Abstract
Transforming growth factor-βs (TGF-βs) play a dual role in carcinogenesis, functioning as tumor suppressors early in the process, and then switching to act as pro-metastatic factors in late-stage disease. We have previously shown that high molecular weight TGF-β antagonists can suppress metastasis without the predicted toxicities (Yang et al., J. Clin. Invest. (2002) 109:1607-1615). To address the underlying mechanisms, we have used the 4T1 syngeneic mouse model of metastatic breast cancer. Treatment of mice with a monoclonal anti-TGF-β antibody (1D11) significantly suppressed metastasis of 4T1 cells to the lungs. When metastatic 4T1 cells were recovered from lungs of 1D11-treated and control mice, the most differentially expressed gene was found to be bone sialoprotein (Bsp). Immunostaining confirmed the loss of Bsp protein in 1D11-treated lung metastases, and TGF-β was shown to regulate and correlate with Bsp expression in vitro. Functionally, knockdown of Bsp in 4T1 cells reduced the ability of TGF-β to induce local collagen degradation and invasion in vitro, and treatment with recombinant BSP protected 4T1 cells from complement-mediated lysis. Finally, suppression of Bsp in 4T1 cells reduced metastasis in vivo. We conclude that Bsp is a plausible mediator of at least some of the tumor cell-targeted prometastatic activity of TGF-β in this model, and that Bsp expression in metastases can be successfully suppressed by systemic treatment with anti-TGF-β antibodies.
Keywords: Transforming growth factor-β, monoclonal anti-TGF-β antibody, metastasis, invasion, bone sialoprotein, matrix metalloproteinases, complement
Introduction
The transforming growth factor-βs (TGF-βs) are multifunctional growth factors that play particularly complex roles in tumorigenesis. Clinical and mouse model data show that the TGF-β pathway clearly has tumor suppressor activity, and reduction or loss of TGF-β receptors or downstream signaling components is seen in many human tumors (reviewed in (1)). However, late-stage human tumors frequently show a paradoxical increase in expression of TGF-βs that is associated with metastasis and poor prognosis (2). The unifying hypothesis is that TGF-βs have tumor suppressor activity early in the carcinogenic process, but that in the later stages, suppressor activity is lost and prooncogenic activities prevail (1, 3).
The dual role for TGF-β in carcinogenesis poses a major therapeutic challenge, as strategies must be sought to specifically target the pro-metastatic activities of TGF-βs while sparing the desirable effects on normal homeostasis and tumor suppression. We have previously shown that prolonged exposure to a high molecular weight TGF-β antagonist of the receptor:Fc fusion protein class could suppress metastasis without significant side-effects in a mouse model system, raising the possibility that this goal might be achievable under certain circumstances (4). Other studies have also provided evidence that targeting the TGF-β pathway could be a powerful approach to the treatment or prevention of metastasis (5-9).
Successful application of TGF-β antagonists to prevent or suppress metastatic disease will depend on the ability to stratify patients in such a way as to exclude those who might show an adverse response to treatment. In addition, it will be useful to have molecular biomarkers that correlate with response to antagonist treatment. We believe that a better understanding of mechanisms underlying both the tumor suppressor and pro-metastatic effects of TGF-β in vivo will be critical for the achievement of these goals. A large body of experimental data has suggested that TGF-β has the potential to promote metastasis through effects both on the tumor cell itself and on other cellular compartments. Direct effects on the tumor cell that might promote metastasis include induction of an epithelial -to-mesenchymal transition, promotion of migration and invasion, and enhanced tumor cell survival (reviewed in (1)). By contrast, indirect effects of TGF-β that could increase metastatic efficiency include its ability to suppress the immune surveillance system, and to promote angiogenesis (reviewed in (1)). While the existing experimental data have been helpful in establishing the spectrum of possible activities of TGF-β in vivo, it is currently less clear which of these various activities are actually engaged by a developing tumor, and more specifically which activities might be accessible to modulation by TGF-β antagonists.
Here we have used the 4T1 mouse model of metastatic breast cancer to investigate mechanisms underlying metastasis suppression when the TGF-β system is antagonized using an anti-TGF-β monoclonal antibody (1D11) that recognizes all three isoforms of TGF-β. The 4T1 model, which is syngeneic to BALB/c mice, is widely considered to be one of the best models of post-operative stage IV breast cancer, and the 4T1 cells can metastasize to lung, liver and bone following tail-vein injection or orthotopic implantation (10, 11). In this study, we have focused on the possible effects of TGF-β antagonism on the tumor cells themselves. We present evidence that bone sialoprotein (Bsp) may be a key mediator of the pro-metastatic effects of TGF-β in this model of breast cancer, and that anti-TGF-β antibodies may act in part through the down-regulation of Bsp in the metastatic cells.
Materials and Methods
Cell culture and reagents.
67NR, 4TO7, and 4T1 cell lines were provided by Dr. Fred Miller at the Barbara Ann Karmanos Cancer Institute, Detroit, MI., and were cultured as described previously (10). TGF-β1 and TGF-β Type I receptor kinase inhibitor (ALK5 Inhibitor I) were purchased from R&D Systems (Minneapolis, MN) and Calbiochem (La Jolla, CA), respectively. The anti-TGF-β murine monoclonal antibody, 1D11, which neutralizes all three isoforms of TGF-β (12), and an isotype-matched IgG1 monoclonal antibody 13C4, which was raised against Shigella toxin and serves as a control, were provided by Genzyme Corp., Framingham, MA.
In vivo metastasis study.
All animals were maintained according to the National Cancer Institute’s Animal Care and Use Committee guidelines, under approved animal study protocols. For the spontaneous metastasis format, the left thoracic (#2) mammary glands of anesthetized 7-week-old female BALB/cANCr mice (National Cancer Institute-Frederick, Frederick, MD) were surgically exposed, and 4 X 104 4T1 cells were inoculated into the mammary fat pad (m.f.p.) in a volume of 40 μl. After inoculation, the mice were randomized into two treatment groups, with 17-20 animals/group. Anti-TGF-β antibody (1D11, 5 mg/kg body weight) was administered three times per week i.p., starting one day after cell inoculation. The control group received the same dosage and volume of the control monoclonal antibody 13C4. Primary tumors were surgically excised on day 10. Mice were euthanized by carbon dioxide narcosis on day 28, and the lungs were removed, inflated and fixed in 10% buffered formalin. The relative lung weight was calculated using the formula: lung weight/body weight X 100 (%). Macroscopic quantitation of metastases was performed by counting the number of nodules on the surface of the lung. For microscopic quantitation of lung metastases, each lobe of the lung was processed for hematoxylin-eosin staining and evaluated by a board-certified veterinary pathologist (Miriam R. Anver, DVM, PhD). For the experimental metastasis format, 5,500 4T1 cells were injected into the tail-vein of 7-week-old female BALB/c mice. Lungs were harvested on day 21 and analyzed as above.
Recovery of metastatic cells from lungs.
Lungs were harvested from tumor-bearing mice treated with 1D11 (anti-TGF-β) or 13C4 (control) antibodies, minced and digested for 1 hour with 1 mg/ml type IV collagenase (Sigma-Aldrich, St. Louis, MO) suspended in Dulbecco’s modified Eagle Medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS). After spinning out debris, the cell digests were placed in culture medium containing 10 μg/ml of 6-thioguanine (Sigma-Aldrich) for several days in order to enrich for 4T1 cells.
Oligonucleotide microarray analysis.
RNA was prepared from five independent isolates of metastatic 4T1 cells recovered from the lungs of 1D11-treated and control mice, using RNeasy Mini kit according to manufacturer’s instructions (Qiagen, Valencia, CA). The Affymetrix Gene Chip MOE430A (Affymetrix, Santa Clara, CA) was used for analysis. cDNA synthesis and cRNA in vitro transcription, labeling and linear amplification were performed using the Two-cycle cDNA Synthesis Kit and GeneChip IVT Labeling kit (Affymetrix). The in vitro transcription products were purified, fragmented and hybridized to the oligonucleotide arrays as recommended by the manufacturer. Raw data were processed with Robust Multiarray Average (RMA) algorithm and quantile normalization to obtain gene summary measures (13). Differences in gene expression levels between the two treatment groups were identified using univariate two-sample t test (P<0.001). The statistical computations were done using the R and Affy package of the Bioconductor software project (http://www.bioconductor.org).
Quantitative reverse-transcription polymerase chain reaction (RTQ-PCR).
To validate the microarray results, real-time quantitative PCR was performed using the iCycler iQ Real-time PCR Detection System (Bio-Rad) using SYBR green dye (Stratagene, Cedar Creek, TX). First-strand cDNA was prepared from total RNA using a SuperScript III first strand synthesis kit (Invitrogen). The quantitative RT-PCR was done in triplicate. Mouse Bsp mRNA levels were normalized to mouse 28S rRNA. The primer sets used in this study were as follows: Bsp, 5′-TTCCCAGGTGTGTCATTGAAGA-3′ (forward primer) and 5′-GGTATGTTTGCGCAGTTAGCAA-3′ (reverse primer); and 28S rRNA, 5′-GGGTGGTAAACTCCATCTAA-3′ (forward primer) and 5′-AGTTCTTTTCAACTTTCCCT -3′ (reverse primer).
Immunoblotting, immunohistochemistry and ELISA assays for Bsp and TGF-β1.
Immunoblotting was performed as described previously (14). Membranes were probed with anti-Bsp polyclonal antibody LF-84 (1:1,000 dilution) (15), and anti-β-actin monoclonal antibody (Clone AC-15, 1:5,000 dilution, Sigma-Aldrich). For immunostaining of formalin-fixed samples for Bsp, the avidin-biotin-peroxidase complex method was used, with the anti-Bsp polyclonal antibody LF-84, as above, at a final dilution of 1:100. Lung metastases were individually evaluated for Bsp expression using a semiquantitative score system as follows: 0, no Bsp-positive 4T1 cells in the metastasis; 1, < 30% positive cells; 2, 30-60% positive cells; 3, >60% positive cells. Metastases were scored for three mice from each treatment group, for a total of 152 metastases. The difference in score between the two treatment groups was assessed by the likelihood ratio test of the β binomial model, grouping metastases with a score of 0 and 1 or 2 and 3 for each mouse. Circulating Bsp levels in serum were determined using a competitive ELISA assay following separation of Bsp from complement Factor H by denaturation, reduction and column chromatography, as described (16). Total TGF-β1 levels in clarified serum-free cell-conditioned medium were determined without sample acidification, using a Quantikine TGF-β1 ELISA kit ( R&D Systems, Minneapolis MN), according to manufacturer’s instructions.
Promoter-reporter assays.
4T1 cells at 50% confluence in 12-well plates were transfected with a total of 100 ng/well of promoter-reporter plasmid, together with 10 ng/well of pRL-TK (Renilla) plasmid DNA for normalization, using Fugene 6 transfection reagent (Roche, Indianapolis, IN) according to the manufacturer’s instructions. The promoter-reporter constructs used were pCAGA12LUC, containing 12 repeats of the Smad3 binding element CAGA12 driving luciferase (17), or p1.4kbBsp-LUC, with the proximal 1.4kb of the mouse Bsp promoter driving luciferase (18). After transfection, cells were incubated for 16-18 hours in the presence or absence of TGF-β1 (5 ng/ml), and cell lysates were harvested for assay using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Luminescence was measured by VICTOR2 (PerkinElmer Life and Analytical Sciences, Boston, MA).
Transfection of cells with siRNA.
Four siRNA sequences were chosen to target mouse Bsp gene (GeneBank accession no. NM_008318), according to the Dharmacon’s siGENOME protocol (Dharmacon, Lafayette, CO). The position of the target sequences for these 4 siRNA duplexes, designated as siB1, siB2, siB3, and siB4, are as follows: siB1, bases 203-221; siB2, bases 1023-1041; siB3, bases 975-993; siB4, bases 909-927 in the nucleotide sequence of Bsp. GFP siRNA (GFP duplex I, Dharmacon) was used as a non-specific siRNA control. siRNA was introduced into the cells by reverse transfection, using Silentfect lipid reagent (Bio-Rad, Hercules, CA). Briefly, serum free DMEM containing Silenfect lipid reagent (Bio-Rad) was added to the culture flask. Next, serum free DMEM containing siRNA was added. The lipid/siRNA mixture was incubated for 30 minutes at ambient temperature. 4T1 cells were trypsinized and washed three times with DMEM. Cells were then resuspended in DMEM supplemented with 10% FBS. Cell suspension were added to the lipid/siRNA mixture and incubated for 45 minutes at ambient temperature followed by incubation at 37 °C (CO2, humidified). The final siRNA concentration was 50 nM.
Alternate complement-mediated cell lysis assay.
The susceptibility of 4T1 cells to complement-mediated cell lysis was determined as described previously (19). Briefly, trypsinized cells were preincubated with 10 μg/ml of purified human BSP protein for 10 minutes at 37 °C, followed by addition of normal human serum to a final dilution of 1:5 to 1:20. After an additional 2 hour incubation at 37 °C, cell viability was evaluated using a colorimetric MTT assay (Chemicon, Temecula, CA).
Matrigel invasion assay.
Breast cancer cell invasion was assayed in 24-well Biocoat Matrigel invasion chambers (8 μm; BD Biosciences, Bedford, MA) according to the manufacturer’s protocol. Briefly, the top chamber was seeded with 1 X 105 viable tumor cells in a serum free medium (AIM-V medium, Invitrogen). The bottom chamber was filled with DMEM supplemented with 10% FBS as a chemoattractant. After 20 hours incubation, the noninvasive cells that remained on the upper surface of the membrane were removed with a cotton swab. Cells that had migrated through the membrane were then fixed with methanol and stained with hematoxylin (Vector, Burlingame, CA). Migratory cells per field were counted for three random fields for each membrane, using a light microscope at X200 magnification. Invasion assays were performed in triplicate.
Conventional and in-situ zymography.
Conventional zymography of cell-free culture supernatants harvested from cells grown on type I collagen was performed using 10% polyacrylamide, 0.1% gelatin gels as described (20). In-situ zymography was also performed as described previously (21). In brief, cells grown on glass coverslips were washed twice with PBS, overlaid with a solution of 50 μg/ml fluorescein-labeled collagen I or collagen IV (DQ collagen: Molecular Probes, Eugene, OR), 1% (w/v) low-melting agarose (BME, Rockland, ME), and 5 μg/ml DAPI (Molecular Probes) in PBS, and incubated on ice for 15 minutes, followed by incubation in a humidified chamber at room temperature for 3 hours. Specimens were fixed with 10% buffered formalin (Sigma-Aldrich), and were examined by confocal microscopy (Leica DM IRBE confocal microscopy, X630 magnification). Nuclei and collagen degradation were visualized as blue and green fluorescence respectively. The calculation of collagen degradation is based on the green fluorescence area and density divided by the cell number, as determined from the number of DAPI-stained nuclei, in three randomly selected fields for each specimen from a total of three independent experiments, using the Image-Pro Plus program (MediaCybernetics, Silver Spring, MD). For the quantitation, an arbitrary threshold was set to distinguish specific from background staining, and this same threshold setting was applied to all the samples analyzed.
Statistical analysis.
Unpaired parametric Student t test and non-parametric Mann-Whitney U tests were used to analyze the data, unless otherwise indicated in the text.
Results
The 1D11 anti-TGF-β monoclonal antibody suppresses metastasis of 4T1 cells to the lung.
First we evaluated whether the anti-TGF-β antibody could regulate metastasis of 4T1 cells in syngeneic BALB/c mice, following orthotopic implantation into the mammary fat pad (Fig. 1A). When initiated one day after tumor cell implantation, 1D11 treatment significantly reduced metastasis to the lung as evidenced by the decreased relative lung weight (Fig. 1B), and the reduction (∼2-fold) in number of macroscopically visible metastatic lung nodules in 1D11-treated mice as compared with the control group (Fig. 1C). Pathological examination confirmed that the macroscopic nodules represented lung metastases (data not shown). Taken together, these results clearly show that the anti-TGF-β antibody can suppress metastasis of 4T1 cells to the lungs from the orthotopic site.
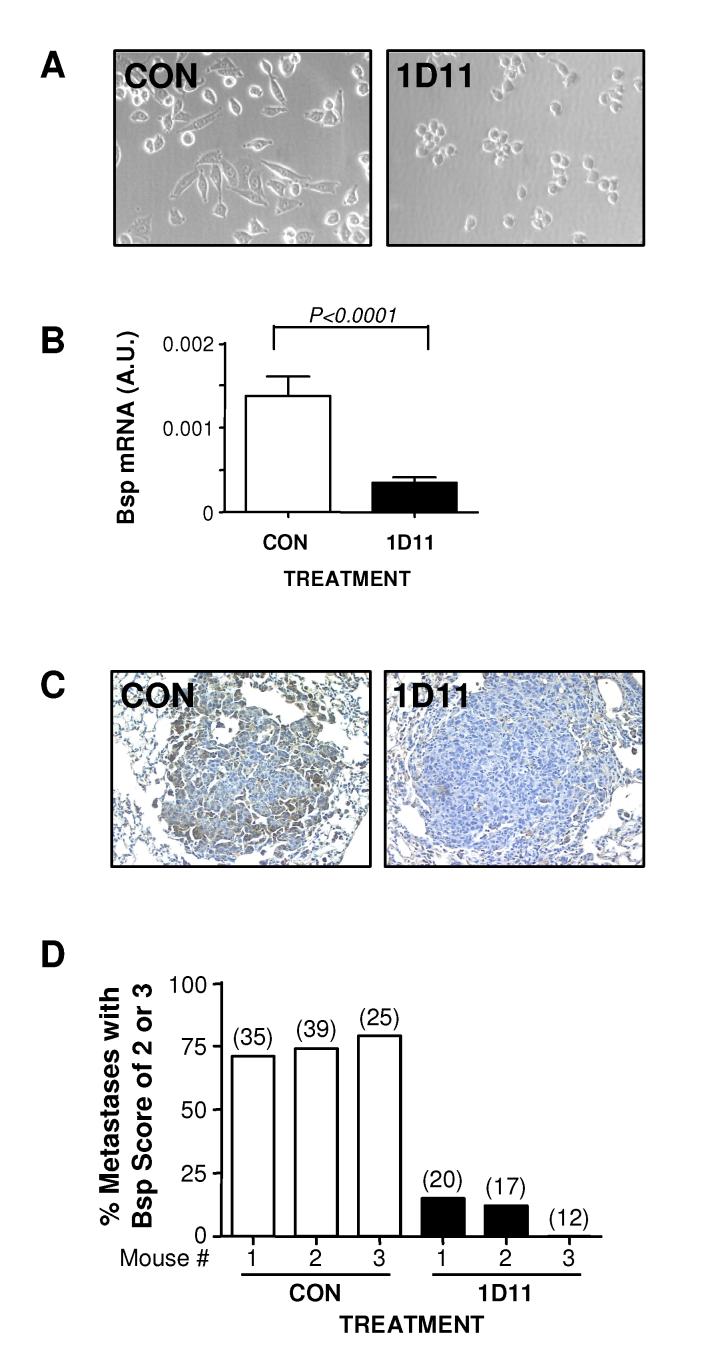
Figure 1. 1D11 decreases 4T1 metastasis to the lung following orthotopic implantation.
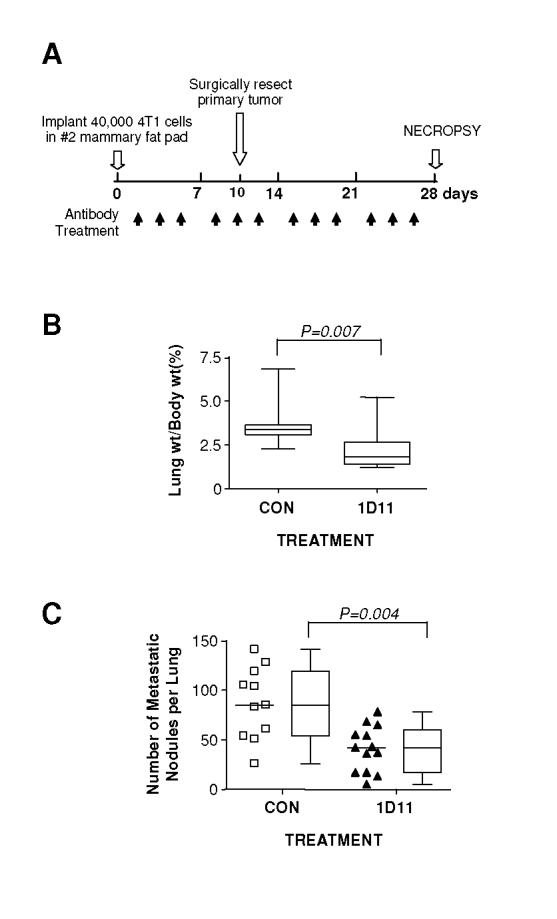
A. Experimental schema. 4T1 cells (4 X 104 cells) were inoculated into the left thoracic mammary fat pad of BALB/c mice. After the inoculation, either anti-TGF-β antibody 1D11 (5 mg/kg) or control 13C4 antibody, CON, (5 mg/kg) was administered three times per week i.p. to the mice. The primary tumors were removed on day 10 after the inoculation and mice were euthanized for necropsy on day 28. B. Relative lung weight at necropsy. C. Total number of grossly visible metastatic nodules in the lung. Boxes show median values with upper and lower quartiles, and whiskers indicate range (control group = 11 mice, 1D11 group = 13 mice).
Metastatic cell cultures recovered from 1D11-treated lungs show altered morphology and gene expression patterns.
Previous studies have shown that TGF-β can have pro-metastatic effects that target the tumor cell directly (6, 22). We hypothesized that part of the therapeutic efficacy of the TGF-β antibody might be due to changes in gene expression in the tumor cells themselves. Recovery of high quality RNA from lung metastases by laser capture microdissection has proven to be a major technical challenge, so instead we decided to recover and briefly culture metastatic cells from treated and untreated lungs prior to assay. To do this, we digested the tumor-bearing lungs with collagenase, and placed the resulting cells in culture under selection with 6-thioguanine to enrich for metastatic tumor cells, as the 4T1 cells are resistant to this drug (10). We observed morphological differences between metastatic cell cultures derived from 1D11-treated or control antibody-treated lungs, that persisted for up to 10 days in culture. Metastatic 4T1 cells cultured from the lungs of mice treated with 1D11 appeared rounder and less spindled, compared with their counterparts from animals treated with control antibody (Fig. 2A). In agreement with this observation, it has previously been shown that 4T1 cells treated with a murine TGF-β receptor:Fc fusion protein are less spindled in vivo when observed in a dorsal skin window assay (5).
Figure 2. Metastatic cells recovered from 1D11-treated lungs show persistent morphological changes in culture and elevated Bsp expression in vitro and in vivo.
4T1 cells were recovered from lungs harvested on day 28 as in Fig. 1 and cultured as described in Methods. A. Morphological appearance of the metastatic 4T1 cells cultured from the lungs of mice treated with anti-TGF-β (1D11) or control antibody (CON) after 7 days in culture. B. RTQ-PCR validation of differential expression of Bsp mRNA between metastatic cell cultures derived from treated and control mice. Values represent mean ± SD for 5 independent cell isolates/treatment group. Bsp mRNA expression was normalized to the 28S rRNA in each case. C. Immunohistochemical staining for Bsp in lung metastases from treated and control mice. D. Graphical representation of semi-quantitative scoring for Bsp expression in lung metastases. Individual metastases were scored for Bsp expression on a scale of 0-3 as detailed in methods. Data represent the % metastases with a score of ⩾ 2 for each of 3 mice/treatment group, for a total of 152 metastases evaluated. The number of metastases/mouse is indicated in parentheses above the bar.
Since the morphologic effects of anti-TGF-β antibody treatment in vivo persisted in the cultured cells for several days, we reasoned that gene expression changes induced by in vivo treatment might also persist. We therefore compared gene expression patterns of the metastatic cells from treated and untreated mice after 7 days in culture, using the Affymetrix GeneChip MOE430A. Forty genes differed at the p=0.001 level between cultures from 1D11 or CON treated mice (Supplemental Table 1). Of these, the most differentially expressed gene was bone sialoprotein (Bsp), also known as integrin binding sialoprotein (Ibsp), or bone sialoprotein II (BspII). Using RTQ-PCR, we confirmed a >3-fold reduction of Bsp mRNA level in metastatic 4T1 cells cultured from the lungs of mice treated with 1D11, as compared with their control counterparts (Fig. 2B). The other genes on the list had no known or proposed association with the metastatic process, and were not pursued further.
Bsp expression is reduced in 1D11-treated metastases in vivo.
BSP is a member of the SIBLING family of secreted integrin-binding proteins (23). It is overexpressed in many tumor types, including breast cancer, where overexpression is associated with clinical severity and poor survival (24). Overexpression of BSP in the human breast cancer cell line MDA-MB-231 has recently been shown to promote metastasis to bone and visceral organs (25). Thus BSP is a plausible candidate to mediate some of the prometastatic activity of TGF-β in breast cancer.
To investigate whether the observed decrease of Bsp mRNA expression in cultured metastatic cells reflected a loss of Bsp in the metastases in vivo, we immunostained lungs from treated and untreated mice. 1D11 treatment resulted in a substantial reduction in Bsp staining in the lung metastases, when compared with the control counterparts (Fig. 2C). Bsp expression was scored semiquantitatively on a scale of 0-3 (see Methods) for a total of 152 metastases in 6 mice, and the fraction of metastases with a score of 2 or 3 is shown for each animal (Fig. 2D). The difference between the two treatment groups was highly significant.
Bsp expression is regulated by TGF-β, and correlates with TGF-β expression and metastic potential in cells in vitro
TGF-β regulates Bsp expression in a rat osteosarcoma cell line by transcriptional and post-transcriptional mechanisms (26). Here we showed that TGF-β treatment increased the expression of Bsp mRNA in 4T1 cells in vitro, while treatment with a TGF-β receptor kinase inhibitor (ALK5 Inhibitor I) decreased it (Fig. 3A). The ability of the Alk5 inhibitor I to reduce basal Bsp expression in the absence of added TGF-β suggests that there is a functional TGF-β autocrine loop operating in the 4T1 cells. However, TGF-β treatment did not upregulate transcription from the a Bsp promoter-reporter construct containing a previously-described TGF-β response element, although it clearly enhanced transcription of a SMAD3-dependent CAGA12-luciferase reporter construct (Fig. 3B). Accordingly, these results suggest either that TGF-β upregulates Bsp in 4T1 cells in vitro exclusively through post-transcriptional mechanisms, or that any transcriptional response in the 4T1 cells involves a novel TGF-β regulatory element that is not present in the 1.4 kb of promoter sequence present in this construct.
Figure 3. TGF-β regulates and is correlated with Bsp expression in 4T1-related cell lines in vitro.
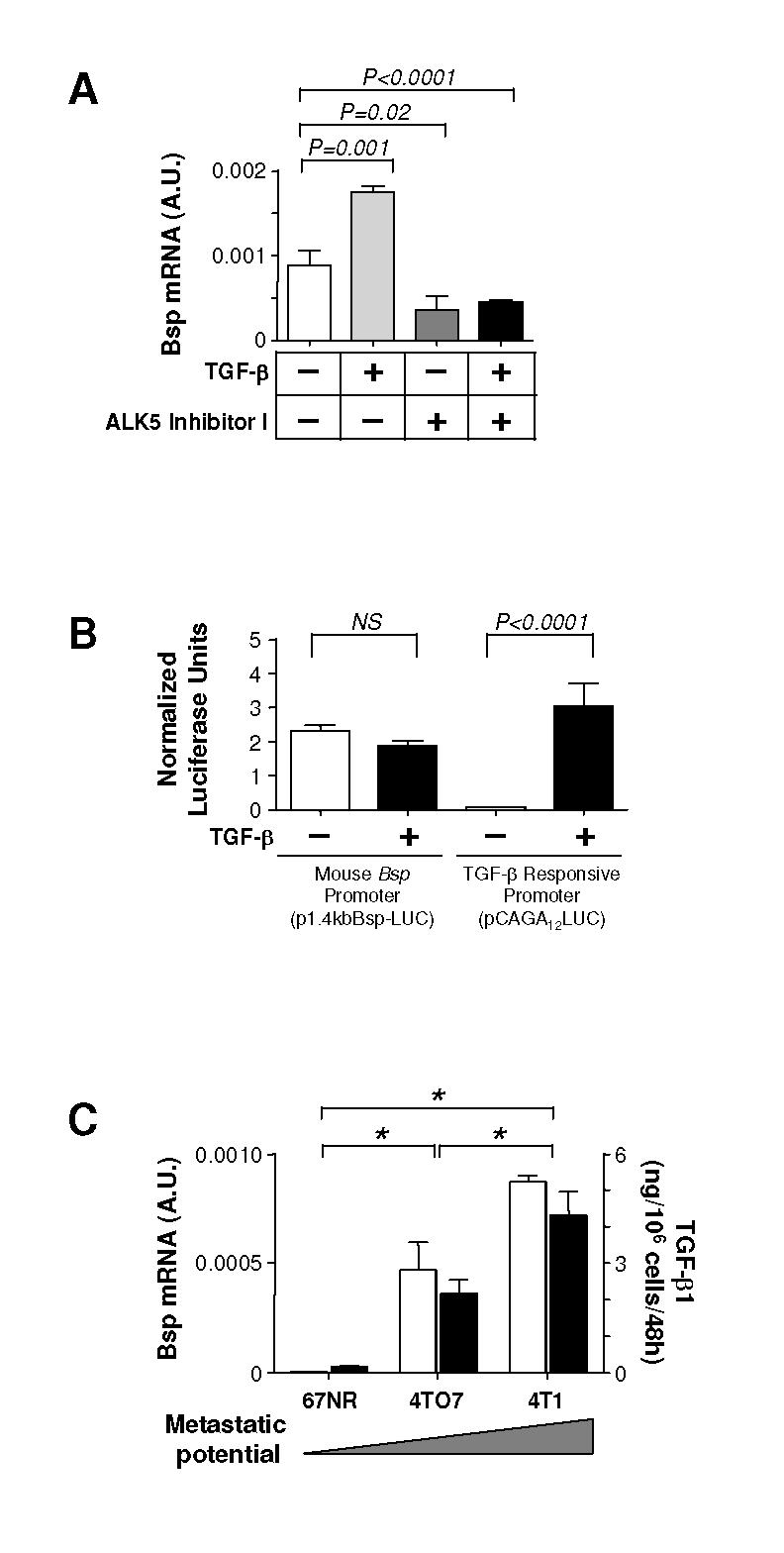
A. Effect of TGF-β1 or a TGF-β type I receptor kinase inhibitor on the expression of Bsp mRNA by 4T1 cells in vitro. Cells were exposed to either TGF-β1 (5 ng/ml) or the ALK5 inhbitor I (10 μM) for 48 hours prior to analyzing Bsp expression by RTQ-PCR. B. Effect of TGF-β treatment on transcription from the mouse Bsp promoter was assessed by measuring luciferase activity in 4T1 cells transiently transfected with promoter-reporter constructs. P1.4kbBsp-LUC is the mouse Bsp promoter-luciferase construct and pCAGA12LUC is a synthetic TGF-β-responsive promoter-luciferase construct. Cells were treated with 5 ng/ml TGF-μ1 for 18 hours prior to assay. NS, no significance. C. Correlation between endogenous Bsp and TGF-β expression. Bsp mRNA expression ([box1]) was determined by RTQ-PCR, and secreted total (latent plus active) TGF-β1 protein levels ([square7]) were determined by ELISA assay in a series of 4T1-related cells of increasing metastatic potential. *P < 0.001. All values represent the mean ± SD for 3 determinations.
We next examined Bsp and TGF-β expression in cell lines of increasing metastatic potential. 67NR, 4TO7, and 4T1 cell lines were derived from a single mammary tumor that arose spontaneously in a wild-type BALB/c mouse, and while they form primary tumors with similar kinetics, the cell lines differ dramatically in their metastatic potential (19). We observed a >100-fold increase of Bsp mRNA level in the 4TO7 cells, which can spread to but not colonize the lung, as compared with the nonmetastatic 67NR cells which do not leave the primary site. Bsp expression was further increased by ∼2-fold in the fully metastatic 4T1 cells (Fig. 3C). Interestingly, expression of total TGF-μ1 protein showed a parallel increase (Fig. 3C). Taken together, these results clearly show that TGF-β and Bsp expression correlate with each other, and with increasing metastatic ability, in this graded series of cell lines.
Bsp mediates TGF-β effects on invasion and collagen degradation in vitro
To address the possible importance of Bsp in mediating pro-metastatic activities of TGF-β, we developed siRNAs to reduce Bsp expression. We tested four siRNAs (siB1-4) designed from the mouse Bsp gene sequence. Overall, siB3 resulted in the greatest inhibition of Bsp mRNA expression (∼3-fold) and was selected for further study (Fig. 4A). Reduced Bsp expression persisted for at least 7 days in culture (data not shown). The expression of Bsp protein was also significantly suppressed by siB3, but not control siRNA (Fig. 4B). Knockdown of Bsp had no effect on proliferation or survival of the 4T1 cells in culture (data not shown).
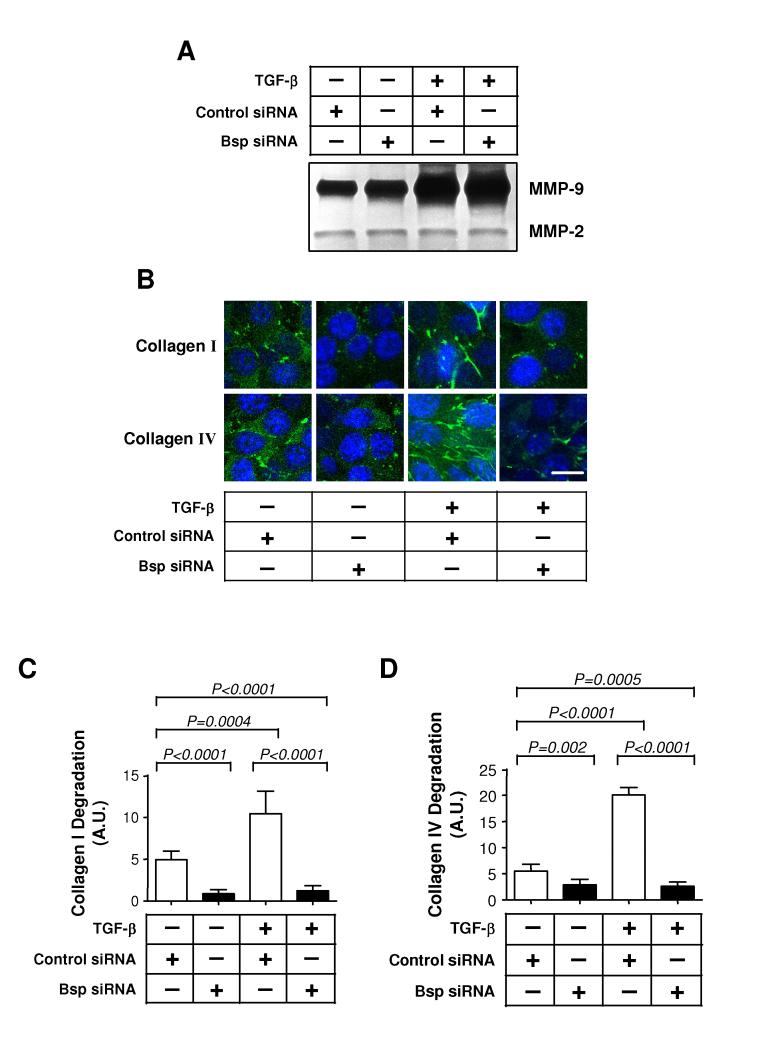
Figure 4. Bsp knockdown suppresses basal and TGF-β-induced invasion through Matrigel.
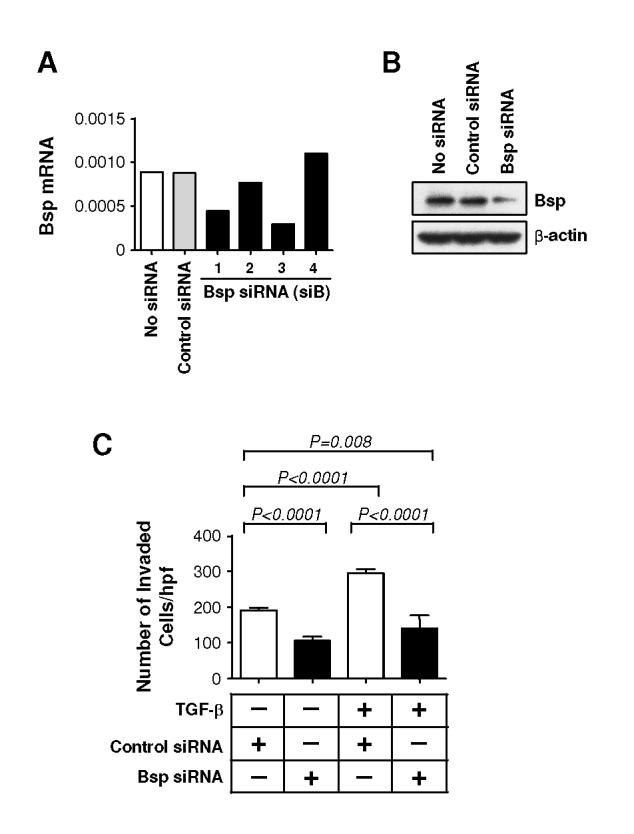
A. RTQ-PCR analysis of Bsp expression following transfection of 4T1 cells with four different siRNA sequences targeting Bsp. An siRNA to GFP was used as a negative control. B. Western blot analysis of Bsp protein levels in 4T1 cells 48 hours following transfection with the siB3 Bsp siRNA. C. Effect of Bsp knockdown on invasiveness of 4T1 cells. 4T1 cells transfected with siB3 were assessed for their ability to invade through Matrigel using a Transwell assay system, with and without added TGF-β1 (5 ng/ml). Results are the mean ± SD for 3 determinations.
BSP has been shown to promote the invasiveness of many cancer cell lines (27), and might therefore be a critical mediator of TGF-β-induced invasion. In a Matrigel invasion assay, addition of TGF-β caused in a 1.5-fold increase in invasiveness of 4T1 cells. Knockdown of Bsp significantly reduced both basal and TGF-β induced invasion to a similar level, suggesting that Bsp is necessary for the pro-invasive activity of both autocrine and exogenously added TGF-β (Fig. 4C).
TGF-β is thought to increase the invasiveness and metastatic behavior of cancer cells in part by stimulating the degradation of extracellular matrix (ECM) (28), and we did see a strong induction of MMP-9 on treatment of 4T1 cells with TGF-β (Fig. 5A, lanes 1 and 3). Members of the SIBLING family can interact with and activate specific matrix-metalloproteinases (MMPs) (29), an activity that is critical for BSP to promote invasion (27). We saw no effect of Bsp knockdown on the ability of TGF-β to induce pro-MMP synthesis and secretion as assayed by conventional zymography of cell supernatants, and there was no evidence for detectable activation of either MMP-2 or MMP-9 in the cell-conditioned medium or cellular lysates (Fig. 5A, and data not shown). However, we did see a significant impact of the loss of Bsp on local matrix degradation induced by TGF-β at the cell surface, as assessed by in-situ zymography using DQ collagen I and IV; presumably this level of MMP activation was below the detection limit of conventional zymography. Addition of TGF-β resulted in a >2-fold increase in local degradation of both collagen I and IV by 4T1 cells. Knockdown of Bsp significantly reduced local degradation of collagen both in the basal state and following TGF-β treatment (Fig. 5, B, C and D). Taken together, these results demonstrate that TGF-β induced expression of Bsp could play a critical role in increasing invasion of the cancer cell by stimulating local ECM degradation.
Figure 5. Bsp knockdown does not affect induction of MMPs by TGF-β, but does reduce local activation of matrix degrading enzymes.
A. MMP expression assessed by conventional zymography. The effect of Bsp knockdown on basal and TGF-β-induced MMP expression was determined by zymography on gelatin-impregnated polyacrylamide gels of conditioned medium harvested from cells, cultured on type I collagen, following treatment with 5 ng/ml TGF-β1 or vehicle for 48 hours. B. Effect of Bsp knockdown on local matrix degradation as assessed by in-situ zymography of cells grown within agarose impregnated with type I or type IV DQ collagen. 4T1 cells transfected with Bsp siRNA or control siRNA were treated with TGF-β1 for 48 hours and analyzed by confocal microscopy. Local matrix degradation is visualized by release of the green fluorophore. Cell nuclei are visualized by DAPI staining. Scale bar = 20 μm C and D. Quantitation of extracellular matrix degradation in the in-situ zymography assay. The intensity of green fluorescence, representing degraded collagen, was quantitated for 9 random high power fields and normalized to the number of cells within the field, determined by DAPI staining (Blue), as detailed in Methods. C, type I collagen matrix; D, type IV collagen matrix.
Bsp protects 4T1 cells from complement-mediated cell lysis.
Three members of the SIBLING family of proteins, including BSP, can confer short-range protection of tumor cells from complement-mediated attack (19, 30). Increasing concentrations of human serum led to decreased viability of the 4T1 cells, an effect that was lost if the serum was heat-treated to inactivate the complement (Fig. 6A). However, incubation of the cells with purified recombinant BSP prior to the addition of normal human serum preserved cell viability (Fig. 6B). Thus by inducing Bsp, TGF-β could promote metastasis both through enhanced invasion, and through evasion of lysis by the alternative complement pathway.
Figure 6. Bsp protects 4T1 cells from complement-mediated cell lysis.
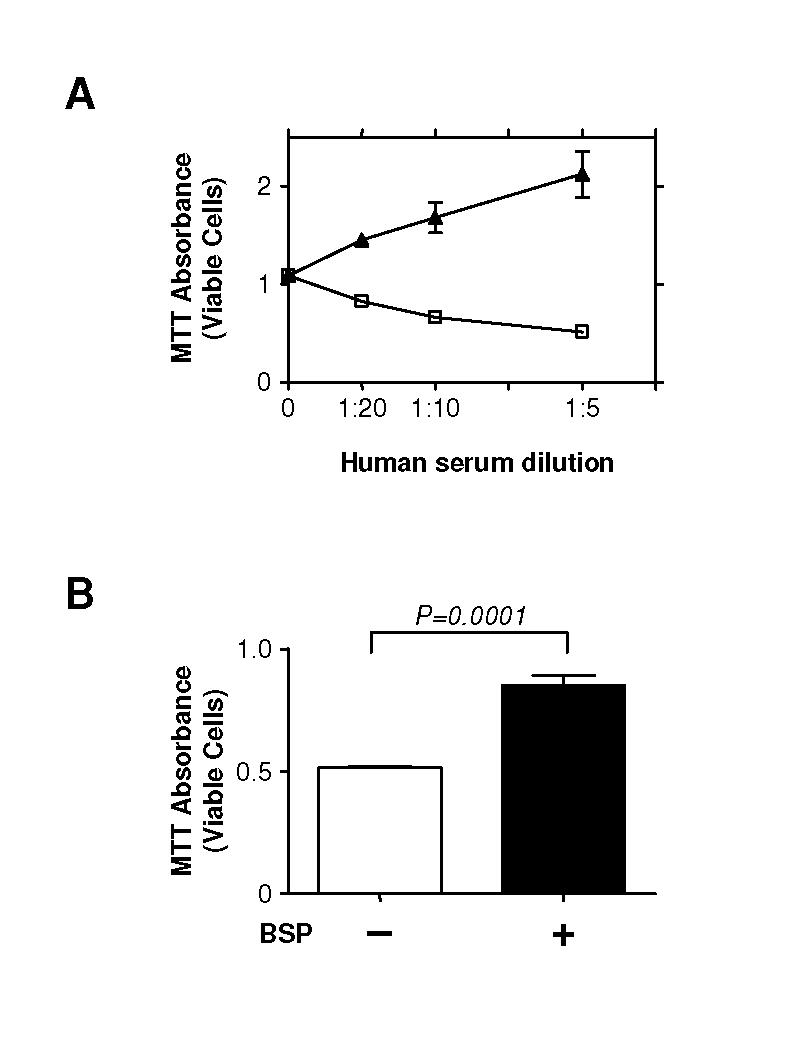
A. 4T1 cells are susceptible to complement-mediated cell lysis. 4T1 cells (5 X 106 cells/ml) were suspended in GVB-MgEGTA buffer and incubated with different concentrations of normal human serum, with ([triangleup]) or without ([box1]) prior heat treatment to inactivate the complement. After 2 hours, cell viability was evaluated by colorimetric MTT assay. B. Bsp protects against complement-mediated lysis. 4T1 cells were incubated with purified human BSP protein (10 μg/ml) for 10 minutes prior to incubation with normal human serum diluted 1:5 for the lysis assay as above. Results are the mean ± SD for 3 determinations.
Knockdown of Bsp reduces metastasis of 4T1 cells to the lung
Experimental overexpression of BSP can promote metastasis of the MDA-MB-231 human breast cancer cell line (25), but to date no one has shown that endogenous Bsp is important for visceral metastasis. In order to separate effects of Bsp on metastasis from any possible effects on the primary tumor, we addressed this question using the tail-vein injection (Fig 7A), rather than the orthotopic implantation route for introducing the 4T1 cells. In this format, fewer but larger metastases are formed than with orthotopic implantation, but treatment with 1D11 antibody still caused an ∼2-fold reduction in the number of metastases (Fig. 7B). Bsp knockdown significantly reduced the number of metastatic lung nodules, to an extent that was similar to that induced by treatment with 1D11 anti-TGF-β antibody (Fig. 7C).
Figure 7. Knockdown of Bsp suppresses metastasis of 4T1 cells to the lung.
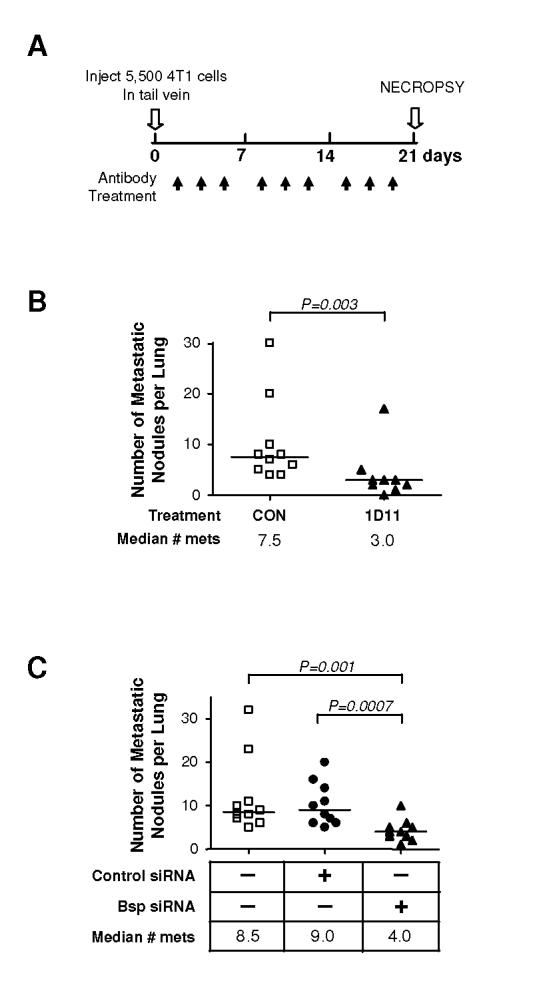
A. Experimental schema. 4T1 cells (5.5 X 103 cells) were inoculated into tail vein of BALB/c mice. Where relevant, either 1D11 (5 mg/kg) or control 13C4 antibody, CON, (5 mg/kg) was administered three times per week i.p., starting one day after cell innoculation. Mice were euthanized for necropsy on day 21. B. Effect of 1D11 on metastatic efficiency. Mice were injected with 4T1 cells and then treated with 1D11 or control antibody. Grossly visible lung metastases were quantitated at necropsy range (control group = 10 mice, 1D11 group = 9 mice). C. Effect of Bsp knockdown on metastatic efficiency. Mice were injected with parental 4T1 cells, or 4T1 cells that had been transfected with either Bsp siRNA or control siRNA. Metastases were assessed as in B. All experimental groups contained 10 mice. Median numbers of metastases for each group are indicated (n = 10 for each group).
Discussion
Many advanced human tumors markedly overexpress TGF-β, which allows the tumor cell to generate a more permissive stromal environment through paracrine mechanisms such as enhanced angiogenesis and reduced immune surveillance. However, due to the accumulation of genetic and epigenetic defects, in the later stages of the carcinogenic process the tumor cell itself can also respond to TGF-β in a manner that would promote progression, by reactivating developmentally inappropriate migratory and invasive programs. Therapeutic application of TGF-β antagonists could potentially suppress metastasis by interfering with either or both of these types of mechanisms. While it is clear that bulky TGF-β antagonists such as antibodies could readily interfere with the longer-range effects of tumor-derived TGF-β, it is less clear whether these agents would be able to impact on very short-range effects of TGF-β on the tumor cell itself. To address this question, we recovered metastatic cells from the lungs of tumor-bearing mice treated with an anti-TGF-β antibody, and looked for direct effects of the therapeutic intervention on the metastatic tumor cell.
Applying this approach to the 4T1 mouse model of metastatic breast cancer, we have shown for the first time that integrin-binding sialoprotein (Bsp) is an important mediator of the direct pro-metastatic effects of TGF-β on the tumor cell. In vivo, treatment with TGF-β antibody was associated with reduced expression of Bsp in lung metastases, and the functional signficance of this observation was confirmed when we showed that experimental knockdown of Bsp in the 4T1 cells significantly reduced their metastatic efficiency. Furthermore, Bsp and TGF-β expression were tightly correlated with each other, and with metastatic ability in a series of related breast cancer cell lines of differing metastatic potential, suggesting that TGF-β is a key regulator of Bsp expression during progression.
BSP is a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family of glycoproteins that also includes osteopontin (OPN). Both BSP and OPN are major constitutents of the non-collagenous matrix in skeletal tissues, where they play important roles in bone turnover (23), and both are regulated by TGF-β (26, 31). In addition to its role in bone physiology, OPN has recently been implicated as an important player in metastasis to multiple sites (32, 33), but BSP is less well studied in this regard. However, a large body of clinical data shows that BSP protein is overexpressed by many malignant tissues, including breast (34-39). Serum levels of BSP are increased in colon, breast and prostate cancer patients (16), and elevated serum BSP in primary breast cancer patients is prognostic for bony metastasis (40). Moreover, by in silico analysis of large clinical microarray studies of breast cancer (www.oncomine.org), we have found that BSP mRNA expression in the primary tumor increases with increasing tumor grade, as had been previously been shown in a smaller scale study (39), and furthermore that BSP mRNA levels correlate significantly with the presence of metastatic disease ((41, 42): Supplemental Fig. S1). These correlative clinical studies are all consistent with an important role for BSP in breast cancer metastasis. Indeed, forced overexpression of BSP in the MDA-MB231 human breast cancer cell line can enhance invasion and migration in vitro, and promote metastasis in vivo (25, 43), and anti-BSP antibody treatment can suppress the formation of osteolytic metastases by these cells (44). Our study is the first to demonstrate a role for endogenous Bsp in metastasis to visceral organs, and to place Bsp as a downstream mediator of TGF-β in this process.
Metastasis is a multi-step process, and Bsp appears to contribute to the prometastatic effects of TGF-β at more than one of these steps. Members of the SIBLING family bind to integrins on the cell surface and mediate formation of tertiary complexes with other effector molecules that then modulate cell behaviour. BSP, OPN and dentin matrix protein 1 can form tertiary complexes on the cell surface with αvβ3 integrin and specific metalloproteinases, thereby enhancing MMP activation (29). BSP specifically binds to and activates MMP-2, and was the only SIBLING that could enhance invasion in multiple cancer cell lines (27). TGF-βs are also thought to enhance invasiveness through a mechanism that is dependent on MMPs (28, 45). Here we have shown that the ability of TGF-β to promote invasion is completely dependent on the presence of Bsp. Knockdown of Bsp had no effect on the ability of TGF-β to enhance the synthesis or secretion of pro-MMPs, and it did not cause detectable activation of MMPs in cell culture medium. However, it did significantly reduce basal and TGF-β stimulated activation of the MMPs locally at the cell surface, as evidenced by a reduction in the degradation of type I or type IV collagen in the immediate vicinity of the cell. Thus the presence of Bsp is critical for TGF-β to cause local matrix degradation, a key step for invasion and metastasis.
BSP can also form a tertiary complex with αvβ3 integrin and complement Factor H (30). Metastasizing cells as they travel through the blood stream are exposed to the complement system, and must control complement activity on their surfaces so as to avoid direct lysis, opsonization or macrophage activation. The BSP-mediated sequestration of Factor H on the tumor cell surface can inhibit the anti-tumor effects of alternate complement pathway for several cell types (19), by activating Factor I and inhibiting the lytic pathway of complement (30). We showed that treatment with BSP can also protect 4T1 cells against complement-mediated cell lysis. Since Bsp must interact with the integrin prior to binding Factor H in order to be effective, this action of Bsp, like its effect on invasion, is likely to be very local (30). Thus there are at least two distinct mechanisms whereby locally increased levels of Bsp on the cell surface could mediate the promotion of metastasis by TGF-β.
BSP and OPN are thought to play particularly important roles in osteotropic metastasis. In the 4T1 model in the format in which we have used it, the predominant metastatic site is the lung, and the tumor burden in the bone is relatively low (data not shown), but our data clearly show that Bsp can significantly impact on metastatic efficiency to visceral sites in this model. We had hoped that our experimental approach might lead to identification of a circulating biomarker that would aid in the selection of patients for TGF-β antibody treatment, and allow monitoring of the efficacy of TGF-β antagonism. However, when we determined serum levels of Bsp in this model, they were not significantly increased by the presence of tumor (Supplemental Fig. S2). Others have found that BSP expression is lower in visceral metastases than in skeletal ones (46) and osteolytic bone metastases are likely to liberate additional BSP from the bone matrix, which may explain why increases in circulating BSP in breast cancer patients are only prognostic for bone metastases (40). In preliminary experiments using intracardiac injection of 4T1 cells, we have found that 1D11 is efficacious in reducing the burden of osteolytic bone metastases (JP and SL, unpublished data), and work is in progress to assess BSP status in this experimental setting. Thus it remains possible that TGF-β antibody treatment might impact on circulating BSP levels for patients with bone metastases, but nevertheless, it is clear that therapeutic benefit can be obtained even in settings where BSP is only locally increased in the tumor. Since BSP mRNA is elevated in metastatic compared with non-metastatic breast cancers (Supplemental Fig. S1B), BSP mRNA levels in the tumor might form a useful component of a patient stratification scheme even if circulating protein levels do not.
In summary, in this study we have identified Bsp as a novel mediator of the prometastatic effects of TGF-β on the tumor cell. Since Bsp expression in metastases is down-regulated by systemic treatment with anti-TGF-β antibodies, local suppression of Bsp in the tumor cell may contribute to the efficacy of this therapeutic approach. In other model systems, systemic antagonism of TGF-β has been shown to enhance immune surveillance and suppress angiogenesis (7, 8, 47). Treatment with anti-TGF-β antibodies enhanced the ability of dendritic cell-based vaccines to inhibit the growth of 4T1 primary tumors (48), so immune mechanisms are likely also to contribute to the anti-metastatic efficacy of TGF-β antagonism in the 4T1 model. Indeed, we have preliminary data suggesting that efficacy is at least partially dependent on the presence of CD8+ T-cells (J-SN and LW, unpublished observations). The relative contributions of the tumor- and stroma-targeted mechanisms are likely to vary among tumor types, depending on which steps in the metastatic cascade pose the biggest barrier to efficient metastasis, and the extent to which they are TGF-β-dependent. A more complete understanding of the molecular mediators of the various pro-metastatic effects of TGF-β should help guide the clinical application of TGF-β antagonists.
Supplementary Material
Acknowledgements
We would like to thank Dr. Miriam Anver for her pathology expertise, Dr. Mario Anzano and Jeff Nyswaner for excellent technical support in the animal husbandry, Drs. Seth Steinberg and David Venzon for help with the statistical analyses, and Drs. Glenn Merlino and Anita Roberts for critical reading of the manuscript. We thank Dr. Jaro Sodek for provision of the p1.4kbBsp-LUC plasmid, and for his insight and many helpful discussions. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. The National Cancer Institute has a Cooperative Research and Development Agreement with Genzyme Corporation.
References
- 1.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 2.Gold LI. The role for transforming growth factor-β (TGF-β) in human cancer. Crit Rev Oncog. 1999;10:303–60. [PubMed] [Google Scholar]
- 3.Wakefield LM, Roberts AB. TGF-β signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–9. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 4.Yang YA, Dukhanina O, Tang B, et al. Lifetime exposure to a soluble TGF-β antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–15. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muraoka RS, Dumont N, Ritter CA, et al. Blockade of TGF-β inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–9. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang B, Vu M, Booker T, et al. TGF-β switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116–24. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. Anti-transforming growth factor (TGF)-β antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-β interactions in human breast cancer progression. J Clin Invest. 1993;92:2569–76. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandyopadhyay A, Lopez-Casillas F, Malik SN, et al. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res. 2002;62:4690–5. [PubMed] [Google Scholar]
- 9.Rowland-Goldsmith MA, Maruyama H, Matsuda K, et al. Soluble type II transforming growth factor-β receptor attenuates expression of metastasis-associated genes and suppresses pancreatic cancer cell metastasis. Mol Cancer Ther. 2002;1:161–7. [PubMed] [Google Scholar]
- 10.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–405. [PubMed] [Google Scholar]
- 11.Lelekakis M, Moseley JM, Martin TJ, et al. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17:163–70. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- 12.Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor β2 affinity purification. J Immunol. 1989;142:1536–41. [PubMed] [Google Scholar]
- 13.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 14.Nam JS, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res. 2002;8:2430–6. [PubMed] [Google Scholar]
- 15.Fisher LW, Stubbs JT, III, Young MF. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995;266:61–5. [PubMed] [Google Scholar]
- 16.Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001;7:4060–6. [PubMed] [Google Scholar]
- 17.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu Q, Yamauchi M, Pageau SC, Chen JJ. Autoregulation of bone sialoprotein gene in pre-osteoblastic and non-osteoblastic cells. Biochem Biophys Res Commun. 2004;316:461–7. doi: 10.1016/j.bbrc.2004.02.068. [DOI] [PubMed] [Google Scholar]
- 19.Fedarko NS, Fohr B, Robey PG, Young MF, Fisher LW. Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. J Biol Chem. 2000;275:16666–72. doi: 10.1074/jbc.M001123200. [DOI] [PubMed] [Google Scholar]
- 20.Tester AM, Ruangpanit N, Anderson RL, Thompson EW. MMP-9 secretion and MMP-2 activation distinguish invasive and metastatic sublines of a mouse mammary carcinoma system showing epithelial-mesenchymal transition traits. Clin Exp Metastasis. 2000;18:553–60. doi: 10.1023/a:1011953118186. [DOI] [PubMed] [Google Scholar]
- 21.Stuelten CH, DaCosta BS, Arany PR, Karpova TS, Stetler-Stevenson WG, Roberts AB. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-α and TGF-β. J Cell Sci. 2005;May:15–118. doi: 10.1242/jcs.02334. [DOI] [PubMed] [Google Scholar]
- 22.McEarchern JA, Kobie JJ, Mack V, et al. Invasion and metastasis of a mammary tumor involves TGF-beta signaling. Int J Cancer. 2001;91:76–82. doi: 10.1002/1097-0215(20010101)91:1<76::aid-ijc1012>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–5. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 24.Bellahcene A, Menard S, Bufalino R, Moreau L, Castronovo V. Expression of bone sialoprotein in primary human breast cancer is associated with poor survival. Int J Cancer. 1996;69:350–3. doi: 10.1002/(SICI)1097-0215(19960822)69:4<350::AID-IJC19>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Sharp JA, Waltham M, Williams ED, Henderson MA, Thompson EW. Transfection of MDA-MB-231 human breast carcinoma cells with bone sialoprotein (BSP) stimulates migration and invasion in vitro and growth of primary and secondary tumors in nude mice. Clin Exp Metastasis. 2004;21:19–29. doi: 10.1023/b:clin.0000017167.17065.61. [DOI] [PubMed] [Google Scholar]
- 26.Ogata Y, Niisato N, Furuyama S, et al. Transforming growth factor-β 1 regulation of bone sialoprotein gene transcription: identification of a TGF-β activation element in the rat BSP gene promoter. J Cell Biochem. 1997;65:501–12. doi: 10.1002/(sici)1097-4644(19970615)65:4<501::aid-jcb6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.Karadag A, Ogbureke KU, Fedarko NS, Fisher LW. Bone sialoprotein, matrix metalloproteinase 2, and αvβ3 integrin in osteotropic cancer cell invasion. J Natl Cancer Inst. 2004;96:956–65. doi: 10.1093/jnci/djh169. [DOI] [PubMed] [Google Scholar]
- 28.Welch DR, Fabra A, Nakajima M. Transforming growth factor β stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci U S A. 1990;87:7678–82. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedarko NS, Jain A, Karadag A, Fisher LW. Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J. 2004;18:734–6. doi: 10.1096/fj.03-0966fje. [DOI] [PubMed] [Google Scholar]
- 30.Jain A, Karadag A, Fohr B, Fisher LW, Fedarko NS. Three SIBLINGs (small integrin-binding ligand, N-linked glycoproteins) enhance factor H’s cofactor activity enabling MCP-like cellular evasion of complement-mediated attack. J Biol Chem. 2002;277:13700–8. doi: 10.1074/jbc.M110757200. [DOI] [PubMed] [Google Scholar]
- 31.Noda M, Yoon K, Prince CW, Butler WT, Rodan GA. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type β transforming growth factor. J Biol Chem. 1988;263:13916–21. [PubMed] [Google Scholar]
- 32.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 34.Bellahcene A, Merville MP, Castronovo V. Expression of bone sialoprotein, a bone matrix protein, in human breast cancer. Cancer Res. 1994;54:2823–6. [PubMed] [Google Scholar]
- 35.Waltregny D, Bellahcene A, Van Riet I, et al. Prognostic value of bone sialoprotein expression in clinically localized human prostate cancer. J Natl Cancer Inst. 1998;90:1000–8. doi: 10.1093/jnci/90.13.1000. [DOI] [PubMed] [Google Scholar]
- 36.Bellahcene A, Maloujahmoum N, Fisher LW, et al. Expression of bone sialoprotein in human lung cancer. Calcif Tissue Int. 1997;61:183–8. doi: 10.1007/s002239900320. [DOI] [PubMed] [Google Scholar]
- 37.Bellahcene A, Albert V, Pollina L, Basolo F, Fisher LW, Castronovo V. Ectopic expression of bone sialoprotein in human thyroid cancer. Thyroid. 1998;8:637–41. doi: 10.1089/thy.1998.8.637. [DOI] [PubMed] [Google Scholar]
- 38.Riminucci M, Corsi A, Peris K, Fisher LW, Chimenti S, Bianco P. Coexpression of bone sialoprotein (BSP) and the pivotal transcriptional regulator of osteogenesis, Cbfa1/Runx2, in malignant melanoma. Calcif Tissue Int. 2003;73:281–9. doi: 10.1007/s00223-002-2134-y. [DOI] [PubMed] [Google Scholar]
- 39.Fisher LW, Jain A, Tayback M, Fedarko NS. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10:8501–11. doi: 10.1158/1078-0432.CCR-04-1072. [DOI] [PubMed] [Google Scholar]
- 40.Diel IJ, Solomayer EF, Seibel MJ, et al. Serum bone sialoprotein in patients with primary breast cancer is a prognostic marker for subsequent bone metastasis. Clin Cancer Res. 1999;5:3914–9. [PubMed] [Google Scholar]
- 41.van ’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 42.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JH, Tang J, Wang J, et al. Over-expression of bone sialoprotein enhances bone metastasis of human breast cancer cells in a mouse model. Int J Oncol. 2003;23:1043–8. [PubMed] [Google Scholar]
- 44.Bauerle T, Adwan H, Kiessling F, Hilbig H, Armbruster FP, Berger MR. Characterization of a rat model with site-specific bone metastasis induced by MDA-MB-231 breast cancer cells and its application to the effects of an antibody against bone sialoprotein. Int J Cancer. 2005;115:177–86. doi: 10.1002/ijc.20840. [DOI] [PubMed] [Google Scholar]
- 45.Kim ES, Kim MS, Moon A. Transforming growth factor (TGF)-β in conjunction with H-ras activation promotes malignant progression of MCF10A breast epithelial cells. Cytokine. 2005;29:84–91. doi: 10.1016/j.cyto.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Waltregny D, Bellahcene A, de L,X, Florkin B, Weidle U, Castronovo V. Increased expression of bone sialoprotein in bone metastases compared with visceral metastases in human breast and prostate cancers. J Bone Miner Res. 2000;15:834–43. doi: 10.1359/jbmr.2000.15.5.834. [DOI] [PubMed] [Google Scholar]
- 47.Bandyopadhyay A, Zhu Y, Malik SN, et al. Extracellular domain of TGFβ type III receptor inhibits angiogenesis and tumor growth in human cancer cells. Oncogene. 2002;21:3541–51. doi: 10.1038/sj.onc.1205439. [DOI] [PubMed] [Google Scholar]
- 48.Kobie JJ, Wu RS, Kurt RA, et al. Transforming growth factor β inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.