What? Salt and hypertension? Again? If your attention drifted and you haven’t been able to follow the debate for the last 30 years, I’d highly recommend reading the 1998 Science article appropriately entitled “The (political) science of salt” (1). Both the original article and the heated responses it generated are more entertaining than a ringside seat at a Saturday night wrestling match. Why can’t we agree on whether dietary salt is good or bad for people (or neither)? Some have suggested that the difficulty lies in the fact that the general population should be subclassified as salt “responders” and “non-responders”. Another possibility, strengthened by the work of Ni et al. (2) in this issue of the JCI, is that additional hormonal factors modulate our salt sensitivity, making it a moving target.
A tough nut to crack
Hormonal modulation of salt balance and blood volume is an indisputable truth in physiology. But does it play a critical role in hypertension? Genetic evidence certainly supports the possibility that it can. In humans, rare genetic disorders of salt transport in the kidney have been shown to be responsible for several forms of hyper- and hypotension including Liddle, Bartter, and Gitelman syndromes (reviewed in ref. 3). Nevertheless, there’s no clear evidence to date that the same genes that cause these disorders play a significant role in essential hypertension. But wait, maybe we just need to do a better job of subclassifying patients and regulating their diets when we do genetic screens. As an alternative and presumably simpler approach, animal models have been used. In the early 1960s, Dahl and colleagues reported that they had bred rats characterized by a salt-sensitive form of hypertension (4). These rats have been the subject of hundreds of research studies aimed at defining the genes and proteins responsible for salt-sensitivity. In spite of the fact that genetic heterogeneity and diet can be taken out of the equation with this approach, as of 3 years ago 24 chromosomal regions spread over 19 chromosomes had been found to contribute to hypertension in rats (5), with at least 8 of these regions having effects on blood pressure in the Dahl salt-sensitive strain. It now seems clear that although this approach still holds promise to uncover new targets in hypertension, it’s anything but simple. This is where the ability to knockout genes in mice comes in handy. In the current issue, Ni et al. show that mice having an impaired ability to make γ-melanocyte–stimulating hormone (γ-MSH) exhibit salt-sensitive hypertension (2).
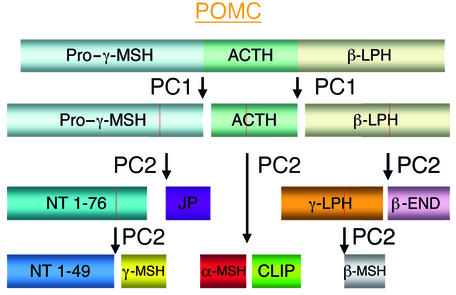
γ-MSH is a small peptide hormone that is clipped out of the middle of the larger protein precursor proopiomelanocortin (POMC) by prohormone convertase 2 (PC2) (Figure 1). Expression of POMC in the pituitary gland results in the release of several hormones, including the melanocortins, into the circulation while its expression in the hypothalamus and brainstem leads to production of melanocortinergic neurotransmitters. In normal mice, γ-MSH increases in the circulation when the animals are placed on a high-salt diet. In contrast, in PC2-knockout mice γ-MSH does not increase and the mice develop hypertension. Of course this alone would not constitute strong evidence for the role of γ-MSH since PC2 is involved in generating a lot of other peptide and protein hormones including insulin, glucagon, and somatostatin. However, the authors also show that the hypertension seen in the high-salt–fed PC2-knockout animals can be corrected by administration of a synthetic γ-MSH analogue and that the same results are seen in mice in which the γ-MSH receptor, melanocortin receptor 3 (MC3-R), is knocked out. So why hasn’t γ-MSH been implicated in salt-sensitive hypertension until now? Part of the reason is certainly the multitude of neuroendocrine signals mediated by the other peptides produced along with γ-MSH when POMC is processed (Figure 1), making it hard to isolate γ-MSH–specific actions in a physiological setting. In fact, 5 receptors have been identified to date — MC1-R, MC2-R, MC3-R, MC4-R, and MC5-R — for the melanocortin hormones alone, with more possibly to come. These receptors have a broad tissue distribution and they mediate a range of physiological responses depending on their location. These include: (a) pigmentation (MC1-R); (b) modulation of corticosterone levels (MC2-R); (c) appetite suppression and metabolic activation (MC4-R); (d) thermoregulation and water repulsion (MC5-R); and (e) inflammation (MC1-R and MC3-R). It’s easy to imagine that several of these responses might have masked or complicated the analysis of blood pressure effects of γ-MSH.
Figure 1.
Schematic representation of the cleavage of pituitary proopiomelanocortin (POMC) by prohormone convertase (PC) enzymes 1 and 2. Generated peptides include α-, β-, and γ-melanocyte–stimulating hormone (MSH), β- and γ- lipotropic hormone (LPH), adrenocorticotropic hormone (ACTH), β-endorphin (β-END), and the amino-terminal (NT), joining (JP), and CLIP peptides. Note that the release of γ-MSH requires a cleavage by PC2 at both ends.
Does this mean that γ-MSH is the cause of salt-induced hypertension in humans? Not by a long shot! The knockout of other mouse genes, including those for atrial natriuretic peptide (6) and its receptor (7), the prostaglandin receptor EP2 (8), and the bradykinin receptor (9), also leads to salt-sensitive hypertension. However, none of these as yet has been linked to the condition in humans. Moreover, the existence of γ-MSH in humans is still a matter of some debate. Nevertheless, the possibility that hormones with both central and peripheral actions like γ-MSH could cause our salt-sensitivity to fluctuate is an attractive hypothesis to test in a field where salient explanations are scarce.
Footnotes
See the related article beginning on page 1251.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: γ-melanocyte–stimulating hormone (γ-MSH); proopiomelanocortin (POMC); prohormone convertase (PC); melanocortin receptor (MC-R).
References
- 1.Taubes G. The (political) science of salt. Science. 1998;281:898–901, 903–907. doi: 10.1126/science.281.5379.898. [DOI] [PubMed] [Google Scholar]
- 2.Ni X-P, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of γ-melanocyte–stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J. Clin. Invest. 2003;111:1251–1258. doi:10.1172/JCI200316993. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 1998;281:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 4.Dahl LK, Heine M, Tassinari L. Role of genetic factors in susceptibility to experimental hypertension due to chronic excess salt ingestion. Nature. 1962;194:480–482. doi: 10.1038/194480b0. [DOI] [PubMed] [Google Scholar]
- 5.Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol. Rev. 2000;80:135–172. doi: 10.1152/physrev.2000.80.1.135. [DOI] [PubMed] [Google Scholar]
- 6.John SW, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 7.Oliver PM, et al. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2547–2551. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy CR, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat. Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 9.Alfie ME, Yang XP, Hess F, Carretero OA. Salt-sensitive hypertension in bradykinin B2 receptor knockout mice. Biochem. Biophys. Res. Commun. 1996;224:625–630. doi: 10.1006/bbrc.1996.1076. [DOI] [PubMed] [Google Scholar]