A knowledge of the biochemical loci of action of 6-MP in the inhibition of nucleic acid synthesis is not sufficient to explain the effects of the thiopurines on the immune system.
—Gertrude B. Elion (Winner of 1988 Nobel Prize in Medicine for “important principals of drug development”; codiscovered 6-MP and azathioprine with George Hitchings) (1)
Azathioprine is among the oldest pharmacologic immunosuppressive agents in use today. Initially developed as a long-lived prodrug of 6-mercaptopurine (6-MP), it was quickly found to have a more favorable therapeutic index. It was soon found that 6-MP could produce remissions in childhood acute leukemia (1), and later, that azathioprine could prolong renal allograft survival (2). Over the past 50 years, azathioprine has been used in the treatment of hematologic malignancies, rheumatologic diseases, solid organ transplantation, and inflammatory bowel disease.
The drug is a purine analog, and the accepted mechanism of action is at the level of DNA (1, 3). Both in vitro and in vivo, azathioprine is metabolized to 6-MP through reduction by glutathione and other sulphydryl-containing compounds and then enzymatically converted into 6-thiouric acid, 6-methyl-MP, and 6-thioguanine (6-TG) (1, 3). Ultimately, azathioprine can then become incorporated into replicating DNA and can also block the de novo pathway of purine synthesis. It is this action that is thought to contribute to its relative specificity to lymphocytes due to their lack of a salvage pathway. However, the effects on the blockade of DNA replication have never fully explained all of the laboratory and clinical findings of azathioprine-induced immunosuppression.
Optimal T cell activation requires a costimulatory signal
T lymphocytes play a primary role in many autoimmune disorders and in allograft rejection. Optimal activation of T lymphocytes requires two signals: ligation of the T cell receptor (TCR) as well as a second costimulatory signal (4–6). Anergy, or T cell unresponsiveness to antigen encounter (7), can be induced by stimulation through the TCR in the absence of costimulation (8). TCR stimulation without a costimulatory signal can also result in apoptosis or programmed cell death (9). Apoptosis has been shown to be important for induction of peripheral tolerance in a model of transplant rejection (10). During the past decade, it has become clear that ligation of the CD28 transmembrane protein can induce this costimulatory signal (9, 11).
Since the identification of CD28 as a costimulatory molecule, intense effort has been focused on understanding the signal transduction pathways that are induced following its crosslinking. Despite this effort, controversy still exists as to whether CD28 merely augments TCR-generated signals or induces a separate set of signals. A YMNM motif in the cytoplasmic tail of CD28 allows the recruitment of PI3K and the downstream activation of the protein kinase Atk/PKB (12). This same YMNM motif also mediates CD28 interaction with the adaptor protein Grb2 (12). Recently, the interaction with Grb2 has been proposed to link CD28 to the activation of the small GTPase Rac1 via the guanine nuclear exchange factor Vav and the adaptor protein SLP-76 (12, 13). Ligation of CD28 results in increased activity of the transcription factors NF-κB and nuclear factor of activated T cells (NFAT) via this second pathway (13, 14). Once translocated to the nucleus, NF-κB can take part in the upregulation of the antiapoptotic Bcl-xL gene (9, 15). Transgenic and retroviral reconstitution of CD28–/– mice has shown that the YMNM motif is required for upregulation of Bcl-xL and for survival following TCR/CD28 stimulation (16, 17). These studies suggest that pharmacologic inhibitors that specifically interfere with CD28 signaling without affecting antigen-specific signals from the TCR may be found.
Azathioprine and CD28 signaling
The report by Tiede et al. (18) in this issue of the JCI brings together these two areas of research and may help explain Elion’s observation. The authors show that in vitro stimulation of primary human T lymphocytes in the presence of azathioprine or 6-MP results in an increased percentage of apoptotic cells. They go on to investigate the molecular mechanisms responsible and find that 6-MP interacts directly with the small GTP-binding protein Rac1, thus blocking upregulation of Bcl-xL mRNA and protein. Specifically, 6-thioguanine triphosphate (6-ThioGTP) binds to Rac1 but does not bind to another small GTP binding protein, Ras. The authors also present in vivo data indicating that inflammatory bowel disease patients treated with azathioprine have more apoptotic mononuclear cells than untreated controls, indicating that this mechanism may be responsible for the in vivo response to the drug in this disease.
Antigen-specific tolerance has been shown in experimental systems using azathioprine and 6-MP dating back to 1958 (ref. 1 and references therein). In these early experiments, 6-MP administration was shown to prevent an anti-BSA–antibody response in rabbits. Not only was the primary response suppressed, but the animals then showed long-term antigen-specific tolerance to reexposure to the antigen after the drug was discontinued. The mechanism of this tolerance now has a potential explanation. In retrospect, it is likely that CD28 costimulation was blocked — thus inducing either T cell anergy or apoptosis. Unfortunately, the tolerizing effect of azathioprine has been less robust in human solid organ transplants, resulting in movement toward newer, more potent immunosuppressive agents such as calcineurin inhibitors (cyclosporin A and tacrolimus) and the antiproliferative agents mycophenylate mophetil and rapamycin. While the clinical trend has been to increase global immunosuppression, the goal of solid organ transplantation remains long-term, allograft-specific tolerance.
Bench back to bedside?
The findings of Tiede et al. reopen the possibility that an old drug, azathioprine, holds promise for the development of drugs that could induce allograft-specific tolerance (18). Blockade of TCR-induced or costimulatory signals are among current strategies of immunosuppression (Figure 1). CD28 inhibition using the fusion protein CTLA4-Ig is currently undergoing clinical trials. However, the findings of Tiede et al. provide a potential adjunctive or alternative therapeutic approach to block the costimulatory signals that result from CD28 ligation (18). Certainly, targeting of intracellular signal transduction is not a new idea (19). In fact, the targeting of another signal transduction pathway has already been successfully translated from bench to bedside with the development of imatinib in the treatment of chronic myelogenous leukemia (20). As more effective immunosuppressive medications have become available, azathioprine has lost its place as first-line therapy in solid organ transplantation. With the knowledge that an azathioprine metabolite can block CD28 signaling via Rac1, one could envision that chemical modifications may result in a more specific compound that alone, or in combination with others, could induce long-lived antigen-specific tolerance.
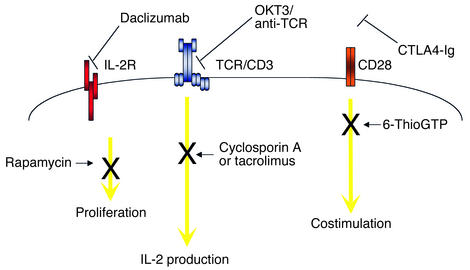
Figure 1.
Schematic of extracellular and intracellular T cell–signaling pathways targeted by immunosuppressive medications. The IL-2 receptor (IL-2R) and CD28 molecules are shown; daclizumab and CTLA4-Ig, respectively, block interaction of these cell-surface receptors with their ligands. OKT3 interacts with the TCR-associated CD3 complex. Rapamycin inhibits cell cycle progression through its interaction with mTOR. Cyclosporin A and tacrolimus inhibit calcineurin, thereby inhibiting NFAT and IL-2 synthesis. Azathioprine, through its 6-ThioGTP metabolite, inhibits CD28 signals.
Acknowledgments
We are grateful to J. Rathmell and L. Turka for their comments. J.S. Maltzman is supported by a grant from the American Society of Transplantation.
Footnotes
See the related article beginning on page 1133.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: 6-mercaptopurine (6-MP); 6-thioguanine (6-TG); T cell receptor (TCR); nuclear factor of activated T cells (NFAT); 6-thioguanine triphosphate (6-ThioGTP).
References
- 1.Elion GB. The George Hitchings and Gertrude Elion Lecture. The pharmacology of azathioprine. Ann. N. Y. Acad. Sci. 1993;685:400–407. doi: 10.1111/j.1749-6632.1993.tb35897.x. [DOI] [PubMed] [Google Scholar]
- 2.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N. Engl. J. Med. 1963;268:1315–1323. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 3.Aarbakke J, Janka-Schaub G, Elion GB. Thiopurine biology and pharmacology. Trends Pharmacol. Sci. 1997;18:3–7. doi: 10.1016/s0165-6147(96)01007-3. [DOI] [PubMed] [Google Scholar]
- 4.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 5.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust. J. Exp. Biol. Med. Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 6.Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat. Rev. Immunol. 2002;2:439–446. doi: 10.1038/nri823. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RH. T Cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J. Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 9.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109(Suppl):S97–S107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 10.Wells AD, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat. Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 11.Turka LA, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J. Clin. Invest. 2002;109:295–299. doi:10.1172/JCI200214941. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raab M, Pfister S, Rudd CE. CD28 signaling via VAV/SLP-76 adaptors: regulation of cytokine transcription independent of TCR ligation. Immunity. 2001;15:921–933. doi: 10.1016/s1074-7613(01)00248-5. [DOI] [PubMed] [Google Scholar]
- 14.Marinari B, et al. Vav cooperates with CD28 to induce NF-kappaB activation via a pathway involving Rac-1 and mitogen-activated kinase kinase 1. Eur. J. Immunol. 2002;32:447–456. doi: 10.1002/1521-4141(200202)32:2<447::AID-IMMU447>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Khoshnan A, et al. The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J. Immunol. 2000;165:1743–1754. doi: 10.4049/jimmunol.165.4.1743. [DOI] [PubMed] [Google Scholar]
- 16.Burr JS, et al. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J. Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 17.Okkenhaug K, et al. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat. Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 18.Tiede I, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J. Clin. Invest. 2003;111:1133–1145. doi:10.1172/JCI200316432. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sausville EA, Elsayed Y, Monga M, Kim G. Signal transduction-directed cancer treatments. Annu. Rev. Pharmacol. Toxicol. 2003;43:199–231. doi: 10.1146/annurev.pharmtox.43.100901.135813. [DOI] [PubMed] [Google Scholar]
- 20.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]