Abstract
Death receptor–mediated activation-induced apoptosis of antigen-specific T cells is a major mechanism of peripheral tolerance induction and immune homeostasis. Failure to undergo activation-induced cell death (AICD) is an important underlying cause of many autoimmune diseases. Thus, enhancing the T cell’s own suicide mechanism may provide an efficient therapy for the treatment of autoimmune diseases. Bisindolylmaleimide VIII (Bis VIII), a PKC inhibitor, can sensitize T cells for death receptor–induced apoptosis and thus can inhibit the development of T cell–mediated autoimmune disease in vivo. In this study, we have analyzed the functional consequences of accelerated suicide for a protective CD8+ T cell–mediated immune response. Our data indicate that CD8+ T cells are sensitized by Bis VIII to AICD, both in vitro and in vivo. The sensitizing effect of Bis VIII appears to be mediated by specific downmodulation of the antiapoptotic molecule cellular FLICE-like inhibitory protein (cFLIPL). Importantly, Bis VIII administration during an acute lymphocytic choriomeningitis virus (LCMV) infection causes the depletion of virus-specific CD8+ T cells and subsequently impaired cytotoxicity and virus clearance. We conclude that resistance to death receptor–induced apoptosis is crucial for the efficient induction of a protective immune response, and that Bis VIII–based immunotherapies have to be applied under well-controlled conditions to avoid the induction of immune incompetence and the inability to respond to pathogen infection.
Introduction
Death receptors of the TNF receptor family are crucially involved in immune homeostasis, cytotoxicity, and immune privilege (1–3). In particular, autoreactive T and B cells that escape central deletion are efficiently depleted by death receptor–induced apoptosis (2). Thus, chronic activation of T lymphocytes induces expression of death receptor and corresponding ligand and may result in cell-autonomous suicide (4–7). In contrast, autoreactive B cells are killed through interaction with death ligand–expressing T cells (8, 9). Failure to eliminate such autoreactive lymphocytes, as is the case in Fas-defective (CD95/Apo-1–defective) lpr mice and Fas ligand–defective gld mice, causes the development of severe autoimmune diseases (10). Defective elimination of autoreactive lymphocytes appears to be a major cause of many autoimmune diseases, in human patients as well as in experimental animal models (2, 11). Thus, mechanisms that prevent death receptor–mediated suicide are a major target for autoimmune disease therapy.
Recently, Zhou and colleagues reported that the PKC inhibitor bisindolylmaleimide VIII (Bis VIII) sensitizes cells specifically to death receptor–mediated apoptosis but not to other mechanisms of apoptosis induction — for example, DNA damage — or glucocorticoid-induced cell death (12). Thus, such a compound would fulfill the expectation of a therapeutic drug targeting the life of autoreactive T and B cells. Most interestingly, in vivo administration of Bis VIII strongly reduces the onset and clinical symptoms of autoimmune diseases on two different experimental models. The Bis VIII–based therapy appears to be specific for activated lymphocytes, since activated T and B cells express high cell-surface levels of death receptors, such as Fas, but also express abundant levels of diverse antiapoptotic molecules that prevent the premature demise of these cells upon antigen-specific activation. It is this prolonged survival that impedes an efficient control of autoreactive T and B cells by death receptor–mediated peripheral deletion. Therefore, Bis VIII–based therapies may offer an attractive treatment for the mostly CD4+ T cell–mediated autoimmune diseases in particular but also for uncontrolled and destructive T cell–mediated immune responses in general (13).
Although uncontrolled activation of autoreactive T cells is destructive, activation and expansion of normal T and B cells is crucial for mounting an efficient immune response against life-threatening infections by pathogens. In this study, we have investigated whether CD8+ T cells can be sensitized by Bis VIII like CD4+ T cells, and whether sensitization for accelerated suicide might affect the normal protective immune response against pathogens. Using the lymphocytic choriomeningitis virus (LCMV) infection model in mice, we found that the number of virus-specific T cells is strongly reduced upon Bis VIII treatment and that the decrease in the number of virus-specific cytotoxic T cells leads to inefficient anti-LCMV immune response and virus clearance. We conclude that resistance to death receptor–mediated apoptosis is an important element in the initiation phase of normal T cell–mediated immune responses and that Bis VIII–based therapies for the treatment of autoimmune diseases must be applied under well-controlled conditions to avoid immune deficiency against opportunistic viral or bacterial infections.
Methods
Mice and reagents.
C57BL/6 (H-2b) (Biological Research Laboratories, Fuellinsdorf, Switzerland) and LCMV gp33 peptide T cell receptor (TCR) transgenic mice on the C57BL/6 background (line 318; kindly provided by H.P. Pircher, Freiburg, Germany, and H. Hengartner, Zurich, Switzerland) were held and bred at the animal facility of the Department of Medicine, University of Bern. All animal experiments were performed in compliance with the laws and guidelines of the State of Bern. Bis VIII was purchased from Alexis (Läufelingen, Switzerland). Anti–CD8α Cy-chrome, anti–CD8β FITC, anti–CD8β phycoerythrin(PE), anti–CD4 PE, anti–Vα2 PE, and neutralizing anti-FasL (MFL-3) were obtained from PharMingen (La Jolla, California, USA). Goat anti-rat FITC was obtained from Southern Biotechnology Associates (Birmingham, Alabama, USA) and neutralizing anti–TNF-α (IP-400) from R&D Systems (Minneapolis, Minnesota, USA). Cell culture media Iscove’s Modified Dulbecco’s Medium (IMDM) and MEM were obtained from Invitrogen Life Technologies (Carlsbad, California, USA). Recombinant human IL-2 was obtained from Chiron (Amsterdam, the Netherlands). The hybridoma producing the VL4 antibody (recognizing LCMV-infected cells), the LCMV strain WE, the virus propagation cell line MC57G, and the thymoma target cell line RMA were kindly provided by R. Zinkernagel, S. Oehen, and D. Kägi (Zurich, Switzerland). Concanavalin A (Con A) was obtained from Sigma-Aldrich (St. Louis, Missouri, USA).
Assessment of activation-induced cell death in vitro.
Spleen cells from C57BL/6 mice were isolated by dissociation of the spleen between frosted microscopy slides, which was followed by hypotonic lysis of erythrocytes. CD4+ and CD8+ T cells were then isolated by negative selection using antibody-coated magnetic beads. Briefly, cells were stained with either anti–B220-biotin and anti–CD8β-biotin for the isolation of CD4+ cells or anti–B220-biotin and anti–CD4-biotin for the isolation of CD8+ cells. Then streptavidin-coated magnetic beads (MACS; Miltenyi, Bergisch Gladbach, Germany) were added, and contaminating cells were removed on iron mesh–containing columns placed in a strong magnetic field (MACS). Negatively selected cells consisted mostly (>95%) of CD4+, respectively CD8+ T cells, as determined by flow cytometry. Purified T cells were then mixed and cocultured with irradiated C57BL/6 spleen cells and stimulated for 2 days with 1 μg/ml Con A, washed, and further cultured for 3 days with 80 U/ml recombinant IL-2. Viable cells were then isolated by Ficoll gradient and resuspended in RPMI containing 10% FCS. To induce activation-induced apoptosis, CD4+ or CD8+ T cells were cultured overnight on flat-bottom, 96-well tissue culture plates, previously coated with 2 μg/ml anti-CD3, in the presence or absence of various concentrations of Bis VIII. FasL or TNF-α dependency of activation-induced apoptosis was determined by adding neutralizing anti-FasL (10 μg/ml) or anti–TNF-α (1:100). Cells were then harvested and apoptosis was detected by assessment of DNA fragmentation in individual nuclei, as described by Nicoletti et al. (14).
Adoptive transfer and viral infection.
Splenic T cells were isolated from mice expressing the TCR specific for the gp33–41 (gp33) peptide of LCMV (line 318) (15). The relative distribution of CD8+ T cells expressing the transgenic TCR (Vα2+ Vβ8+) was determined by flow cytometry, and 0.5 × 106 Vα2+ CD8α+ cells were injected intraperitoneally into 7- to 10-week-old C57BL/6 mice. The day after, mice were infected by intraperitoneal injection of 2 × 104 PFU of LCMV strain WE in a total volume of 100 μl of MEM/2% FCS. Bis VIII (220 μg per mouse) or PBS/10% DMSO control was administered on days –2, 0, 2, 4, and 6 before and after infection by intraperitoneal injection, as described previously (12). In some experiments, one group of mice was also treated intraperitoneally every other day with 25 mg of cyclosporin A (CsA) per kilogram of body weight (Sandimmun, Novartis, Basel, Switzerland). On day 7, at the time point of maximal antiviral CTL expansion, mice were euthanized, and tissue samples and sera were collected for further analysis.
Assessment of virus-specific T cells.
Spleen cells were isolated as described above. Intestinal intraepithelial lymphocytes (IELs) were isolated as described previously (16). Briefly, the small intestine of infected mice was isolated and Peyer’s patches were removed. The intestine was then opened longitudinally, rinsed in HEPES-buffered salt solution (HBSS; 10 mM HEPES [pH 7.2], 25 mM NaHCO3, 5.4 mM KCl, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 137 mM NaCl, 5.6 mM D-glucose), and cut in 5-mm-long pieces. IELs were then dissociated by incubation of tissue in HBSS containing 1 mM DTT and separated by a 40%/70% Percoll gradient. IEL fractions usually consisted of 30–70% CD8+ T cells. Spleen cells and IELs were then stained with anti–CD8α Cy-chrome and anti–Vα2 PE to detect the expansion of T cells carrying the gp33-specific transgenic TCR. Samples were analyzed by flow cytometry on a FACScan (Becton Dickinson, San Jose, California, USA) using Cell Quest software. Electronic gates were set around lymphocyte populations based on forward-side scatter properties, and the percentage of CD8+/Vα2+ T cells was analyzed among total CD8+ T cells.
LCMV cytotoxicity assay.
The 51Cr release assay was performed as described previously (17). Briefly, RMA targets were labeled with 75 μCi 51Cr (Amersham Life Sciences, Aylesbury, United Kingdom) per 106 cells in IMDM/2% FCS for 1 hour at 37°C and 5% CO2. Labeled cells were then washed with medium and resuspended at 3 × 104 cells per milliliter in IMDM/2% FCS. Before use, target cells were pulsed with 1 μg/ml of the immunodominant LCMV peptide gp33 or the adenovirus-derived nonapeptide adn5 (kindly provided by H.P. Pircher) (17). Effector cells were then resuspended at 3.2 × 106 cells per milliliter in IMDM/5% FCS containing 1 mM L-glutamine (Seromed, Berlin, Germany), 1 μM β-mercapto-ethanol, and nonessential amino acids (Roche, Basel, Switzerland). Effector cells were serially diluted in 96-well V-bottom tissue culture plates (Costar, Cambridge, Massachusetts, USA), and 3,000 target cells per well were added. After a quick spin, cells were incubated at 37°C and 5% CO2 for 5 hours. Cell-free supernatant was harvested, and 51Cr released was measured in a TopCount scintillation counter (Canberra Packard, Meriden, Connecticut, USA). Maximum counts were determined by lysing cells in 0.5% Nonided P40, spontaneous release by culturing target cells in the absence of effector cells. Specific lysis was calculated according to the following equation: ([experimental counts – spontaneous counts]/[maximum counts – spontaneous counts]) × 100.
Immunohistochemistry of virus-infected tissue.
Tissue samples were embedded in Tissue Tek cryosection medium (Sakura Finetek, Zoeterwoude, the Netherlands). Five-micrometer sections were made, mounted on poly-L-lysine–coated glass slides, and fixed in acetone for 15 minutes. Sections were then rehydrated and blocked for unspecific binding in antibody dilution buffer (Tris-buffered saline containing 5% goat serum, 0.5% casein, 0.05% sodium azide). LCMV-infected cells were detected by staining with VL-4 antibody (18) and secondary goat anti-rat FITC antibody. Rat IgG was used as an isotype control. Sections were then analyzed by fluorescence microscopy.
Plaque assay.
Viral replication in infected tissues and sera was determined by plaque assay as described previously (17, 18). Briefly, upon euthanization, tissue samples of equal size and weight were removed and frozen in MEM/2% FCS until further use. After thawing, samples were homogenized and cell-free supernatants were added in serial dilutions to MC57G cells in 24-well tissue culture plates (TPP, Trasadingen, Switzerland). Virus was allowed to infect MC57G cells for 4 hours before 50% methylcellulose (Methocel, Sigma-Aldrich) in MEM was added to prevent virus budding. After 48 hours of incubation at 37°C and 5% CO2, cells were fixed in PBS/4% paraformaldehyde and permeabilized in HBSS/1% Triton X-100 (Fluka, Buchs, Switzerland). Virus-infected cells (plaques) were then detected by staining with the VL4 antibody, peroxidase-conjugated goat anti-rat (DAKO Diagnostics, Zug, Switzerland), and o-phenylenediamine dichloride (Sigma-Aldrich) according to the manufacturer’s instructions. Viral titers were calculated by counting plaques and multiplying with the dilution factor.
In vivo proliferation assay.
Upon transfer of TCR transgenic T cells, mice were infected with LCMV and treated with Bis VIII as described above. On day 5 after infection, control mice and Bis VIII–treated mice were injected intraperitoneally with 25 μCi of [3H]thymidine (Amersham Life Sciences). Four hours later, mice were sacrificed and spleen cells were isolated and stained for CD8 and Vα2. Virus-specific CD8+Va2+ T cells were then sorted on a FACS Vantage (Becton Dickinson, Franklin Lakes, New Jersey, USA) directly onto microscope slides. Slides were then dipped into autoradiography photoemulsion (Kodak, Denges, Switzerland), dried, and exposed for 2 weeks. Upon development, cells were counterstained with nuclear fast red, and the percentage of proliferating cells was calculated (cells with seven or more silver grains were considered to be positive). Experiments were done with at least three mice per group.
Detection of antiapoptotic gene products by RT-PCR.
Freshly isolated cells or Con A blasts (day 5) were stimulated with plate-bound anti-CD3 (2 μg/ml) for 8 hours, and Bis VIII (5 μM) was added as indicated. Cells were harvested and lysed in TRI-Reagent (Sigma-Aldrich). RNA was extracted according to the manufacturer’s protocol. cDNA was synthesized from 1.5 μg of total RNA using a commercial cDNA kit (Promega, Madison, Wisconsin, USA), oligo-dT primers, and the manufacturer’s suggested conditions. The PCR reaction mixture consisted of 36.7 μl of nuclease-free water, 5 μl of 10× reaction buffer, 1 μl of dNTP (10 μM), 0.3 μl of Taq polymerase (5 U/μl), 0.5 μl of each primer (100 μM), 2 μl of MgCl2 (50 mM) (all from Invitrogen), and 4 μl of serially diluted RT product (1:1, 1:3, 1:9). The following amplification conditions were used: 1 minute at 95°C, 1 minute at 55°C, and 1 minute at 72°C for 20 cycles for actin; 1 minute at 95°C, 1 minute at 55°C, and 2 minutes at 72°C for 43 cycles for cellular FLICE-like inhibitory protein (cFLIPL); 1 minute at 95°C, 1 minute at 52°C, and 1 minute at 72°C for 35 cycles for cellular inhibitor of apoptosis-1 (cIAP-1) and -2; 1 minute at 95°C, 1 minute at 55°C, and 1 minute at 72°C for 32 cycles for Fas and FasL; 1 minute at 95°C, 1 minute at 50°C, and 1 minute at 72°C for 32 cycles Bcl-xL; and a final extension of 10 minutes at 72°C. All reactions were performed in a Biometra T3 Thermocycler (Whatman Biometra, Goettingen, Germany). The primers used were murine β-actin forward (5′-TGGAATCCTGTGGCATCCATGAAAC-3′) and reverse (5′-TAAAACGCAGCTCAGTAACAGTCCG-3′), murine cFLIPL forward (5′-GTCACATGACATAACCCAGATTGT-3′) and reverse (5′-GTACAGACTGCTCTCCCAAGCACT-3′), murine cIAP-1 forward (5′-GCCATTGTCTTTTCTGTCACC-3′) and reverse (5′-CTGCGTCTGCATTCTCATC-3′), murine cIAP-2 forward (5′-ACCTAGTGTTCCTGTTCAGC-3′) and reverse (5′-CCTTCTCCTCTTCTCTTCTCTC-3′), murine FasL forward (5′-CAGCAGTGCCACTTCATCTTGG-3′) and reverse (5′-TTCACTCCAGAGATCAGAGCGG-3′), murine Fas forward (5′-GAGGACTGCAAAATGAATGGGG-3′) and reverse (5′-ACAACCATAGGCGATTTCTGGG-3′), and murine Bcl-xL forward (5′-TAGTCCAGCCAGGCACGT-3′) and reverse (5′-GGCTGATATCATACTGCAT-3′). PCR products were separated on a 4% polyacrylamide–Tris-borate-EDTA gel and stained with ethidium bromide.
Alternatively, Fas, FasL, and cFLIPL mRNA expression was also detected by real-time RT-PCR. Briefly, 1 μg of total RNA was treated with DNase for 30 minutes at 37°C and then reverse transcribed as described above. One microliter of 1:2 diluted RT product was then added to the PCR mix consisting of 12.5 μl of SYBR green mix (Applied Biosystems, Foster City, California, USA), 1.5 μl of forward primer (5 μM), 1.5 μl of reverse primer (5 μM), and 8.5 μl of H2O. For Fas and FasL, the same primers as described above were used; for cFLIPL and GAPDH, the following primers were used: cFLIPL forward (5′-GGAGGTAGATGGGCCATCAA-3′) and reverse (5′-TCCACGCATACACTTTGTCCA-3′), and GAPDH forward (5′-TTCACCACCATGGAGAAGGC-3′) and reverse (5′-GGCATGGACTGTGGTCATGA-3′). The DNA was amplified in a TaqMan PCR machine (Applied Biosystems) using the following conditions: 2 minutes at 50°C, 10 minutes at 95°C, and 45 cycles of 15 seconds at 95°C and 2 minutes at 60°C. Fold increase in gene expression was calculated using the Sequence Detection System Version 1.7 from Applied Biosystems.
Western blot for cFLIPL.
Con A–stimulated T cell blasts were generated as described above. Viable cells were then purified by Ficoll gradient centrifugation, washed, and resuspended in culture medium. Cells were then stimulated by plate-bound anti-CD3 in the presence or absence of 5 μM Bis VIII for 4 hours. Cells were then harvested, and cellular proteins were harvested by trichloroacetic acid precipitation as described previously (19). The proteins of 3 × 106 cells were then resolved on a 12% SDS-PAGE and blotted on nitrocellulose. cFLIPL was detected using a polyclonal rabbit anti-cFLIP antibody (anti-I-FLICE, 1:500; Becton Dickinson) and a goat anti-rabbit horseradish peroxidase conjugate (Biorad, Hercules, California, USA). Equal loading of proteins was confirmed by detection of α-tubulin (anti–α-tubulin, Sigma-Aldrich). The intensity of the bands was measured densitometrically, and ratios between cFLIPL and α-tubulin were calculated and normalized with the unstimulated control.
Statistical analysis.
The differences in the percentage of virus-specific T cells in the control group versus the Bis VIII–treated group were analyzed by Student’s t test, the differences in viral titers by the Wilcoxon signed ranks test, the differences in in vivo proliferation by a univariate ANOVA, and the differences in gene expression measured by real-time PCR by an ANOVA with a Bonferroni post hoc test.
Results
Bis VIII enhances activation-induced cell death of CD4+ and CD8+ T cells.
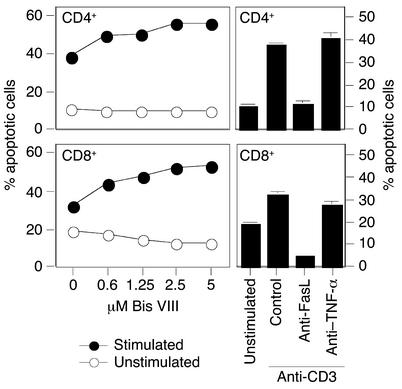
Death receptor–mediated activation-induced cell death (AICD) is a major mechanism of peripheral tolerance induction. Restimulation of primed CD4+ T cells causes the rapid expression of Fas ligand, and subsequent interaction with its receptor Fas, on the same cell (autocrine) or on neighboring cells (paracrine), induces rapid apoptotic cell death (4–7, 20). In CD8+ T cells, AICD may, in addition to Fas-FasL, involve TNF-α/TNF receptor interaction (21, 22). Since the sensitizing effect of Bis VIII has been only described so far in CD4+ T cells (12), we investigated whether AICD in CD8+ T cells can also be enhanced by Bis VIII treatment. Highly purified CD4+ and CD8+ T cell blasts, respectively, were restimulated with plate-bound anti-CD3, in the presence or absence of increasing concentrations of Bis VIII. Figure 1 shows that both CD4+ and CD8+ T cell blasts were relatively insensitive to induction of AICD; however, apoptosis was significantly enhanced by Bis VIII in a dose-dependent manner. Thus, Bis VIII can sensitize both CD4+ and CD8+ T cells to AICD. The Fas/FasL dependency of AICD in CD4+ T cells was confirmed by the efficient inhibition of apoptosis with a neutralizing anti-FasL antibody but not with an anti–TNF-α antibody. Interestingly, AICD in CD8+ T cells was also found to be mostly Fas/FasL-dependent and only minimally reduced by anti–TNF-α (Figure 1).
Figure 1.
CD4+ and CD8+ T cells are sensitized by Bis VIII for AICD. Highly purified CD4+ and CD8+ T cell blasts (day 5) were stimulated with plate-bound anti-CD3 in the presence or absence of increasing concentrations of Bis VIII. Apoptosis (AICD) was assessed by detection of fragmented apoptotic nuclei, as described in Methods. The Fas/FasL dependency of AICD was confirmed by adding neutralizing anti-FasL or anti–TNF-α antibodies. Mean values ± SD of triplicates are shown.
Bis VIII preferentially downregulates cFLIPL.
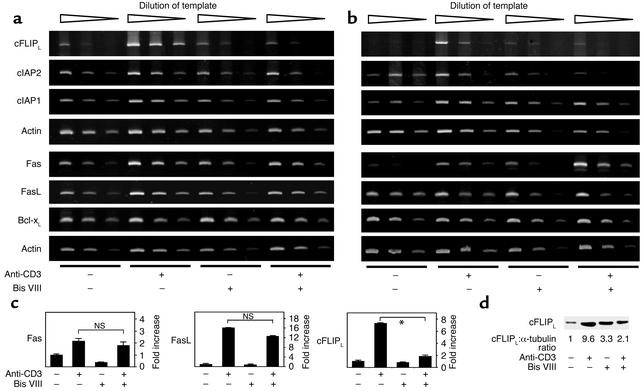
cFLIPL is a molecule with strong sequence homology to procaspase 8; however, it lacks the active site of the caspase (23, 24). It thus competes for Fas and TNF receptor complex binding and inhibits death receptor–induced apoptosis. Recent reports suggested that cFLIPL is an NF-κB–regulated gene product and that cell treatment with cycloheximide or the PKC inhibitor Bis III or VIII can downmodulate cFLIPL expression and sensitize cells to Fas-induced apoptosis (25–27). We thus analyzed the effect of Bis VIII on activation-induced cFLIPL expression in freshly isolated T cells and day 4 T cell blasts. Figure 2 (a and b) shows that cell activation caused the rapid expression of Fas and FasL; however, apoptosis induction appeared to be prevented by the simultaneous induction of cFLIPL and other antiapoptotic molecules, such as cIAP-1, cIAP-2, and Bcl-xL. Treatment of T cells with Bis VIII resulted in a strong inhibition of activation-induced cFLIPL expression. Surprisingly, the expression of cIAP-1, cIAP-2, and Bcl-xL, was less affected by Bis VIII treatment, although all of these genes are known to be transcriptionally regulated by NF-κB (28, 29). In addition, Bis VIII did not appear to inhibit T cell activation in general, since activation-induced Fas and FasL expression was unaffected by Bis VIII treatment. The data were further confirmed using quantitative real-time PCR for cFLIPL, Fas, and FasL (Figure 2c) and Western blotting for cFLIPL (Figure 2d). Thus, activation-induced Fas and FasL expression in the absence of specific apoptosis inhibitors promotes AICD in T cells.
Figure 2.
Effect of Bis VIII on antiapoptotic gene expression. Freshly isolated spleen cells (a) or day 5 T cell blasts (b) were stimulated with plate-bound anti-CD3 for 8 hours in the presence or absence of 5 μM Bis VIII. RNA was isolated and reverse transcribed, and gene expression was detected by PCR using serially diluted cDNA templates. (c) Detection of Fas, FasL, and cFLIPL expression by real-time PCR. Day 5 T cell blasts were treated as indicated in (b). Fold induction of gene expression (mean values ± SD of triplicates) as compared with unstimulated controls are shown. *P < 0.001. (d) Detection of cFLIPL protein by Western blot. T cell blasts were treated as described above. Equal protein loading was confirmed by anti–α-tubulin Western blot. Ratios between cFLIPL and α-tubulin expression, normalized to the unstimulated control, are indicated.
Bis VIII induces the premature depletion of antigen-specific CD8+ T cells in vivo.
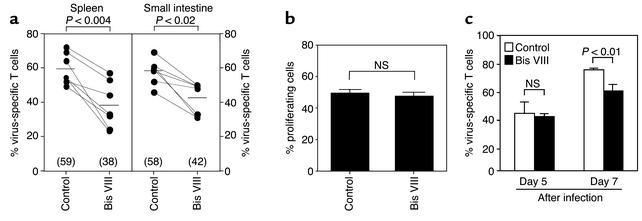
In order to investigate the effect of Bis VIII–based sensitization on peripheral deletion of antigen-specific CD8+ T cells in vivo, we established a mouse model of viral infection. LCMV infection results in the rapid induction of an antiviral immune response and efficient elimination of the virus by CD8+ cytotoxic T cells (17, 30). To follow the fate of antigen-specific T cells, 0.5 × 106 TCR transgenic T cells (recognizing the gp33 peptide from LCMV) (15, 31) were transferred into a wild-type mouse. Although these cells were undetectable in uninfected mice (data not shown), they strongly expanded during LCMV infection and comprised up to 70% of all CD8+ in the spleen on day 7 after infection (Figure 3a). As reported previously, infection of mice with high numbers of virus particles (2 × 104 PFU) also results in the infection of the intestinal mucosa (17). In agreement with this observation, we observed that virus-specific T cells (transgenic TCR) also dramatically expanded in the intestinal epithelium and comprised approximately 60% of all lymphocytes in this tissue (Figure 3a).
Figure 3.
Increased depletion of virus-specific CD8+ T cells in Bis VIII–treated animals. (a) TCR transgenic T cells were transferred into WT C57Bl/6 animals before infection with LCMV. Mice were then treated intraperitoneally every other day with either PBS or 220 μg of Bis VIII. At day 7, mice were sacrificed and the relative distribution of virus-specific T cells (percent Vα2+ CD8+ of total CD8+ T cells) in the spleen and the epithelial layer of the small intestine was analyzed by flow cytometry. Corresponding values from the same experiment are connected by lines; seven or eight experiments were analyzed. Statistical differences were analyzed by Student’s t test. Numbers in brackets and bars indicate mean values. (b) In vivo proliferation of LCMV-specific T cells. Control- or Bis VIII–treated animals were injected with [3H]thymidine at day 5 after viral infection, virus-specific T cells (Vα2+ CD8+) were isolated, and the percentage of DNA-synthesizing cells was analyzed by autoradiography. Mean values ± SD of three mice per group are shown. No statistically significant difference between the control group and the Bis VIII group was found. (c) Effect of Bis VIII on the in vivo expansion of virus-specific T cells. In the same experiment as shown in (b), the number of virus-specific T cells in the spleen was assessed at day 5 and day 7 after infection. At day 5, no significant differences were observed, whereas at day 7 significantly reduced levels of virus-specific T cells were detected in Bis VIII–treated versus control animals (P < 0.01). Mean values ± SD are shown (n = 3 per time point and group).
We next examined whether treatment of LCMV-infected mice with Bis VIII every other day would cause the sensitization and death receptor–mediated depletion of the virus-specific T cells. Figure 3a shows that in Bis VIII–treated mice, virus-specific CTL expansion was significantly reduced in both spleen and intestinal epithelium. This reduction most likely reflects the accelerated peripheral deletion in response to TCR stimulation due to the downmodulation of antiapoptotic molecules. This significant alteration in the levels of virus-specific T cells was not only observed when the expansion of T cells carrying the transgenic TCR (Vα2) was examined but also when total numbers of CD8+ T cells were monitored. LCMV infection caused a strong increase in the total number of CD8+ T cells (17, 30), which was significantly reduced in LCMV-infected mice treated with Bis VIII (data not shown).
To exclude that the reduced number of virus-specific T cells in Bis VIII–treated mice at day 7 after viral infection was due to inhibition of T cell activation and reduced expansion, we analyzed the effect of Bis VIII on T cell proliferation in vivo. Bis VIII– or control-treated mice were therefore injected with [3H]thymidine 5 days after LCMV infection, at a stage when considerable virus-specific T cell proliferation is observed. After 4 hours, virus-specific T cells were isolated, and [3H]thymidine incorporation was assessed by autoradiography. Figure 3b shows that Bis VIII at the concentrations used did not significantly affect the proliferation of antigen-specific T cells in vivo. In addition, we observed no significant changes in the numbers of Vα2+ CD8+ (TCR transgenic) T cells in peripheral blood and spleen of control and Bis VIII–treated animals at day 5 after LCMV infection (data not shown and Figure 3c), indicating that over a period of 5 days Bis VIII did not inhibit the in vivo expansion of virus-specific T cells. In marked contrast, in the same experiment virus-specific T cells were significantly (P = 0.01) reduced in Bis VIII–treated animals versus control animals 7 days after viral infection (Figure 3c), suggesting that virus-specific T cells had started to disappear by accelerated cell death. These data support the conclusion that Bis VIII does not inhibit the proliferation but enhances the apoptotic cell death of antigen-specific T cells in vivo.
Antiviral cytotoxicity is impaired in Bis VIII–treated mice.
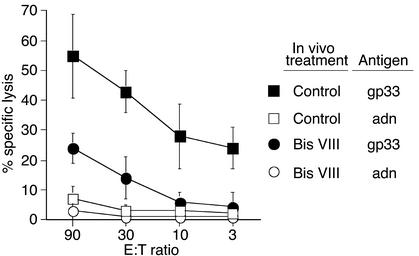
In immunocompetent mice, an LCMV infection is usually resolved after about a week because of the elimination of virus-infected cells by cytotoxic T cells. Impaired cytotoxic responses — for example, due to perforin deficiency or immune suppression — result in impaired virus elimination and persisting high viral titers (30, 32). We thus examined whether Bis VIII–mediated premature deletion of virus-specific T cells leads to reduced antiviral cytotoxicity. Figure 4 shows that total spleen cells isolated at day 7 after LCMV infection induced efficient lysis of target cells pulsed with the LCMV-derived antigenic peptide gp33, even at low effector/target ratios. In contrast, no target cell lysis was observed when targets were pulsed with a control peptide. Although antigen-specific cytotoxicity was still detected in spleen cells isolated from LCMV-infected Bis VIII–treated mice, it was strongly reduced, and approximately eight times more effector cells were required for a comparable cytotoxic response. This reduced cytotoxic response strongly correlated with the reduced numbers of virus-specific (TCR transgenic) T cells found in the spleen of Bis VIII–treated animals (Figure 3a). Thus, Bis VIII treatment causes the loss of virus-specific CD8+ T cells, resulting in reduced antiviral cytotoxicity.
Figure 4.
Impaired antiviral cytotoxicity in Bis VIII–treated mice. Animals were infected with LCMV and treated with PBS (open and filled squares) or Bis VIII (open and filled circles) as described in Methods. On day 7 after infection, spleen cells were isolated and cocultured at various effector/target (E:T) ratios with LCMV peptide–labeled (gp33, filled squares and circles) or control peptide–labeled (adn, open squares and circles) target cells. Virus-specific cytotoxicity was assessed by target cell lysis (51Cr release). Mean values ± SD from a typical experiment of three performed are shown.
Bis VIII causes uncontrolled viral replication.
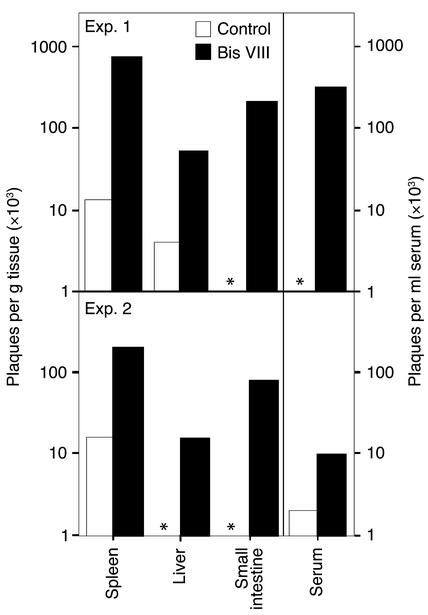
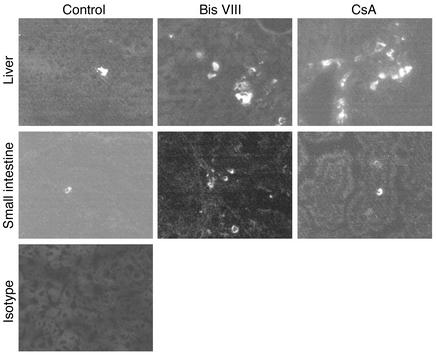
Our experiments described above demonstrated reduced numbers of virus-specific T cells and a reduced antigen-specific cytotoxicity in Bis VIII–treated versus control mice. We thus examined whether this reduction in effector T cells would result in uncontrolled viral replication and persistence. Under normal circumstances, an LCMV infection is resolved at day 7, and few virus particles are detected at this time point (17, 33) because of efficient elimination of virus-infected host cells. In agreement with this observation, no or very limited numbers of virus particles were detected in the various tissues and serum of control-treated animals (Figure 5), as determined by plaque assay. Similarly, we observed by immunohistochemistry only very few virus-infected cells in the liver and small intestine tissue sections of control-treated mice (Figure 6). In marked contrast, virus production in Bis VIII–treated animals strongly exceeded that of control animals. Although in control mice the number of virus particles was often below the detection limit, in Bis VIII–treated animals we always observed high virus titers in all tissues assessed, often several log higher than in control mice. The statistical analysis of the virus titers in control mice versus Bis VIII–treated mice revealed that the differences were always significant (spleen, P < 0.012; liver, P < 0.012; small intestine, P < 0.043; serum, P < 0.012; n = 8 per group). This inefficient elimination of virus-infected cells and consequent extensive spreading of virus particles was also reflected by immunohistochemistry (Figure 6). In particular, in the liver of Bis VIII–treated animals, high frequencies of virus-infected cells were observed. These frequencies were comparable to those in CsA-treated mice, which are known to have a suppressed antiviral immune response and uncontrolled viral spreading (32).
Figure 5.
Uncontrolled viral replication in Bis VIII–treated animals. Animals were infected with LCMV and treated with PBS (white bars) or Bis VIII (black bars) as described in Methods. On day 7 after infection, sera and samples from spleen, liver, and small intestine were collected, and levels of infectious virus particles were determined by plaque assay. Two typical experiments of nine performed are shown. An asterisk indicates viral particles below the detection limit (103 per gram of tissue or milliliter of serum).
Figure 6.
Increased frequencies of virus-infected cells in Bis VIII–treated animals. Animals were infected with LCMV and treated with PBS, Bis VIII, or CsA as described in Methods. On day 7 after infection, tissue samples from liver and small intestine were embedded in cryosection medium, and tissue sections were either stained with VL4 antibody, recognizing virus-infected cells, or isotype control and analyzed by fluorescence microscopy. Magnification, ×400. A typical experiment of four performed is shown.
Discussion
The importance of death receptor–mediated apoptosis in the regulation of immune homeostasis and the prevention of autoimmune diseases has been illustrated in many experimental animal models as well as in human patients. Death receptor–mediated apoptosis in T cells is tightly regulated by the timed expression of antiapoptotic gene products. The early induction of apoptosis resistance genes and the degradation of these molecules at a later stage of the immune response ensure appropriate initial cell activation and immune effector functions and prevent chronic T cell activation and subsequent tissue destruction by induction of T cell apoptosis, respectively. Most autoimmune diseases appear to be mediated by CD4+ T cells; however, CD8+ T cells are also involved in excessive and destructive immunopathologies, such as acute graft-versus-host disease, hepatitis, or graft rejection, and may also be involved in the exacerbation of autoimmune disorders, such as type I diabetes or inflammatory bowel disease (34–36). Thus, enhancing the cells’ own suicide program offers an attractive target for the treatment of such diseases (13, 37, 38).
Our data clearly show that a Bis VIII–based strategy of T cell apoptosis induction is operational in vitro and in vivo and that antigen-specific immune responses can be efficiently attenuated by sensitizing T cells for accelerated apoptosis. However, they also demonstrate that resistance to early death receptor–mediated apoptosis is crucial for mounting an efficient and protective immune response. Failure to resist premature cell death results in immune incompetence and the impaired elimination of the pathogen. Thus, apoptosis resistance in autoreactive T cells is regulated by similar molecules as in “normal” T cells, and the fine-tuned expression or degradation of apoptosis-resistant gene products in individual cells determines their final fate.
Since Bis VIII is known as a PKC inhibitor, it is tempting to believe that the inhibitory effect of Bis VIII on the in vivo expansion of virus-specific T cells may be due to inhibition of T cell activation rather than sensitization of these cells for accelerated suicide. However, several points support the latter possibility. At early time points (days 3–5), we always observed similar onsets of virus-specific T cell expansion, indicating that early T cell activation signals were not affected by Bis VIII treatment. Similarly, we observed no differences in the extent of in vivo proliferation in control or Bis VIII–treated mice as measured by [3H]thymidine incorporation (Figure 3b). However, since Bis VIII may have affected the in vivo expansion and proliferation already before this time window when [3H]thymidine incorporation was assessed, we also monitored the total numbers of virus-specific T cells in peripheral blood and spleen during the entire course of the infection. Our results clearly show that Bis VIII did not affect the in vivo expansion of virus-specific T cells until day 5, and only at day 7 after infection were significantly reduced levels of virus-specific T cells found in Bis VIII–treated versus control animals due to induction of premature cell death (Figure 3, a and c). Finally, our in vitro data show that TCR signaling per se is not blocked by Bis VIII, since Fas and FasL expression were still induced, and AICD was even enhanced (Figures 1 and 2, a–c). Thus, Bis VIII appears to affect antiviral immune responses by enhancing death receptor–mediated apoptosis in virus-specific T cells.
Interestingly, Bis VIII sensitizes cells only for death receptor–induced apoptosis, not for dexamethasone- and irradiation-induced cell death (12). This strongly suggests that Bis VIII induces the downregulation of a specific inhibitor of death receptors. Since cIAPs are general inhibitors of caspases (39) and Bcl-xL protects from apoptosis induction at the level of the mitochondria (40), these antiapoptotic molecules can be excluded as potential targets of Bis VIII activity. This suggestion is also supported by our observation that Bis VIII did not efficiently downregulate cIAP-1, cIAP-2, and Bcl-xL expression in T cells. In contrast, cFLIPL is a potent inhibitor of death receptor–induced apoptosis in many cell types. cFLIPL binds to the adaptor molecule Fas-associated death domain and prevents the formation of the death-inducing signaling complex (DISC) (41). cflip is an NF-κB–regulated target gene (25, 26), and its induction may in part explain the potent antiapoptotic activity of NF-κB activation (28, 42, 43). Specifically, cFLIPL is a short-lived molecule and is efficiently downregulated by Bis VIII and cycloheximide treatment (Figure 2, a and b) (25, 27).
There is increasing evidence that cFLIPL plays a crucial role in a T cell’s life cycle. cFLIPL is expressed in resting T cells (41, 44) or induced upon initial stimulation (45, 46), and appears to prevent early death receptor–mediated T cell apoptosis. T cell activation also leads to the induction of IL-2, which in turn downregulates cFLIPL expression and sensitizes T cells for cell death (44). Constitutive overexpression of cFLIPL in T cells causes refractory deletion of peripheral T cells upon superantigen stimulation and supports the accumulation of autoreactive lymphocytes (47). Thus, deregulated expression of cFLIPL and resistance to death receptor–mediated suicide may be crucially involved in the inappropriate survival of autoreactive and cytotoxic T cells. Alternatively, high levels of cFLIPL expression may shift death receptor signaling from apoptosis induction toward cell-activating signals and enhanced proliferation through activation of NF-κB and MAP kinases (48). Thus, in addition to enhancing death receptor–mediated apoptosis, downmodulation of FLIP by Bis VIII treatment in T cells may also limit the proinflammatory, activating effect of death receptor signaling.
Although the identification of apoptosis resistance genes and their inhibition by Bis VIII–based strategies may lead to the development of successful immunotherapies, our data also provide strong evidence that apoptosis resistance is crucial for the rapid induction of an immune response and the development of protective T cell effector functions. In particular, the premature demise of antiviral T cells may lead to uncontrolled virus replication and persistent infection of the host, with devastating consequences for the health of the individual and the community. Thus, while Bis VIII–based immunotherapies may provide interesting alternatives to conventional immunosuppression — for example, steroids and CsA — their use is strongly limited by the potential induction of immune incompetence.
Acknowledgments
The authors wish to thank S. Muller for stimulating advice and discussions and H. Hengartner, R. Zinkernagel, H.P. Pircher, D. Kägi, S. Oehen, and D. Dobbelaere for valuable reagents. This work is supported by grants from the Swiss National Science Foundation (31-65021.01, 3100-05079.98) and Oncosuisse (OCS-01161-09-2001, SKL 617-2-1998) to T. Brunner.
Footnotes
Diana Arnold’s present address is: Institute of Immunology, Insel Hospital, Bern, Switzerland.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: bisindolylmaleimide VIII (Bis VIII); lymphocytic choriomeningitis virus (LCMV); T cell receptor (TCR); phycoerythrin (PE); Iscove’s modified Dulbecco’s medium (IMDM); concanavalin A (Con A); cyclosporin A (CsA); intestinal intraepithelial lymphocyte (IEL); HEPES-buffered salt solution (HBSS); cellular FLICE-like inhibitory protein (cFLIPL); cellular inhibitor of apoptosis (cIAP); activation-induced cell death (AICD); death-inducing signaling complex (DISC).
References
- 1.Green DR, Ferguson TA. The role of Fas ligand in immune privilege. Nat. Rev. Mol. Cell. Biol. 2001;2:917–924. doi: 10.1038/35103104. [DOI] [PubMed] [Google Scholar]
- 2.Lenardo M, et al. Mature T lymphocyte apoptosis — immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 3.Pinkoski MJ, Brunner T, Green DR, Lin T. Fas and Fas ligand in gut and liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G354–G366. doi: 10.1152/ajpgi.2000.278.3.G354. [DOI] [PubMed] [Google Scholar]
- 4.Brunner T, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 5.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/Fas (CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 6.Ju ST, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 7.Alderson MR, et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J. Exp. Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott DW, Grdina T, Shi Y. T cells commit suicide, but B cells are murdered! J. Immunol. 1996;156:2352–2356. [PubMed] [Google Scholar]
- 9.Ju ST, Matsui K, Ozdemirli M. Molecular and cellular mechanisms regulating T and B cell apoptosis through Fas/FasL interaction. Int. Rev. Immunol. 1999;18:485–513. doi: 10.3109/08830189909088495. [DOI] [PubMed] [Google Scholar]
- 10.Nagata S, Suda T. Fas and fas ligand: lpr and gld mutations. Immunol. Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat. Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 12.Zhou T, et al. Bisindolylmaleimide VIII facilitates Fas-mediated apoptosis and inhibits T cell–mediated autoimmune diseases. Nat. Med. 1999;5:42–48. doi: 10.1038/4723. [DOI] [PubMed] [Google Scholar]
- 13.Brunner T, Mueller C. Is autoimmunity coming to a Fas(t) end? Nat. Med. 1999;5:19–20. doi: 10.1038/4695. [DOI] [PubMed] [Google Scholar]
- 14.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 15.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 16.Brunner T, Arnold D, Wasem C, Herren S, Frutschi C. Regulation of cell death and survival in intestinal intraepithelial lymphocytes. Cell. Death Differ. 2001;8:706–714. doi: 10.1038/sj.cdd.4400854. [DOI] [PubMed] [Google Scholar]
- 17.Muller S, Buhler-Jungo M, Mueller C. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection. J. Immunol. 2000;164:1986–1994. doi: 10.4049/jimmunol.164.4.1986. [DOI] [PubMed] [Google Scholar]
- 18.Battegay M, et al. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates [errata 1991, 35:115 and 1992, 38:263] J. Virol. Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 19.Niggli V, Keller H. On the role of protein kinases in regulating neutrophil actin association with the cytoskeleton. J. Biol. Chem. 1991;266:7927–7932. [PubMed] [Google Scholar]
- 20.Zhang X, et al. Unequal death in T helper cell (Th) 1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng L, et al. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 22.Speiser DE, et al. Tumor necrosis factor receptor p55 mediates deletion of peripheral cytotoxic T lymphocytes in vivo. Eur. J. Immunol. 1996;26:3055–3060. doi: 10.1002/eji.1830261235. [DOI] [PubMed] [Google Scholar]
- 23.Irmler M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 24.Tschopp J, Irmler M, Thome M. Inhibition of fas death signals by FLIPs. Curr. Opin. Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 25.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-κB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol. Cell. Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willems F, et al. Expression of c-FLIP(L) and resistance to CD95-mediated apoptosis of monocyte-derived dendritic cells: inhibition by bisindolylmaleimide. Blood. 2000;95:3478–3482. [PubMed] [Google Scholar]
- 28.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Edelstein LC, Gelinas C. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol. Cell. Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kagi D, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 31.Reich A, Korner H, Sedgwick JD, Pircher H. Immune down-regulation and peripheral deletion of CD8 T cells does not require TNF receptor-ligand interactions nor CD95 (Fas, APO-1) Eur. J. Immunol. 2000;30:678–682. doi: 10.1002/1521-4141(200002)30:2<678::AID-IMMU678>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Huegin AW, Cerny A, Hengartner H, Zinkernagel RM. Suppression by cyclosporin A of murine T-cell-mediated immunity against viruses in vivo and in vitro. Cell Immunol. 1985;90:464–473. doi: 10.1016/0008-8749(85)90211-4. [DOI] [PubMed] [Google Scholar]
- 33.Muller S, et al. Role of an intact splenic microarchitecture in early lymphocytic choriomeningitis virus production. J. Virol. 2002;76:2375–2383. doi: 10.1128/jvi.76.5.2375-2383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vizler C, Bercovici N, Cornet A, Cambouris C, Liblau RS. Role of autoreactive CD8+ T cells in organ-specific autoimmune diseases: insight from transgenic mouse models. Immunol. Rev. 1999;169:81–92. doi: 10.1111/j.1600-065x.1999.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 35.Wong FS, Janeway CA., Jr The role of CD4 versus CD8 T cells in IDDM. J. Autoimmun. 1999;13:290–295. doi: 10.1006/jaut.1999.0322. [DOI] [PubMed] [Google Scholar]
- 36.Kappeler A, Mueller C. The role of activated cytotoxic T cells in inflammatory bowel disease. Histol. Histopathol. 2000;15:167–172. doi: 10.14670/HH-15.167. [DOI] [PubMed] [Google Scholar]
- 37.Brunner T, Arnold D, Wasem C, Laissue JA, Mueller C. Death receptor–mediated suicide: a novel target of autoimmune disease treatment. Exp. Opin. Invest. Drugs. 1999;8:1–14. doi: 10.1517/13543784.8.9.1359. [DOI] [PubMed] [Google Scholar]
- 38.Carroll HP, Ali S, Kirby JA. Accelerating the induction of Fas-mediated T cell apoptosis: a strategy for transplant tolerance? Clin. Exp. Immunol. 2001;126:589–597. doi: 10.1046/j.1365-2249.2001.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deveraux QL, Reed JC. IAP family proteins — suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 40.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell. Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- 41.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 42.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 43.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 44.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 45.Di Somma MM, et al. TCR engagement regulates differential responsiveness of human memory T cells to Fas (CD95)-mediated apoptosis. J. Immunol. 1999;162:3851–3858. [PubMed] [Google Scholar]
- 46.Kirchhoff S, Muller WW, Krueger A, Schmitz I, Krammer PH. TCR-mediated up-regulation of c-FLIPshort correlates with resistance toward CD95-mediated apoptosis by blocking death-inducing signaling complex activity. J. Immunol. 2000;165:6293–6300. doi: 10.4049/jimmunol.165.11.6293. [DOI] [PubMed] [Google Scholar]
- 47.Van Parijs L, Refaeli Y, Abbas AK, Baltimore D. Autoimmunity as a consequence of retrovirus-mediated expression of C-FLIP in lymphocytes. Immunity. 1999;11:763–770. doi: 10.1016/s1074-7613(00)80150-8. [DOI] [PubMed] [Google Scholar]
- 48.Budd RC. Death receptors couple to both cell proliferation and apoptosis. J. Clin. Invest. 2002;109:437–442. doi:10.1172/JCI200215077. doi: 10.1172/JCI15077. [DOI] [PMC free article] [PubMed] [Google Scholar]