Recruitment of circulating monocytes to the arterial intima contributes to the formation of atherosclerotic lesions and may participate in their destabilization. Leukocyte emigration from blood into tissues is mediated by multiple adhesion molecules and chemokines, which orchestrate specific steps of emigration and regulate preferential recruitment of different leukocytes depending on their expression patterns of chemokine receptors. Over the last several years, a number of adhesion molecules, including VCAM-1, P-selectin and ICAM-1, the chemokines MCP-1 (also known as CCL2) and IL-8 (also known as CXCL8), and their respective receptors CCR2 and CXCR2, have been functionally implicated in atherosclerosis. Two studies — one recently published in the JCI (1), and the second reported in this issue (2) — expand this list to include the chemokine receptor CX3CR1, the receptor for fractalkine (also known as CX3CL1).
Fractalkine structure and functions
Among more than 50 known chemokines, fractalkine is the sole member of the CX3C family, and has unique structural and functional attributes (3, 4). In contrast to many other chemokines, whose presentation on the cell surface requires interaction with proteoglycans, the N-terminal chemokine domain of fractalkine is anchored to the cell membrane through a contiguous extended mucin-like stalk, transmembrane and cytoplasmic domains (Figure 1). Fractalkine binding to its seven-transmembrane domain G protein–coupled receptor triggers signaling, but it also directly mediates cell adhesion (5). Fractalkine binds CX3CR1 rapidly and firmly, which leads to tethering and arrest of leukocytes under conditions of physiological flow independent of CX3CR1 signaling (5). TNF-α–converting enzyme (also known as ADAM17) can cleave the mucin stalk of fractalkine and release soluble chemokine (6, 7). CX3CR1 has two common coding polymorphisms, namely V249I and T280M, that are in strong linkage disequilibrium (almost always occurring on the same allele) and have been associated with interindividual differences in susceptibility to both HIV infection and atherosclerosis (8–10). If replicated, these findings may have clinical relevance.
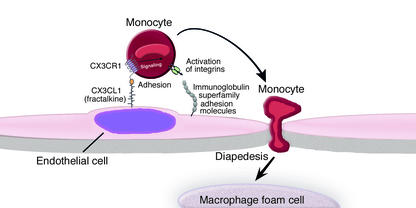
Figure 1.
Functions of fractalkine and its receptor CX3CR1 in monocyte recruitment during atherosclerosis. The recruitment of blood monocytes to the arterial intima is one of the earliest events in the formation of an atherosclerotic lesion and persists even in advanced lesions. Monocyte emigration is a multistep process that includes tethering and rolling, arrest, stable adhesion, and diapedesis (transendothelial migration). Shortly after initial tethers are established through binding of selectins or VCAM-1 on activated endothelium, monocytes encounter chemokines bound to endothelial cell–surface proteoglycans. Chemokines initiate signaling via chemokine G protein–coupled receptors, which then activates monocyte integrins and subsequently leads to reorganization of the cytoskeleton. Activated integrins mediate arrest and stable adhesion, and contribute to diapedesis by binding immunoglobulin gene superfamily adhesion molecules, including VCAM-1 and ICAM-1. Fractalkine, like other chemokines, activates multiple intracellular signaling pathways via its receptor. However, it is unique, since it is a transmembrane protein that binds to CX3CR1 rapidly and firmly, which may directly contribute to monocyte tethering and arrest. During atherogenesis, monocytes recruited to the arterial intima transform into macrophages, engulf lipids, and exhibit morphological features of foam cells.
The role of CX3CR1 in experimental atherogenesis
The article by Lesnik et al. (1) demonstrated that fractalkine expression was upregulated in atherosclerotic lesions of apolipoprotein E–/– (apoe–/–) mice, primarily in intimal smooth muscle cells, which is consistent with the expression pattern observed previously in human atherosclerosis. The function of CX3CR1 in atherosclerosis was assessed by crossing CX3CR1–/– mice into the apoe–/– background and feeding these mice a Western-type diet for 5, 10, or 15 weeks. Lesion formation throughout the aorta, including the aortic root, was significantly reduced in the CX3CR1–/– groups. These elegant data provide convincing evidence that CX3CR1 plays an important role in experimental atherogenesis, are consistent with a recent study by Combadiere et al. (11), and establish CX3CR1 as a potential therapeutic target.
In the field of experimental atherosclerosis, sophisticated transgenic and bone marrow transplantation approaches have been used to modulate the expression of candidate genes. However, the analysis, for the most part, consists of simple determination of atherosclerotic lesion area and/or volume and assessment of histological features. Similarly, Lesnik et al. (1) did not provide direct evidence of reduced monocyte recruitment. It is reasonable to assume that deficiency of a chemokine, chemokine receptor, or adhesion molecule will result in decreased monocyte recruitment to the arterial intima, which will translate into reduced lesion area and volume. Yet, one must keep in mind that there may be significant redundancy in molecular pathways contributing to leukocyte recruitment, that expression of adhesion molecules and chemokines is not restricted to the arterial endothelium, and that these molecules have functions outside of inflammation, for example in development. Intimal cells within atherosclerotic lesions express fractalkine and VCAM-1 as well as other chemokines and adhesion molecules, which may influence monocyte and/or macrophage biology, including survival, migration, gene expression or efflux. Unfortunately, little is known about the fate of individual monocytes and macrophages in atherosclerotic lesions and new approaches are required to elucidate this in transgenic mice. Similarly, new practical approaches are required to directly assess monocyte recruitment to atherosclerotic lesions.
Effects of CX3CR1 polymorphisms on function and atherogenesis
The growing catalogue of genetic association studies of atherosclerosis has tended to curb enthusiasm regarding the role of any particular gene or polymorphism in this complex phenotype (12). The paper by McDermott et al. (2), reporting association between CX3CR1 coding polymorphisms and various atherosclerosis end points in the Framingham Offspring Cohort, contrasts with most association studies principally because the authors included functional evaluation of the T280M/V249I polymorphisms. Relative to T280, the atheroprotective M280/I249 variant when expressed by transfected HEK 293 cells exhibited a lower on-rate of fractalkine binding. Transfected K562 cells or primary leukocytes from homozygous M280/I249 donors revealed markedly decreased adhesive function, signaling (calcium flux), and chemotaxis. These are important and novel insights into the significance of CX3CR1 coding polymorphisms. The association between reduced risk of atherosclerotic cardiovascular disease and CX3CR1-M280/I249 polymorphisms was based on a dominant model, since the majority of individuals were heterozygotes with a fully functional CX3CR1-T280 allele. Considering that heterozygotes have only a partial deficiency in CX3CR1 function and that redundant pathways for monocyte recruitment may exist, assessment of intermediate phenotypes, such as monocyte recruitment or abundance within lesions, is required to bolster confidence in the idea that the M280/I249 polymorphisms are truly responsible for the significant association with reduced atherosclerotic disease. Other possibilities should be explored. There are several common single nucleotide polymorphisms (SNPs) that lie within the CX3CR1 promoter (13) and it is necessary to determine if these affect CX3CR1 transcription and are in linkage disequilibrium with the M280/I249 polymorphisms. Furthermore, as is usually the case in genetic association studies, extra complexity arises from the variability of these linkage relationships among different ethnic groups and population substrata, not to mention the possibility that the observed associations resulted from linkage disequilibrium with unmeasured functional variants in closely linked genes, such as CCR8 (13). Additional studies will be needed to replicate the associations in other samples and the trend in the future should be towards analysis of all possible SNPs at a locus.
Conclusion
So where do we stand today? We now appreciate that CX3CR1 and presumably fractalkine are important players in atherogenesis, and that CX3CR1 contains polymorphisms that modulate its adhesive and signaling functions and are associated with reduced risk of atherosclerotic cardiovascular disease. But to truly understand the mechanism of CX3CR1 and other genes in a highly complex and chronic disease process such as atherosclerosis, new practical intermediate markers are required in both experimental models and clinical studies.
Footnotes
See the related article beginning on page 1241.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: single nucleotide polymorphism (SNP).
References
- 1.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1–/– mice reveals a role for fractalkine in atherogenesis. J. Clin. Invest. 2003;111:333–340. doi:10.1172/JCI200315555. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott DH, et al. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in man. J. Clin. Invest. 2003;111:1241–1250. doi:10.1172/JCI200316790. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazan JF, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 5.Imai T, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 6.Tsou CL, Haskell CA, Charo IF. Tumor necrosis factor-alpha-converting enzyme mediates the inducible cleavage of fractalkine. J. Biol. Chem. 2001;276:44622–44626. doi: 10.1074/jbc.M107327200. [DOI] [PubMed] [Google Scholar]
- 7.Garton KJ, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J. Biol. Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- 8.Faure S, et al. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–2277. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
- 9.McDermott DH, et al. Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ. Res. 2001;89:401–407. doi: 10.1161/hh1701.095642. [DOI] [PubMed] [Google Scholar]
- 10.Moatti D, et al. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- 11.Combadiere C, et al. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 12.Hegele RA. SNP judgments and freedom of association. Arterioscler. Thromb. Vasc. Biol. 2002;22:1058–1061. doi: 10.1161/01.atv.0000026801.56080.14. [DOI] [PubMed] [Google Scholar]
- 13.DeVries ME, et al. Genomic organization and evolution of the CX3CR1/CCR8 chemokine receptor locus. J. Biol. Chem. 2003;278:11985–11994. doi:10.1074/jcb.M211422200. doi: 10.1074/jbc.M211422200. [DOI] [PubMed] [Google Scholar]