Abstract
A 66-year-old woman presented with new-onset complete left bundle branch block and congestive heart failure. She had had chronic paranoid schizophrenia for 35 years and had been taking medications to control her psychiatric disorder for the past 10 years. A 2-dimensional echocardiogram performed at the onset of congestive heart failure showed dilated cardiomyopathy with global impairment of left ventricular function (ejection fraction <0.25). Despite withdrawal of the medications most likely responsible for the heart problems (perphenazine, 2 mg; and amitriptyline, 25 mg), the patient died of refractory congestive heart failure 2 years later. Histologic examination at autopsy showed evidence of persistent toxic myocarditis with fibrosis of the heart and persistent chronic hepatitis. These autopsy findings were considered to be drug related. (Tex Heart Inst J 2003;30:76–9)
Key words: Cardiomyopathy, congestive/pathology; myocarditis, toxic/complications/diagnosis/etiology/pathology/therapy
The incidence of myocarditis in patients with idiopathic dilated cardiomyopathy varies widely—from 13% to 63%—as shown by endomyocardial biopsy. 1 The incidence is probably higher when the entire myocardium is microscopically examined at autopsy. We present an illustrative case and discuss toxic myocarditis on the basis of autopsy findings.
Case Report
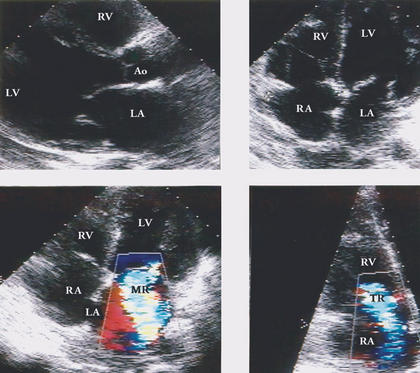
In September 1999, a 66-year-old woman was evaluated for new-onset complete left bundle branch block and congestive heart failure (CHF). She had had chronic paranoid schizophrenia for 35 years and had been controlling the symptoms for 10 years with perphenazine (2 mg) and amitriptyline (25 mg, 3 times per day). An echocardiogram (Fig. 1) showed dilated chambers, severe mitral and tricuspid regurgitation due to annular dilatation, and global impairment of left ventricular function (ejection fraction, <0.25). Toxic myocarditis (TM) related to the above-mentioned drugs was considered the most likely diagnosis. Despite the withdrawal of the perphenazine and amitriptyline and treatment for CHF with salt restriction, inotropic agents (digoxin, 0.25 mg), diuretics (furosemide, 40 mg), enalapril (10 mg per day), and isosorbide dinitrate (10 mg, 4 times per day), the patient's course over the next 2 years was characterized by slow clinical deterioration. The CHF eventually became refractory to medical treatment, and the patient died in September of 2001.
Fig. 1 Two-dimensional echocardiogram shows A) enlarged left atrium and ventricle and B) 4-chamber enlargement. Colorflow Doppler shows C) mitral regurgitation and D) tricuspid regurgitation.
Ao = aorta; LA = left atrium; LV = left ventricle; MR = mitral regurgitation; RA = right atrium; RV = right ventricle; TR = tricuspid regurgitation
At autopsy, the heart weighed 340 g, and there was dilatation of all 4 chambers. The aorta and coronary arteries were free of atherosclerosis and thrombi. Gross examination of the myocardium showed absence of necrosis or scarring. Histologic examination (Fig. 2) of the left ventricle, however, showed vacuolization and disruption of myocardial fibers. These findings were associated with widespread scattered foci of interstitial chronic inflammatory cells consisting of lymphocytes, eosinophils, and plasma cells. Prominent interstitial fibrosis was also present (Fig. 3). Intramural arteries and veins were normal. These histologic findings met the Dallas criteria for toxic myocarditis with fibrosis. 2 Other related histologic findings were confined to the liver, where chronic nonspecific hepatitis was present, probably related to the same drugs (Fig. 4). Serologic tests for hepatitis viruses A, B, and C were negative.
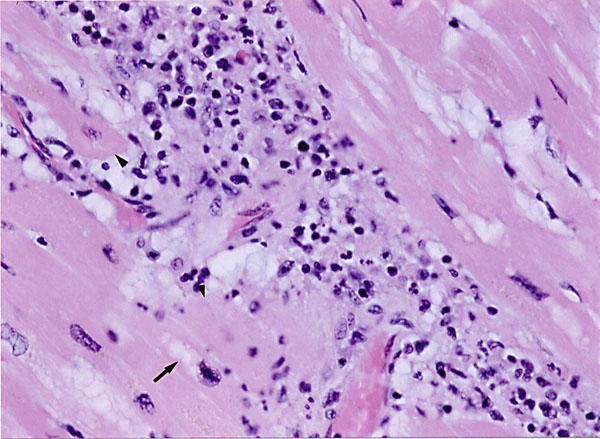
Fig. 2 Histologic examination of left ventricular myocardium shows vacuolization (arrow), disruption of myocardial fibers (arrowheads), and widespread foci of chronic inflammatory cells consisting of lymphocytes, eosinophils, and plasma cells (H&E, orig. ×200).
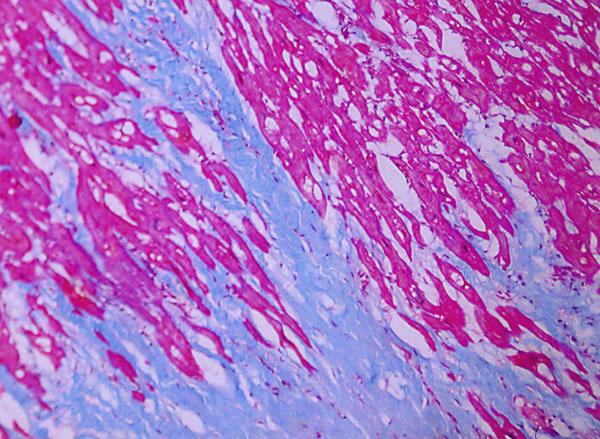
Fig. 3 Left ventricular myocardium shows prominent and widespread interstitial fibrosis (Masson's trichrome stain, orig. ×100).
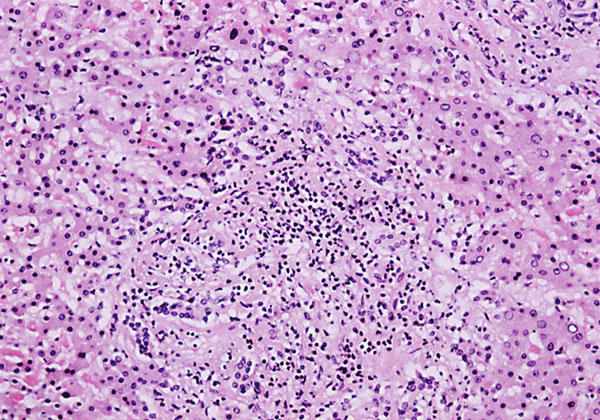
Fig. 4 Histologic examination of the liver shows collection of prominent mixed cellular infiltrate, degenerating hepatocytes, and fatty infiltration (H&E, orig. ×100).
Discussion
Toxic drug-induced myocarditis, simply defined, is an inflammation of heart muscle resulting from drugs used illicitly or as a part of medical treatment. Toxic myocarditis is characterized by either acute or insidious onset and usually runs a protracted course. Frequently, irreversible end-stage disease manifests as dilated cardiomyopathy. 3 This progression is characterized by inflammation and myocytolysis, gradually resulting in death of myocytes and replacement by fibrous tissue. 4
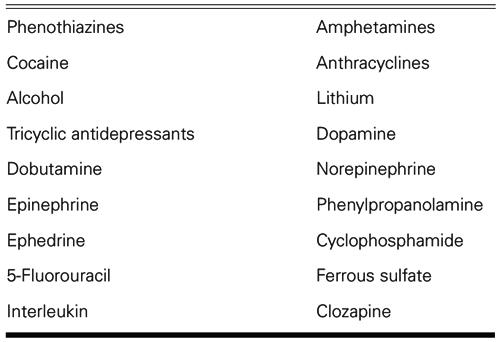
Dilated cardiomyopathy may occur as a consequence of the use of phenothiazines. If TM is the underlying cause, evidence of this can be documented on endomyocardial biopsy or autopsy. Identification of TM also suggests the possibility of remission upon withdrawal of the medications early in the course of disease. Although it is possible that any drug used in medical practice can cause TM, medications known to cause this problem are listed in Table I. The most recent addition to the list is clozapine. 5
TABLE I. Drugs Known to Cause Toxic Myocarditis
Findings of new-onset complete left bundle branch block and CHF in combination are compatible with a diagnosis of idiopathic dilated cardiomyopathy, but also with a diagnosis of myocarditis of infectious (viral, bacterial) or toxic origin. An endomyocardial biopsy is often used to differentiate between cardiomyopathy and myocarditis. Morphologic evidence, including both an inflammatory infiltrate and myocyte damage, are required for the unequivocal diagnosis of myocarditis. 2 These criteria, as described by Aretz and colleagues, 2 are widely accepted. Within their classification system, TM exists when myocyte degeneration occurs, along with a collection of mature lymphocytes or a mixed cellular infiltrate, often with fibrosis. These required constellations of pathologic findings are meant to avoid over-diagnosis of TM by the presence of leukocytic infiltrate alone, because the latter is a common finding in idiopathic dilated cardiomyopathy, as well. 6 In our patient, the presence of drug-induced chronic hepatitis gave further weight to the diagnosis of TM rather than idiopathic dilated cardiomyopathy. When the diagnosis of TM seems likely, additional evaluation by immunofluorescence and electron microscopic evaluation of the biopsies or an autopsy specimen is very helpful. Myofilament loss shown by electron microscopy is a valuable prognostic indicator. 7 Other such indicators include left bundle branch block and left atrial enlargement, which independently predict death. 8
Toxic myocarditis, even if recognized early in its course, may not be reversible. Nonetheless, withdrawal of the offending drug and continued follow-up are prudent for effective management. With these measures, our patient survived 2 years after the clinical diagnosis of toxic myocarditis, which was confirmed at autopsy. The persistence of toxic myocarditis under such conditions may be related to the complex relationship between adaptive and maladaptive genetic and immunologic responses of the affected host.
Acknowledgment
We wish to sincerely thank Ms Marlis Gravley for preparation of this manuscript.
Footnotes
Address for reprints: Azam Ansari, MD, Suite 812, 825 South 8th Street, Minneapolis, MN 55404
E-mail: dr_azam_ansari@hotmail.com
References
- 1.Latham RD, Mulrow JP, Virmani R, Robinowitz M, Moody JM. Recently diagnosed idiopathic dilated cardiomyopathy: incidence of myocarditis and efficacy of prednisone therapy. Am Heart J 1989;117:876–82. [DOI] [PubMed]
- 2.Aretz TH, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ Jr, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1987; 1:3–14. [PubMed]
- 3.Feldman AM, McNamara D. Myocarditis. N Engl J Med 2000;343:1388–98. [DOI] [PubMed]
- 4.Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation 1999;99:1091–100. [DOI] [PubMed]
- 5.La Grenade L, Graham D, Trontell A. Myocarditis and cardiomyopathy associated with clozapine use in the United States. N Engl J Med 2001;345:224–5. [DOI] [PubMed]
- 6.Tazelaar HD, Billingham ME. Leukocytic infiltrates in idiopathic dilated cardiomyopathy. A source of confusion with active myocarditis. Am J Surg Pathol 1986;10(6):405–12. [DOI] [PubMed]
- 7.Hammond EH, Menlove RL, Anderson JL. Predictive value of immunofluorescence and electron microscopic evaluation of endomyocardial biopsies in the diagnosis and prognosis of myocarditis and idiopathic dilated cardiomyopathy. Am Heart J 1987;114:1055–65. [DOI] [PubMed]
- 8.Cianfrocca C, Pelliccia F, Nigri A, Critelli G. Resting and ambulatory ECG predictions of mode of death in dilated cardiomyopathy. J Electrocardiol 1992;25:295–303. [DOI] [PubMed]