Abstract
Age-related resistance (ARR) has been observed in a number of plant species; however, little is known about the biochemical or molecular mechanisms involved in this response. Arabidopsis becomes more resistant, or less susceptible, to virulent Pseudomonas syringae (pv tomato or maculicola) as plants mature (in planta bacterial growth reduction of 10- to 100-fold). An ARR-like response also was observed in response to certain environmental conditions that accelerate Arabidopsis development. ARR occurs in the Arabidopsis mutants pad3-1, eds7-1, npr1-1, and etr1-4, suggesting that ARR is a distinct defense response, unlike the induced systemic resistance or systemic acquired resistance responses. However, three salicylic acid (SA) accumulation-deficient plant lines, NahG, sid1, and sid2, did not exhibit ARR. A heat-stable antibacterial activity was detected in intercellular washing fluids in response to Pst inoculation in wild-type ARR-competent plants but not in NahG. These data suggest that the ability to accumulate SA is necessary for the ARR response and that SA may act as a signal for the production of the ARR-associated antimicrobial compound(s) and/or it may possess direct antibacterial activity against P. syringae.
INTRODUCTION
The relationship between plant age and disease resistance has been investigated in many plant-pathogen systems (Bateman and Lumsden, 1965; Griffey and Leach, 1965; Hunter et al., 1977; Lazarovits et al., 1981; Ward et al., 1981; Miller, 1983; Chase and Jones, 1986; Reuveni et al., 1986; Pretorius et al., 1988; Koch and Mew, 1991; Chang et al., 1992; Heath, 1993; Rupe and Gbur, 1995). Some plants become more susceptible to certain pathogens as they develop (Miller, 1983); however, susceptibility decreases with increasing leaf age in the rice/Xanthomonas campestris pv oryzae (Koch and Mew, 1991) and rice/Pyricularia oryzae (Roumen et al., 1992) interactions. In contrast, older plants (both young and mature leaves) display increased resistance in the wheat/Puccinia recondita f.sp. tritici (Pretorius et al., 1988) and tobacco/Peronospora tabacina (Reuveni et al., 1986) interactions. When older leaves/plants display increased resistance or reduced susceptibility to pathogens, this form of resistance often is referred to as age-related resistance (ARR).
The actual mechanisms responsible for the different forms of ARR have been studied in a preliminary manner in only a few cases. In cowpea/rust and cereal/rust interactions, an ARR response is thought to be controlled by single resistance genes expressed in adult plants (Roelfs, 1984; Heath, 1993). Ward et al. (1981) and Lazarovits et al. (1981) observed a positive correlation between increasing plant age, glyceollin production, and resistance to Phytophthora megasperma var sojae in soybean. A similar correlation was observed for the accumulation of a cotton phytoalexin in response to Verticillium albo-atrum infection (Bell, 1969), constitutive accumulation of terpenoids in older cotton plants (Hunter et al., 1977), or capsidiol accumulation in mature pepper plants in response to Phytophthora capsici (Hwang, 1995). The positive correlation observed between the production of defense-associated compounds in older leaves and plants displaying ARR suggests that the various forms of ARR may be controlled developmentally. It also is possible that, in some cases, ARR may result from the accumulation of toxic compounds during the life cycle of the plant.
A number of genes with possible defense functions are expressed late in plant development, when ARR usually is observed, suggesting that they may be involved in ARR. For example, some pathogenesis-related (PR) and PR-like genes are upregulated during flower development and senescence (Fraser, 1981; Lotan et al., 1989; Buchanan-Wollaston, 1994; Hanfrey et al., 1996; Butt et al., 1998; Quirino et al., 1999). Older leaves of flowering tobacco accumulate specific PR proteins (PR-1, PR-2, and PR-3), and this correlates with increased resistance to viral and fungal pathogens (Fraser, 1972; Takahashi, 1972; Reuveni et al., 1986; Wyatt et al., 1991). A subsequent study demonstrated that the ARR response in tobacco also was associated with a fivefold increase in endogenous salicylic acid (SA) (Yalpani et al., 1993). This example of ARR in tobacco resembles the systemic acquired resistance (SAR) response.
SAR is an inducible defense response that leads to broad-spectrum systemic resistance after an initial “immunizing” infection (Hammerschmidt, 1999) and is associated with SA accumulation and PR-1 expression in both inoculated and systemic tissue (Kuc, 1982; Ward et al., 1991; Yalpani et al., 1991; Uknes et al., 1992, 1993; Cameron et al., 1999). In the course of studying the SAR response, we observed that older Arabidopsis plants become more resistant to normally virulent Pseudomonas syringae pv tomato (Pst) regardless of SAR induction. Preliminary experiments suggested that ARR in the Arabidopsis/Pst system was different from the SAR response. We also considered the possibility that ARR could be a form of induced systemic resistance (ISR) resulting from contact with soil microbes during the course of our experiments. ISR occurs in plants colonized with nonpathogenic plant growth–promoting rhizobacteria such that they become resistant to subsequent infection with virulent pathogens (reviewed by Van Loon et al., 1998). ISR occurs in transgenic NahG plants (Delaney et al., 1994), which cannot accumulate SA, suggesting that, unlike the SAR response, SA accumulation is not required for ISR (Pieterse et al., 1998). Moreover, functional jasmonate and ethylene signaling pathways are necessary for the ISR response in Arabidopsis (Pieterse et al., 1998). In this work, we present data that suggest that ARR is distinct from the ISR and SAR pathways in that the NPR1 gene product is not required. However, our results also suggest that the ability to accumulate SA is a necessary component of the ARR pathway.
RESULTS
In Planta Pst Growth Is Reduced in Older Arabidopsis Plants
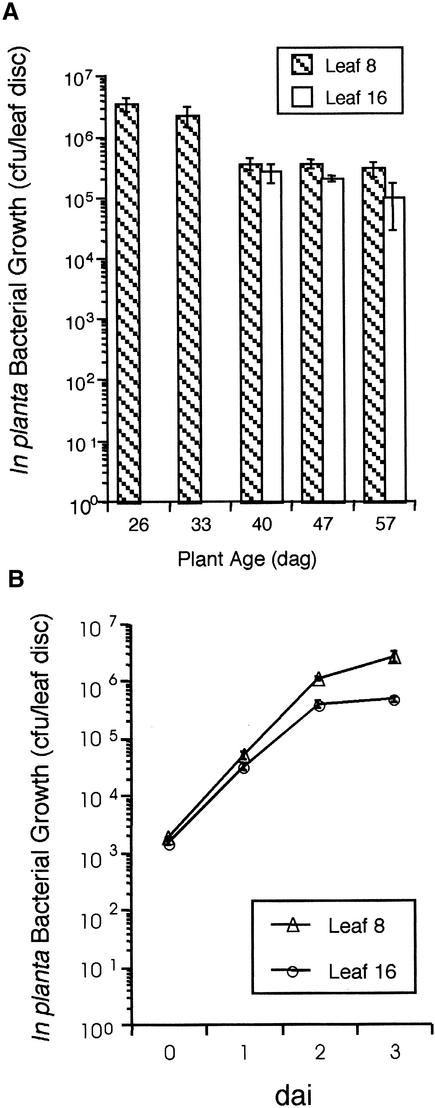
Wild-type Arabidopsis ecotype Columbia (Col-0) plants of different ages were inoculated with 106 colony-forming units (cfu)/mL of virulent Pst. Older plants became less susceptible, or more resistant, to virulent Pst, as demonstrated by a 10-fold reduction in bacterial growth between 30 and 40 days after germination (dag) (Figure 1A). In other experiments, plants displayed a 100-fold reduction in bacterial growth in a more gradual manner over 50 days (Figure 2A). Mature plants exhibiting ARR were symptomless or slightly chlorotic at the site of inoculation compared with young plants, which displayed typical chlorotic water soaking over the entire leaf. This ARR response also has been observed in ecotypes Wassilewskija and Bensheim and in response to a different pathovar, P. syringae pv maculicola (Psm), as demonstrated by a 100-fold reduction in bacterial growth in mature plants compared with young plants (data not shown). All ARR experiments were performed using overnight P. syringae cultures grown to midexponential log phase to ensure that similar bacterial numbers were inoculated into plants at each time point throughout each experiment.
Figure 1.
In Planta Growth of Virulent Pst in Leaves 8 and 16 for 57 Days.
(A) Leaves 8 and 16 were inoculated with 106 cfu/mL virulent Pst at ∼1-week intervals from 26 to 57 dag. In planta bacterial growth was monitored 3 days after inoculation (dai) and is presented as the mean of five samples ±se. In planta bacterial growth in leaf 8 at 26 dag was significantly different from in planta bacterial growth in leaves 8 and 16 at 40, 47, and 57 dag, as determined by Student's t test (P ≤ 0.05).
(B) In planta bacterial growth in mature Col-0 plants (42 dag) was measured over 3 dai (106 cfu/mL virulent Pst) in leaves 8 and 16 and is presented as the mean of five samples ±se.
The experiments in both (A) and (B) were repeated two additional times with similar results.
Figure 2.
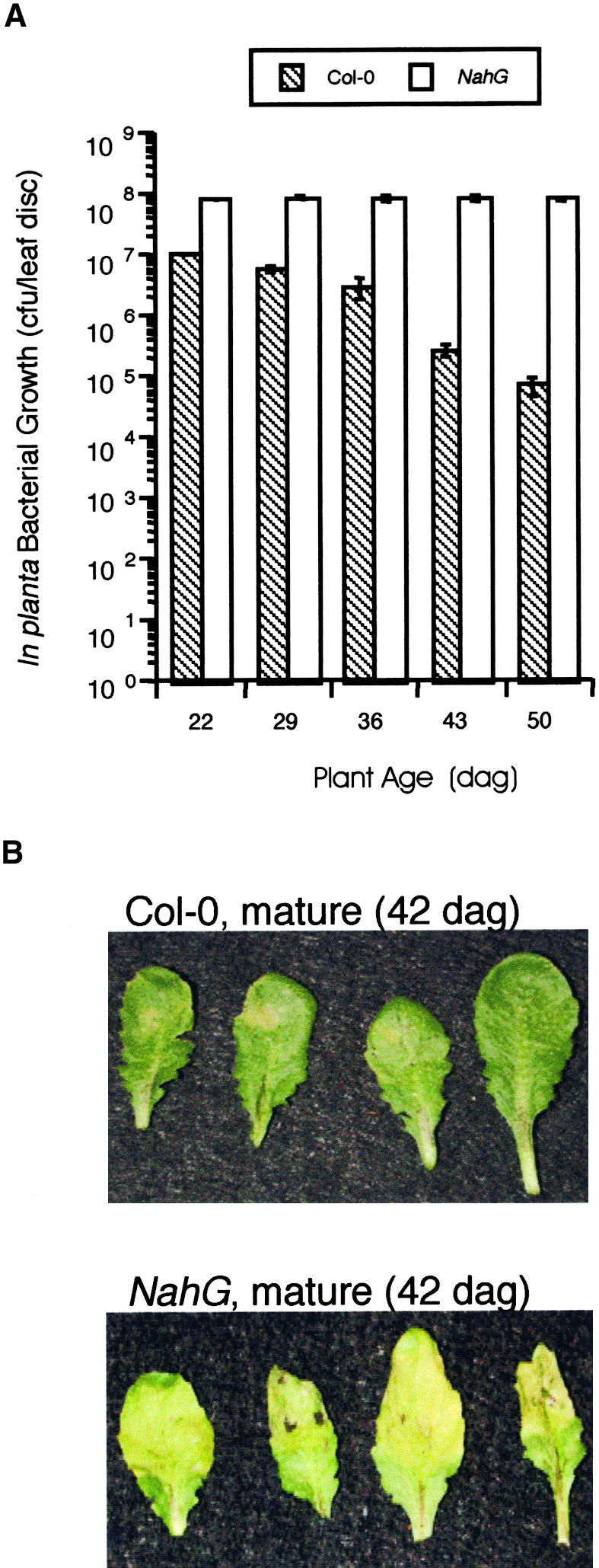
In Planta Bacterial Growth in Arabidopsis for 50 Days in NahG Versus Col-0.
(A) Col-0 and NahG plants were inoculated with 106 cfu/mL virulent Pst at ∼1-week intervals from 22 to 50 dag. In planta bacterial growth was monitored 3 dai and is presented as the mean of five samples ±sd. A significant difference (Student's t test, P < 0.00003) was observed between in planta Pst growth in young Col-0 plants (22 dag) compared with mature Col-0 plants (43 and 50 dag). This experiment was repeated at least three additional times with similar results.
(B) Mature (43 dag) Col-0 and NahG leaves inoculated with 106 cfu/mL virulent Pst and photographed 3 dai.
To prove that the reduction in bacterial growth in older plants is not caused by potential differences in inoculum concentration, ARR experiments were conducted on plants whose growth was synchronized so that young and mature plants were inoculated on the same day with the same inoculum. ARR also was observed, such that mature Col-0 supported at least 10-fold less bacterial growth than young Col-0 inoculated at the same time (Figure 3). Additionally, differences in leaf morphology between young and mature plants could affect the in planta bacterial concentration immediately after inoculation (day 0) of plants of different ages and therefore the final bacterial concentration on day 3 after inoculation. This was not observed, because similar levels of bacteria were detected in leaves 8 to 12 of plants of different ages immediately after inoculation (day 0) with 106 cfu/mL Pst (average of five replicates ±sd [22 dag, 304 ± 48 cfu/leaf disc; 28 dag, 612 ± 54 cfu/leaf disc; 40 dag, 619 ± 63 cfu/leaf disc; 49 dag, 669 ± 49 cfu/leaf disc]). Young plants (22 dag) displayed a twofold lower bacterial concentration immediately after inoculation, perhaps because the cells in young expanding leaves are tightly packed with fewer intercellular spaces (Esau, 1977; Donnelly et al., 1999). It is interesting that young plants still support vigorous bacterial growth (Figures 1A and 2A) even when twofold fewer bacteria are inoculated into leaves. These results indicate that the reduced bacterial growth observed in older plants is not attributable to variation in inoculum concentrations or the number of bacteria infiltrated into young versus mature leaves.
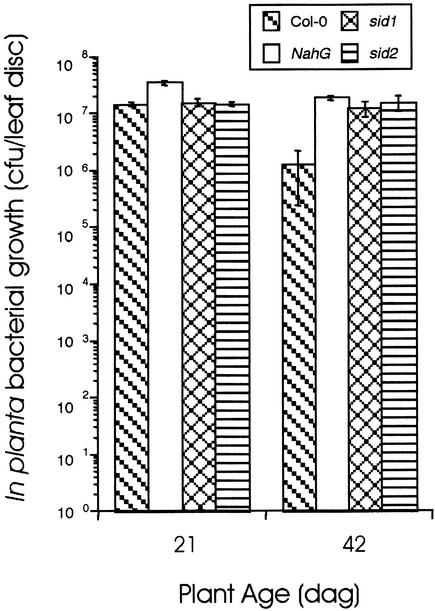
Figure 3.
In Planta Bacterial Growth in Col-0, NahG, sid1, and sid2.
Col-0, NahG, sid1, and sid2 plants were inoculated with 106 cfu/mL virulent Pst at 21 and 42 dag. In planta bacterial growth was monitored 3 dai and is presented as the mean of five samples ±sd. A significant difference (Student's t test, P < 0.001) was observed in Pst growth in corresponding young versus mature Col-0 plants. This experiment was repeated two additional times with similar results.
ARR also could be the result of transplantation stress and the active oxygen species produced by mechanical damage (Yahraus et al., 1995). Therefore, the ARR response was monitored in plants sown directly to soil or in plants that were transplanted at the seedling stage from medium to soil. ARR developed in a similar manner with both growth methods in repeated experiments (data not shown), indicating that transplantation-induced mechanical damage and the associated active oxygen species production are not involved in the ARR response.
Young and mature leaves on older plants were followed in a number of experiments to determine if the ARR response in Arabidopsis is a whole-plant phenomenon affected by the overall age of the plant or whether it is a leaf-specific response pertaining to individual leaf age. For example, if ARR is governed by the developmental state of individual leaves, young leaves on mature plants would not exhibit ARR. Leaf 8 was chosen to represent an older leaf because it is formed well after the transition from juvenile to adult phase (Telfer et al., 1997; Donnelly et al., 1999) and is macroscopically visible at 14 dag (and therefore present throughout the experiment). Additionally, it is one of the leaves (leaves 8 to 12) normally inoculated in our infection experiments. Leaf 16 represents a younger leaf that becomes macroscopically visible at ∼23 dag. In planta bacterial growth was reduced 10-fold in leaf 8 on mature plants (40, 47, and 57 dag) compared with young plants (26 dag) (Figure 1A). At 40 dag, leaf 16 supported similarly low in planta bacterial growth, and this was reduced further at 47 and 57 dag. These data suggest that the age of the plant, rather than individual leaf age, regulates the expression of ARR.
Leaves 8 and 16 are morphologically different: leaf 16 has a narrow leaf blade with more trichomes compared with leaf 8. To determine if these morphological differences could account for the observed reduction in in planta bacterial growth in leaf 16, the growth of Pst during 3 days in leaves 8 and 16 was monitored in mature plants (50 dag). There was no significant difference in leaf 8 and 16 bacterial levels immediately after inoculation (day 0); therefore, differences in leaf morphology did not affect the number of bacteria that were infiltrated successfully into the leaves (Figure 1B). In both leaves 8 and 16, bacterial growth was reduced similarly over 3 days, but to a greater extent in leaf 16 compared with leaf 8 (Figure 1B). These data suggest that a stronger ARR response correlates positively with the length of time a leaf spends on a plant that is expressing ARR.
ARR Response Differs from the SAR and ISR Response Pathways
There is increasing evidence that the signal transduction pathways leading to different types of disease resistance share common components (Dong, 1998; Maleck and Dietrich, 1999). This prompted us to examine a number of Arabidopsis defense response mutants to determine if these gene products are required for a successful ARR response. The npr1-1 mutant is defective for SAR and is more susceptible to virulent pathogens (Cao et al., 1994; Delaney et al., 1995). Interestingly, mature versus young npr1-1 plants exhibited a 10-fold reduction in in planta bacterial growth, even though they were more susceptible to Pst than was Col-0 (Table 1). This finding suggests that an intact SAR pathway is not required for the ARR response. Moreover, functional NPR1 is required for the ISR response and ARR occurs in npr1, suggesting that ARR is not a form of ISR.
Table 1.
In Planta Bacterial Growth in Young and Mature Arabidopsis Mutants
| In Planta Bacterial Growth
|
||
|---|---|---|
| Genotype | Young Plants | Mature Plants |
| Col-0 | 6.1 × 106 ± 1.5 × 105 | a3.0 × 105 ± 1.0 × 104 |
| npr1 | 1.6 × 107 ± 2.7 × 105 | a1.7 × 106 ± 1.7 × 105 |
| Col-0 | 9.9 × 106 ± 4.4 × 105 | b9.5 × 105 ± 2.9 × 104 |
| pad3-1 | 9.8 × 106 ± 1.2 × 106 | b9.3 × 105 ± 3.5 × 104 |
| Col-0 | 6.1 × 106 ± 1.7 × 105 | b2.8 × 105 ± 3.3 × 104 |
| eds7-1 | 1.3 × 107 ± 4.8 × 105 | b2.2 × 105 ± 5.6 × 104 |
| Col-0 | 9.9 × 106 ± 4.4 × 105 | a9.5 × 105 ± 2.9 × 104 |
| etr1-4 | 9.7 × 106 ± 1.1 × 106 | a1.2 × 106 ± 2.8 × 105 |
Various Arabidopsis mutants plus wild type (Col-0) were inoculated with virulent Pst at 106 cfu/mL. In planta bacterial growth was determined at 3 dai and is presented as cfu/leaf disc ±se. Each Col-0/mutant experiment was repeated three times with similar results. Young plants ranged in age from 28 to 30 dag, and mature plants ranged in age from 52 to 62 dag.
a In planta bacterial growth in old plants was significantly different from that in corresponding young plants (P ≤ 0.05, Student's t test).
b In planta bacterial growth in old plants was significantly different from that in corresponding young plants (P ≤ 0.001, Student's t test).
The contribution of the Arabidopsis phytoalexin, camalexin, was determined by testing pad3-1 for the ARR response. The pad3-1 mutant was chosen because it accumulates very little camalexin in response to Pst but exhibits a wild-type response to both avirulent and virulent Pst (Glazebrook and Ausubel, 1994; Glazebrook et al., 1997). The pad3-1 mutant displayed ARR to the same degree as Col-0 (Table 1). The eds7-1 mutant also was tested for the ARR response because it exhibits enhanced disease susceptibility to both Pst and Psm but is wild type for SAR (including PR-1 expression) (Rogers and Ausubel, 1997). As with the npr1-1 and pad3-1 mutants, the eds7-1 mutant plants also displayed wild-type levels of ARR (Table 1).
The etr1-4 mutant (which is defective in ethylene signaling and ISR) was examined to determine if ARR requires a functional ethylene signaling pathway and to confirm that ARR is not an ISR response to the potential accumulation of microbes in the soil during the experiment. The etr1-4 plants also were capable of expressing ARR, as demonstrated by a 10-fold reduction in in planta bacterial growth in mature compared with young etr1-4 (Table 1).
ARR Does Not Occur in NahG Plants
NahG plants accumulate little SA during defense responses, display enhanced disease susceptibility to some virulent pathogens, and are compromised in their ability to establish SAR (Delaney et al., 1994; Vernooij et al., 1994). NahG plants were tested to determine if SA accumulation is required for the ARR response. As demonstrated in Figure 2, NahG plants displayed chlorotic disease symptoms and supported vigorous bacterial growth throughout the 50-day experiment. In contrast, wild-type Col-0 plants were almost completely symptomless and displayed increasing resistance to Pst (100-fold reduction) during the course of the experiment (Figures 2A and 2B). These results demonstrate that NahG plants do not exhibit the ARR response, suggesting that SA accumulation is required to manifest ARR. To confirm these findings and to ascertain whether NahG is ARR defective as a result of the inability to accumulate SA or some other effect on phenylpropanoid metabolism of the NahG transgene (Cameron, 2000; Maleck et al., 2000), two other SA accumulation mutants were tested for their ability to manifest ARR. The sid1 and sid2 (SA induction–deficient) mutants were chosen because they contain single recessive mutations and accumulate little SA in response to pathogen inoculation. Moreover, they are more susceptible to both virulent and avirulent Pst and Peronospora parasitica than wild-type plants but less susceptible than NahG lines (Nawrath and Métraux, 1999), suggesting that these mutants affect fewer aspects of phenylpropanoid metabolism than does NahG.
Both sid1 and sid2 supported vigorous in planta bacterial growth in young and mature plants in a manner similar to NahG, unlike wild-type Col-0, which displayed a typical ARR response (Figure 3). These observations suggest that the ARR-defective phenotype observed in NahG and sid plants is attributable to the inability to accumulate SA.
Accumulation of PR-1 and SAG-13 Transcripts during ARR
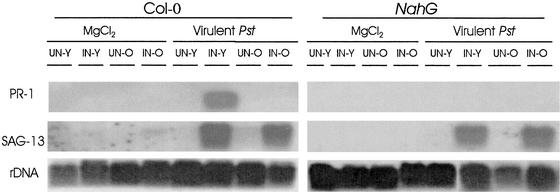
The SAR-defective npr1-1 mutant expresses little PR-1, PR-2, or PR-5 in response to pathogens (Cao et al., 1994) but does exhibit ARR, suggesting that PR gene expression and a functional SAR pathway are not required for the ARR response. Therefore, it was intriguing to discover that NahG, which also is compromised for PR-1 gene expression and SAR, was unable to manifest ARR. To understand these results, PR-1 expression was monitored in young and mature Col-0 and NahG plants. As expected, PR-1 was expressed weakly at 1 dai in young Col-0 plants (data not shown) but was expressed strongly at 3 dai (Figure 4). In contrast, NahG plants did not express PR-1 when young or mature (Figure 4). Interestingly, mature Col-0 plants that displayed ARR (100-fold reduction in Pst growth compared with young Col-0; Figure 2A) did not express PR-1 in response to virulent Pst inoculation at 1 dai (data not shown) or 3 dai (Figure 4). Additionally, there was no expression of PR-1 in mature mock-inoculated plants, indicating that ARR is not caused by constitutive PR-1 gene expression in mature leaves, unlike the ARR response in tobacco (Fraser, 1972; Takahashi, 1972; Reuveni et al., 1986; Wyatt et al., 1991; Yalpani et al., 1993). Similar results also were obtained for PR-5 (data not shown). Clearly, Arabidopsis plants respond differently to the same pathogen at different stages of development. Moreover PR-1 gene expression does not correlate with the ARR response. This may explain why npr1-1 plants exhibited ARR even in the absence of PR-1 expression (Cao et al., 1994).
Figure 4.
PR-1 and SAG-13 Transcript Accumulation in Young and Mature Col-0 and NahG Plants.
Col-0 and NahG plants were mock inoculated or inoculated with 106 cfu/mL virulent Pst at 28 dag (young [Y]) and 50 dag (old [O]). Three days after inoculation, leaves were monitored for PR-1, SAG-13, and rDNA transcript accumulation by RNA gel blot analysis in both uninoculated (UN) and inoculated (IN) leaves collected from mock-inoculated and Pst-inoculated plants. This experiment was repeated three times with similar results.
Because ARR occurs in mature Arabidopsis plants that have not begun to flower, it is possible that ARR is associated with the early stages of senescence. The expression of SAG-13, a molecular marker for the early prechlorotic stages of senescence (Weaver et al., 1998), was monitored in the same Col-0 and NahG plants described above. SAG-13 was not expressed in uninoculated or mock-inoculated leaves of Col-0 or NahG (young or old). Therefore, it appears that mature Col-0 plants have not yet entered even the early stages of the senescence program. Little SAG-13 expression was observed in uninoculated leaves of Pst-infected plants; however, SAG-13 transcripts accumulated in leaves inoculated with Pst in both Col-0 and NahG. This is not unexpected, because it has been observed that some pathogens induce the synthesis of ethylene, and thus senescence (Stall and Hall, 1984), or pathogen-induced chlorophyl destruction (chlorosis) may induce the senescence program (Quirino et al., 2000). These results correspond to the observations that both total protein and carbohydrate (soluble hexoses) levels remain elevated throughout the experimental period (data not shown), suggesting that ARR is not the result of senescence-associated nutrient reduction that could negatively affect in planta bacterial growth.
Stress Induces an ARR-Like Response in Young Plants
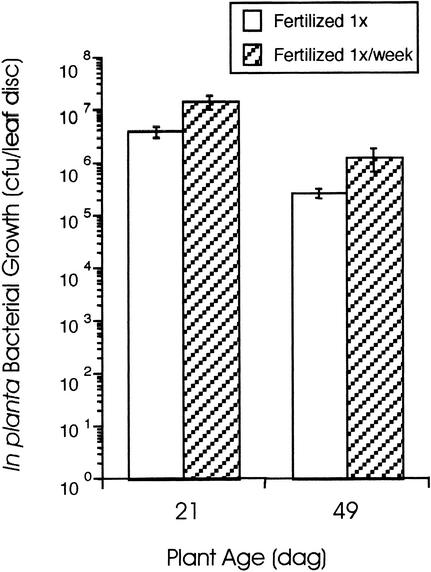
Observations made during the course of these studies led us to hypothesize that certain stressful conditions might induce an ARR-like response in developmentally younger plants. For example, an episode of mild drought, in which the soil dries out in the absence of plant wilting, was correlated with reduced in planta bacterial growth (twofold to sixfold reduction) compared with control plants of the same age (20 to 28 dai; data not shown). Additionally, like many Arabidopsis researchers (Scholl et al., 1998), we fertilize seedlings once during seedling transfer to soil to standardize plant growth procedures in the laboratory. This fertilization regimen produces healthy plants that do not appear to be limited for nutrients and do not initiate flowering during the experimental period. However, it has been reported that poor nutrition causes stress-induced premature flowering (Martinez-Zapater et al., 1994; Scholl et al., 1998). To determine if limited fertilization can accelerate development and affect the timing of the ARR response, the one-time fertilization regimen was compared with a once per week regimen. Plants that received just one application of fertilizer as seedlings supported reduced levels of bacterial growth as both young and mature plants compared with those fertilized once per week; however, plants fertilized once per week still exhibited ARR (Figure 5). These results suggest that even mild nutrient limitation can affect the level of in planta growth of Pst in Arabidopsis. We also observed that constantly wet soil sometimes supports algae growth and that plants grown under these conditions exhibit reduced in planta bacterial growth when young (21 to 28 dai) and often flower prematurely (data not shown). These data suggest that various stresses can accelerate Arabidopsis development, and this correlates with the appearance of an ARR-like response in young plants.
Figure 5.
In Planta Bacterial Growth in Plants Fertilized Once Versus Once per Week.
Young (21 dag) and mature (49 dag) Col-0 plants that received fertilizer once at the seedling stage (1×) or were fertilized once per week (1×/week) were inoculated with 106 cfu/mL virulent Pst. In planta bacterial growth was monitored 3 dai and is presented as the mean of five samples ±sd. A significant difference (Student's t test, P < 0.05) was observed between in planta Pst growth in the corresponding young and mature Col-0 plants. This experiment was repeated two additional times with similar results.
Plants Displaying ARR Produce an Intercellular Antimicrobial Compound
Pseudomonas species reside in plant intercellular spaces (Collmer and Bauer, 1994) and therefore may be subject to attack by secreted plant antimicrobial compounds. An in vitro assay for the detection of antimicrobial activity in intercellular washing fluids (IWFs) was used to determine if ARR-competent plants produce intercellular antimicrobial compounds. An inhibition assay consisting of a short incubation of Pst with IWF followed by plating on appropriate medium was chosen to quantify the number of viable Pst and to make efficient use of the limited quantities of IWF (15 to 25 μL/50 mature leaves), thus allowing sufficient experimental replication. IWFs collected from young Col-0 or NahG leaves (mock or Pst inoculated) did not inhibit the growth of Pst in the in vitro assay (Table 2, experiment 1). Only IWFs collected from mature Col-0 plants inoculated with Pst and displaying ARR significantly inhibited the growth of Pst, by 20% in experiment 1 and 46% in experiment 2 (Table 2). Antibacterial activity was not observed in IWFs from ARR-defective NahG plants (Table 2, experiment 1 or 2). Boiling treatment did not alter the inhibitory activity of IWFs from inoculated mature Col-0 plants (Table 2, experiment 2). Similar levels of bacterial inhibition (20% to 50%) have been observed by others who, like us, used dilute plant IWFs or extracts rather than purified compounds (Smith, 1982; Rauscher et al., 1999; Brader et al., 2001). IWF samples with antibacterial activity were concentrated 10- to 30-fold to increase the level of inhibition observed; unfortunately, antibacterial activity was abolished by this procedure. Overall, these data suggest that ARR is an induced response to Pst infection, because antibacterial activity was observed in IWFs from Pst-inoculated mature Col-0 but not in IWFs collected from mature mock-inoculated plants.
Table 2.
IWFs from Plants Displaying ARR Inhibit the Growth of Pst in Vitro
| In Vitro Pst Growth with or without IWF
|
|||
|---|---|---|---|
| IWF Source | Col-0 | NahG | No IWF |
| Experiment 1 | |||
| Young leaves | |||
| Mock inoculated | 1084 ± 36 | 1101 ± 72 | |
| Pst inoculated | 1048 ± 48 | 1067 ± 54 | |
| Mature leaves | |||
| Mock inoculated | 1134 ± 69 | 1175 ± 186 | |
| Pst inoculated | 914 ± 57a | 1206 ± 53 | |
| 1084 ± 291b | |||
| Experiment 2 | |||
| Mature leaves | |||
| Mock inoculated | 92 ± 6.1 | 89 ± 8.8 | |
| Pst inoculated | 41 ± 9.5a | 90 ± 2.2 | |
| Pst inoculated + B | 41 ± 3.5 | ||
| 88 ± 5.8b | |||
Pst (tetracycline-resistant) bacteria (10 μL) were incubated in King's B (KB) medium ±IWF for 1 hr and then plated on KB plus rifampacin and tetracycline. The number of cfu/plate was determined 3 days later. IWFs were collected from mock-inoculated and Pst-inoculated Col-0 and NahG leaves from 4-week-old (young) and 6.5-week-old (mature) Arabidopsis plants. IWFs collected from mature Pst-inoculated Col-0 leaves were boiled (B) for 10 min before incubation with Pst.
Results are presented as means of five replicates ±sd. Student's t test indicated a significant difference in Col-0 (mock-inoculated) and Col-0 (Pst-inoculated) IWF assays (P < 0.006). Both experiments were repeated twice with similar results.
Pst alone was followed to monitor the number of bacteria present in each sample in the absence of any IWF.
DISCUSSION
Arabidopsis plants grown under short daylengths and fertilized once at the seedling stage exhibited ARR to Pst and Psm in a gradual manner during 8 weeks or more abruptly at 30 to 40 dag. Increasing the fertilization frequency to once per week resulted in a fivefold increase in in planta Pst growth in both young and mature plants, but ARR still was observed in mature plants. Therefore, the fertilization regimen significantly affects the level of in planta bacterial growth in Arabidopsis, suggesting that mild nutrient limitation may contribute to the ARR response. This also may explain why ARR is observed during Pst infection experiments on a regular basis in our laboratory and highlights the dramatic effects that different fertilization regimens can have on Arabidopsis physiology.
We also observed, but never fully documented, that an ARR-like response was seen in young plants (15 to 21 dag) grown in long day conditions (14 to 24 hr of light) or in plants exposed to stresses such as crowded growth conditions, mild drought, or infestation with thrips or algae. Our empirical observations suggest that these environmental conditions induce Arabidopsis to develop more quickly, as demonstrated by premature flowering in some experiments. This may explain why ARR develops gradually as plants mature in some experiments and more abruptly in experiments that include stress-associated accelerated development. These observations are not unexpected, because stress-induced (poor nutrition, crowded growth conditions, algae growth on soil) transition to flowering has been documented previously (Martinez-Zapater et al., 1994; Scholl et al., 1998). In numerous experiments in which an ARR-like response developed in younger stressed plants, reduced PR-1 gene expression also was observed (data not shown), suggesting that this response is similar to ARR observed in mature unstressed Arabidopsis plants. The effect of fertilization and other stresses on in planta Pst growth in Arabidopsis highlights the major impact that environmental factors have on the level of disease observed in a plant/pathogen interaction (classic disease triangle; Agrios, 1997).
Our studies also indicate that ARR is a whole-plant phenomenon, in that both young and older leaves on mature plants exhibit ARR. Thus, ARR appears to be a developmentally regulated and environmentally sensitive response.
Interestingly, of the mutants tested for their ability to exhibit ARR, all exhibited ARR except the SA accumulation–deficient lines NahG, sid1, and sid2. The pad3-1 camalexin-deficient mutant (Glazebrook et al., 1997) displayed ARR, indicating that camalexin accumulation is not required for the ARR response. The eds7-1 mutant was chosen because it is defective in general or horizontal resistance to both Pst and Psm but displays a normal SAR response (Rogers and Ausubel, 1997). The eds7-1 mutant line displayed ARR, suggesting that the ARR response is a distinct defense response that does not require a functional EDS7 protein.
The fact that ARR was observed in npr1-1 plants indicates that PR-1 gene expression is not necessary for ARR, and this is corroborated by the finding that PR-1 gene expression is reduced greatly in plants displaying ARR. Both NPR1 and ETR1 are required for the ISR response (Pieterse et al., 1998), and both npr1-1 and etr1-4 exhibit ARR, strongly suggesting that ARR is not a form of ISR. Moreover, the early senescence–associated molecular marker SAG-13 was not expressed in mature plants, suggesting that ARR begins before the onset of the senescence program.
Given the facts that the ARR response is independent of NPR1 function and that NahG and npr1 are defective for SAR, it was surprising to discover that NahG, unlike npr1, was defective for the ARR response. One could conclude that the ARR response is SA dependent, because NahG plants do not accumulate SA in response to infection as a result of the activity of the salicylate hydroxylase transgene (Delaney et al., 1994). However, as discussed elsewhere (Cameron, 2000; Maleck et al., 2000), other components of the phenylpropanoid pathway also may be affected in NahG plants. It is possible that in an attempt to compensate for the inability to accumulate SA during pathogen attack, the flux through the phenylpropanoid pathway may be altered in NahG plants, reducing the production of other phenylpropanoid-derived compounds, some of which may be important for ARR. However, both sid1 and sid2 are unable to manifest ARR, strongly suggesting that the accumulation of SA is an integral part of the ARR response pathway. Perhaps SA acts as a signal molecule, stimulating the production and secretion of antibacterial compound(s) into the intercellular space, and/or its accumulation contributes to the antibacterial activity observed in plants displaying ARR.
The detection of antibacterial activity in IWFs from plants displaying ARR, but not in ARR-defective NahG plants, suggests that antibacterial compound(s) present in the Arabidopsis intercellular space are responsible for ARR. The heat-resistant nature of the activity further supports the notion that a low-molecular-mass chemical such as SA or a related phenolic, rather than an antibacterial protein(s), is responsible for ARR. The antibacterial activity was detected only in IWFs from mature plants inoculated with Pst, not in IWFs from mature mock-inoculated plants, strongly suggesting that the ARR response in Arabidopsis is a developmentally regulated and pathogen-induced response. On the other hand, it is possible that antibacterial compounds accumulate as Arabidopsis matures and that these compounds are released by the activity of Pst virulence factors secreted into plant cells via the type III secretion system (Galan and Collmer, 1999). This seems unlikely because disease symptoms are suppressed in ARR-competent plants, suggesting that the bacterial type III system has little effect on plant cells displaying ARR.
Pseudomonas species are biotrophs that have a necrotrophic phase after a period of multiplication in the plant (Collmer and Bauer, 1994). Quorum sensing or population density–dependent sensing has been demonstrated to be important for the expression of virulence in a number of plant pathogens (Pierson et al., 1998). We postulate that the reductions in chlorosis, necrosis, and bacterial growth observed in plants expressing ARR may be attributable to the inhibition of bacterial multiplication by intercellular antibacterial compound(s), such that the bacterial density remains too low to initiate quorum sensing and the switch to the necrotrophic phase, which includes the production of Pst virulence factors and disease.
ARR in tobacco is composed of at least two defense signaling pathways that are activated constitutively in a developmentally regulated manner (Hugot et al., 1999). Older tobacco leaves on flowering plants accumulate SA and PR proteins constitutively (Yalpani et al., 1993), and an intercellular compound toxic to Phytophthora parasitica zoospores also is produced, even in NahG transgenic lines (Hugot et al., 1999). Earlier work in soybean, cotton, and pepper demonstrated a correlation between ARR and phytoalexin accumulation (Bell, 1969; Ward et al., 1981; Kim et al., 1989), and in Arabidopsis, ARR to P. syringae appears to be a pathogen-induced response involving an antibacterial activity (this work). Like the complex network of defense pathways available to young plants (Feys and Parker, 2000), it appears that different plant species also use different mechanisms to defend themselves against a variety of pathogens as they mature. Previous studies have not addressed whether ARR provides protection to many pathogens. Future studies will determine if ARR in Arabidopsis extends to pathogens other than Pst and Psm.
ARR appears to be a distinct defense response pathway, unlike SAR or ISR, in that NPR1 and ETR1 functions are not necessary. However, SA accumulation appears to be required for a successful response. Future studies to determine the identity of the ARR-associated antibacterial compound(s) and the role of SA in this developmentally regulated pathogen-induced response will provide insights into this novel pathway and, in addition, contribute to the elucidation of the role of stress in accelerating development and ARR in Arabidopsis.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana ecotype Columbia (Col-0) plants, along with the Col-0 mutants npr1-1 (X. Dong, Duke University, Durham, NC), etr1-4 (E. Meyerowitz, California Institute of Technology, Pasadena, CA), pad3-1 (J. Glazebrook, Novartis Agricultural Discovery Institute, San Diego, CA), eds7-1 (F. Ausubel, Massachusetts General Hospital, Boston), and sid1 and sid2 (C. Nawrath, University of Fribourg, Switzerland) and the transgenic NahG line (K. Lawton, Syngenta, Research Triangle Park, NC), were used in these studies. Seeds were surface sterilized and germinated on Murashige and Skoog (1962) medium. After 10 days under continuous light, seedlings were transferred to soil (Sunshine Mix No. 1 [Sun Gro Horticulture, Bellevue, WA] moistened with 1 g/L 20-20-20 fertilizer) and grown at 22°C under a 9-hr photoperiod with an average light intensity of 150 μEm−2· sec−1 for up to 60 days. Plants treated with a once per week fertilization regimen were grown as described above but received 1 to 2 L of fertilizer (1 g/L 20-20-20) once per week for 3 to 4 weeks and then once every 2 weeks for the remainder of the experiment.
Bacterial Growth and Inoculation Procedures
Avirulent and virulent Pseudomonas syringae pv tomato (Pst) strain DC3000 (rifampicin [rif] and kanamycin [kan] resistant) and P. syringae pv maculicola (Psm) strain 4326 were obtained from Dr. Andrew Bent (University of Wisconsin at Madison) (Whalen et al., 1991). The avirulent Pst strain contained the plasmid pV288 harboring the avrRpt2 gene, whereas the virulent strain contained the same plasmid without the avrRpt2 gene (pVSP61). Another DC3000 strain (rif and tetracycline [tet] resistant [pL6]; J. Dangl, University of North Carolina, Chapel Hill) was used in the intercellular washing fluid (IWF) experiments. In all experiments, Pst or Psm from midlog phase overnight cultures shaken at room temperature were diluted to 106 colony-forming units (cfu)/mL in 10 mM MgCl2 and pressure infiltrated into the abaxial sides of leaves 8 to 12 using a needleless 10-mL syringe, filling the intercellular spaces of the entire leaf. Control plants were mock inoculated with 10 mM MgCl2. In planta bacterial growth was determined as described previously with serial dilutions plated on King's B (KB) agar plates containing 100 μg/mL rif (Sigma) and 50 μg/mL kan (Sigma) (Wolfe et al., 2000).
Age-Related Resistance Experimental Design
The growth of Pst in planta was monitored for 8 weeks beginning at 22 to 25 days after germination (dag). At each time point (∼25, 33, 40, 48, and 57 dag), 20 to 24 plants (four leaves per plant) were inoculated with Pst at 106 cfu/mL and another set of 10 to 14 plants (four leaves per plant) were mock inoculated with 10 mM MgCl2. Leaves were collected at 3 days after inoculation (dai) and frozen for subsequent RNA gel blot analysis. Both inoculated and uninoculated leaves were collected from plants infected with Pst or mock inoculated. Leaves also were collected at 3 dai for determination of in planta bacterial growth (eight leaf discs [4 mm diameter] per replicate, five replicates for Pst-infected samples and three replicates for the mock control treatment). There was very little Pst growth (0 to <102 cfu/leaf disc) observed in the negative mock-inoculated control leaves; therefore, these data are not shown. Replicates were averaged, and the se or sd was calculated. Student's t test was used to determine if differences in Pst growth between young and mature plants were statistically significant. This experiment was performed on plants germinated first on Murashige and Skoog (1962) agar plates with subsequent transplantation to soil after 10 days of growth in continuous light and also on plants sown directly to soil. The experiment was repeated at least three times for each growing regimen, and similar results were obtained each time (for replicate experiments, see Kus, 1999). Experiments also were conducted in which plant growth was synchronized so that young and mature plants were inoculated on the same day with the same inoculum to determine if differences in inoculum dose were responsible for the reduction in in planta bacterial growth observed in mature plants.
After characterizing age-related resistance (ARR) in Col-0 wild-type plants, young (25 to 30 dag) and old (52 to 62 dag) plants were used to study ARR in the Arabidopsis mutants npr1-1, eds7-1, pad3-1, and etr1-4 and the transgenic NahG line. The various mutant lines were always compared with wild-type plants grown at the same time under the same conditions. Each mutant/Col-0 experiment was repeated three times with similar results each time (for replicate experiments, see Kus, 1999).
To determine whether ARR is a whole-plant response affected by the overall age of the plant or whether it is a leaf-specific response pertaining to the age of individual leaves, experiments similar to those described above were performed. Leaves 8 and 16 were monitored for the ARR response and for leaf blade growth every 2 to 3 days. Both leaves expanded rapidly between 23 and 37 dag, at which time leaf 8 did not expand further, whereas leaf 16 stopped expanding at 43 dag. This experiment was repeated two additional times with similar results.
Extraction and Analysis of RNA
Uninoculated and inoculated leaves (three leaves per sample) from mock-inoculated plants and plants inoculated with virulent Pst (106 cfu/mL) were collected at 3 dai and flash frozen in liquid nitrogen. RNA was isolated and analyzed as described previously (Cameron et al., 1999). Arabidopsis cDNA clones for PR-1 (K. Lawton, Syngenta), SAG-13 (R.M. Amasino, University of Wisconsin, Madison), and rDNA (J. Coleman, University of Toronto) were labeled with 32P by random priming (Random Primer Kit; Amersham). Hybridizations and washes were performed according to Church and Gilbert (1984). This experiment was repeated three times, giving similar results each time (for replicate experiments, see Kus, 1999).
Collection of IWFs and in Vitro Bacterial Inhibition Assays
Leaves 8 through 12 from young (20 and 25 dag) and mature (40 and 45 dag) plants were infiltrated with either 10 mM MgCl2 or 106 cfu/mL virulent Pst (rif and kan resistant). Two days later, leaves were harvested and surface sterilized with 50% ethanol. IWFs were obtained by vacuum infiltrating leaves with sterile distilled H2O for 25 to 30 min; leaves were surface dried by blotting with absorbent paper or in a salad spinner, and IWFs were collected by centrifugation at 1000g for 30 min at 4°C (Hammond-Kosack, 1992). Fifty mature leaves produced 15 to 25 μL of IWF depending on how effectively the leaves were dried before centrifugation; therefore, some IWF samples might have been more dilute than others. IWFs were collected from young and mature mock- and Pst-inoculated leaves and frozen at −20°C before use. The IWFs (50 μL) were incubated with 5 to 10 μL of virulent Pst (rif and tet resistant; estimated at OD600 between 200 and 1000 cfu) and KB broth (1 × final concentration) with shaking at room temperature for 1 hr and then plated on KB rif + tet plates. Colonies were counted 3 days after plating, and the percent inhibition of bacterial growth was determined (cfu in IWF from mock-inoculated leaves minus cfu in IWF from Pst-inoculated leaves divided by IWF from mock-inoculated leaves multiplied by 100). Pst bacteria were incubated in KB broth because more than 50% died in 10 mM MgCl2 or sterile water during the 1-hr incubation. Bacterial numbers varied 5- to 10-fold from experiment to experiment (cf. IWFs 1 and 2 in Table 2) because the density of the midlog phase cultures used varied by 5- to 10-fold. A number of controls were included to show that the rif- and kan-resistant Pst present in IWFs from Pst-inoculated leaves did not grow on the KB rif + tet plates. rif + tet Pst growth in the absence of IWF (Pst + 4 × KB) was the baseline for normal Pst growth. IWFs from mature wild-type plants inoculated with Pst were subjected to boiling for 10 min before incubation with rif + tet Pst and plating.
Acknowledgments
We thank Verna Higgins, Michele Heath, Peter McCourt, Nancy Dengler, David Guttman, and Allan Shapiro for helpful discussions. We greatly appreciate the plasmid constructs, Arabidopsis mutants, and bacterial strains provided by a number of helpful scientists (see Methods). This work was supported by a grant to R.K.C. from the Natural Sciences and Engineering Research Council of Canada and the Ontario Research Challenge Fund.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010481.
References
- Agrios, G.N. (1997). Plant Pathology, 4th ed. (New York: Academic Press), pp. 43–62.
- Bateman, D.F., and Lumsden, R.D. (1965). Relationship of calcium content and the nature of the pectic substances in bean hypocotyls of different ages and susceptibility to an isolate of Rhizoctonia solani. Phytopathology 55, 734–738. [Google Scholar]
- Bell, A.A. (1969). Phytoalexin production and Verticillium wilt resistance in cotton. Phytopathology 59, 1119–1127. [Google Scholar]
- Brader, G., Tas, E., and Palva, E.T. (2001). Jasmonate-dependent induction of indole glucosinolates in Arabidopsis by culture filtrates of the nonspecific pathogen Erwinia carotovora. Plant Physiol. 126, 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston, V. (1994). Isolation of cDNA clones for genes that are expressed during leaf senescence in Brassica napus: Identification of a gene encoding a senescence-specific metallothionein-like protein. Plant Physiol. 105, 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt, A., Mousley, C., Morris, K., Beyton, J., Can, C., Holub, E., Greenberg, J.T., and Buchanan-Wollaston, V. (1998). Differential expression of a senescence-enhanced metallothionein gene in Arabidopsis in response to isolates of Peronospora parasitica and Pseudomonas syringae. Plant J. 16, 209–221. [DOI] [PubMed] [Google Scholar]
- Cameron, R.K. (2000). Salicylic acid and its role in plant defense responses: What do we really know? Physiol. Mol. Plant Pathol. 56, 91–93. [Google Scholar]
- Cameron, R.K., Paiva, N.L., Lamb, C.J., and Dixon, R.A. (1999). Accumulation of salicylic acid and PR-1 gene transcripts in relation to the systemic acquired resistance (SAR) response induced by Pseudomonas syringae pv. tomato in Arabidopsis. Physiol. Mol. Plant Pathol. 55, 121–130. [Google Scholar]
- Cao, H., Bowling, S.A., Gordon, A.S., and Dong, X. (1994). Characterization of an Arabidopsis mutant that is non-responsive to inducers of systemic acquired resistance. Plant Cell 6, 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, R.J., Ries, S.M., and Pataky, J.K. (1992). Effects of temperature, plant age, inoculum concentration, and cultivar on the incubation period and severity of bacterial canker of tomato. Plant Dis. 76, 1150–1155. [Google Scholar]
- Chase, A.R., and Jones, J.B. (1986). Effects of host nutrition, leaf age, and preinoculation light levels on severity of leaf spot on dwarf scefflera caused by Pseudomonas cichorii. Plant Dis. 70, 561–563. [Google Scholar]
- Church, G.M., and Gilbert, W. (1984). Genomic sequencing. Proc. Natl. Acad. Sci. USA 81, 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer, A., Bauer, D.W. (1994). Erwinia chrysanthemi and Pseudomonas syringae: Plant pathogens trafficking in virulence proteins. Curr. Top. Microbiol. Immunol. 192, 43–78. [DOI] [PubMed] [Google Scholar]
- Delaney, T.P., Uknes, S., Vernooij, B., Friedrich, L., Weyman, K., Negrotto, D., Gaffney, T., Gut-Rela, M., Kessmann, H., Ward, E., and Ryals, J. (1994). A central role for salicylic acid in plant disease resistance. Science 266, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Delaney, T.P., Friedrich, L., and Ryals, J.A. (1995). Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc. Natl. Acad. Sci. USA 92, 6602–6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, X. (1998). SA, JA, ethylene and disease resistance in plants. Curr. Opin. Plant Biol. 1, 316–323. [DOI] [PubMed] [Google Scholar]
- Donnelly, P., Bonetta, D., Tsukaya, H., Dengler, R., and Dengler, N. (1999). Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev. Biol. 215, 407–419. [DOI] [PubMed] [Google Scholar]
- Esau, K. (1977). Anatomy of Seed Plants. (New York: John Wiley and Sons), pp. 62–63, 341–345.
- Feys, B.J., and Parker, J.E. (2000). Interplay of signaling in plant disease resistance. Trends Genet. 16, 449–455. [DOI] [PubMed] [Google Scholar]
- Fraser, R. (1972). Effects of two strains of tobacco mosaic virus on growth and RNA content of tobacco leaves. Virology 47, 261–269. [DOI] [PubMed] [Google Scholar]
- Fraser, R. (1981). Evidence for the occurrence of “pathogenesis-related” proteins in the leaves of healthy tobacco plants during flowering. Physiol. Plant Pathol. 19, 69–76. [Google Scholar]
- Galan, J.E., and Collmer, A. (1999). Type II secretion machines: Bacterial devices for protein delivery into host cells. Science 284, 1322–1328. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J., and Ausubel, F.M. (1994). Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc. Natl. Acad. Sci. USA 91, 8955–8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J., Zook, M., Mert, F., Kagan, I., Rogers, E.E., Crute, I.R., Holub, E.B., Hammerschmidt, R., and Ausubel, F.M. (1997). Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 146, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffey, R.T., and Leach, J.G. (1965). The influence of age on tissue development of bean anthracnose lesions. Phytopathology 55, 915–918. [PubMed] [Google Scholar]
- Hammerschmidt, R. (1999). Induced resistance: How do induced plants stop pathogens? Physiol. Mol. Plant Pathol. 55, 77–84. [Google Scholar]
- Hammond-Kosack, K. (1992). Preparation and analysis of intercellular fluid. In Molecular Plant Pathology: A Practical Approach, Vol. II, S.J. Gurr, M.J. McPherson, and D.J. Bowles, eds (Oxford, UK: IRL Press/Oxford University Press), pp. 15–30.
- Hanfrey, C., Fife, M., and Buchanan-Wollaston, V. (1996). Leaf senescence in Brassica napus: Expression of genes encoding pathogenesis-related proteins. Plant Mol. Biol. 30, 597–609. [DOI] [PubMed] [Google Scholar]
- Heath, M.C. (1993). Genetics and cytology of age-related resistance in North American cultivars of cowpea (Vigna unguiculata) to cowpea rust fungus (Uromyces vignae). Can. J. Bot. 72, 575–581. [Google Scholar]
- Hugot, K., Aime, S., Conrod, S., Poupet, A., and Galiana, E. (1999). Developmental regulated mechanisms affect the ability of a fungal pathogen to infect and colonize tobacco leaves. Plant J. 20, 163–170. [DOI] [PubMed] [Google Scholar]
- Hunter, R.E., Halloin, J.M., Veech, J.A., and Carter, W.W. (1977). Terpenoid accumulation in hypocotyls of cotton seedlings during aging and after infection by Rhizoctonia solani. Phytopathology 68, 347–350. [Google Scholar]
- Hwang, B.K. (1995). Effects of age-related resistance and metalazyl on capsidiol production in pepper plants infected with Phytophthora capsici. In Handbook of Phytoalexin Metabolism and Action, M. Daniel and R.P. Purkayastha, eds (New York: Marcel Dekker), pp. 503–523.
- Kim, Y.J., Hwang, B.K., and Park, K.W. (1989). Expression of age-related resistance in pepper plants infected with Phytophthora capsici. Plant Dis. 73, 745–747. [Google Scholar]
- Koch, M.F., and Mew, T.W. (1991). Effects of plant age and leaf maturity on the quantitative resistance of rice cultivars to Xanthomonas campestris pv. oryzae. Plant Dis. 75, 901–904. [Google Scholar]
- Kuc, J. (1982). Induced immunity to plant disease. Bioscience 32, 854–860. [Google Scholar]
- Kus, J.V. (1999). Age-Related Resistance in the Arabidopsis thaliana-Pseudomonas syringae pv. tomato System. Master's Thesis (Toronto, Canada: University of Toronto).
- Lazarovits, G., Stössel, R., and Ward, E.W.B. (1981). Age-related changes in specificity and glyceollin production in the hypocotyl reaction of soybeans to Phytophthora megasperma var. sojae. Phytopathology 71, 94–97. [Google Scholar]
- Lotan, T., Ori, N., and Fluhr, R. (1989). Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell 1, 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleck, K., and Dietrich, R. (1999). Defense on multiple fronts: How do plants cope with diverse enemies? Trends Plant Sci. 4, 215–219. [DOI] [PubMed] [Google Scholar]
- Maleck, K., Levine, A., Eulgem, T., Morgan, A., Schmid, J., Lawton, K.A., Dangl, J.L., and Dietrich, R.A. (2000). The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat. Genet. 26, 403–409. [DOI] [PubMed] [Google Scholar]
- Martinez-Zapater, J.M., Coupland, G., Dean, C., and Koornneef, M. (1994). The transition to flowering in Arabidopsis. In Arabidopsis, E.M. Meyerowitz and C.R. Somerville, eds (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 403–411.
- Miller, M.E. (1983). Relationship between onion leaf age and susceptibility to Alternaria porri. Plant Dis. 67, 284–286. [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nawrath, C., and Métraux, J.-P. (1999). Salicylic acid induction deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen infection. Plant Cell 11, 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson, L., Wood, D., and Pierson, E. (1998). Homoserine lactone-mediated gene regulation in plant-associated bacteria. Annu. Rev. Phytopathol. 36, 207–225. [DOI] [PubMed] [Google Scholar]
- Pieterse, C.M.J., van Wees, S.C.M., van Pelt, J.A., Knoester, M., Laan, R., Gerrits, H., Weisbeek, P.J., and van Loon, L.C. (1998). A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10, 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, Z.A., Rijkenberg, F.H.J., and Wilcoxson, R.D. (1988). Effects of growth stage, leaf position, and temperature on adult-plant resistance of wheat infected by Puccinia recondita f.sp. tritici. Plant Pathol. 37, 36–44. [Google Scholar]
- Quirino, B.F., Normanly, J., and Amasino, R.M. (1999). Diverse range of gene activity during Arabidopsis thaliana leaf senescence includes pathogen-independent induction of defense-related genes. Plant Mol. Biol. 40, 267–278. [DOI] [PubMed] [Google Scholar]
- Quirino, F., Noh, Y., Himelblau, E., and Amasino, R.M. (2000). Molecular aspects of leaf senescence. Trends Plant Sci. 5, 278–282. [DOI] [PubMed] [Google Scholar]
- Rauscher, M., Adam, A.L., Wirtz, S., Guggenheim, R., Mendgen, K., and Deising, H.B. (1999). PR-1 protein inhibits the differentiation of rust infection hyphae in leaves of acquired resistant broad bean. Plant J. 19, 625–633. [DOI] [PubMed] [Google Scholar]
- Reuveni, M., Tuzun, S., Cole, J.S., Siegel, M.R., and Kuc, J. (1986). The effects of plant age and leaf position on the susceptibility of tobacco to blue mold caused by Peronospora tabacina. Phytopathology 76, 455–458. [Google Scholar]
- Roelfs, A.P. (1984). Race specificity and methods of study. In The Cereal Rusts, Vol. 1: Origins, Specificity, Structure and Physiology, W.R. Bushnell and A.P. Roelfs, eds (Orlando, FL: Academic Press), pp. 131–164.
- Rogers, E.E., and Ausubel, F.M. (1997). Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell 9, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumen, E.C., Bonman, J.M., and Parlevliet, J.E. (1992). Leaf age related partial resistance to Pyricularia oryzae in tropical lowland rice cultivars as measured by number of sporulating lesions. Phytopathology 82, 1414–1417. [Google Scholar]
- Rupe, J.C., and Gbur, E.E., Jr. (1995). Effects of plant age, maturity group, and environment on disease progress of sudden death syndrome of soybean. Plant Dis. 79, 139–143. [Google Scholar]
- Scholl, R., Rivero-Lepinckas, L., and Crist, D. (1998). Growth of plants and preservation of seeds. In Arabidopsis Protocols, J.M. Martinez-Zapater and J. Salinas, eds (Totowa, NJ: Humana Press), pp. 3–5.
- Smith, D.A. (1982). Toxicity of phytoalexins. In Phytoalexins, J.A. Bailey and J.W. Mansfield, eds (New York: Halstead Press/John Wiley and Sons), pp. 218–252.
- Stall, R.E., and Hall, C.B. (1984). Chlorosis and ethylene production in pepper leaves infected with Xanthomonas campestris pv. vesicatoria. Phytopathology 74, 373–375. [Google Scholar]
- Takahashi, T. (1972). Studies on viral pathogenesis in plant hosts. III. Leaf age-dependent susceptibility to tobacco mosaic virus infection in “Samsun NN” and “Samsun” tobacco plants. Phytopathol. Z. 75, 140–155. [Google Scholar]
- Telfer, A., Bollman, K., and Poethig, S. (1997). Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development 124, 645–654. [DOI] [PubMed] [Google Scholar]
- Uknes, S., Mauch-Mani, B., Moyer, M., Potter, S., Williams, S., Dincher, S., Chandler, D., Slusarenko, A., Ward, E., and Ryals, J. (1992). Acquired resistance in Arabidopsis. Plant Cell 4, 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uknes, S., Winter, A., Delaney, T., Vernooij, B., Morse, A., Friedrich, L., Nye, G., Potter, S., Ward, E., and Ryals, J. (1993). Biological induction of systemic acquired resistance in Arabidopsis. Mol. Plant-Microbe Interact. 6, 692–698. [Google Scholar]
- Van Loon, L.C., Bakker, P.A.H.M., and Pieterse, C.M.J. (1998). Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36, 453–483. [DOI] [PubMed] [Google Scholar]
- Vernooij, B., Friedrich, L., Morse, A., Reist, R., Kolditzjawhar, R., Ward, E., Uknes, S., Kessmann, H., and Ryals, J. (1994). Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell 6, 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, E.R., Uknes, S.J., Williams, S.C., Dincher, S.S., Wiederhold, D.L., Alexander, D.C., Ahl-Goy, P., Métreaux, J.P., and Ryals, J.A. (1991). Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 3, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, E.W.B., Stössel, R., and Lazarovits, G. (1981). Similarities between age-related and race-specific resistance of soybean hypocotyls to Phytophthora megasperma var. sojae. Phytopathology 71, 94–97. [Google Scholar]
- Weaver, L.M., Gan, S., Quirino, B., and Amasino, R.M. (1998). A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol. Biol. 37, 455–469. [DOI] [PubMed] [Google Scholar]
- Whalen, M.C., Innes, R.W., Bent, A.F., and Staskawicz, B.J. (1991). Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 3, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, J., Hutcheon, C.J., Higgins, V.J., and Cameron, R.K. (2000). A functional gene-for-gene interaction is required for the production of an oxidative burst in response to infection with avirulent Pseudomonas syringae pv. tomato in Arabidopsis thaliana. Physiol. Mol. Plant Pathol. 56, 253–261. [Google Scholar]
- Wyatt, S., Pan, S., and Kuc, J. (1991). β-1,3-Glucanase, chitinase and peroxidase activities in tobacco tissues resistant and susceptible to blue mould as related to flowering, age and sucker development. Physiol. Mol. Plant Pathol. 39, 433–440. [Google Scholar]
- Yalpani, N., Silverman, P., Wilson, T.M.A., Kleier, D.A., and Raskin, I. (1991). Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell 3, 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani, N., Shulaev, V., and Raskin, I. (1993). Endogenous salicylic acid levels correlate with accumulation of pathogenesis-related proteins and virus resistance in tobacco. Phytopathology 83, 702–708. [Google Scholar]
- Yahraus, T., Chandra, S., Legendre, L., and Low, P. (1995). Evidence for a mechanically induced oxidative burst. Plant Physiol. 109, 1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]