Abstract
While antibiotic selection has been routinely used for the selection of genetically modified cells, administration of cytotoxic drugs often leads to deleterious effects not only to inert cells but also to transfected or transduced ones. In this study, we propose an Antigen-MEdiated Genetically modified cell Amplification (AMEGA) system employing antibody/receptor chimeras without antibiotic selection. Based on a rational design where the extracellular domains of dimeric erythropoietin receptor (EpoR) or gp130 were substituted with heterodimeric VH/VL regions of anti-hen egg lysozyme (HEL) antibody and EpoR D2 domains, the genes encoding the chimeras as well as a model transgene, enhanced green fluorescent protein (EGFP), were retrovirally infected into IL-3-dependent Ba/F3 cells followed by direct HEL selection in the absence of IL-3. After a single round of selection, EGFP-positive cells were selectively amplified, resulting in a population of almost 100% positive cells. The AMEGA without antibiotic selection will not harm normal cells, which will be especially useful for increasing the efficacy for stem cell-based gene therapy.
INTRODUCTION
Selection of genetically modified cells is a critical step to engineer the cells with desired properties. Antibiotic selection has been commonly used, which is based on the rescue of the transfectant in the presence of a cytotoxic drug and the selective deprivation of the non-transduced cells. However, the selection conditions such as initial cell density and drug concentration should be carefully determined since inappropriate conditions often lead to death of the transfectant or survival of the parental cells (1). Furthermore, the growth rate is decreased even in the optimal concentration due to the cytotoxic drug (2,3). Such a selection procedure has some limitations for cells in which transduction efficiency is low and/or growth induction is difficult (1,4–6).
To overcome these problems, we tried to create an artificial receptor that transduces a growth signal in response to a non-toxic substance. Co-expression of the artificial receptor and the gene of interest would result in a ‘growth advantage’ only for genetically modified cells by simply adding the non-toxic substance in the culture medium. We focused on an antigen– antibody system that has an infinite number of combinations with high specificity. In our previous study, the Fv region of anti-hen egg lysozyme (HEL) antibody HyHEL-10 was chosen as a model, since its VH-VL association is greatly enhanced with addition of HEL (7), which is expected to be suitable for mimicry of ligand-induced dimerization of cytokine receptors in their activation (8,9). Thus, two chimeric erythropoietin receptors whose extracellular domain was replaced with either the VH or VL region of anti-hen egg lysozyme (HEL) antibody HyHEL-10 (HE or LE, respectively) were expressed in interleukin-3 (IL-3)-dependent hematopoietic cell lines, resulting in HEL-dependent cell growth without IL-3 (10). This result demonstrates the utility of our antibody/receptor chimera for functional mimicry of cytokine receptors. In the following experiment, the cytoplasmic domains of HE and LE chimeric receptors were replaced with that of gp130 to create Hg and Lg chimeric receptors, respectively. Co-expression of Hg and Lg or Hg and LE resulted in HEL-dependent cell growth in factor- dependent cell lines (11,12). Furthermore, when the enhanced green fluorescent protein (EGFP) was employed as a model transgene and placed downstream of the LE gene and the internal ribosomal entry site (IRES), HEL induced specific amplification of the cells with high expression level of EGFP (13). However, in all these studies, we used electroporation to transfect plasmids into the cells, which is not an ideal method for many applications due to relatively low transfection efficiency. In addition, in order to enrich the proportion of transfectants, antibiotic selection was always conducted prior to HEL selection, and the possibility of direct HEL selection was not pursued.
In this study, we employed a retroviral vector to attain higher transduction efficiency in a variety of cell types to expand the current approach. Whether or not the genetically modified IL-3-dependent pro-B cells expressing the antibody/receptor chimera could be directly amplified by HEL selection immediately after retroviral infection was examined.
MATERIALS AND METHODS
Vector construction
The ecotropic retroviral vector pMX, and its derivatives pMX-GFP and pMXs-neo (14), were kindly provided by Dr T. Kitamura (Institute of Medical Science, University of Tokyo). The construction of the HE chain gene encoding HyHEL-10 VH, a GSG linker, extracellular D2 and transmembrane/cytoplasmic domains of EpoR, and the LE chain gene encoding HyHEL-10 VL instead of VH in the HE chain, was described previously (10). Construction of the Hg and Lg chain genes encoding the transmembrane and cytoplasmic domains of gp130 instead of those for HE and LE chains, respectively, was as described (11). First, the ‘amplifier’ vectors were constructed. The Hg and Lg genes were digested from pME-Hg and pMEZ-Lg (11) with EcoRI, and ligated into EcoRI-digested pMX to obtain pMX-Hg and pMX-Lg, respectively. pME-HE (11) was digested with NcoI and XbaI to obtain the HE chain fragment, and inserted into NcoI and XbaI-digested pBSN, which is a variant of pBluescriptII SK(–) (Stratagene, La Jolla, CA) having a NcoI site between the SpeI and XbaI sites, in order to make a NotI site downstream of the HE gene, resulting in pBSN-HE. The HE gene fragment was digested from pBSN-HE with NcoI and NotI, and ligated into NcoI and NotI-digested pMX-Hg to produce pMX-HE. pMEZ-LE was digested with NcoI and SacII to obtain the VL fragment, and ligated into NcoI and SacII-digested pMX-HE to obtain pMX-LE. The ‘courier’ vectors were constructed as follows. pMEZ-LEIGFP (13) was digested with XbaI to obtain the IRES-EGFP fragment, and inserted into a XbaI site of pBluescriptII SK(–) to produce pBS-IGFP. The IRES-EGFP fragment from pBS-IGFP was digested with NotI, and inserted into a NotI site of pMX-Hg and pMX-Lg to get pMX-HgIGFP and pMX-LgIGFP, which encode the IRES-EGFP cassette downstream of Hg and Lg, respectively. The LEIGFP fragment was digested from pMEZ-LEIGFP (13) with EcoRI and NotI, and inserted into EcoRI and NotI-digested pMX to create pMX-LEIGFP. To make a drug-resistant control vector, pMX-LgIGFP was digested with EcoRI and blunted with T4 DNA polymerase, followed by XhoI digestion to remove the Lg fragment. pMXs-neo was digested with BglII and blunted, followed by SalI digestion to obtain the neomycin-resistance gene fragment. The fragment was inserted into the digested pMX-LgIGFP to obtain pMX-neoIGFP.
Cell culture
A murine IL-3-dependent pro-B cell line, Ba/F3 (15), was cultured in RPMI 1640 medium (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% FBS (Iwaki, Tokyo, Japan) and 2 ng/ml murine IL-3 (Genzyme/Techne, Cambridge, MA). A retroviral packaging cell line, Plat-E (16), was cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Nissui Pharmaceutical) supplemented with 10% FBS, 1 µg/ml puromycin (Sigma, St Louis, MO) and 10 µg/ml blasticidin (Kaken Pharmaceutical, Tokyo, Japan).
Retroviral infection and selection of the transfectants
Plat-E cells were inoculated into a 60 mm diameter dish at 5 × 105 cells/ml in 4 ml DMEM, and cultured for 20 h. An aliquot of 9 µl Fugene6 (Roche Diagnostics, Basel, Switzerland) was diluted with 100 µl of serum-free F12 medium (Gibco BRL, Rockville, MD) and added to 3 µg of the retroviral vector solubilized in 6 µl of sterile water. After 15 min incubation at room temperature, the vector/Fugene6 mixture was added to the Plat-E cells. After 24 h incubation, the culture medium was refreshed with 4 ml DMEM, followed by an additional 24 h incubation. After the supernatant was centrifuged at 1000 g for 5 min at 20°C, Ba/F3 cells (105 cells) were infected with 500 µl of the viral supernatant in the presence of 10 µg/ml polybrene (Sigma) and 4 ng/ml IL-3 in 24-well plates. In the case of co-infection, 500 µl each of the viral supernatants was mixed and used to infect Ba/F3 cells. After 5 h incubation, the same volume of RPMI 1640 as the viral supernatant was added to reduce the toxicity of polybrene. The approximate multiplicity of infection (MOI) ranged from 1.0 to 4.2%, as estimated from the flow cytometric analysis on day 3 or 4 after infection. On day 6 or 7 after infection, the cells were washed with PBS once, and inoculated into 24-well plates at 2 × 105 cells/ml. Selection was performed in the medium containing no factor, 1 µg/ml HEL (Seikagaku Corporation, Tokyo, Japan) or 2 ng/ml IL-3.
For the cloning experiment, cells before selection were washed with PBS once, and inoculated into 96-well plates at 5 × 103 cells/ml (103 cells/well) with 1 µg/ml HEL. Parental Ba/F3 cells were washed with PBS three times and used to supplement each well at 105 cells/ml as feeder cells to effectively rescue limiting-diluted transfectant cells in their early growth phase. For comparison, retrovirally packaged pMX-neoIGFP was infected into Ba/F3 cells (MOI ∼35%), followed by inoculation into 96-well plates at 5 cells/ml (1 cell/well) with 2 ng/ml IL-3 and 800 µg/ml G418 (Calbiochem, Darmstadt, Germany). Washed Ba/F3 cells (105 cells/ml) were used to supplement each well as feeders.
Western blotting
The cells (106 cells) were washed with PBS, lysed with 100 µl of lysis buffer [20 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 µg/ml aprotinin, 10 µg/ml leupeptin] and incubated on ice for 10 min. After centrifugation at 16 000 g for 5 min, the supernatant was mixed with Laemmli’s sample buffer and boiled. The lysate was resolved by SDS–PAGE and transferred to a nitrocellulose membrane (Millipore, Bedford, MA). After the membrane was blocked with 5% skimmed milk, the blot was probed with 160 ng/ml of rabbit anti-mouse EpoR antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or 160 ng/ml of anti-mouse gp130 antibody (Santa Cruz Biotechnology) followed by 560 ng/ml of HRP-conjugated anti-rabbit IgG (Biosource, Camarillo, CA), and detection was performed using the ECL system (Amersham-Pharmacia, Little Chalfont, UK).
Cell proliferation assay
The HEL-selected cells were washed three times with PBS and seeded in 24-well plates containing various concentrations of HEL. The initial cell concentration was adjusted to 104 cells/ml. Cell number and viability were determined using a hemocytometer and the trypan blue exclusion assay.
Flow cytometry
Cells were washed with PBS and resuspended in Isoflow (Beckman Coulter, Fullerton, CA). Green fluorescence intensity was measured using an EPICS-ALTRA flow cytometer (Beckman Coulter) at 488 nm excitation and fluorescence detection at 525 ± 20 nm.
RESULTS
Direct HEL selection amplified the transfectants expressing chimeric receptors
We first designed a selection scheme of the direct antigen-mediated selection without the antibiotic selection step (Fig. 1). An ‘amplifier’ vector encodes a chimeric receptor gene, while a ‘courier’ vector encodes the other partner of the functional chimeric receptor pair and an IRES-transgene cassette. The IRES sequence was used to couple a growth signal derived from chimeric receptors with transgene expression, since a correlation was observed between the expression levels of the two cistrons placed upstream and downstream of the IRES sequence (17,18). Therefore, transgene-expressing cells are expected to preferentially proliferate in the antigen-containing medium.
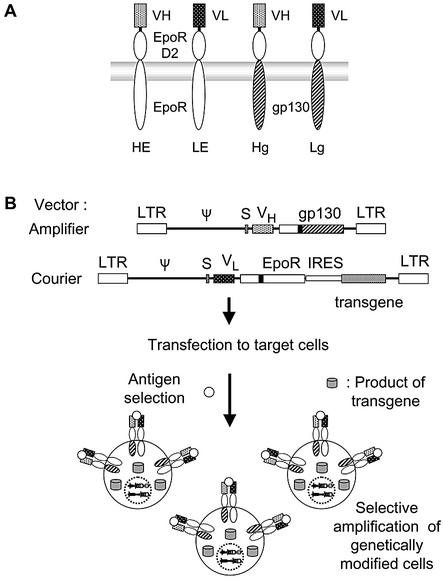
Figure 1.
Scheme of the AMEGA system. (A) Chimeric receptor constructs used in this study. The extracellular N-terminal half of EpoR was replaced with either the VH or VL region of HyHEL-10 with a Gly-Ser-Gly tripeptide linker between VH/VL and Val118 of the EpoR D2 domain. The transmembrane and intracellular domains could be easily replaced at the EcoRV site inserted at the C-terminus of the EpoR D2 domain. (B) Schematic diagram of the selection procedure. Chimeric receptor combination of Hg and LE is depicted in the scheme as a representative. Retroviral vectors with long- terminal repeats (LTRs) and a packaging signal (ψ) are used for the efficient transcription of the Hg and LE-IRES-transgene. An immunoglobulin heavy-chain secretion signal sequence (S) is located upstream of the chimeric receptor genes to enable their cell surface expression.
In this study, the EGFP gene was used as a model transgene, and an IRES-EGFP cassette was designated as IGFP. Four types of ‘amplifier’ vectors (pMX-HE, pMX-LE, pMX-Hg and pMX-Lg) and three types of ‘courier’ vectors (pMX-LEIGFP, pMX-HgIGFP and pMX-LgIGFP) were constructed. IL-3-dependent Ba/F3 cells were infected with either one of the courier vectors or with one of the amplifier–courier combinations, resulting in Ba/LEIGFP, Ba/HgIGFP, Ba/LgIGFP, Ba/HgIGFP+Lg, Ba/HE+LgIGFP, Ba/Hg+LEIGFP and Ba/HgIGFP+LE cells. On day 6 or 7 after infection, the cells were washed with PBS once, and selected in the medium containing no factors, 1 µg/ml HEL or 2 ng/ml IL-3. Ba/LEIGFP, Ba/HgIGFP and Ba/LgIGFP cells died immediately in the selection medium without IL-3 (data not shown), indicating that these courier vectors have no activity for selective amplification of the transfectants on their own. On the other hand, all transfectants with amplifier–courier combinations gave proliferating cells with HEL selection. Ba/HgIGFP+Lg and Ba/HE+LgIGFP cells proliferate even without any factors, indicating the background growth-stimulating activity of the Hg+Lg and HE+Lg combinations.
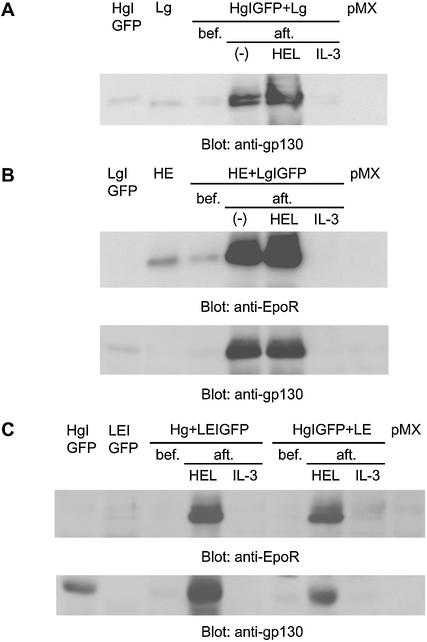
To investigate whether cell proliferation in the selection medium with HEL or no factors was induced by the expressed chimeric receptors, cell lysates before and after the selection were prepared and the amounts of expressed chimeric receptors was compared by western blotting using anti-EpoR or anti-gp130 antibodies (Fig. 2). In all amplifier–courier combinations, IL-3-selected cells expressed ∼2-fold less amounts of chimeric receptors than the cells before selection. On the other hand, HEL-selected cells expressed considerably higher amounts of chimeric receptors than the cells before selection, indicating the efficient amplification of the transduced cells over the non-transduced cells. Furthermore, Ba/HgIGFP+Lg and Ba/HE+LgIGFP cells selected without any factors also expressed high amounts of chimeric receptors comparable to the HEL-selected cells, further confirming the background growth signal from these chimeric receptor combinations. These results demonstrate the preferential amplification of the transfectants expressing chimeric receptor combinations by direct HEL selection without antibiotic selection.
Figure 2.
Antigen-mediated expansion of the transfectants with chimeric receptor expression. The expression level of the chimeric receptor was compared before (bef.) and after (aft.) selection. Selection was performed in the culture medium containing either no factors (–), 1 µg/ml HEL or 2 ng/ml IL-3. pMX means Ba/F3 cells transfected with pMX vector without any inserts (named Ba/pMX) as a negative control. (A) Hg+Lg combinations, (B) HE+Lg combinations and (C) Hg+LE combinations were analyzed with anti-gp130 and anti-EpoR C-terminus antibodies. In each lane, cell lysate from 1.1 × 105 cells was loaded. The actual dates for the harvest were as follows. In (A), day 6 for HgIGFP, Lg and HgIGFP+Lg bef.; day 39 for aft. (–) and HEL; day 36 for IL-3. In (B), day 7 for LgIGFP, HE and HE+LgIGFP bef.; day 67 for aft. (–); day 37 for HEL; day 56 for IL-3. In (C), day 6 for HgIGFP; day 7 for LEIGFP and Hg+LEIGFP bef.; day 40 for aft. HEL; day 56 for IL-3; day 6 for HgIGFP+LE bef.; day 39 for aft. HEL; day 36 for IL-3.
HEL selection induced selective expansion of EGFP-positive cells
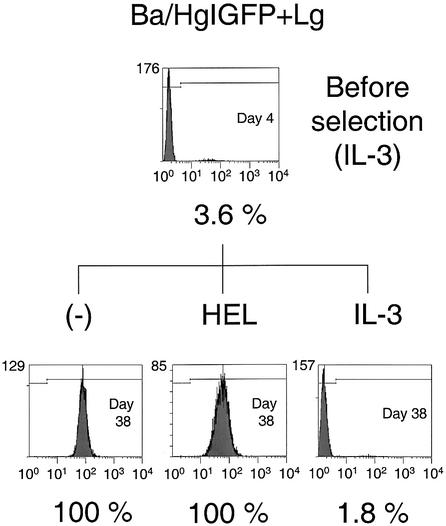
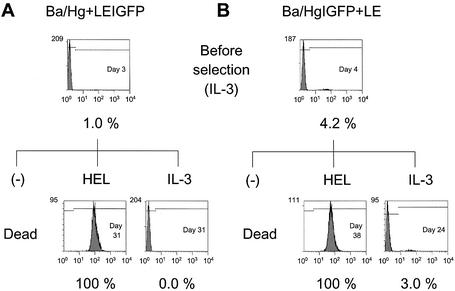
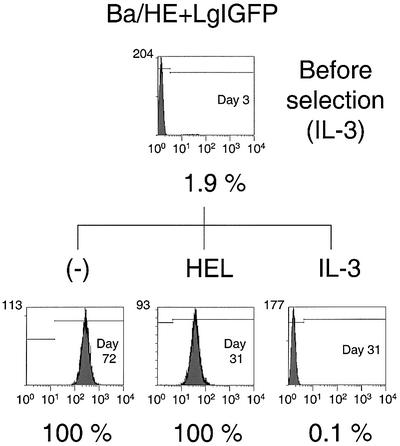
To examine whether HEL selection induced selective expansion of the cells with transgene expression as well as chimeric receptor expression, flow cytometric analysis was performed to determine the EGFP-positive cell population before and after selection. On day 3 or 4 after infection, all the transfectants showed an EGFP-positive cell ratio of <5% (Figs 3–5). After IL-3 selection, the selected cells exhibited similar EGFP-positive ratios to the cells before selection. On the other hand, the HEL-selected cells were 100% EGFP-positive for all amplifier–courier combinations. Furthermore, Ba/HgIGFP+Lg and Ba/HE+LgIGFP cells cultured without any factors also exhibited an EGFP-positive ratio of 100% (Figs 3 and 4). The EGFP-positive ratio was closely related to the expression level of the chimeric receptors, indicating efficient coupling of the two cistrons located upstream and downstream of the IRES sequence. These results clearly demonstrate that the chimeric receptor-derived growth signal induced highly efficient amplification of EGFP-positive cells.
Figure 3.
Selective expansion of EGFP-positive cells with Hg+Lg combinations. Cells (104) were analyzed with flow cytometry, and cell number was plotted against log green fluorescence intensity. EGFP-negative and EGFP-positive regions were determined by taking Ba/pMX cells as a negative control. The day from retroviral infection and the EGFP-positive ratio are indicated.
Figure 5.
Selective expansion of EGFP-positive cells with Hg+LE combinations. Cell number is plotted against log green fluorescence intensity. The day from retroviral infection and the EGFP-positive ratio are indicated. (A) Ba/Hg+LEIGFP cells. (B) Ba/HgIGFP+LE cells.
Figure 4.
Selective expansion of EGFP-positive cells with HE+Lg combinations. Cell number is plotted against log green fluorescence intensity. The day from retroviral infection and the EGFP-positive ratio are indicated.
HEL-dependent cell growth of the transfectants
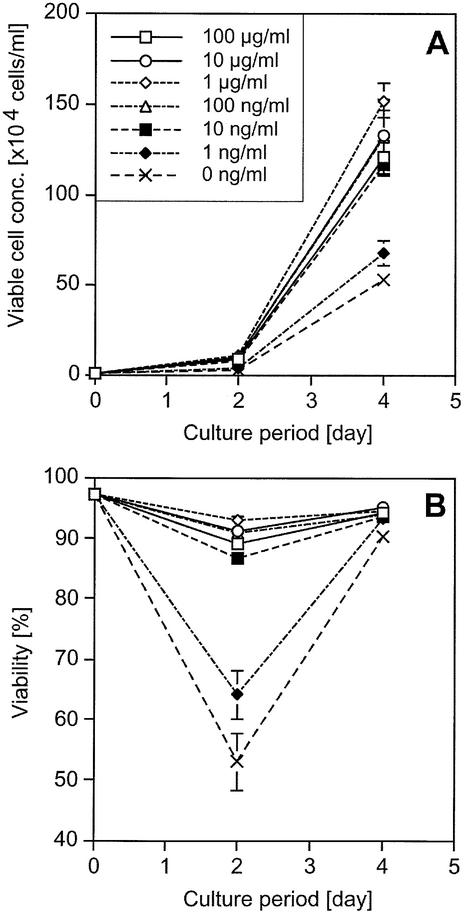
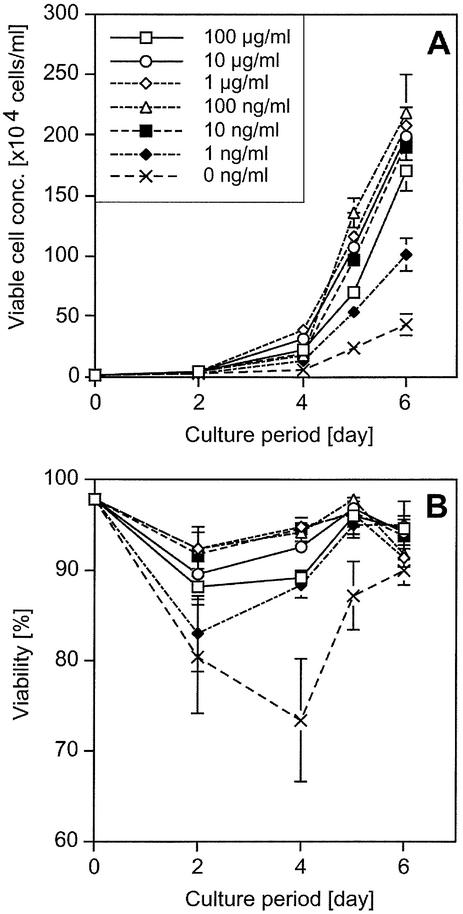
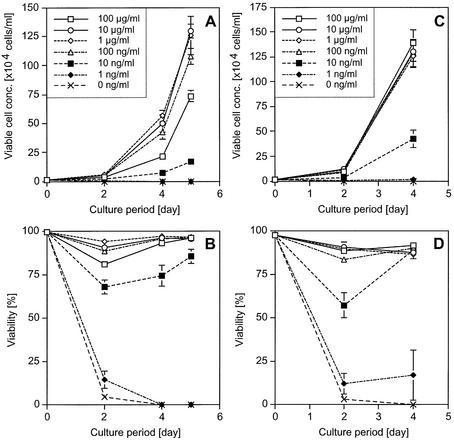
To confirm the characteristics of HEL-dependent cell growth of the transfectants after HEL selection, a cell proliferation assay of the transfectants was performed. Cells were washed and cultured in various concentrations of HEL. During the culture period, cell density and viability were measured. Ba/HgIGFP+Lg cells showed HEL-dependent cell growth with a lower limit of 10 ng/ml, although considerable background growth without HEL was observed (Fig. 6). In the case of Ba/HE+LgIGFP cells, the lower limit of HEL-dependent cell growth was 1 ng/ml, which was most sensitive of all the amplifier–courier combinations (Fig. 7). However, background cell growth without HEL, which was less efficient than that induced by Hg+Lg combinations, was also observed. On the other hand, Ba/HgIGFP+LE and Ba/Hg+LEIGFP showed strict HEL-dependent cell growth without background growth (Fig. 8). The lower limit of HEL required for growth was 10 ng/ml, although 1 ng/ml HEL showed some anti-apoptotic effect. From these analyses, the Hg+LE combination was found to be the best one as an inducible selective amplifier in Ba/F3 cells.
Figure 6.
Growth and viability curves of Ba/HgIGFP+Lg cells. Cells (104 cells/ml) were inoculated into 24-well plates at day 0. Viable cell concentration and viability of triplicates are plotted with average and 1 SD. (A) HEL-dependent growth of Ba/HgIGFP+Lg cells. (B) Viability of the same cells, where the keys are as in (A).
Figure 7.
Growth and viability curves of Ba/HE+LgIGFP cells. Cells (104 cells/ml) were inoculated into 24-well plates at day 0. Viable cell concentration and viability of triplicates are plotted with average and 1 SD. (A) HEL-dependent growth of Ba/HE+LgIGFP cells. (B) Viability of the same cells, where the keys are as in (A).
Figure 8.
Growth and viability curves of the transfectants with Hg+LE combinations. Cells (104 cells/ml) were inoculated into 24-well plates at day 0. Viable cell concentration and viability of triplicates are plotted with average and 1 SD. (A) HEL-dependent growth of Ba/Hg+LEIGFP cells. (B) Viability of the same cells, where the keys are as in (A). (C) HEL-dependent growth of Ba/HgIGFP+LE cells. (D) Viability of the same cells.
Comparison of the selection efficiency with antibiotic selection
To investigate the selection efficiency of our Antigen MEdiated Genetically modified cell Amplification (AMEGA) system, a cloning experiment was performed. Ba/HE+LgIGFP and Ba/Hg+LEIGFP cells before selection were inoculated into two 96-well plates at a cell concentration of 103 cells/well. From the EGFP-positive cell ratio before selection, given that the gene transfer efficiencies of both courier and amplifier vectors are the same, the populations of co-transfectants are calculated to be 0.036% (= 1.9%2) and 0.01% (= 1.0%2) for Ba/HE+LgIGFP and Ba/Hg+LEIGFP cells, respectively. As shown in Table 1, Ba/HE+LgIGFP and Ba/Hg+LEIGFP showed selection efficiencies of up to 30 and 26%. Although the values were not more than that with antibiotic selection of 45%, the data suggests that our AMEGA system could be almost as efficient as antibiotic selection as a selection method.
Table 1. Comparison of the selection efficiency.
| Cells | No. positive wells | Expected clone no. | Selection efficiency (%) |
|---|---|---|---|
| Ba/HE+LgIGFP | 21 | 69 | 30 |
| Ba/Hg+LEIGFP | 5 | 19 | 26 |
| Ba/neoIGFP | 30 | 67 | 45 |
The cells were inoculated into two 96-well plates with an initial cell density of 1 cell/well (for Ba/neoIGFP) or 103 cells/well (for the others). Number of positive wells out of 192 wells for cell growth is shown. Expected clone number was calculated from the EGFP-positive cell ratio before selection on the assumption that transduction efficiency of an amplifier vector was the same as that of a cognate courier vector in each combination.
DISCUSSION
In this study, we have described an AMEGA system without any antibiotic selection steps. Antibiotic selection induces some deleterious effects such as growth inhibition (2,3), mutations in the antibiotic resistance gene and cell membrane permeability (19,20) and morphological changes (21). In contrast, in our system only one-step antigen selection resulted in 100% transgene-positive cells without any deleterious effects, indicating the utility of our method over conventional antibiotic selection.
Compared with antibiotic selection, the selection efficiency of our AMEGA system was almost comparable. In addition, the AMEGA system could be potentially used for in vivo selection of transduced cells, which could not be attained by cytotoxic drug selection. We believe that this is a particular advantage of the AMEGA system, and that the selection efficiency of our system is sufficiently high for practical use.
Whereas Ba/HgIGFP and Ba/LgIGFP cells did not exhibit background growth, Hg+Lg and HE+Lg combinations elicited some background growth without HEL, indicating preformed Hg-Lg or HE-Lg heterodimers in the transfectant expressing Hg+Lg or HE+Lg combinations, respectively. Although the background growth of the transfectant expressing Hg+Lg was observed in previous studies (11,12), the background growth of Ba/HE+LgIGFP was an unexpected finding, since the Hg+LE combination exhibited strict HEL-dependent cell growth. The distinct characteristics of Hg+LE and HE+Lg imply that the small conformational difference has an effect on signal transduction in the artificially induced EpoR-gp130 heterodimer. Recent X-ray crystallographic analyses revealed the importance of the conformation of the extracellular domain in EpoR signaling (8,9,22–24). In addition, recent studies using mutagenesis or chimeric receptors demonstrated the contribution of association between receptor transmembrane domains in EpoR signaling (25,26). In the model proposed in the study (26), although EpoR takes two dimeric conformations with association in the extracellular domains or the transmembrane domains in the absence of Epo, the latter conformation is stabilized by Epo binding, thereby triggering signal transduction. Therefore, the different characteristics of Hg+LE and HE+Lg might imply that the stabilization effect of the transmembrane domain was changed by the extracellular construct of the chimera. The alteration to the mutated transmembrane domain with weaker association might realize more strictly regulated antigen-dependent cell amplification (25–28).
Since antibiotic selection inhibits cell growth, it may take more time to select and expand the transfectant. The long selection period is problematic for the selection of primary culture cells, especially for the ones in which transduction efficiency is low, since the cells might be unexpectedly differentiated during the selection. In contrast, our method has the advantage that the cell growth could be rather stimulated. Stem cells, due to their pluripotency, are promising targets for gene therapy to correct various diseases (29–31). Since hematopoietic stem cells were reported to be expanded with gp130 and EpoR signaling (32,33), Hg+LE combinations might be applied to the ex vivo or in vivo amplification of genetically modified hematopoietic stem cells. Generalization of this approach including the use of other functional antigen–antibody pairs and alternative intracellular domains will provide wide applications of our selection system.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Dr K. Todokoro (RIKEN) and Dr M. Hibi (Osaka University) for murine EpoR and gp130 expression vectors, respectively, and Dr T. Kitamura for pMX and pMX-GFP. We thank Y. Masago (University of Tokyo) for instruction and troubleshooting of the flow cytometer EPICS-ALTRA. M.K. was supported by research fellowships of the Japan Society for the Promotion of Science for Young Scientists. This work was supported by Grants-in-Aid for Scientific Research (S13854003 and 14030017) from MEXT, Japan.
REFERENCES
- 1.Brielmeier M., Bechet,J.M., Falk,M.H., Pawlita,M., Polack,A. and Bornkamm,G.W. (1998) Improving stable transfection efficiency: antioxidants dramatically improve the outgrowth of clones under dominant marker selection. Nucleic Acids Res., 26, 2082–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu M.B., Kern,J.A., Todd,P. and Kompala,D.S. (1992) Effect of amplification of dhfr and lacZ genes on growth and beta-galactosidase expression in suspension cultures of recombinant CHO cells. Cytotechnology, 9, 237–245. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.J., Kim,N.S., Ryu,C.J., Hong,H.J. and Lee,G.M. (1998) Characterization of chimeric antibody producing CHO cells in the course of dihydrofolate reductase-mediated gene amplification and their stability in the absence of selective pressure. Biotechnol. Bioeng., 58, 73–84. [PubMed] [Google Scholar]
- 4.Berardi A.C., Wang,A., Levine,J.D., Lopez,P. and Scadden,D.T. (1995) Functional isolation and characterization of human hematopoietic stem cells. Science, 267, 104–108. [DOI] [PubMed] [Google Scholar]
- 5.Miller D.G., Adam,M.A. and Miller,A.D. (1990) Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol., 10, 4239–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roe T., Reynolds,T.C., Yu,G. and Brown,P.O. (1993) Integration of murine leukemia virus DNA depends on mitosis. EMBO J., 12, 2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueda H., Tsumoto,K., Kubota,K., Suzuki,E., Nagamune,T., Nishimura,H., Schueler,P.A., Winter,G., Kumagai,I. and Mohoney,W.C. (1996) Open sandwich ELISA: a novel immunoassay based on the interchain interaction of antibody variable region. Nat. Biotechnol., 14, 1714–1718. [DOI] [PubMed] [Google Scholar]
- 8.Syed R.S., Reid,S.W., Li,C., Cheetham,J.C., Aoki,K.H., Liu,B., Zhan,H., Osslund,T.D., Chirino,A.J., Zhang,J. et al. (1998) Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature, 395, 511–516. [DOI] [PubMed] [Google Scholar]
- 9.Livnah O., Stura,E.A., Middleton,S.A., Johnson,D.L., Jolliffe,L.K. and Wilson,I.A. (1999) Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science, 283, 987–990. [DOI] [PubMed] [Google Scholar]
- 10.Ueda H., Kawahara,M., Aburatani,T., Tsumoto,K., Todokoro,K., Suzuki,E., Nishimura,H., Schueler,P.A., Winter,G., Mahoney,W.C. et al. (2000) Cell-growth control by monomeric antigen; the cell surface expression of lysozyme-specific Ig V-domains fused to truncated Epo receptor. J. Immunol. Methods, 241, 159–170. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara M., Ueda,H., Tsumoto,K., Kumagai,I., Mahoney,W. and Nagamune,T. (2001) A growth signal with an artificially induced erythropoietin receptor-gp130 cytoplasmic domain heterodimer. J. Biochem., 130, 305–312. [DOI] [PubMed] [Google Scholar]
- 12.Kawahara M., Natsume,A., Terada,S., Kato,K., Tsumoto,K., Kumagai,I., Miki,M., Mahoney,W., Ueda,H. and Nagamune,T. (2001) Replacing factor-dependency with that for lysozyme: affordable culture of IL-6-dependent hybridoma by transfecting artificial cell surface receptor. Biotechnol. Bioeng., 74, 416–423. [DOI] [PubMed] [Google Scholar]
- 13.Kawahara M., Ueda,H., Tsumoto,K., Kumagai,I., Mahoney,W. and Nagamune,T. (2002) Selection of highly productive mammalian cells based on an inducible growth advantage using an antibody/receptor chimera. J. Biosci. Bioeng., 93, 399–404. [DOI] [PubMed] [Google Scholar]
- 14.Kojima T. and Kitamura,T. (1999) A signal sequence trap based on a constitutively active cytokine receptor. Nature Biotechnol., 17, 487–490. [DOI] [PubMed] [Google Scholar]
- 15.Palacios R. and Steinmetz,M. (1985) IL-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration and generate B lymphocytes in vivo. Cell, 41, 727–734. [DOI] [PubMed] [Google Scholar]
- 16.Morita S., Kojima,T. and Kitamura,T. (2000) Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther., 7, 1063–1066. [DOI] [PubMed] [Google Scholar]
- 17.Persons D.A., Allay,J.A., Allay,E.R., Smeyne,R.J., Ashmun,R.A., Sorrentino,B.P. and Nienhuis,A.W. (1997) Retroviral-mediated transfer of the green fluorescent protein gene into murine hematopoietic cells facilitates scoring and selection of transduced progenitors in vitro and identification of genetically modified cells in vivo. Blood, 90, 1777–1786. [PubMed] [Google Scholar]
- 18.Sugimoto Y., Aksentijevich,I., Murray,G.J., Brady,R.O., Pastan,I. and Gottesman,M.M. (1995) Retroviral coexpression of a multidrug resistance gene (MDR1) and human alpha-galactosidase A for gene therapy of Fabry disease. Hum. Gene Ther., 6, 905–915. [DOI] [PubMed] [Google Scholar]
- 19.Assaraf Y.G. and Schimke,R.T. (1987) Identification of methotrexate transport deficiency in mammalian cells using fluoresceinated methotrexate and flow cytometry. Proc. Natl Acad. Sci. USA, 84, 7154–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haber D.A., Beverley,S.M., Kiely,M.L. and Schimke,R.T. (1981) Properties of an altered dihydrofolate reductase encoded by amplified genes in cultured mouse fibroblasts. J. Biol. Chem., 256, 9501–9510. [PubMed] [Google Scholar]
- 21.Chung J.Y., Kim,T.K. and Lee,G.M. (2000) Morphological selection of parental Chinese hamster ovary cell clones exhibiting high-level expression of recombinant protein. Biotechniques, 29, 768–774. [DOI] [PubMed] [Google Scholar]
- 22.Livnah O., Stura,E.A., Johnson,D.L., Middleton,S.A., Mulcahy,L.S., Wrighton,N.C., Dower,W.J., Jolliffe,L.K. and Wilson,I.A. (1996) Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 Å. Science, 273, 464–471. [DOI] [PubMed] [Google Scholar]
- 23.Livnah O., Johnson,D.L., Stura,E.A., Farrell,F.X., Barbone,F.P., You,Y., Liu,K.D., Goldsmith,M.A., He,W., Krause,C.D. et al. (1998) An antagonist peptide-EPO receptor complex suggests that receptor dimerization is not sufficient for activation. Nature Struct. Biol., 5, 993–1004. [DOI] [PubMed] [Google Scholar]
- 24.Remy I., Wilson,I.A. and Michnick,S.W. (1999) Erythropoietin receptor activation by a ligand-induced conformation change. Science, 283, 990–993. [DOI] [PubMed] [Google Scholar]
- 25.Constantinescu S.N., Keren,T., Socolovsky,M., Nam,H., Henis,Y.I. and Lodish,H.F. (2001) Ligand-independent oligomerization of cell-surface erythropoietin receptor is mediated by the transmembrane domain. Proc. Natl Acad. Sci. USA, 98, 4379–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubatzky K.F., Ruan,W., Gurezka,R., Cohen,J., Ketteler,R., Watowich,S.S., Neumann,D., Langosch,D. and Klingmuller,U. (2001) Self assembly of the transmembrane domain promotes signal transduction through the erythropoietin receptor. Curr. Biol., 11, 110–115. [DOI] [PubMed] [Google Scholar]
- 27.Gurezka R., Laage,R., Brosig,B. and Langosch,D. (1999) A heptad motif of leucine residues found in membrane proteins can drive self-assembly of artificial transmembrane segments. J. Biol. Chem., 274, 9265–9270. [DOI] [PubMed] [Google Scholar]
- 28.Lemmon M.A., Flanagan,J.M., Treutlein,H.R., Zhang,J. and Engelman,D.M. (1992) Sequence specificity in the dimerization of transmembrane alpha-helices. Biochemistry, 31, 12719–12725. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Serrano A., Rubio,F.J., Navarro,B., Bueno,C. and Villa,A. (2001) Human neural stem and progenitor cells: in vitro and in vivo properties and potential for gene therapy and cell replacement in the CNS. Curr. Gene Ther., 1, 279–299. [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi H., Smith,K.A., Mosier,D.E., Verma,I.M. and Torbett,B.E. (1999) Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science, 283, 682–686. [DOI] [PubMed] [Google Scholar]
- 31.Thomson J.A., Itskovitz-Eldor,J., Shapiro,S.S., Waknitz,M.A., Swiergiel,J.J., Marshall,V.S. and Jones,J.M. (1998) Embryonic stem cell lines derived from human blastocysts. Science, 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- 32.Kirby S.L., Cook,D.N., Walton,W. and Smithies,O. (1996) Proliferation of multipotent hematopoietic cells controlled by a truncated erythropoietin receptor transgene. Proc. Natl Acad. Sci. USA, 93, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui X., Tsuji,K., Tanaka,R., Tajima,S., Muraoka,K., Yasukawa,K., Taga,T., Kishimoto,T. and Nakahata,T. (1995) gp130 and c-Kit signalings synergize for ex vivo expansion of human primitive hemopoietic progenitor cells. Proc. Natl Acad. Sci. USA, 92, 2859–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]