It has long been known that estrogen deficiency in animal models and postmenopausal women is associated with increased osteoclastic bone resorption and bone loss (1–5). In the past decade, several important discoveries of some of the key factors involved in osteoclast formation, survival, function, and regulation by estrogen have been made.
A number of hormones and cytokines modulate osteoclastogenesis by enhancing osteoclast differentiation, activation, lifespan, and function. These include parathyroid hormone (PTH), calcitriol, PTH-related protein, prostaglandin E2, thyroxine, and IL-11 (3–5). The formation of active osteoclasts requires M-CSF (1, 3–6) and involves cell-to-cell contact between precursors of the monocyte/macrophage lineage and osteoblasts, marrow stromal cells, and T and B cells. These cells express the receptor activator of NF-κB ligand (RANKL), a member of the TNF ligand family, which is essential for this process. RANKL attaches to RANK, a receptor on the cell surface of osteoclasts and osteoclast precursors, to stimulate proliferation and differentiation of cells to form the osteoclast phenotype and inhibit apoptosis. Osteoprotegerin (OPG), a soluble decoy receptor produced by osteoblasts, marrow stromal cells, and other cells, profoundly modifies the effects of RANKL by inhibiting RANKL/RANK interaction (1, 3–5).
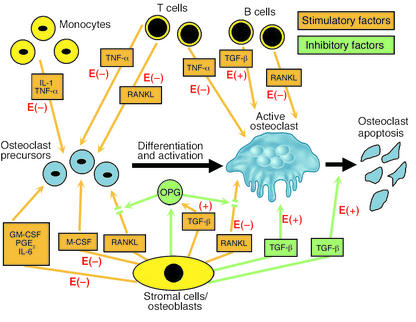
In laboratory animals, estrogen deficiency leads to increased osteoclastogenesis and bone loss, and inhibition of osteoclastogenesis is the major means by which estrogen prevents the loss of bone. This is accomplished by diminishing production of IL-1, IL-6, and TNF-α — cytokines that enhance production of M-CSF and RANKL (1, 3–5) — and downregulating NF-κB and RANKL-induced activation of JNK1 and osteoclastogenic activator protein–1 transcription factors c-Fos and c-Jun (7). Estrogen upregulates OPG (8) and TGF-β (9), and TGF-β increases OPG expression by osteoblasts and stromal cells (10) and inhibits bone resorption by increasing apoptosis of osteoclasts (11) (Figure 1).
Figure 1.
Regulation of osteoclast formation, function, and apoptosis by cytokines produced by bone marrow stromal cells, osteoblasts, monocytes, T cells, and B cells. Stimulatory factors are shown in orange and inhibitory factors in green. The effects of E to enhance (+) and inhibit (–) the factors are shown in red.
RANKL and postmenopausal bone loss
Whether the OPG/RANKL/RANK system is involved in bone loss caused by estrogen deficiency in humans was not known until now. In this issue of the JCI (12), Eghbali-Fatourechi and colleagues employ an elegant set of experiments to examine the possible role of RANKL in postmenopausal bone loss. The authors obtained bone marrow mononuclear cells and used surface markers and flow cytometry to isolate and identify preosteoblastic marrow stromal cells, T lymphocytes, and B lymphocytes in groups of premenopausal women, untreated postmenopausal women, and postmenopausal women treated with estrogen. Characteristically, serum 17β-estradiol was reduced, and serum and urinary markers of bone resorption were increased in postmenopausal compared to premenopausal women and postmenopausal women treated with estrogen. Concentrations of serum OPG and RANKL were not different in the three groups. It was found that the levels of RANKL per cell, preosteoblasts, T cells, and B cells were increased by two- to three-fold in the untreated postmenopausal women and correlated positively with serum and urinary markers of bone resorption and negatively with serum 17β-estradiol in the three groups. As discussed in this article, the fact that serum RANKL was not different in the three groups indicates the necessity for investigating its concentration in the microenvironment of marrow. These results are important because they provide strong evidence that: (i) it is the upregulation of RANKL on bone marrow cells as opposed to increases in the number of T or B cells (as occurs in rodents) that plays a pathogenetic role in increased postmenopausal skeletal remodeling; (ii) the immune system is intimately involved in this process; and (iii) estrogen directly or indirectly modifies this process. The study also provides a rationale for the use of drugs that modify osteoclastogenesis in the treatment of estrogen deficiency–related bone loss.
Effects of OPG and RANK gene mutations
The importance of the OPG/RANKL/RANK system in regulating osteoclastogenesis is underscored by the findings that OPG-deficient mice develop profound osteoclastogenesis and osteoporosis with fractures (13), and that mutations in OPG and RANK in humans cause unrestrained bone resorption and generalized bone disease. Inactivating mutations in TNFRSF11B, the TNF receptor superfamily member 11b gene encoding OPG, causes a high turnover bone disorder variously called hereditary hyperphosphatasia, hyperostosis corticalis deformans juvenilis, craniotubular dysostosis with hyperphosphatasia, or juvenile Paget’s disease (14, 15). The disorder is characterized by increased susceptibility to fractures, marked increases in skeletal remodeling with widened diaphyses and progressive deformities of long bones, deformities of the pelvis and vertebrae, and massive thickening of the calvarium. Histologically, the number of osteoclasts and osteoblasts is greatly increased. Activating mutations in TNFRSF11A, the TNF receptor superfamily member 11a gene encoding RANK, causes two bone diseases. These are familial expansile osteolysis, which is characterized by focal expansile osteolytic bone lesions and generalized osteopenia (16), and expansile skeletal hyperphosphatasia, which is characterized by deafness, premature loss of teeth, progressive hyperostotic widening of long bones, enhanced bone remodeling, and intermittent hypercalcemia (17). Since OPG has been shown to reverse the bone disease in OPG-deficient mice (18), OPG should be a made-to-order means of therapy for these clinical disorders.
Treatment of postmenopausal osteoporosis
To date, drugs and hormones that act by inhibiting osteoclast-mediated bone resorption have been the mainstay of osteoporosis treatment and prevention of fractures. In addition to estrogen, these include bisphophonates, selective estrogen receptor modulators (SERMs), and calcitonin (19). Although estrogen reduces the incidence of fractures and colorectal cancer, it increases the incidence of coronary artery disease, stroke, breast cancer, and thromboembolic events (20). An increased incidence of uterine cancer is prevented by coadministration of progestins. Raloxifene, a SERM which prevents bone loss and fractures in postmenopausal women, inhibits the growth of uterine tissue and reduces the incidence of breast cancer but increases the incidence of thromboembolic phenomena (19, 21). Calcitonin inhibits osteoclastic bone resorption, an effect mediated by calcitonin receptors. However, calcitonin downregulates calcitonin receptors, and this may reduce its effectiveness (19). Bisphosphonates act by inactivating osteoclasts to increase bone mineral density and prevent fractures whereas long-term treatment with bisphosphonates produces microdamage accumulation and increased susceptibility to fractures in dogs (22). This potential complication of bisphosphonate therapy has not been reported in patients with osteoporosis but could occur as a consequence of inhibition of bone formation rate and remains a concern (21).
New drugs that are under investigation to treat bone-resorbtion diseases include inhibitors of αvβ3 integrin, an adhesion receptor that mediates attachment of osteoclasts to bone surface (5, 19), and OPG. Indeed, in dose-response studies lasting for two to three months, single doses of OPG, which inhibits both differentiation and activation of osteoclasts, were shown to profoundly inhibit bone resorption in postmenopausal women (23) and in patients with multiple myeloma or skeletal metastases caused by breast cancer (24).
Despite the breadth and depth of these seminal discoveries, there is still much more to be learned about basic bone biology and the mechanisms by which estrogen modulates bone metabolism.
Footnotes
See the related article beginning on page 1221.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: parathyroid hormone (PTH); receptor activator of NF-κB (RANK); RANK ligand (RANKL); osteoprotegerin (OPG); selective estrogen receptor modulator (SERM).
References
- 1.Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 2.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J. Bone Miner. Res. 1996;11:1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 3.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 4.Suda T, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 5.Duong LT, Rodan GA. Regulation of osteoclast formation and function. Rev. Endocr. Metab. Disord. 2001;2:95–104. doi: 10.1023/a:1010063225902. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka S, et al. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J. Clin. Invest. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strivastava S, et al. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-κB ligand (RANKL)-induced JNK activation. J. Biol. Chem. 2001;276:8836–8840. doi: 10.1074/jbc.M010764200. [DOI] [PubMed] [Google Scholar]
- 8.Hofbauer LC, et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 9.Finkelman RD, Bell NH, Strong DD, Demers LM, Baylink DJ. Ovariectomy selectively reduces the concentration of transforming growth factor beta in rat bone: implications for estrogen deficiency-associated bone loss. Proc. Natl. Acad. Sci. U. S. A. 1992;89:12190–12193. doi: 10.1073/pnas.89.24.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thirunavukkarasu KT, et al. Stimulation of osteoprotegerin (OPG) gene expression by transforming growth factor-β (TGF-β) J. Biol. Chem. 2001;276:36241–36250. doi: 10.1074/jbc.M104319200. [DOI] [PubMed] [Google Scholar]
- 11.Weitzmann MN, et al. B lymphocytes inhibit human osteoclastogenesis by secretion of TGFβ. J. Cell. Biochem. 2000;78:318–324. doi: 10.1002/(sici)1097-4644(20000801)78:2<318::aid-jcb13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Eghbali-Fatourechi G, et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Invest. 2003;111:1221–1230. doi:10.1172/JCI200317215. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucay N, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whyte MP, et al. Osteoprotegerin deficiency and juvenile Paget’s disease. N. Engl. J. Med. 2002;347:175–184. doi: 10.1056/NEJMoa013096. [DOI] [PubMed] [Google Scholar]
- 15.Cundy T, et al. A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum. Mol. Genet. 2002;11:2119–2127. doi: 10.1093/hmg/11.18.2119. [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Pais TL, et al. Identification of a novel tandem duplication in exon I of the TNFRSF11A gene in two unrelated patients with familial expansile osteolysis. J. Bone Miner. Res. 2002;18:376–380. doi: 10.1359/jbmr.2003.18.2.376. [DOI] [PubMed] [Google Scholar]
- 17.Whyte MP, Hughes AE. Expansile skeletal hyperphosphatasia is caused by a 15-base pair tandem duplication in TNFRSF11A encoding RANK and is allelic to familial expansile osteolysis. J. Bone Miner. Res. 2002;18:376–380. doi: 10.1359/jbmr.2002.17.1.26. [DOI] [PubMed] [Google Scholar]
- 18.Min H, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J. Exp. Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 20.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Post-menopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 21.Ott SM, Oleksik A, Lu Y, Harper K, Lips P. Bone histomorphometric and biochemical markers: results of a 2-year placebo-controlled trial of raloxifene in post-menopausal women. J. Bone Miner. Res. 2002;17:341–348. doi: 10.1359/jbmr.2002.17.2.341. [DOI] [PubMed] [Google Scholar]
- 22.Mashiba T, et al. Effect of suppressed bone turnover by bisphosphonate on microdamage and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/s8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 23.Bekker PJ, et al. The effect of a single dose of osteoprotegerin in postmenopausal women. J. Bone Miner. Res. 2001;16:348–360. doi: 10.1359/jbmr.2001.16.2.348. [DOI] [PubMed] [Google Scholar]
- 24.Body JJ, et al. A Phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97(Suppl. 3):887–892. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]