are depolymerized (Gibbon et al., 1999).
Role of the Cytoskeleton in the Basipetal Movement of the Nucleus When Growth Terminates
In growth-terminating root hairs, the basipetal movement of the nucleus occurred normally in the absence of microtubules (oryzalin treatment). In the absence of subapical fine F-actin (CD treatment), the nucleus behaved as in normal growth-terminating root hairs and migrated from its fixed position near the hair tip backward toward the hair base. To determine whether filamentous actin is required for the backward movement of the nucleus during growth arrest, we completely depolymerized the actin cytoskeleton by the application of LA (Figure 5G). LA binds 1:1 to G-actin and prevents the incorporation of the protein into filaments, leading to a change in the equilibrium between G- and F-actin, thus inducing actin depolymerization (Gibbon et al., 1998). The application of 1 μM LA to Arabidopsis root hairs led to the arrest of cell growth and cytoplasmic streaming within 1 min. After complete actin depolymerization, the position of the nucleus did not change (mean change in position from the tip from the moment of LA application to 10 min after LA application was 0.23 ± 0.16 μm [n = 15]). From these data, it can be concluded that the movement of the nucleus in root hairs is based on filamentous actin. More importantly, at growth arrest, the backward movement of the nucleus from its position in the subapex of the hair did not occur in the absence of bundles of actin filaments caused by LA treatment. Together, the data obtained with actin drugs show that during root hair growth arrest, the movement of the nucleus away from its position in the subapex is related to the disappearance of the subapical fine F-actin and that the bundles of actin filaments in the root hair tube are required for this process.
Protein Synthesis Is Required to Initiate Basipetal Movement of the Nucleus When Growth Terminates
The speed of the movement of the nucleus toward the root hair base when growth terminates in naturally growth-terminating root hairs varies between 0 and 1 μm/s. The initial release takes place when the root hair is still growing but the length of the cytoplasm-dense area is decreasing. Once basipetal movement is initiated, the nucleus moves randomly throughout the root hair, alternating with intervals during which it does not move, as has been demonstrated by Chytilova et al. (2000).
To determine whether the release of the nucleus from its fixed position in relation to the apex at growth arrest is an active process, we added to the medium either 10 μg/mL cycloheximide to block mRNA translation or 20 μM actinomycin D to block gene transcription. Two minutes after the application of cycloheximide and 20 min after the application of actinomycin D, root hair growth had terminated (Figure 7), but cells remained alive for the 5 h during which they were observed and cytoplasmic streaming continued. During protein synthesis inhibition in growing root hairs, we observed a slight flattening of the root hair tip, which we cannot explain. Within the first hour, the subapical cytoplasm-dense area disappeared. The nucleus did not move in the basipetal direction but maintained its fixed distance from the root hair tip (62 ± 10 μm [17%; n = 20]) (Figure 7). In root hairs that had terminated growth before the application of the drugs, the normal basipetal movement of the nucleus was not inhibited (Figure 7). Figure 7A shows a root hair that had been treated for 4 h with 10 μg/mL of the translation blocker cycloheximide. The nucleus is still located at the fixed distance from the tip, which is normal for a growing cell. The root hair shown in Figure 7B had been treated for 4 h with 20 μM actinomycin D, an inhibitor of gene transcription. The nucleus is in a position that is normal for a growing cell. The photograph of the growing root hair shown in Figure 7C was taken immediately after the application of 20 μM actinomycin D, and the one shown in Figure 7D was taken 2 h later. The nucleus is still located at the same distance from the tip. Figures 7E (photograph taken immediately after application) and 7F (photograph taken after 2 h) are images of a fully grown root hair undergoing the same treatment. In fully grown root hairs, the nucleus continues to migrate when the formation of new proteins is blocked. These results show two interesting phenomena. First, when growth is inhibited, the accumulation of subapical fine F-actin stops, but its bundling continues without new protein synthesis. Second, protein synthesis seems to be required for the release of the nucleus from its fixed position when growth terminates.
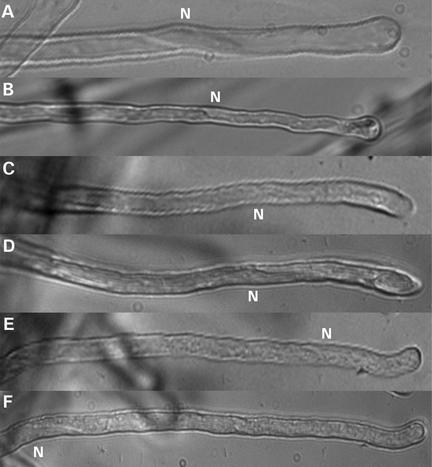
Figure 7.
The Nucleus Does Not Migrate Basipetally during or after Growth Termination if Inhibition of Gene Transcription or Protein Synthesis Starts during Cell Growth, but It Migrates if Inhibition Starts after Growth Has Stopped.
In root hairs that had terminated growth before the inhibition of gene transcription or translation, the basipetal movement of nuclei was not inhibited.
(A) A root hair treated for 4 h with 10 μg/mL of the translation blocker cycloheximide. The nucleus (N) was still located at the fixed distance from the tip.
(B) A root hair treated for 4 h with 20 μM actinomycin D, an inhibitor of gene transcription.
(C) and (D) Micrographs of a growing root hair immediately after (C) and 2 h after (D) the application of 20 μM actinomycin D. The nucleus was still located at the same distance from the tip.
(E) and (F) Micrographs of a fully grown root hair immediately after (E) and 2 h after (F) the application of 20 μM actinomycin D. In fully grown root hairs, the nucleus continued to migrate when the formation of new proteins was blocked. During protein synthesis inhibition in growing root hairs, we observed a slight flattening of the root hair tip that we cannot explain.
Actin Filament Bundling Is Involved in Maintaining the Distance between the Nucleus and the Tip
Because subapical fine F-actin is required to keep the nucleus at a fixed distance from the growing hair tip, because for its movement away from the tip at growth arrest the production of new protein is needed, and because for the actual backward movement bundles of actin filaments are required, we wondered whether actin bundling is involved in keeping the nucleus in place in growing hairs and/or keeping its movement away from the tip during growth arrest.
In common with V. sativa root hairs (De Ruijter et al., 1999), progressive bundling of actin filaments, from the apex to the nucleus, occurred in the subapical area of growing Arabidopsis root hairs (Figures 5A to 5E). The elongated nucleus was surrounded by actin filaments and located in the basal part of this area. To determine whether bundling of actin filaments is involved in maintaining the fixed distance between the nucleus and the growing tip in root hairs, we injected an antibody against a villin-like actin binding protein derived from plants.
Villin-like actin binding proteins are expressed ubiquitously in Arabidopsis (Klahre et al., 2000). Yokota and Shimmen (1999) raised an antibody against one such protein of 135 kD from lily. Injection of this antibody in Hydrocharis root hairs demonstrated that the protein has actin-bundling activity. After injection of the antibody against lily villin-like protein, fusion of transvacuolar cytoplasmic strands into one transvacuolar strand was inhibited, leading to numerous transvacuolar cytoplasmic strands (Tominaga et al., 2000). Because actin filaments are the backbones of cytoplasmic strands (Valster et al., 1997), one can infer from this result that lily villin-like protein bundles actin filaments. The lily anti-villin antibody recognized specifically a band of ∼135 kD in Arabidopsis root protein extracts (Figure 8). We tested whether actin bundling is involved in determining the position of the nucleus in growing root hairs by injecting lily anti-villin antibody into Arabidopsis root hairs and studying the positioning of the nucleus. Injection of anti-villin antibody into Arabidopsis root hairs increased the number of cytoplasmic strands, as did injection of this antibody into Hydrocharis root hairs (Figure 9). Interestingly, during the unbundling process after anti-villin antibody injection, the nucleus moved forward 10 to 20 μm at 3 to 5 min after injection, after which it oscillated around that position and moved slightly backward but did not reach its original position (Figure 10). Injection of preimmune rabbit serum did not lead to significant relocation of the nucleus or other cellular changes. During injections of either anti-villin antibody or preimmune rabbit serum, growth continued in most root hairs (80%; n = 10) during and after injection. The root hairs that terminated growth after injection were distributed evenly among lily anti-villin antibody and control injections (one in six for the experiment, one in four for the preimmune serum control), indicating that the anti-villin antibody does not inhibit growth. Figure 11 shows the positions of nuclei in relation to the actin cytoskeleton in growing root hairs and after anti-villin antibody microinjection.
Figure 8.
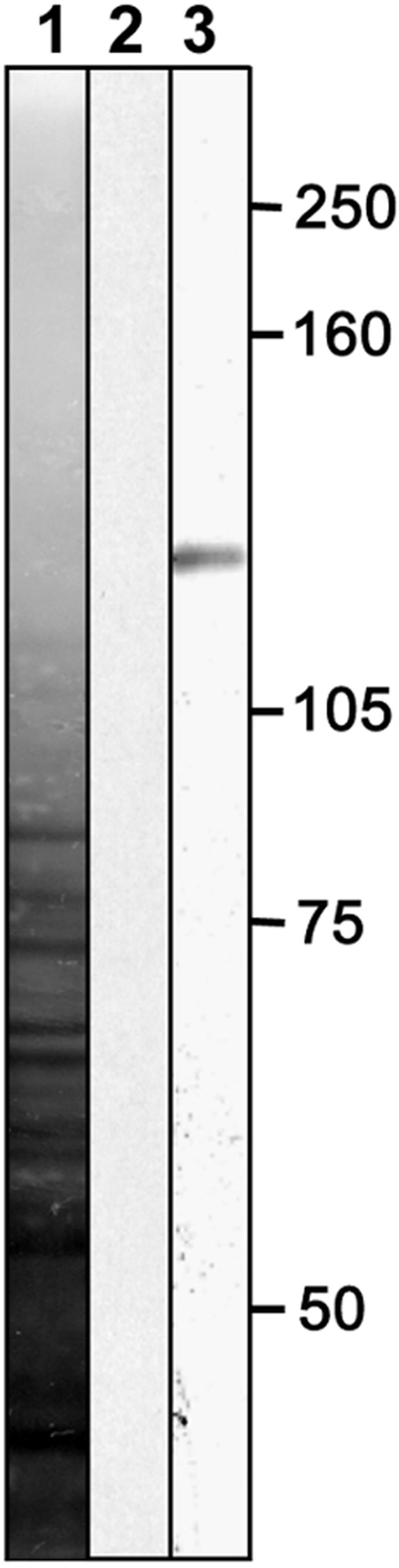
Protein Gel Blot of an Arabidopsis Root Extract Labeled with Lily Anti-Villin Antibody.
On Arabidopsis root extract, the antibody gave a band of ∼135 kD (lane 3), comparable to the mass of lily villin. Lane 1, silver-stained nitrocellulose membrane; lane 2, labeled with rabbit preimmune serum.
Figure 9.
Injection of Lily Anti-Villin Antibody Causes an Increase in the Number of Cytoplasmic Strands.
During the first minutes after injection, newly formed cytoplasmic strands emerged through the vacuolated area of the root hair as a result of the unbundling of the bundles of actin filaments. Three minutes after injection, the nucleus moved toward the apex, until it stopped approaching the apex after 6 to 8 min (see Figure 10). Nuclei (N) are indicated. Bar = 10 μm.
Figure 10.
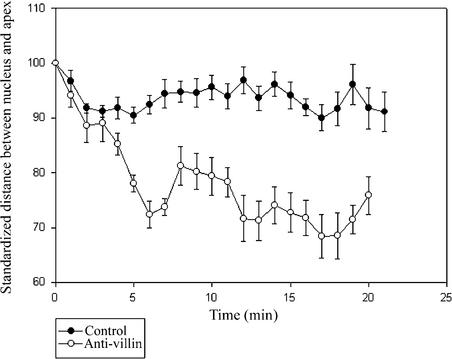
Injection of Lily Anti-Villin Antibody Leads to a Decrease in the Distance between the Nucleus and the Tip.
The distance between the nucleus and the apex after injection of a 1:10 dilution of anti-villin antibody into growing Arabidopsis root hairs (n = 6). As a control, a 1:10 dilution of rabbit preimmune serum was used (n = 4). The distance between the nucleus and the tip was standardized by setting the distance before injection to 100 in all observed root hairs. Error bars indicate standard errors.
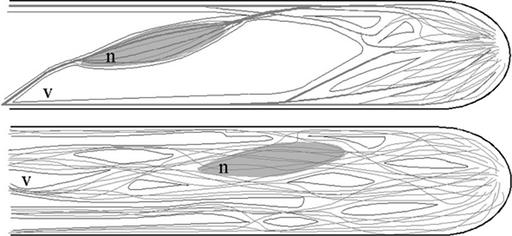
Figure 11.
Effects of Unbundling of the Actin Filaments.
Actin cytoskeleton configuration and the location of the nucleus (n) in control root hairs (top) and after the injection of anti-villin antibody (bottom).
V, vacuole.
These results led us to conclude that the actin binding villin-like protein bundles the subapical fine F-actin into thicker bundles of actin filaments in the root hair tube in a gradient away from the hair tip. The forward movement of the nucleus during the inhibition of actin bundling shows that actin bundling is involved in maintaining a fixed distance between the nucleus and the growing apex. Actin filament bundling appears to prevent the nucleus from approaching the growing root hair tip.
DISCUSSION
In tip-growing root hairs, growth occurs abundantly at one side of the cell and the nucleus follows the growing cell tip at a certain distance. This fact has been observed many times, and the advantage for a cell to keeping the nucleus in the vicinity of the site of abundant protein production for plasma membrane growth and new wall deposition is obvious. As in other species studied, in growing Arabidopsis wild-type root hairs, the nucleus moves relative to the root hair base but does not move relative to the root hair tip; rather, it keeps pace with the advancing tip. How is this distance maintained? An obvious possibility could involve the microtubule cytoskeleton.
It has been shown that without microtubules (oryzalin treatment [Bibikova et al., 1999] and mor1 mutant [Whittington et al., 2001]), Arabidopsis root hairs grow normally, although with a wavy morphology. Here, we also show that the nucleus persists in following the growing tip. The physical link that keeps the nucleus tethered in the subapex does not seem to involve the microtubule cytoskeleton in Arabidopsis. However, data obtained with legume root hairs indicate that microtubules play a role in nuclear positioning. Lloyd et al. (1987) reported that microtubules are responsible for the connection of the nucleus with the tip in growing root hairs of Vicia hirsuta. More recently, Sieberer et al. (2002) reported endoplasmic microtubules in the subapex of Medicago truncatula root hairs. After depolymerization of these microtubules, they observed decreased growth speeds and backward movement of the nucleus. These observations indicate that differences between species may occur.
Subapical Fine F-Actin as the Agent That Keeps the Nucleus in the Vicinity of the Growing Hair Tip
One specific type of actin configuration that could be a good candidate for maintaining the nuclear position is subapical fine F-actin. Subapical fine F-actin has been described previously as a configuration of fine bundles of actin filaments, which are bundled progressively farther and farther away from the hair tip (V. sativa [Miller et al., 1999] and Arabidopsis [Figures 5A to 5E]). Subapical fine F-actin is enhanced by lipochitooligosaccharides from Rhizobium bacteria (De Ruijter et al., 1999), molecules that reinitiate growth in growth-terminating legume root hairs (De Ruijter et al., 1998). During root hair growth, the subapical area with fine F-actin maintains a more-or-less constant length of 63 ± 17 μm in Arabidopsis. This is achieved by constant bundling between the actual subapical fine F-actin and the nucleus and constant elongation of the subapical fine F-actin at the hair tip, where the Golgi-derived vesicles accumulate. From this, we deduce that in subapical fine F-actin, the filaments point with their plus sides toward the hair tip. However, this idea remains to be proven.
Pharmacological experiments provide good evidence that the maintenance of the nucleus with the growing root hair tip is a process involving the subapical fine F-actin cytoskeleton. CD, which caps growing actin filaments (Cooper, 1987) at low concentration (1 to 5 μM), depolymerizes the subapical fine F-actin only, as has been shown for V. sativa root hairs (Miller et al., 1999). This results initially in cessation of the delivery of Golgi-derived vesicles and consequently in an arrest of cell elongation, although cytoplasmic streaming continues. Similarly, LA, a G-actin binding molecule, at low concentration initially depolymerizes subapical fine F-actin and arrests root hair growth (data not shown), but this drug also depolymerizes the thicker bundles of actin filaments in the remainder of the hair and consequently stops cytoplasmic streaming. These results indicate that subapical F-actin and its continuation of actin filaments around the nucleus is (part of) the physical link between the nucleus and the hair tip.
The link between the nucleus and the tip is severed, or “snaps,” when the distance between the nucleus and the tip is increased experimentally by trapping the nucleus. However, this direct physical link may include the subapical longitudinal ER, which also encircles the nucleus (Miller et al., 2000). This type of ER configuration is supported and probably produced and configured by the subapical fine F-actin. The snapping occurs after the nucleus is trapped for 10 to 15 min, which is exactly the time in which a root hair grows 10 to 15 μm. When a root hair elongates 10 to 15 μm, the distance between the hair tip and the nucleus just exceeds the maximal distance known from growing control hairs. This suggests that the trapping of the nucleus causes the disappearance of the subapical area containing fine F-actin in a way that is comparable to physiological growth arrest and is not an unphysiological rupture of the actin cytoskeleton. Detailed analysis of the actin cytoskeleton during trapping would be advantageous but is not yet possible because of the limited ability to visualize the subapical fine F-actin in living cells (GFP-talin [Baluška et al., 2000]).
We do not know if and where fine F-actin attaches to the plasma membrane of the hair tip. To our knowledge, no work has reported the presence of filamentous actin in the extreme tip of growing root hairs, but with the procedures used here, the thinnest bundles or single filaments may be invisible by confocal laser scanning microscopy. Therefore, we do not know whether actin polymerization takes place at the plasma membrane, as has been suggested for animal cells (for a recent review, see Sechi and Wehland, 2000). Interestingly, using the new technique of single-molecule speckle analysis of actin filament turnover in lamellipodia, Watanabe and Mitchison (2002) have shown that in these cells, contrary to previous expectations, the majority of actin filaments are generated away from the very tip. Another possibility would be that the subapical fine F-actin attaches to and/or polymerizes from the Golgi-derived vesicles in the cell's apex or that the subapical longitudinal ER is the site of actin filament nucleation. These are important questions to answer in future work.
Actin Bundling as the Agent That Inhibits the Nucleus from Reaching the Hair Tip
As discussed above, at the location of the hair tip, the subapical fine F-actin elongates constantly in growing root hairs, and on the other side, actin filaments are bundled constantly. Therefore, we asked whether actin bundling also is involved in keeping the nucleus in place. Villin is an actin-bundling protein. Villin-like proteins are known to be present in Arabidopsis (Klahre et al., 2000); therefore, we attempted to increase actin bundling in our system by injecting an antibody against a plant villin-like protein into the Arabidopsis root hair. The injection of lily anti-villin antibody has been shown to unbundle bundles of actin filaments in Hydrocharis root hairs (Tominaga et al., 2000), and lily anti-villin antibody cross-reacted with a polypeptide of similar molecular mass in an Arabidopsis root extract (Figure 8). Indeed, injection of this antibody unbundled the subapical cytoplasmic strands of growing Arabidopsis root hairs further and increased the length of this area such that the subapical fine F-actin–containing cytoplasmic strands between the original subapical fine F-actin and the nucleus also became unbundled. Consequently, the nucleus moved forward toward the hair tip. This result is direct proof that bundling of the actin cytoskeleton plays a role in nuclear positioning, keeping the nucleus at a fixed distance from the growing root hair tip.
In vivo, the actin cytoskeleton is under the control of many actin-interacting proteins, some of which are sensitive to the concentration of specific ions (e.g., calcium). Therefore, as has been shown in pollen tubes, ion gradients may play an indirect or direct role in the organization of the actin cytoskeleton (for review, see Hepler et al., 2001) and thus the positioning of the nucleus.
Requirement of Bundles of Actin Filaments and New Protein Synthesis for Nuclear Movement during Growth Arrest
What could happen during physiological growth arrest? During growth arrest, the subapical fine F-actin area becomes progressively shorter because fine F-actin no longer elongates on the tip side, whereas bundling of actin filaments and, consequently, cytoplasmic strands, continues on the other side. After the subapical fine F-actin has disappeared, no new vesicles are delivered and the residual Golgi-derived vesicles are consumed at the plasma membrane of the root hair tip, after which cell growth stops. Thus, actin filament polymerization stops, although actin filament bundling continues. The study of the cow1-2 mutant supports this hypothesis. In this mutant with branched root hairs, the nucleus oscillates between the two tips. The tip it is closest to is the one that elongates, although with a certain lag time. The tip from which the nucleus moves still elongates slightly, possibly because the Golgi-derived vesicles already present in the vesicle-rich area still are being used. Similarly, it could take time before a newly approached tip elongates, because first the Golgi-derived vesicles needed for cell elongation may have to be delivered. If this hypothesis is true, the results from the cow1-2 mutant would demonstrate that the nucleus approaches the tip for vesicle delivery, but not for vesicle insertion, the growth process per se. This phenomenon also is seen when cell growth is terminated by subapical fine F-actin breakdown by CD or when the nucleus is kept artificially at a greater distance from the tip by trapping it in a laser beam.
The experiments in which LA was used show that a functional actin cytoskeleton in the root hair tube, consisting of the thicker bundles of actin filaments, is required for the movement of the nucleus at growth arrest. The experiments with transcription/translation inhibitors provide an interesting clue to the mechanism of growth arrest. They show that for the hair base–directed movement of the nucleus during growth arrest, gene transcription and translation is required. Because we found that the bundling of actin filaments and the fusion of Golgi-derived vesicles occurs during growth arrest but the subapical fine F-actin area becomes shorter and vanishes, the newly formed protein should stop, directly or indirectly, the polymerization of fine F-actin at the plasma membrane/ER/Golgi-derived vesicle membrane.
A Tip-Growth Scenario
The purpose of our study was to investigate how the cell accomplishes its persistent localization of the nucleus at a set distance from the advancing cell tip and, more specifically, the role of the cytoskeleton in this process. Our results indicate the following: (1) Fine F-actin, a subset of actin filaments in the cell's subapex between the nucleus and the hair tip (i.e., distal from the nucleus), functions in keeping the nucleus following the growing hair tip. (2) Actin bundling between subapical fine F-actin and the nucleus inhibits the nucleus from reaching the hair tip. (3) New protein synthesis is required to move the nucleus back from the tip at root hair growth arrest. (4) Bundles of actin filaments in the hair's tube are needed for this movement of the nucleus away from the hair tip at growth arrest.
A possible scenario to be studied further is the following. Actin filament polymerization is nucleated at the plasma membrane/ER/Golgi-derived vesicle membranes in the vesicle-rich area of the root hair tip. An actin-bundling protein produces a gradient of more and more bundled actin filaments between ER cisternae away from the growing tip. In these fine F-actin–supported ER cisternae, the nucleus is caught. The nucleus follows the tip because it is connected to the elongating fine F-actin at the hair tip, but bundling of actin filaments at the base of the subapical fine F-actin keeps the nucleus from approaching the very tip. Our study has clarified some details of the mechanisms of nuclear positioning during tip growth and has laid the basis for further studies leading to the discovery of proteins involved in plant cell (tip) growth.
METHODS
Plant Growth and Drug Treatment
Arabidopsis thaliana seeds were germinated on a chamber between a glass slide and a cover slip, so that the roots grew into the chamber. Germination was carried out in half-strength Murashige and Skoog (1962) medium (Duchefa, Haarlem, The Netherlands) for 5 days at 25°C with a light regimen of 16 h of light and 8 h of darkness. Drugs were applied by gradually replacing the medium with medium containing drugs in the growth room. Oryzalin (Greyhound, Birkenhead, UK), cytochalasin D (Sigma, St. Louis, MO), and latrunculin A (Molecular Probes, Leiden, The Netherlands) stocks were made in DMSO and diluted at least 1000-fold in the medium. Cycloheximide and actinomycin D stocks were made in methanol.
Immunocytochemistry
For immunofluorescence, plants were fixed by rapid freezing in liquid propane. Freeze substitution was performed in methanol plus 0.5% glutaraldehyde and, for actin labeling, in 0.4 mM m-maleimidobenzoyl N-hydroxysuccinimide ester (Sigma) at −90°C. After fixation, the temperature was increased stepwise (with steps at −60 and −30°C) over 10 h to 0°C. The freeze-substitution medium was replaced stepwise (10, 30, 50, 70, 90, and 100% [v/v]) by buffer (for actin labeling, actin-stabilizing buffer [50 mM Pipes, pH 6.8, 0.5 mM MgCl2, 0.5 mM CaCl2, and 37 mM KCl] [Miller et al., 1999], and for microtubules, PBS) with 2% paraformaldehyde and 0.05% glutaraldehyde. After washing in buffer, the material was incubated in a saturated suspension of driselase (Kyowa Hakko Kogyo, Tokyo, Japan) and macerozyme R10 (Serva, Heidelberg, Germany) in Mes (30°C at pH 5.8 for 40 min). The enzymes were washed away in buffer, and the samples were blocked in 0.1% acetylated BSA (Aurion, Wageningen, The Netherlands) for at least 3 min. As a primary antibody, either the monoclonal anti-plant actin clone 3H11 (Andersland et al., 1994) or DM1a, a monoclonal anti-α-tubulin antibody (diluted 1:300; Sigma), was used; as a secondary antibody, GaM-IgG-Cy3 (Molecular Probes) was used. Before microscopic analysis, the material was washed in PBS-citifluor (Citifluor, Canterbury, UK) and mounted in Prolong antifade (Molecular Probes).
Staining of Actin Filaments after Improved Ester-Aldehyde-Choline Fixation
Five-day-old roots of Arabidopsis were fixed for 2 min with 100 μM m-maleimidobenzoyl N-hydroxysuccinimide ester in 1% freshly prepared paraformaldehyde and 0.025% glutaraldehyde in demineralized water, followed by 200 μM ester in 2% paraformaldehyde and 0.025% glutaraldehyde in demineralized water for 10 min. Roots were postfixed for 20 min in a final concentration of 3% paraformaldehyde and 0.075% glutaraldehyde in actin-stabilizing buffer. Subsequently, root hairs were permeabilized for 2 to 3 min with 100 μg/mL l-α-lysophosphatidylcholine in actin-stabilizing buffer. Actin filaments were stained with 0.33 μM Bodipy-FL (505/512)–phallacidin (Molecular Probes) in actin-stabilizing buffer supplemented with 0.05% acetylated BSA (Aurion) to decrease nonspecific binding. Roots were mounted in glycerol.
Microinjection
For antibody injection, growing root hairs were pressure microinjected with a 1:10 dilution of lily anti-villin antibody or preimmune serum in PBS, and for actin visualization, root hairs were injected with 0.66 μM Alexa-488–phalloidin (Molecular Probes) in half-strength PBS. Root hairs were grown in a thin layer of 0.1% Phytagel (Sigma) in half-strength Murashige and Skoog (1962) medium, injected with micropipettes, and pulled with a vertical pipette puller (model 700C; David Kopf Instruments, Tujunga, CA) from borosilicate glass capillaries containing an internal filament (World Precision Instruments, Sarasota, FL). Root hairs were injected at the base with a water pressure microinjection device (water pressure device from Gilmont [Barrington, IL], and needle holder from Eppendorf, Merck Eurolab BV [Amsterdam, The Netherlands]).
Protein Gel Blot Analysis
Arabidopsis plants were grown on half-strength Murashige and Skoog (1962) medium (Duchefa) containing 0.6% agar for 3 weeks at 20°C with a light regimen of 16 h of light and 8 h of darkness. Immediately after they had been cut from the plants, roots were frozen in liquid nitrogen, ground with pestle and mortar, and transferred to boiling SDS sample buffer (0.0625 M Tris-HCl, pH 6.8, 2.3% [w/v] SDS, 5% 3-mercaptoethanol, 10% glycerol, and 0.00125% bromphenol blue) for 3 min. SDS-PAGE was performed on a 6% acrylamide gel (Laemmli, 1970), after which the proteins were transferred electrophoretically to a nitrocellulose blotting membrane (British Drug House).
The blot was probed with either antiserum against ABP135 (the lily villin) diluted 1:1000 or rabbit preimmune serum diluted 1:100, followed by anti-rabbit IgG horseradish peroxidase diluted 1:5000 (Amersham). The signal was detected with enhanced chemiluminescence (Amersham).
Microscopy and Image Processing
For measurement of the distance between the apex and the nucleus, plants were prefixed with 1 μM m-maleimidobenzoyl N-hydroxysuccinimide ester to arrest cytoplasmic streaming and fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in medium. Nuclei did not displace during this procedure. After fixation, nuclei were stained with 1 μg/mL 4′,6-diamidino-2-phenylindole, and the distance from the middle of the nucleus to the apex was measured. Images were acquired with a Nikon Microphot FXA microscope (Tokyo, Japan) equipped with a Photometrics Quantix cooled charge-coupled device camera combined with IP-Lab spectrum 3.1 image-analysis software (Scanalytics, Fairfax, VA) or with a Bio-Rad MRC-600 confocal laser scanning microscope equipped with an argon-krypton laser with a ×60 magnification, 1.4–numerical aperture oil-immersion objective. A neutral density filter was set to obtain 10% transmission intensity of a 5-mW, 488-nm laser beam to excite the Bodipy-FL or a 5-mW, 568-nm laser beam to excite the Cy3. For imaging, a DM 488 BA 522 DF 35 filter was used for Bodipy-FL and a DM 568 BA 585 EFLP filter was used for Cy3. Images were obtained as z-series with 0.5- to 1-μm intervals with a moderately closed pinhole (setting 3 or 4), with high-gain settings (80 to 100) and an average of two to three scans (Kalman filter). Images were processed with Confocal Assistant (Bio-Rad Software, Hertfordshire, UK) and Adobe Photoshop 5.5 (Adobe Systems, Mountain View, CA). Time-lapse sequences were acquired by placing the plant growth chamber in an inverted Nikon Diaphot 200 microscope equipped with Hoffman modulation contrast.
Trapping of the Nucleolus with Optical Tweezers
A Nd:YVO4 laser beam (1064-nm continuous wave; Spectra Physics, Mountain View, CA) was introduced into a ×100 magnification, 1.3–numerical aperture oil-immersion objective (DMIRB inverted microscope; Leica, Wetzlar, Germany), for which a dichroic mirror was used, positioned below the objective. The trap position within the specimen plane was controlled using a lens telescope mounted on a XYZ translation stage in a conjugate plane with the back focal plane of the objective. The multiple laser spots were created by time-sharing the laser beam with two acousto-optical deflectors (IntraAction, Bellwood, IL) digitally controlled by computer (Visscher et al., 1996). The standard laser power used to hold the nucleolus was ∼300 to 400 mW in the sample.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
We thank T. Shimmen (Department of Life Science, Himeji Institute of Technology) for providing the antibody against lily villin, R. Cyr (Biology Department, Pennsylvania State University) for providing the antibody against plant actin, Astrid van der Horst (Institute for Atomic and Molecular Physics) for technical assistance in setting up the optical tweezers, and Ellen Allwood (University of Durham, UK) for editing of the text and discussion.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.005892.
References
- Andersland, J.M., Fisher, D.D., Wymer, C.L., Cyr, R.J., and Parthasarathy, M.V. (1994). Characterization of a monoclonal antibody prepared against plant actin. Cell Motil. Cytoskeleton 29, 339–344. [DOI] [PubMed] [Google Scholar]
- Anthony, R.G., Waldin, T.R., Ray, J.A., Bright, S.W., and Hussey, P.J. (1998). Herbicide resistance caused by spontaneous mutation of the cytoskeletal protein tubulin. Nature 393, 260–263. [DOI] [PubMed] [Google Scholar]
- Baluška, F., Salaj, J., Mathur, J., Braun, M., Jasper, F., Šamaj, J., Chua, N.-H., Barlow, P.W., and Volkman, D. (2000). Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol. 227, 618–632. [DOI] [PubMed] [Google Scholar]
- Bibikova, T.N., Blancaflor, E.B., and Gilroy, S. (1999). Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17, 657–665. [DOI] [PubMed] [Google Scholar]
- Chytilova, E., Macas, J., Sliwinska, E., Rafelski, S.M., Lambert, G.M., and Galbraith, D.W. (2000). Nuclear dynamics in Arabidopsis thaliana. Mol. Biol. Cell 11, 2733–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, J.A. (1987). Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 105, 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen, J., and Emons, A.M. (1990). Microtubules in tip growth systems. In Tip Growth in Plant and Fungal Cells, I.B. Heath, ed (San Diego, CA: Academic Press), pp. 147–181.
- De Ruijter, N.C.A., Bisseling, T., and Emons, A.M.C. (1999). Rhizobium nod factors induce an increase in sub-apical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol. Plant-Microbe Interact. 12, 829–832. [Google Scholar]
- De Ruijter, N.C.A., Rook, M.B., Bisseling, T., and Emons, A.M.C. (1998). Lipochito-oligosaccharides reinitiate root hair tip growth in Vicia sativa with high calcium and spectrin-like antigen at the tip. Plant J. 13, 341–350. [Google Scholar]
- Emons, A.M.C. (1987). The cytoskeleton and secretory vesicles in root hairs of Equisetum and Limnobium and cytoplasmic streaming in root hairs of Equisetum. Ann. Bot. 60, 625–632. [Google Scholar]
- Emons, A.M.C., and De Ruijter, N.C.A. (2000). Actin: A target of signal transduction in root hairs. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluška, D. Volkmann, and P.W. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 373–390.
- Galway, M.E., Heckman, J.W., Jr., and Schiefelbein, J.W. (1997). Growth and ultrastructure of Arabidopsis root hairs: The rhd3 mutation alters vacuole enlargement and tip growth. Planta 201, 209–218. [DOI] [PubMed] [Google Scholar]
- Gibbon, B.C., Kovar, D.R., and Staiger, C.J. (1999). Latrunculin B has different effects on pollen germination and tube growth. Plant Cell 11, 2349–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon, B.C., Zonia, L.E., Kovar, D.R., Hussey, P.J., and Staiger, C.J. (1998). Pollen profilin function depends on interaction with proline-rich motifs. Plant Cell 10, 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson, C.S., Roberts, K., Feldmann, K.A., and Dolan, L. (1997). The COW1 locus of Arabidopsis acts after RHD2, and in parallel with RHD3 and TIP1, to determine the shape, rate of elongation, and number of root hairs produced from each site of hair formation. Plant Physiol. 115, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlandt, G. (1883). Physiologische Pflanzenanatomie. (Jena, Germany: Verlag von Gustav Fischer), pp. 150–151.
- Haberlandt, G. (1887). Ueber die Beziehungen zwischen Function und Lage des Zellkernes bei den Planzen. (Jena, Germany: Verlag von Gustav Fischer).
- Hepler, P.K., Vidali, L., and Cheung, A.Y. (2001). Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 17, 159–187. [DOI] [PubMed] [Google Scholar]
- Klahre, U., Friederich, E., Kost, B., Louvard, D., and Chua, N.-H. (2000). Villin-like actin-binding proteins are expressed ubiquitously in Arabidopsis. Plant Physiol. 122, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost, B., Spielhofer, P., and Chua, N.-H. (1998). A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 16, 393–401. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lloyd, C.W., Pearce, K.J., Rawlins, D.J., Ridge, R.W., and Shaw, P.J. (1987). Endoplasmic microtubules connect the advancing nucleus to the tip of legume root hairs, but F-actin is involved in basipetal migration. Cell Motil. Cytoskeleton 8, 27–36. [Google Scholar]
- Miller, D.D., De Ruijter, N.C.A., Bisseling, T., and Emons, A.M.C. (1999). The role of actin in root hair morphogenesis: Studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 17, 141–154. [Google Scholar]
- Miller, D.D., De Ruijter, N.C.A., and Emons, A.M.C. (1997). From signal to form: Aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J. Exp. Bot. 48, 1881–1896. [Google Scholar]
- Miller, D.D., Leferink-ten Klooster, H.B., and Emons, A.M.C. (2000). Lipochito-oligosaccharide nodulation factors stimulate cytoplasmic polarity with longitudinal endoplasmic reticulum and vesicles at the tip in vetch root hairs. Mol. Plant-Microbe Interact. 13, 1385–1390. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Ridge, R.W. (1988). Freeze-substitution improves the ultrastructural preservation of legume root hairs. Bot. Mag. Tokyo 101, 427–441. [Google Scholar]
- Sechi, A.S., and Wehland, J. (2000). The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P(2) influences cytoskeletal protein activity at the plasma membrane. J. Cell Sci. 113, 3685–3695. [DOI] [PubMed] [Google Scholar]
- Sieberer, B.J., Timmers, A.C.J., Lhuissier, F.G.P., and Emons, A.M.C. (2002). Endoplasmic microtubules configure the subapical cytoplasm and are required for fast growth of Medicago truncatula root hairs. Plant Physiol., in press. [DOI] [PMC free article] [PubMed]
- Tominaga, M., Yokota, E., Vidali, L., Sonobe, S., Hepler, P.K., and Shimmen, T. (2000). The role of plant villin in the organization of the actin cytoskeleton, cytoplasmic streaming and the architecture of the transvacuolar strand in root hair cells of Hydrocharis. Planta 210, 836–843. [DOI] [PubMed] [Google Scholar]
- Valster, A.H., Pierson, E.S., Valenta, R., Hepler, P.K., and Emons, A.M.C. (1997). Probing the plant actin cytoskeleton during cytokinesis and interphase by profilin microinjection. Plant Cell 9, 1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher, K., Gross, S.P., and Block, S.M. (1996). Construction of a multiple-beam optical trap with nanometer-resolution position sensing. IEEE J. Selected Top. Quant. Elect. 2, 1066–1076. [Google Scholar]
- Watanabe, N., and Mitchison, T.J. (2002). Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science 295, 1083–1086. [DOI] [PubMed] [Google Scholar]
- Whittington, A.T., Vugrek, O., Wei, K.J., Hasenbein, N.G., Sugimoto, K., Rashbrooke, M.C., and Wasteneys, G.O. (2001). MOR1 is essential for organizing cortical microtubules in plants. Nature 411, 610–613. [DOI] [PubMed] [Google Scholar]
- Yokota, E., and Shimmen, T. (1999). The 135-kDa actin-bundling protein from lily pollen tubes arranges F-actin into bundles with uniform polarity. Planta 209, 264–266. [DOI] [PubMed] [Google Scholar]