Abstract
Gene targeting through homologous recombination in murine embryonic stem (ES) cells is already strongly suppressed by DNA mismatch-repair (MMR)-dependent anti-recombination when targeting construct and target locus differ at <1% of the nucleotide positions. We demonstrate that MMR activity also raises a strong impediment to gene modification mediated by small synthetic DNA oligonucleotide sequences. In the absence of the DNA MMR gene MSH2, synthetic single-stranded deoxyribo-oligonucleotides can be used to site-specifically modify the ES cell genome. We show that PCR-based procedures can be used to identify and clone modified cells. By this method we have substituted a single codon in the retinoblastoma gene.
INTRODUCTION
Gene targeting in embryonic stem (ES) cells is widely used to introduce specific genetic modifications into the mouse germline (1). In its simplest form, the technology involves the generation of a so-called targeting construct in which a selectable marker gene (e.g. neo, hyg, pur) is flanked by DNA sequences that are largely identical to the specific chromosomal locus to be modified. On entry of the targeting construct into the cell, homologous recombination between the flanking sequences and their chromosomal counterparts will result in the integration of the marker gene into the chromosome, thereby disrupting the gene of interest (2). In addition, protocols have been developed to introduce subtle modifications as small as the insertion, deletion or substitution of a single base pair. For example, in a two-step protocol, homologous recombination is used to introduce the subtle mutation into the gene of interest concomitantly with a dominantly/negatively selectable marker gene, the latter subsequently being removed via intra-chromosomal homologous or Cre/lox-mediated site-specific recombination (3,4). An alternative approach to introduce subtle gene modifications may be the use of synthetic single-stranded DNA oligonucleotides, as has been proven successful in the yeast Saccharomyces cerevisiae (5). Single-stranded oligonucleotides have also been used in human cells to modify an episomally-located gene; however, this approach has not been followed up by targeting of chromosomally-located genes (6). Instead, oligonucleotide-directed gene modification in mammalian cells has made use of chemically-modified single-stranded oligonucleotides, chimeric RNA/DNA oligonucleotides or triple-helix-forming oligonucleotides (7–10), all containing phosphorothioate linkages or 2′-O-methyl-RNA residues. While the enhanced resistance of these oligonucleotides to intracellular nucleolytic degradation has been considered critical to the success of these approaches (11), the mechanism of transfer of genetic information from the oligonucleotide to the target remains largely elusive. Somewhat surprisingly, no reports have appeared describing the successful use of chemically-modified oligonucleotides in ES cells.
We have previously demonstrated that the efficiency of gene targeting in ES cells is strongly suppressed by DNA mismatch-repair (MMR)-dependent anti-recombination, which is already activated by sequence dissimilarities between targeting construct and target locus as small as 0.6% (12,13). We reasoned that this phenomenon could also raise a strong impediment to gene modification mediated by small synthetic DNA oligonucleotide sequences which differ from the target locus by one or a few nucleotides. To test this hypothesis, we have introduced into MMR-proficient and -deficient ES cells [the latter carrying a homozygous disruption in the central MMR gene Msh2 (13)] a recombination reporter construct consisting of a neo gene that was rendered inactive by either a two base pair insertion disrupting the open reading frame or a base pair substitution in the start codon. We then investigated whether these mutations could be restored by sequence-specific single-stranded deoxyribo-oligonucleotides.
MATERIALS AND METHODS
The neo gene was essentially derived from plasmid pMC1neo (14) in which the sequence around the start codon (between the PstI site and the fifth codon) was replaced by sequences depicted in Figure 1b and c (target 1 and 2, respectively). The Rosa26-targeting construct consisted of a 13 kb Rosa26 fragment (15) (J.-H.Dannenberg, personal communication) carrying a promoterless histidinol-resistance gene with an upstream splice acceptor site inserted into the first intron. Defective neo genes were placed immediately downstream of his. Targeting vectors were introduced into ES cells by electroporation as described (12). Over 90% of histidinol-resistant clones (selected in 2.0 mM histidinol) were the result of homologous recombination at Rosa26 without additional random integrations as verified by Southern blotting.
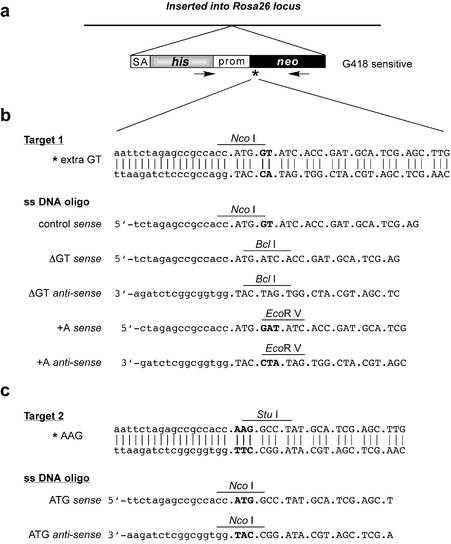
Figure 1.
A selectable target for oligonucleotide-directed gene modification. (a) Single-copy integration of a defective neo gene into the Rosa26 locus. The targeting vector contains a promoterless histidinol-resistance gene (his) preceded by a splice acceptor site (SA); the neo gene is driven by the MC1 promoter (prom). The asterisk indicates the position of an inactivating mutation in neo. The arrows represent PCR primers used to amplify a diagnostic 1200 bp fragment. (b) Target 1 contains a GT frameshift mutation immediately downstream of the start codon. This mutation was restored by deletion of GT using single-stranded DNA oligonucleotides ΔGT sense and ΔGT anti-sense, or insertion of A using oligonucleotides +A sense and +A anti-sense. Restoration of neo leads to loss of NcoI and gain of BclI (ΔGT) or to gain of EcoRV (+A). Lower-case letters represent non-coding bases. (c) Target 2 contains a T to A point mutation in the start codon. AAG was restored to ATG using oligonucleotides ATG sense and ATG anti-sense leading to loss of StuI and gain of NcoI.
Deoxyribo-oligonucleotides were obtained from Sigma-Genosys Ltd. For oligo-targeting experiments, cells were seeded in 6-well plates at a density of 7 × 105 per well. The next day, cells were exposed for 1 h to 1.4 ml of serum-free medium containing 3–7 µg of oligonucleotide plus Tfx™-50 (31.5 µl) or TransFast™ (63 µl) lipofection reagent (Promega Corporation). After addition of 4 ml of medium plus serum, cells were incubated overnight. The next day, cells were counted and replated in selective medium containing 400 μg (target 1 cells) or 600 µg (target 2 cells) of G418. After 8–10 days, G418-resistant colonies were counted. For DNA extraction, colonies were picked and expanded in 96-well plates. DNA was analyzed by PCR as described in the legend to Figure 2 (primer sequences are available upon request).
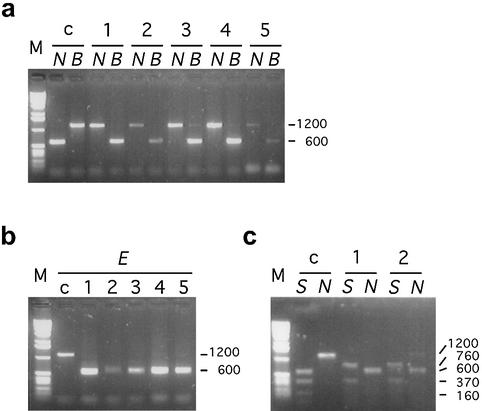
Figure 2.
Confirmation of oligonucleotide-mediated restoration of neo by restriction site analyses. DNA from target 1 or target 2 cells and G418-resistant clones obtained by exposure to DNA oligonucleotides was amplified using the primer pair indicated in Figure 1a. The resulting 1200-bp fragments were digested with diagnostic restriction enzymes. (a) The 1200 bp fragment of target 1 cells (lane c) contains NcoI (giving two 600-bp fragments) and lacks BclI. G418-resistant clones resulting from exposure to ΔGT sense (lanes 1–5) have lost NcoI and gained BclI. (b) The 1200 bp fragment of target 1 cells (lane c) lacks an EcoRV site. G418-resistant clones resulting from exposure to +A sense (lanes 1–5) have gained an EcoRV site. (c) The 1200 bp fragment of target 2 cells (lane c) contains a StuI site at the AAG mutation and several others upstream (giving a 600 bp fragment and several smaller fragments) and lacks NcoI. G418-resistant clones resulting from exposure to ATG sense (lanes 1 and 2) have lost the diagnostic 600 bp StuI fragment and gained a central NcoI site. B, BclI; E, EcoRV; N, NcoI; S, StuI.
Rb-1 mRNA was amplified by reverse transcription using a primer in exon 22, followed by PCR using primers in exons 19 and 20. The exon 19 primer was used for sequencing (primer sequences are available upon request).
RESULTS
Oligonucleotide-mediated deletion of two base pairs
To generate a selectable target for oligonucleotide-directed gene modification (‘oligo targeting’), we constructed a defective neo gene (neos) by inserting a GT frameshift mutation immediately downstream of the start codon (target 1, Fig. 1b). The neos allele was placed adjacent to a histidinol-resistance gene into a gene-targeting vector that allowed single-copy integration at the same chromosomal locus (Rosa26) in MMR-proficient and -deficient (Msh2–/–) (13) ES cells (Fig. 1a). To restore the neo open-reading frame in target 1, a single-stranded oligonucleotide sequence was used, consisting of 35 (non-chemically-modified) deoxyribonucleotides and differing from the target locus by the absence of the GT dinucleotide (ΔGT sense, Fig. 1b). Oligonucleotides were introduced into ES cells by liposome-mediated DNA transfer and cells were selected for restoration of neo in G418-containing medium. Tables 1 and 2 show the results of several experiments demonstrating that G418-resistant colonies were readily obtained with ΔGT sense but not with a control oligonucleotide carrying the GT frameshift mutation (control sense, Fig. 1b). Strikingly, G418-resistant colonies were only obtained in Msh2-deficient ES cells but not in wild-type cells, indicating that MMR imposes a strong barrier to oligonucleotide-mediated gene modification. To verify whether restoration of neo activity was indeed the result of deletion of the GT frameshift mutation, DNA from target 1 cells and G418-resistant clones was amplified by PCR using the primer pair indicated in Figure 1a. The resulting 1200 bp DNA fragments were analyzed for the presence of NcoI (indicative of the neos allele) and BclI (indicative of reactivation of neo by deletion of GT) restriction sites. G418 resistance corresponded in all cases with loss of NcoI and gain of BclI (Fig. 2a).
Table 1. Oligonucleotide-mediated correction of a GT frameshift mutation in Msh2–/– cells.
| Oligonucleotide | No. of G418-resistant colonies | |||
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | |
| ΔGT sense | 22 | 36 | 37 | 0 |
| ΔGT anti-sense | 60 | 24 | 39 | ND |
| ΔGT ds | 1 | 1 | ND | ND |
| Control sense | NDa | 0 | 0 | ND |
ES cells were plated at 7 × 105 cells/10 cm2. The next day cells were exposed for 1 h to lipofection medium consisting of 1.4 ml of medium (without serum), 7 µg of DNA plus 31.5 µl Tfx-50 (Experiment 1); 21 µl Transfast (Experiment 2); 63 µl Transfast (Experiment 3); without lipofection reagent (Experiment 4). After addition of 4 ml of serum-containing medium, cells were incubated overnight. The next day, cells were trypsinized, plated on 10 cm plates and grown in the presence of G418 (400 µg/ml). Colonies were counted after 8–10 days.
aND, not determined.
Table 2. Oligonucleotide-mediated correction of a GT frameshift mutation in wild-type cells.
| Oligonucleotide | No. of G418-resistant colonies | |||
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | |
| ΔGT sense | 0 | 0 | 0 | 0 |
| ΔGT anti-sense | NDa | 0 | 0 | ND |
See footnote to Table 1.
Tables 1 and 2 also show that ΔGT sense and the complementary sequence ΔGT anti-sense (Fig. 1b) performed equally well in generating G418-resistant colonies. However, when both sequences were allowed to anneal prior to introduction into cells (ΔGT ds), only a few G418-resistant colonies were obtained, indicating that gene modification can be mediated by single- but not double-stranded DNA oligonucleotides.
To optimize the efficiency of oligo targeting, we varied the length of the oligonucleotide sequence (Table 3), the amount of oligonucleotide (Table 4), the number of lipofected cells, the duration of exposure to DNA/lipofection reagent and the effect of chemical modifications in the oligonucleotide (Table 5). In order to better compare the efficiency of oligo targeting for different protocols, we counted the number of cells that had survived the lipofection procedure and were reseeded into selective medium. The length of the oligonucleotide did not strongly affect the frequency of gene modification: Table 3 shows that ΔGT sense oligonucleotides of 35, 47 or 60 residues were almost equally effective, while a sequence of 20 residues was slightly less effective. Taken together these experiments revealed that a 1 h exposure of 7 × 105 cells to 3 µg of a 35 nt sequence gave an optimal targeting frequency of 2.6 per 105 cells (Tables 4 and 5, and results not shown). The actual oligo-targeting frequency may be somewhat higher as the plating efficiency of ES cells in these experiments was ∼20–50% (not shown). Strikingly, the presence of phosphorothioate linkages or 2′-O-methyl-RNA residues decreased the efficiency of oligo targeting (Table 5).
Table 3. Optimization of oligo-targeting protocol using target 1: effect of oligonucleotide length.
| Oligonucleotide | No. of cells plateda | No. of G418-resistant colonies | Efficiencyb (/105) | |
|---|---|---|---|---|
| ΔGT sense | 20 nt | 2.3 × 106 | 24 | 1.0 |
| ΔGT sense | 35 nt | 2.2 × 106 | 42 | 1.9 |
| ΔGT sense | 47 nt | 2.3 × 106 | 45 | 2.0 |
| ΔGT sense | 60 nt | 2.4 × 106 | 40 | 1.7 |
7 × 105 Msh2–/– ES cells/10 cm2 were exposed for 1 h to 7 µg of oligonucleotide.
aCells were counted prior to reseeding in selective medium.
bNo. of G418-resistant cells per no. of cells plated.
Table 4. Optimization of oligo-targeting protocol using target 1: effect of amount of oligonucleotide.
| Oligonucleotide | µg | No. of cells plateda | No. of G418-resistant colonies | Efficiencyb (/105) |
|---|---|---|---|---|
| ΔGT anti-sense | 3 | 1.7 × 106 | 44 | 2.6 |
| ΔGT anti-sense | 7 | 2.2 × 106 | 39 | 1.8 |
| ΔGT anti-sense | 14 | 2.5 × 106 | 36 | 1.4 |
| ΔGT anti-sense | 28 | 1.8 × 106 | 27 | 1.5 |
7 × 105 Msh2–/– ES cells/10 cm2 were exposed for 1 h to 3, 7, 14 or 28 µg of oligonucleotide plus Transfast (27, 63, 128 or 256 µl, respectively).
aCells were counted prior to reseeding in selective medium.
bNo. of G418-resistant cells per no. of cells plated.
Table 5. Optimization of oligo-targeting protocol using target 1: effect of chemical modifications.
| Oligonucleotide | No. of cells plateda | No. of G418-resistant colonies | Efficiencyb (/105) |
|---|---|---|---|
| ΔGT sense | 2.8 × 106 | 72 | 2.6 |
| ΔGT sense-U | 2.9 × 106 | 34 | 1.2 |
| ΔGT sense-S | 2.8 × 106 | 21 | 0.75 |
| ΔGT sense-SS | 2.7 × 106 | 10 | 0.37 |
7 × 105 Msh2–/– ES cells/10 cm2 were exposed for 1 h to 3 µg of oligonucleotide plus 27 µl of Transfast. ΔGT sense-U contains three 2′-O-methyl-uracil residues added to its 5′ end; ΔGT sense-S contains three phosphorothioate linkages at its 5′ end; ΔGT sense-SS contains three phosphorothioate linkages at its 5′ end and three at its 3′ end.
aCells were counted prior to reseeding in selective medium.
bNo. of G418-resistant cells per no. of cells plated.
Insertion and substitution of a single base pair
Instead of deleting the GT dinucleotide, the neo open-reading frame in target 1 could also be restored by inserting a single A between G and T using +A sense and anti-sense oligonucleotides (Fig. 1b). The two oligonucleotides performed equally well, giving G418-resistant colonies at a frequency of 1/105 (Table 6). In all cases tested, G418 resistance was associated with the gain of an EcoRV restriction site in the diagnostic 1200 bp PCR fragment (Fig. 2b). Correction of target 1 by +A sense was, similar to ΔGT sense, extremely inefficient in wild-type cells (Table 7). Thus, MSH2 activity suppressed the efficiency of oligo targeting ∼100-fold.
Table 6. Oligonucleotide-mediated GT→GAT using target 1.
| Oligonucleotide | No. of cells plateda | No. of G418-resistant colonies | Efficiencyb (/105) |
|---|---|---|---|
| +A sense | 2.5 × 106 | 27 | 1.1 |
| +A anti-sense | 2.3 × 106 | 26 | 1.1 |
7 × 105 Msh2–/– ES cells/10 cm2 were exposed for 1 h to 7 µg of oligonucleotide plus Transfast (63 µl).
aCells were counted prior to reseeding in selective medium.
bNo. of G418-resistant cells per no. of cells plated.
Table 7. Oligonucleotide-mediated GT correction in wild-type cells.
| Oligonucleotide | No. of cells lipofected | No. of cells plateda | No. of G418-resistant colonies | Efficiencyb (/107) |
|---|---|---|---|---|
| ΔGT sense | 4.2 × 106 | 2.0 × 107 | 1 | 0.5 |
| +A sense | 4.2 × 106 | 2.2 × 107 | 3 | 1.4 |
4.2 × 106 wild-type ES cells on a 6 well plate (6 × 10 cm2) were exposed for 1 h to 7 µg of DNA plus Transfast (63 µl) per well.
aCells were counted prior to reseeding in selective medium.
bNo. of G418-resistant cells per no. of cells plated.
To study oligonucleotide-mediated base substitution, we generated target 2 carrying a point mutation in the start codon: AAG (Fig. 1c). ATG sense and anti-sense oligonucleotides (Fig. 1c) performed equally well in restoring neo activity (Table 8), which in all cases tested was accompanied by loss of StuI and gain of NcoI restriction sites (Fig. 2c).
Table 8. Oligonucleotide-mediated AAG→ATG using target 2.
| Oligonucleotide | No. of cells plateda | No. of G418-resistant colonies | Efficiencyb (/105) |
|---|---|---|---|
| ATG sense | 4.3 × 106 | 53 | 1.2 |
| ATG anti-sense | 4.4 × 106 | 61 | 1.4 |
| PBS | 4.5 × 106 | 0 | 0 |
See footnote to Table 6.
Oligo targeting without selection
We next tested whether the efficiency of oligo targeting was sufficiently high to identify cells that had undergone oligonucleotide-directed gene modification without applying positive selection. Msh2–/–/target 1 ES cells exposed to ΔGT sense were seeded into two 96-well plates at a density of 1000 cells per well. Cells were cultured in non-selective medium and then split into new wells for further growth in non-selective or G418-containing medium. DNA was extracted from cell pools grown in non-selective medium and subjected to the PCR protocol depicted in Figure 3a. Primers 1 and 2 were used to amplify a 1200 bp fragment that was subjected to a second PCR amplification round using the nested primers 3 and 4. Primer 4 is specific for the planned modification giving an 82 bp PCR product, while non-modified DNA would not give a product due to improper annealing of the two 3′ nucleotides of primer 4. Efficient amplification of an 82 bp fragment was found in 8 out of 192 wells (Fig. 3b). In each case, efficient amplification corresponded to the presence of G418-resistant cells in parallel pools grown under selective conditions (Table 9), thereby validating this procedure to identify pools in which part of the cells contained the planned modification. Repeated rounds of this procedure will readily lead to enrichment and finally clonal purification of the oligonucleotide-mediated modification, alleviating the need for a selectable phenotype.
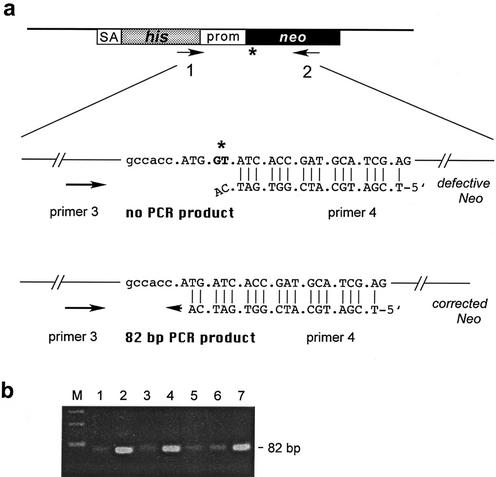
Figure 3.
Procedure to identify oligo-targeted cells without selection. (a) Primers 1 and 2 were used to amplify 1200-bp fragments from pools of cells, which were subsequently used as templates in a second PCR round using the nested primers 3 and 4. Primer 4 is specific for the planned modification giving an 82 bp fragment. (b) Efficient amplification of the 82 bp fragment was observed in pools 2, 4 and 7, indicating the presence of modified cells.
Table 9. Oligonucleotide-mediated GT correction in Msh2–/– cells.
| Oligonucleotide | No. of cells plated | No. of wells with G418-resistant cells | Efficiencya (/105) |
|---|---|---|---|
| ΔGT sense | 2 × 96-well plates | 8 | 4.2 |
| 1000 cells/well |
7 × 105 Msh2–/– ES cells were exposed for 1 h to 7 µg of DNA plus Transfast (63 µl). Cells were seeded into two 96-well plates at a density of 1000 cells/well. After 2 days, cells in each well were split into two new wells: one was used for DNA isolation and one for growth in selective medium.
aNo. of G418-resistant cells per no. of cells plated.
We used this procedure to substitute a single codon CGA for TGG in the retinoblastoma gene Rb-1 (Fig. 4a). Msh2–/– ES cells were exposed to the single-stranded deoxyribo-oligonucleotide Rb-TGG and seeded into 96-well plates at a density of 1000 cells per well. Cells were cultured and each well was split in two. With PCR primers specific for the planned modification (Fig. 4a), we identified two wells giving a strong PCR product and eight giving a weaker signal out of 192 wells. Cells from the strongly positive wells were reseeded in a 96 well plate at a density of 100 cells per well. After culturing, two positive wells were identified (Fig. 4b and c). From these wells, single cell clones were prepared yielding cell cultures that were clonal for the planned oligonucleotide-mediated modification. Finally, from these clones, Rb-1 mRNA was prepared and sequenced. From each clone the sequence showed a superposition of the wild-type and modified message without any additional alteration (Fig. 4d).
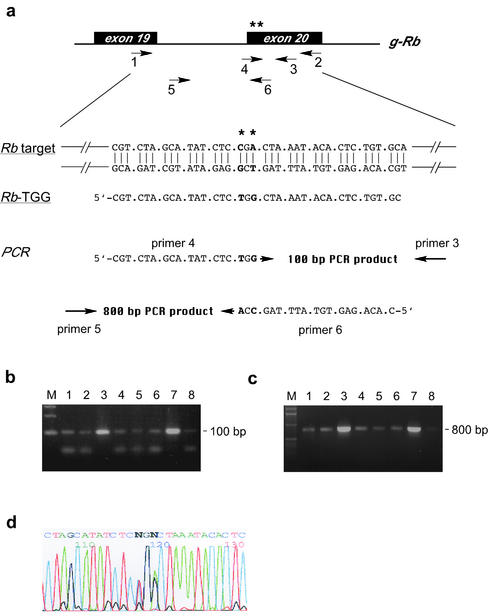
Figure 4.
Oligonucleotide-mediated substitution of a single codon CGA for TGG in the retinoblastoma gene. (a) Base pairs to be substituted are located in exon 20 of Rb-1 and are indicated by asterisks. Primers 1 and 2 were used to amplify 1500 bp fragments from pools of cells, which were subsequently used as templates in a second PCR round using the nested primers 3 and 4 or 5 and 6; primers 4 and 6 are specific for the planned modification. After exposure to the single-stranded DNA oligonucleotide Rb-TGG, cells were seeded in 96-well plates at a density of 1000 cells per well. Cells from wells giving a strong PCR signal were reseeded in 96-well plates at a density of 100 cells per well. (b) Positive wells (lanes 3 and 7) after the second round of enrichment identified with primer pair 3,4 giving a 100 bp PCR product or (c) primer pair 5,6 giving an 800 bp PCR product. Cells from positive wells were clonally expanded giving pure cultures of modified cells. (d) Sequencing reveals the presence of wild-type and modified nucleotides in Rb-1 mRNA in purified clones.
DISCUSSION
Our results demonstrate that small non-chemically-modified single-stranded deoxyribonucleotide sequences can be used to site-specifically introduce subtle alterations into the ES cell genome. However, this procedure appeared only effective in the absence of the central MMR protein MSH2. We speculate that the single-stranded oligonucleotide upon annealing to its chromosomal complement can serve as a primer for DNA synthesis. However, the presence of the genetic alteration leads to a mismatch in the primer-template heteroduplex which, in wild-type cells, activates the MMR machinery to remove the newly synthesized strand including the oligonucleotide thereby preventing the modification to become fixed into the genome. At present it is unclear whether annealing of the single-stranded oligonucleotide to its chromosomal complement occurs in the context of a replication fork or requires the assistance of proteins involved in homologous recombination. Further insight into the mechanism of oligo targeting requires its frequency to be determined in cells with defects in other MMR genes and in recombination-defective backgrounds.
Oligo targeting may provide a valuable addendum to current gene-targeting procedures basically only requiring sequence information, and find an application in the modification of industrially and medically important cell types and organisms. However, we do realize that its general applicability may be hampered by the mutator phenotype associated with MSH2 deficiency. This may lead to inadvertent genetic alterations on top of the desired oligonucleotide-mediated modification. One approach to circumvent this problem may be transient inactivation of MSH2. Interestingly, several small molecule inhibitors of MMR activity in vitro have been identified such as adriamycin (16) and vanadate (17). While at the effective concentrations these compounds are highly toxic to cells, they may serve as lead compounds for the development of reversable inhibitors of MMR in vivo. Under such conditions, oligo targeting may occur sufficiently effective while the accumulation of spontaneous mutations remains below an acceptable level.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Barbara Blouw for help at initial stages of this work, Jan-Hermen Dannenberg for providing the Rosa26-targeting vector, Jacob Hansen for designing the Rb modification, our collegues Nanna Claij, Jan-Hermen Dannenberg, Floris Foijer, Jacob Hansen and Sandra de Vries for fruitful discussions, and Anton Berns and Piet Borst for valuable comments on the manuscript. This work was financially supported by grants from the Dutch Cancer Society (NKI 2000-2233) and the European Committee (ENV4-CT97-0469).
REFERENCES
- 1.The Jackson Laboratory (2003) The Transgenic/Targeted Mutation Database. http://tbase.jax.org/.
- 2.Capecchi M.R. (1989) Altering the genome by homologous recombination. Science, 244, 1288–1292. [DOI] [PubMed] [Google Scholar]
- 3.Hasty P., Ramirez-Sollis,R., Krumlauf,R. and Bradley,A. (1991) Introduction of a subtle mutation into the Hox-2.6 locus in embryonic stem cells. Nature, 350, 243–246. [DOI] [PubMed] [Google Scholar]
- 4.Gu H., Zou,Y.R. and Rajewski,K. (1993) Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-LoxP-mediated gene targeting. Cell, 73, 1155–1164. [DOI] [PubMed] [Google Scholar]
- 5.Moerschell R.P., Tsunasawa,S. and Sherman,F. (1988) Transformation of yeast with synthetic oligonucleotides. Proc. Natl Acad. Sci. USA, 85, 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell P.P., Keown,W., Lowe,L., Kirschling,D. and Kucherlapati,R. (1989) Homologous recombination involving small single-stranded oligonucleotides in human cells. New Biol., 1, 223–227. [PubMed] [Google Scholar]
- 7.Igoucheva O., Alexeev,V. and Yoon,K. (2001) Targeted gene correction by small single-stranded oligonucleotides in mammalian cells. Gene Ther., 8, 391–399. [DOI] [PubMed] [Google Scholar]
- 8.Liu L., Rice,M.C. and Kmiec,E.B. (2001) In vivo gene repair of point and frameshift mutations directed by chimeric RNA/DNA oligonucleotides and modified single-stranded oligonucleotides. Nucleic Acids Res., 29, 4238–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole-Strauss A., Yoon,K., Xiang,Y., Byrne,B.C., Rice,M.C., Gryn,J., Holloman,W.K. and Kmiec,E.B. (1996) Correction of the mutation responsible for sickle cell anemia by an RNA–DNA oligonucleotide. Science, 273, 1386–1389. [DOI] [PubMed] [Google Scholar]
- 10.Wang G., Seidman,M.M. and Glazer,P.M. (1996) Mutagenesis in mammalian cells induced by triple helix formation and transcription-coupled repair. Science, 271, 802–805. [DOI] [PubMed] [Google Scholar]
- 11.Oh T.J. and May,G.D. (2001) Oligonucleotide-directed plant gene targeting. Curr. Opin. Biotechnol., 12, 169–172. [DOI] [PubMed] [Google Scholar]
- 12.te Riele H., Maandag,E.R. and Berns,A. (1992) Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc. Natl Acad. Sci. USA, 89, 5128–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Wind N., Dekker,M., Berns,A., Radman,M. and te Riele,H. (1995) Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination and predisposition to cancer. Cell, 82, 321–330. [DOI] [PubMed] [Google Scholar]
- 14.Thomas K.R. and Capecchi,M.R. (1987) Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell, 51, 503–512. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich G. and Soriano,P. (1991) Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev., 5, 1513–1523. [DOI] [PubMed] [Google Scholar]
- 16.Larson E.D. and Drummond,J.T. (2001) Human mismatch repair and G.T mismatch binding by hMutSα in vitro is inhibited by adriamycin, actinomycin D and nogalamycin. J. Biol. Chem., 276, 9775–9783. [DOI] [PubMed] [Google Scholar]
- 17.Pezza R.J., Villarreal,M.A., Montich,G.G. and Argaraña,C.E. (2002) Vanadate inhibits ATPase activity and DNA binding capability of bacterial MutS. A structural model for the vanadate–MutS interaction at the Walker A motif. Nucleic Acids Res., 30, 4700–4708. [DOI] [PMC free article] [PubMed] [Google Scholar]