Abstract
Advances in bioinorganic chemistry since the 1970s have been driven by three factors: rapid determination of high-resolution structures of proteins and other biomolecules, utilization of powerful spectroscopic tools for studies of both structures and dynamics, and the widespread use of macromolecular engineering to create new biologically relevant structures. Today, very large molecules can be manipulated at will, with the result that certain proteins and nucleic acids themselves have become versatile model systems for elucidating biological function.
During June 16–20, 1976, several hundred chemists and biologists assembled at the University of British Columbia (UBC) to listen to 13 lectures and discuss recent developments at the interface of inorganic chemistry and biology. To be sure, there had been many previous meetings at which this new science was featured, notably one in Blacksburg, Virginia (1), and several others on special topics that were held during the 1950s and 1960s. As the old timers will remember, the Gordon Research Conference on Metals in Biology (MIB GRC, originally called Metals and Metal Binding in Biology) had its inaugural meeting in August 1962, at the New Hampton School, New Hampshire. Interest in the conference from the inorganic side grew rapidly, and in 1970, with Paul Saltman running the show, the MIB GRC tradition of close interactions among biologists, biochemists, and inorganikers was firmly established.
With apologies to the organizers and participants of earlier gatherings of the faithful, I will start with the UBC meeting, because it was the immediate precursor of the now famous International Conference on Bioinorganic Chemistry (ICBIC) series (and, accordingly, often called ICBIC-0!), created by Ivano Bertini and held first in Florence in 1983, with highly successful encores in Portugal, The Netherlands, Boston, Oxford, San Diego, Japan, Germany, and Minneapolis. On its 10th anniversary, in 2001, the ICBIC returned to Florence, as well it should have done, and on it goes, with ICBIC-11 set for Cairns, Australia, in July of this year.
Manuscripts based on 11 of the 13 lectures at UBC were published in a book called Biological Aspects of Inorganic Chemistry edited by the organizers of the meeting, Tony Addison, Bill Cullen, Dave Dolphin, and Brian James. Seven of the 11 dealt more or less with biological oxidation–reduction processes, with heavy emphasis on model systems. I could pick any of the seven papers to illustrate how far we have come in the field since the 1970s, but I will use J. H. Wang's “On the coupling of oxidation to phosphorylation in biological systems” as an example (2). Neither Wang nor anyone else knew the structures of the multisubunit redox protein complexes in the mitochondrial respiratory chain, so model work was the only game in town. Suffice it to say that the mechanistic proposals in this area have changed completely since the 1970s, owing in part to the onslaught of modern protein crystallography, which has given us the structures of cytochrome oxidase and other key protein complexes in the respiratory machinery. Bo Malmström, along with Ben Ramirez, Jay Winkler, and yours truly, wrote about these advances 8 years ago in this journal, in a perspective entitled “The currents of life: The terminal electron-transfer complex of respiration” (3).
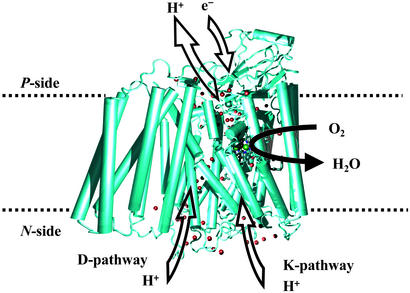
We not only know the structures of many of the respiratory enzymes (4, 5), we know a great deal about their mechanisms of action, owing to advances in methods for studying reaction dynamics, including optical triggering and detection techniques that allow even the earliest events of reactions to be observed directly (6, 7). Matters of great current interest include the mechanism of proton pumping across the mitochondrial membrane during the reduction of dioxygen by the oxidase (Fig. 1; refs. 8–14); the coupling of cytochrome c to CuA (15–17), as well as CuA to cytochrome a (18, 19); and the redox activity, if any, during turnover, of the covalently coupled tyrosine-histidine copper ligand in the binuclear heme a3-CuB active site (20). In this issue of PNAS, both Ken Karlin and Yi Lu report experiments that shed light on intermediates that likely are involved in the course of dioxygen activation/reduction by heme-copper pairs (21, 22), and Bill Tolman contributes to the discussion of dioxygen activation by metals in his density functional theoretical (DFT) analysis of the extent of electron transfer in side-bonded complexes (23).
Figure 1.
View of membrane-bound cytochrome c oxidase showing two of the proposed proton-pumping pathways as well as the site where dioxygen is reduced to water (adapted from ref. 14).
Oxidation–reduction processes continue to be a central theme of biological inorganic chemistry. Well over half of the papers in this special feature deal with biological redox reactions in one way or another. What is particularly striking in these as well as the other reports is the widespread use of powerful methods for structure determination in combination with protein engineering to obtain results that we could only have dreamt about in the 1970s.
The Metals of Biology
Iron.
Hemes have been one of the centers of attention since the earliest days of biological inorganic chemistry (24–42); indeed, work on the absorption spectra of heme proteins began in the 19th century, as the distinctive colors of some cells intrigued investigators (24). At UBC, Bob Williams, who began to address questions in the biological inorganic realm as early as the 1950s, gave the featured heme protein lecture. Some of the proposals in his paper (41), especially the ones on protein mobility and function, can now be examined quantitatively, owing to the development of rapid triggering and ultrafast detection methods to probe polypeptide folding dynamics (43, 44).
In this issue, Brian Hoffman and Jay Groves discuss experiments that have led to the elucidation of the structures of intermediates in the catalytic cycle of cytochrome P450 (33, 45); and, in other recent work, the role of axial ligands in tuning P450 redox states has been investigated by computational methods (46). Indeed, as emphasized in reports by John Dawson and Ann Walker (47, 48), axial interactions with hemes continue to be pursued vigorously (29, 37, 38), as they are involved in the binding and release of nitric oxide, the subject of Walker's paper. How times have changed: NO, the molecule of the year in 1992 (49), was mainly used to teach chemical bonding when we met in Vancouver!
Business is booming in the nonheme iron area (50–65). There are exciting new developments that deal with the binding of iron by transferrin and a possible mechanism for its release by ferritin. Liz Theil has found that pores in the ferritin structure can readily be opened both thermally and chemically and suggests that in living systems the required partial unfolding could be effected by regulatory molecules so that iron can be delivered to cells when called for (66). Phil Aisen reports evidence that the binding of iron to transferrin involves conformational searching akin to some of the gyrations in protein folding events; in other words, he gives us a glimpse of a possible energy landscape for iron binding to the protein (67). Anions play a major role in this binding, as documented in a paper coauthored with Al Crumbliss (68).
Nonheme iron enzymes catalyze an impressive array of redox transformations throughout the biosphere (50, 63). One of the highlights in this issue is Larry Que's report of an authentic nonheme ferryl complex, a long-sought species thought by many to play a key role in oxygenation catalytic cycles (69); and other important contributions to ongoing discussions of nonheme iron structures and reactivities are in the Perspectives (60, 62, 63) as well as in research papers from the laboratories of Marcetta Darensbourg, Brian Fox, Bob Hausinger, Mike Johnson (NO again!), Julie Kovacs, Don Kurtz, Mike Marletta, and Guenther Winkelmann (70–77).
At Vancouver, Dick Holm talked about his work on the core units of iron-sulfur proteins, especially the two-iron and four-iron clusters (78). Work in the area has grown explosively since that time, as recent reviews thoroughly document (51, 53, 79). Both Alex Shilov and Joseph Chatt discussed metal-mediated dinitrogen reduction at UBC, with emphasis on model systems (80, 81). Nitrogenase, the enzyme that coverts dinitrogen to ammonia under mild conditions, contains nonheme iron and molybdenum (82–87), and it was logical to assume that one of these metals likely is the locus for the activation of this frustratingly inert first row diatomic.
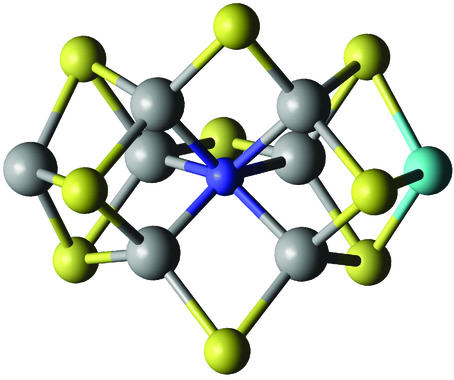
Chatt pioneered the redox chemistry of well characterized molybdenum and tungsten dinitrogen complexes, based on his feeling that molybdenum was critical for the enzymatic reaction, owing to its wide range of accessible oxidation states. Following an analysis of optimal d-electronic structures for dinitrogen reduction in protic media, Shilov, as is his custom, explored several functional models, with an emphasis on vanadium systems. Although Chatt, Shilov, and many other investigators made enormous contributions to the development of dinitrogen inorganic redox chemistry in the last century, the plain fact is that we know little more about the nitrogenase mechanism today than we did in the 1970s, a situation that is all the more perplexing in view of the high-powered armamentarium of structural and kinetics methods that has been brought to bear on the question over the last decade or so. Most of us thought that when the structure of the enzyme was solved in the early 1990s (82–84) that mechanistic understanding would soon follow. Sadly, although there has been real progress in defining the stoichiometric mechanism (85), virtually nothing is known about the intimate mechanism of dinitrogen activation (86). Of course, we can expect that the recent report of a revised active site structure (Fig. 2; ref. 87) will reinvigorate the troops.
Figure 2.
Structure of the nitrogenase active site core showing the location of a nitrogen (or possibly a carbon or oxygen) atom (blue) embedded in a cluster of seven iron atoms (gray), one molybdenum (cyan), and nine bridging sulfurs (yellow) (adapted from ref. 87).
With 20/20 hindsight, we can say that we should have expected the problem to be highly refractory, as the redox reaction in question is the Godzilla of proton-coupled electron transfer processes. Getting each one of the six electrons and six protons to the right place at the right time is a tall order! But I confidently predict that this problem will yield in this century to the young investigators who are now entering the field, and I sincerely hope that the dream of converting the most inert molecule in the air to useful nitrogen-containing substances under very mild conditions will finally be realized.
Copper.
My second favorite metal, which took a back seat at the UBC meeting, is clearly on the move (88–94), as discussed by Bertini and A. Rosato (95). It is featured in several papers in this issue, including one by Bertini and Lucia Banci on a copper protein, CopC, which along with a multicopper oxidase, CopA, may be responsible for copper resistance in Gram-negative bacteria (96). Copper also is involved in aerobic oxidation of methane to methanol by certain bacteria. Based on her investigations of the particulate methane monooxygenase from Methylococcus capsulatus (Bath), Amy Rosenzweig reports EPR, extended x-ray absorption fine structure (EXAFS), and x-ray absorption near-edge structure (XANES) spectra that shed new light on the structures of copper complexes that reside in the active center of this membrane-bound enzyme, including evidence that one of the species is a copper cluster (97). Adding to the parade is a report by Steve Ragsdale that copper is required for the proper functioning of the CO dehydrogenase/acetyl CoA synthase from Moorella thermoacetica, where a structure shows that there is a binuclear Cu-Ni unit bridged to a 4Fe–4S cluster at the ACS active site (98).
Zinc.
Charlie Riordan has shown that intramolecular NH—S hydrogen bonding affects the rates of alkylation reactions of zinc thiolates, suggesting that such interactions could modulate the activities of functionally related zinc enzymes (99), and Peter Sadler has identified binding sites in albumin that could function in the transport and delivery of zinc by blood (100). Indeed, the dipositive zinc ion, although redox-inactive, is still a hot item in biological inorganic chemistry (101–104). At UBC, as many will recall, Bert Vallee taught us the basics of zinc biochemistry (105). Some of us can even remember his “all Zn” periodic table. He showed this table of arguably one of life's more boring essential elements toward the end of many of his lectures. After one of these lectures, delivered at Caltech, students and faculty created a human periodic table of copper in a prank response to his famous “Zn table” (106). Now we learn from Marc Fontecave and Britt-Marie Sjöberg that zinc may play a role in the generation of the glycyl radical in the ribonucleotide reductase (RNR) from bacteriophage T4 (107). Interest in RNR and other radical enzymes is growing rapidly in the bioinorganic community (89, 108–114), in part because posttranslationally modified aromatic amino acids in many metalloproteins have turned out to be functionally relevant redox centers themselves.
Manganese.
The structure of photosystem II is finally yielding to an unrelenting attack by dedicated macromolecular crystallographers (115). It is very likely that full elucidation of the structure will stimulate many new assaults on the mechanism of the solar-driven oxidation of water to dioxygen, one of the holy grails of chemistry. Bioinorganic chemists have invested much time and effort in developing structural and functional models for this reaction, with emphasis on multinuclear manganese complexes (116–119). In this issue, Chuck Dismukes and George McLendon report the kinetics of proton-coupled electron transfer reactions of synthetic tetranuclear manganese-oxo clusters that bear on the question of stepwise or concerted electron/proton flow during the generation of the highly oxidized intermediates that are formed before dioxygen release from the PS II OEC (120).
Cobalt and Nickel.
A major thrust in the 1970s was work on the mechanism of vitamin B12, the subject of Bob Abeles' lecture at UBC (121). Cobalt has received much attention since the early days of bioinorganic chemistry, owing to its geometrically sensitive spectroscopic signatures as well as its rich ligand substitution and redox chemistry (24). In Vancouver, David Buckingham discussed the mechanisms of cobalt(III)-mediated hydrolyses with connections to certain zinc(II) enzymatic reactions (122). In this issue, Bob Scott reports experiments that establish relationships among the coordination structures of Co(II), Ni(II), and Zn(II) and the nature of allosteric regulation in certain DNA binding proteins (123). Although nickel was not featured at UBC, it has become a regular at more recent bioinorganic conferences, and its future in the field is bright (124, 125).
Molybdenum and Tungsten.
Nitrogenase is not the only enzyme containing molybdenum. Many oxo transfers in living organisms are catalyzed by enzymes in which we are certain that molybdenum is required for function (126, 127). In this issue, John Enemark and Dennis Lichtenberger make good use of photoelectron spectroscopy in combination with DFT calculations to investigate the electronic structures of molybdenum arene dithiolates that serve as models for the active centers of certain of these enzymes (128). Tungsten is a relative newcomer, but is making its presence known at a rapid pace, owing in part to the growing interest in life under extreme conditions (127). Activity in this area is lively, as can be judged by the talks and enthusiastic discussions at recent Molybdenum and Tungsten Enzymes GRCs.
Vanadium and Chromium.
Vanadium is another metal that has appeared on the radar screen since the UBC meeting (129), especially in the context of work on the structures and reactions of haloperoxidases containing oxovanadium(V) active centers (130). And chromium also is in the news, especially the toxicity of the hexavalent state, thanks to Julia Roberts; but, until recently, we thought trivalent chromium was okay, as people use tris-picolinatochromium(III), commonly called chromium picolinate, as a dietary supplement, in the hope that it will lead to loss of unwanted fat. More research on this complex is needed, because John Vincent has shown that it causes lethal mutations in fruit flies (131).
Alkali and Alkaline Earth Cations.
Sodium, potassium, magnesium, and calcium are four of the most important constituents of living systems; indeed, I always check to make sure that they are present (with a K/Na ratio that is good for me) in my favorite cereals. Jack Dunitz discussed the binding of sodium and potassium as well as other cations both to natural and synthetic ionophores in his lecture at UBC (132). Although there are sections and even chapters on alkali and alkaline earth metal cations in books on biological inorganic chemistry (24–28), card-carrying bioinorganic chemists have not been among the leaders in this area in recent years; make no mistake, there is a lot of terrific work underway on the biological roles of these cations (133–136), especially on the structures of potassium ion channels (137), but the lion's share of this research is being done by neurobiologists and other hard-core biologists, often in collaboration with macromolecular crystallographers and NMR spectroscopists. I guess that those folks are gutsy enough to make progress working with colorless diamagnetic metal ions.
In a thought-provoking perspective, Steve Lippard (with coauthor S. C. Burdette) argues that bioinorganic chemists are well positioned in 2003 to make major contributions to the quest for understanding the roles of potassium, calcium, and other metal ions in neurobiology, and, to drive his point home, calls the area metalloneurobiology (137). With the powerful tools that are now available to study both coordination structures and polypeptide dynamics, I am inclined to agree with him, and I firmly believe that there are great opportunities in this currently hot area for metal-oriented chemists who are willing and able to join forces with neurobiologists.
Probes and Drugs
Optical and magnetic resonance spectroscopic probes have played a major role in the rapid growth and development of biological inorganic chemistry over the last two decades. More recently, photophysical and photochemical probes have become popular as well, as can be seen from inspection of papers in this issue: Sonya Franklin reports that tryptophan fluorescence in combination with NMR and CD can be used to probe the structures of complexes between designed peptides and lanthanide ions (138); Eichii Kimura has used a fluorescent zinc complex to detect cancer cell apoptosis (139); and Jackie Barton has used rhodium complexes as photocleavage agents to detect mismatched base pairs in DNA (140).
In addition to Barton's findings with rhodium probes, Tom Tullius reports directionally bent structures in DNA duplexes with a single nucleoside gap (141); Bernhard Lippert discusses unusual structures for platinum–nucleobase complexes (142); and Jan Reedijk gives an account of platinum binding to DNA in his perspective on bioinorganic anticancer research (143). Work on metals in medicine is not confined to platinum anticancer drugs, as a quick look at the program for the inaugural (June 2002) Metals in Medicine GRC will confirm. Interest in metal-based radiopharmaceuticals (144) and MRI contrast agents (145) is exceptionally strong.
The current understanding of electron flow through proteins has been achieved mainly through the use of photoprobes, with photoactive ruthenium(II)-diimine complexes in widespread use (146–149). Timetables for electron tunneling through proteins have been validated experimentally by measurements of electron transfer kinetics in structurally characterized single crystals of Ru-modified azurins (150) as well as crystals of Zn,Fe-cytochrome c (151). In these experiments, the tunneling distances are known exactly, in contrast to the situation in the 1970s when we could only guess how far electrons could travel between donors and acceptors in biological redox reactions (152).
Metals in the Environment
With Ed Stiefel and Francois Morel leading the way, the first Environmental Bioinorganic Chemistry GRC was held less than a year ago. And why not have a separate conference on the role of inorganic substances in the environment? Metals are everywhere, in the air, on land, and in lakes, rivers, and the oceans. Much of the chemistry of natural waters by necessity involves metal complex equilibria (153), with the solubilization of ferric iron long a matter of intense interest. In this issue, Alison Butler reports a new class of amphiphilic siderophores, called amphibactins, which associate with bacterial cells, presumably facilitating the mobilization of iron from sea water (154).
The environment is not a new subject for us; indeed, it was the focus of one of the talks in 1976 at UBC (155). Dealing with toxic heavy metals is a matter of great public interest, and much of the work on the coordination chemistry of lead (156) and mercury (157) has been done with human health as a principal motivating factor. And now, as judged by the great success of the inaugural GRC, the role of metals in the environment has assumed a place as a mainstream area of our discipline. In my view, its rise to prominence should be embraced with enthusiasm; indeed, if we do not make a sustainable environment one of our highest priorities, it is not likely that we will be able to live happily on our planet for many more years.
Folding Around Metals
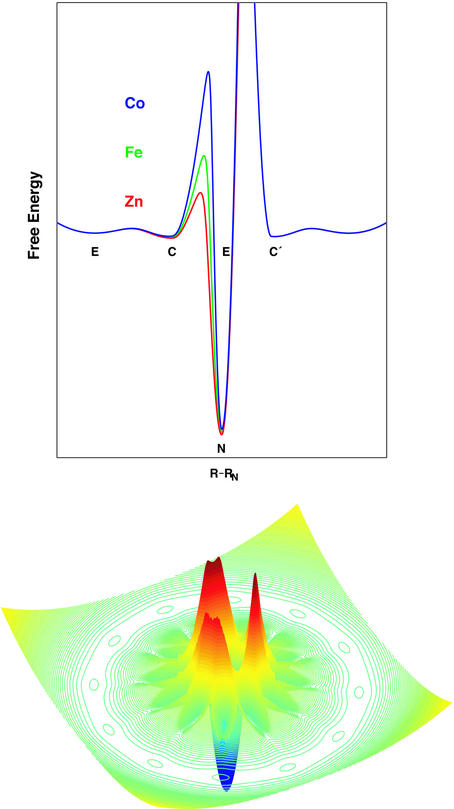
One of the grand challenges in 21st century science is understanding noncovalent assembly of complex macromolecular structures, especially those involving protein–protein, protein–ligand, and protein–nucleic acid interactions. The attack on this problem worldwide currently is centered on the physics and chemistry of protein folding (158), and the part that rightfully belongs in our field is folding around metals, as here ligand substitution processes and metal redox reactions often play a key role (159). In this issue, Vince Pecoraro discusses the folding of polypeptides around mercury (160), and other papers have discussions of the role of conformational dynamics on binding and release of metals (66–68). It is apparent that this will be one of the major growth areas for inorganic biochemistry, as in a few years it has evolved from a subject owned by the biophysics community to one in which we are players. In 1996, Jay Winkler and I showed that laser-induced electron transfer can be used to trigger folding on nanosecond timescales (43), and in this issue our coworkers I-Jy Chang and Jennifer Lee demonstrate that the folding speed limit for cytochrome c is ≈100 ns; this early event, which involves polypeptide loop formation by intrachain diffusion, can be detected by monitoring excited-state redox reactions (161). Experimentally derived maps of the folding energy landscapes for native and metal-substituted proteins (Fig. 3) show that the ligand substitution step that is required for folding is greatly inhibited when the central metal is cobalt(III), allowing early events in the search for topologically productive conformations to be examined thoroughly (162).
Figure 3.
Cytochrome c folding energy landscapes: (Upper) profiles illustrating different ligand substitution barriers for the Co, Fe, and Zn proteins; (Lower) idealized three-dimensional representation of the landscape for the native protein (adapted from refs. 44 and 162).
The many disease states that can be traced directly or indirectly to protein misfolding include Alzheimer's disease, Parkinson's disease, type II diabetes, and Huntington's disease (163, 164). In some cases, notably Lou Gehrig's disease (ALS), altered metal binding may be involved (165). Without question, the stakes are high; we must intensify our efforts to understand in detail how polypeptides are able to form native conformations, and, most importantly, how to intervene when they fail.
Acknowledgments
Many thanks to Peter Brzezinski, Angelo Di Bilio, Alex Dunn, Jenn Lee, Ed Stiefel, Akif Tezcan, and Jay Winkler for assistance with references and figures as well as helpful discussions; and to the National Science Foundation, the National Institutes of Health, and the Arnold and Mabel Beckman Foundation for research support.
References
- 1.Dessy R, Dillard J, Taylor L. Bioinorganic Chemistry, Advances in Chemistry. Vol. 100. Washington, DC: Am. Chem. Soc.; 1971. [Google Scholar]
- 2.Wang J H. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 1–36. [Google Scholar]
- 3.Ramirez B E, Malmström B G, Winkler J R, Gray H B. Proc Natl Acad Sci USA. 1995;92:11949–11951. doi: 10.1073/pnas.92.26.11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malmström B G. In: Electron Transfer in Chemistry. Balzani V, editor. Vol. 3. Weinheim, Germany: Wiley; 2001. pp. 39–55. [Google Scholar]
- 5.Svensson-Ek M, Rodgers L, Abramson J, Brzezinski P, Iwata S. J Mol Biol. 2002;321:329–339. doi: 10.1016/s0022-2836(02)00619-8. [DOI] [PubMed] [Google Scholar]
- 6.Sharp R E, Chapman S K. Biochim Biophys Acta. 1999;1432:143–158. doi: 10.1016/s0167-4838(99)00109-0. [DOI] [PubMed] [Google Scholar]
- 7.Winkler J R, Malmström B G, Gray H B. Biophys Chem. 1995;54:199–209. doi: 10.1016/0301-4622(94)00156-e. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei M J, Libeu C P, Mizushima T, et al. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 9.Verkhovsky M I, Jasaitis A, Verkhovskaya M L, Morgan J E, Wikström M. Nature. 1999;400:480–483. doi: 10.1038/22813. [DOI] [PubMed] [Google Scholar]
- 10.Michel H. Biochemistry. 1999;38:15129–15140. doi: 10.1021/bi9910934. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa S, Shinzawa-Itoh K, Tsukihara T. J Inorg Biochem. 2000;82:1–7. doi: 10.1016/s0162-0134(00)00137-9. [DOI] [PubMed] [Google Scholar]
- 12.Ruitenberg M, Kannt A, Bamberg E, Fendler K, Michel H. Nature. 2002;417:99–102. doi: 10.1038/417099a. [DOI] [PubMed] [Google Scholar]
- 13.Wikström M, Verkhovsky M I. Biochim Biophys Acta. 2002;1555:128–132. doi: 10.1016/s0005-2728(02)00267-0. [DOI] [PubMed] [Google Scholar]
- 14.Namslauer A, Aagaard A, Katsonouri A, Brzezinski P. Biochemistry. 2003;42:1488–1498. doi: 10.1021/bi026524o. [DOI] [PubMed] [Google Scholar]
- 15.Roberts V, Pique M E. J Biol Chem. 1999;274:38051–38060. doi: 10.1074/jbc.274.53.38051. [DOI] [PubMed] [Google Scholar]
- 16.Zhen Y, Hoganson C W, Babcock G T, Ferguson-Miller S. J Biol Chem. 1999;274:38032–38041. doi: 10.1074/jbc.274.53.38032. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Zhen Y, Sadoski R, Grinnell S, Geren L, Ferguson-Miller S, Durham B, Millett F. J Biol Chem. 1999;274:38042–38050. doi: 10.1074/jbc.274.53.38042. [DOI] [PubMed] [Google Scholar]
- 18.Zhen Y, Schmidt B, Kang U G, Antholine W, Ferguson-Miller S. Biochemistry. 2002;41:2288–2297. doi: 10.1021/bi0114628. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Geren L, Zhen Y, Ma L, Ferguson-Miller S, Durham B, Millet F. Biochemistry. 2002;41:2298–2304. doi: 10.1021/bi0114630. [DOI] [PubMed] [Google Scholar]
- 20.Proshlyakov D A, Pressler M A, DeMaso C, Leykam J F, DeWitt D L, Babcock G T. Science. 2000;290:1588–1591. doi: 10.1126/science.290.5496.1588. [DOI] [PubMed] [Google Scholar]
- 21.Kim E, Helton M E, Wasser I M, Karlin K D, Lu S, Huang H-w, Moënne-Loccoz P, Incarvito C D, Rheingold A L, Honecker M, et al. Proc Natl Acad Sci USA. 2003;100:3623–3628. doi: 10.1073/pnas.0737180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigman J A, Kim H K, Zhao X, Carey J R, Lu Y. Proc Natl Acad Sci USA. 2003;100:3629–3634. doi: 10.1073/pnas.0737308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer C J, Tolman W B, Theopold K H, Rheingold A L. Proc Natl Acad Sci USA. 2003;100:3635–3640. doi: 10.1073/pnas.0535926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertini I, Gray H B, Lippard S J, Valentine J S. Bioinorganic Chemistry. Mill Valley, CA: University Science Books; 1994. [Google Scholar]
- 25.Kaim W, Schwederski B. Bioinorganic Chemistry: Inorganic Chemistry in the Chemistry of Life. Chichester, U.K.: Wiley; 1994. [Google Scholar]
- 26.Lippard S J, Berg J M. Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books; 1994. [Google Scholar]
- 27.Cowan J A. Inorganic Biochemistry. New York: Wiley; 1997. [Google Scholar]
- 28.Frausto da Silva J J R, Williams R J P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. Oxford: Oxford Univ. Press; 2001. [Google Scholar]
- 29.Chan M K. Curr Opin Chem Biol. 2001;5:216–222. doi: 10.1016/s1367-5931(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 30.Dawson J H. J Inorg Biochem. 2002;88:241–419. [Google Scholar]
- 31.Garner C D. J Chem Soc Dalton Trans. 1997;21:3903–4126. [Google Scholar]
- 32.Gray H B, Gross Z. Israel J Chem. 2000;40:1–70. [Google Scholar]
- 33.Groves J T. Proc Natl Acad Sci USA. 2003;100:3569–3574. doi: 10.1073/pnas.0830019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holm R H, Solomon E I. Chem Rev. 1996;96:2237–3042. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]
- 35.Mazumdar S, Mitra S. Indian J Chem. 2002;41A:11–142. [Google Scholar]
- 36.Ortiz de Montellano P R. Curr Opin Chem Biol. 2000;4:221–227. doi: 10.1016/s1367-5931(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 37.Poulos T L, Li H, Raman C S. Curr Opin Chem Biol. 1999;3:131–137. doi: 10.1016/s1367-5931(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 38.Rodgers K R. Curr Opin Chem Biol. 1999;3:158–167. doi: 10.1016/S1367-5931(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 39.Smith A T, Veitch N C. Curr Opin Chem Biol. 1998;2:269–278. doi: 10.1016/s1367-5931(98)80069-0. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe Y. Curr Opin Chem Biol. 2002;6:208–216. doi: 10.1016/s1367-5931(02)00301-0. [DOI] [PubMed] [Google Scholar]
- 41.Williams R J P, Moore G R, Wright P E. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 369–401. [Google Scholar]
- 42.Wong L-L. Curr Opin Chem Biol. 1998;2:263–268. doi: 10.1016/s1367-5931(98)80068-9. [DOI] [PubMed] [Google Scholar]
- 43.Pascher T, Chesick J P, Winkler J R, Gray H B. Science. 1996;271:1558–1560. doi: 10.1126/science.271.5255.1558. [DOI] [PubMed] [Google Scholar]
- 44.Lyubovitsky J G, Gray H B, Winkler J R. J Am Chem Soc. 2002;124:5481–5485. doi: 10.1021/ja017399r. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman B M. Proc Natl Acad Sci USA. 2003;100:3575–3578. doi: 10.1073/pnas.0636464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green M T. J Am Chem Soc. 2000;122:9495–9499. [Google Scholar]
- 47.Perera R, Sono M, Sigman J A, Pfister T D, Lu Y, Dawson J H. Proc Natl Acad Sci USA. 2003;100:3641–3646. doi: 10.1073/pnas.0737142100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shokhireva T Kh, Berry R E, Uno E, Balfour C A, Zhang H, Walker F A. Proc Natl Acad Sci USA. 2003;100:3778–3783. doi: 10.1073/pnas.0536641100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Culotta E, Koshland D E. Science. 1992;258:1862–1865. doi: 10.1126/science.1361684. [DOI] [PubMed] [Google Scholar]
- 50.Lange S J, Que L. Curr Opin Chem Biol. 1998;2:159–172. doi: 10.1016/s1367-5931(98)80057-4. [DOI] [PubMed] [Google Scholar]
- 51.Johnson M K. Curr Opin Chem Biol. 1998;2:173–181. doi: 10.1016/s1367-5931(98)80058-6. [DOI] [PubMed] [Google Scholar]
- 52.Aisen P, Wessling-Resnick M, Leibold E A. Curr Opin Chem Biol. 1999;3:200–206. doi: 10.1016/S1367-5931(99)80033-7. [DOI] [PubMed] [Google Scholar]
- 53.Beinert H, Kiley P J. Curr Opin Chem Biol. 1999;3:152–157. doi: 10.1016/S1367-5931(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 54.Adams M W W, Stiefel E I. Curr Opin Chem Biol. 2000;4:214–220. doi: 10.1016/s1367-5931(99)00077-0. [DOI] [PubMed] [Google Scholar]
- 55.Kimura E. Curr Opin Chem Biol. 2000;4:207–213. doi: 10.1016/s1367-5931(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 56.Westerheide L, Pascaly M, Krebs B. Curr Opin Chem Biol. 2000;4:235–241. doi: 10.1016/s1367-5931(99)00081-2. [DOI] [PubMed] [Google Scholar]
- 57.Andrews N C. Curr Opin Chem Biol. 2002;6:181–186. doi: 10.1016/s1367-5931(02)00307-1. [DOI] [PubMed] [Google Scholar]
- 58.Ryle M J, Hausinger R P. Curr Opin Chem Biol. 2002;6:193–201. doi: 10.1016/s1367-5931(02)00302-2. [DOI] [PubMed] [Google Scholar]
- 59.Barnes C M, Petoud S, Cohen S M, Raymond K N. J Biol Inorg Chem. 2003;8:195–205. doi: 10.1007/s00775-002-0409-4. [DOI] [PubMed] [Google Scholar]
- 60.Baker H M, Anderson B F, Baker E N. Proc Natl Acad Sci USA. 2003;100:3579–3583. doi: 10.1073/pnas.0637295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Granier T, Langlois d'Estaintot B, Gallois B, Chevalier J-M, Precigoux G, Santambrogio P, Arosio P. J Biol Inorg Chem. 2003;8:105–111. doi: 10.1007/s00775-002-0389-4. [DOI] [PubMed] [Google Scholar]
- 62.Raymond K N, Dertz E A, Kim S S. Proc Natl Acad Sci USA. 2003;100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solomon E I, Decker A, Lehnert N. Proc Natl Acad Sci USA. 2003;100:3589–3594. doi: 10.1073/pnas.0336792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soum E, Drapier J-C. J Biol Inorg Chem. 2003;8:226–232. doi: 10.1007/s00775-002-0412-9. [DOI] [PubMed] [Google Scholar]
- 65.Volner A, Zoidakis J, Abu-Omar M M. J Biol Inorg Chem. 2003;8:121–128. doi: 10.1007/s00775-002-0395-6. [DOI] [PubMed] [Google Scholar]
- 66.Liu X, Jin W, Theil E C. Proc Natl Acad Sci USA. 2003;100:3653–3658. doi: 10.1073/pnas.0636928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Navati M S, Samuni U, Aisen P, Friedman J M. Proc Natl Acad Sci USA. 2003;100:3832–3837. doi: 10.1073/pnas.262526399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dhungana S, Taboy C H, Anderson D S, Vaughan K G, Aisen P, Mietzner T A, Crumbliss A L. Proc Natl Acad Sci USA. 2003;100:3659–3664. doi: 10.1073/pnas.0536897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim M H, Rohde J-U, Stubna A, Bukowski M R, Costas M, Ho R Y N, Münck E, Nam W, Que L., Jr Proc Natl Acad Sci USA. 2003;100:3665–3670. doi: 10.1073/pnas.0636830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Darensbourg M Y, Lyon E J, Zhao X, Georgakaki I P. Proc Natl Acad Sci USA. 2003;100:3683–3688. doi: 10.1073/pnas.0536955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitchell K H, Rogge C E, Gierahn T, Fox B G. Proc Natl Acad Sci USA. 2003;100:3784–3789. doi: 10.1073/pnas.0636619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryle M J, Koehntop K D, Liu A, Que L, Jr, Hausinger R P. Proc Natl Acad Sci USA. 2003;100:3790–3795. doi: 10.1073/pnas.0636740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clay M D, Cosper C A, Jenney F E, Jr, Adams M W W, Johnson M K. Proc Natl Acad Sci USA. 2003;100:3796–3801. doi: 10.1073/pnas.0636858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shearer J, Fitch S B, Kaminsky W, Benedict J, Scarrow R C, Kovacs J A. Proc Natl Acad Sci USA. 2003;100:3671–3676. doi: 10.1073/pnas.0637029100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emerson J P, Cabelli D E, Kurtz D M., Jr Proc Natl Acad Sci USA. 2003;100:3802–3807. doi: 10.1073/pnas.0537177100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spiering M M, Ringe D, Murphy J R, Marletta M A. Proc Natl Acad Sci USA. 2003;100:3808–3813. doi: 10.1073/pnas.0737977100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hantke K, Nicholson G, Rabsch W, Winkelmann G. Proc Natl Acad Sci USA. 2003;100:3677–3682. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holm R H. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 71–111. [Google Scholar]
- 79.Lee S C, Holm R H. Proc Natl Acad Sci USA. 2003;100:3595–3600. doi: 10.1073/pnas.0630028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chatt J. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 229–243. [Google Scholar]
- 81.Shilov A E. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 197–228. [Google Scholar]
- 82.Kim J S, Rees D C. Nature. 1992;360:553–560. doi: 10.1038/360553a0. [DOI] [PubMed] [Google Scholar]
- 83.Kim J S, Rees D C. Science. 1992;257:1677–1682. doi: 10.1126/science.1529354. [DOI] [PubMed] [Google Scholar]
- 84.Bolin J T, Campobasso N, Muchmore S W, Morgan T V, Mortenson L E. Am Chem Soc Symp Ser. 1993;535:186–195. [Google Scholar]
- 85.Thorneley R N F, Lowe D J. J Biol Inorg Chem. 1996;1:576–580. [Google Scholar]
- 86.Ferguson S J. Curr Opin Chem Biol. 1998;2:182–193. doi: 10.1016/s1367-5931(98)80059-8. [DOI] [PubMed] [Google Scholar]
- 87.Einsle O, Tezcan F A, Andrade S L A, Schmid B, Yoshida M, Howard J B, Rees D C. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 88.Malmström B G, Leckner J. Curr Opin Chem Biol. 1998;2:286–292. doi: 10.1016/s1367-5931(98)80071-9. [DOI] [PubMed] [Google Scholar]
- 89.McGuirl M A, Dooley D M. Curr Opin Chem Biol. 1999;3:138–144. doi: 10.1016/S1367-5931(99)80025-8. [DOI] [PubMed] [Google Scholar]
- 90.Gray H B, Malmström B G, Williams R J P. J Biol Inorg Chem. 2000;5:551–559. doi: 10.1007/s007750000146. [DOI] [PubMed] [Google Scholar]
- 91.Mahadevan V, Klein Gebbink M, R J, Stack T D P. Curr Opin Chem Biol. 2000;4:228–234. doi: 10.1016/s1367-5931(99)00080-0. [DOI] [PubMed] [Google Scholar]
- 92.Rosenzweig A C, O'Halloran T V. Curr Opin Chem Biol. 2000;4:140–147. doi: 10.1016/s1367-5931(99)00066-6. [DOI] [PubMed] [Google Scholar]
- 93.Szilagyi R K, Solomon E I. Curr Opin Chem Biol. 2002;6:250–258. doi: 10.1016/s1367-5931(02)00304-6. [DOI] [PubMed] [Google Scholar]
- 94.Wernimont A K, Huffman D L, Finney L A, Demeler B, O'Halloran T V, Rosenzweig A C. J Biol Inorg Chem. 2003;8:185–194. doi: 10.1007/s00775-002-0404-9. [DOI] [PubMed] [Google Scholar]
- 95.Bertini I, Rosato A. Proc Natl Acad Sci USA. 2003;100:3601–3604. doi: 10.1073/pnas.0736657100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arnesano F, Banci L, Bertini I, Mangani S, Thompsett A R. Proc Natl Acad Sci USA. 2003;100:3814–3819. doi: 10.1073/pnas.0636904100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lieberman R L, Shrestha D B, Doan P E, Hoffman B M, Stemmler T L, Rosenzweig A C. Proc Natl Acad Sci USA. 2003;100:3820–3825. doi: 10.1073/pnas.0536703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seravalli J, Gu W, Tam A, Strauss E, Begley T P, Cramer S P, Ragsdale S W. Proc Natl Acad Sci USA. 2003;100:3689–3694. doi: 10.1073/pnas.0436720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiou S-J, Riordan C G, Rheingold A L. Proc Natl Acad Sci USA. 2003;100:3695–3700. doi: 10.1073/pnas.0637221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stewart A J, Blindauer C A, Berezenko S, Sleep D, Sadler P J. Proc Natl Acad Sci USA. 2003;100:3701–3706. doi: 10.1073/pnas.0436576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coleman J E. Curr Opin Chem Biol. 1998;2:222–234. doi: 10.1016/s1367-5931(98)80064-1. [DOI] [PubMed] [Google Scholar]
- 102.Hightower K E, Fierke C A. Curr Opin Chem Biol. 1999;3:176–181. doi: 10.1016/s1367-5931(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 103.Cox E H, McLendon G L. Curr Opin Chem Biol. 2000;4:162–165. doi: 10.1016/s1367-5931(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 104.Jaffe E K. J Biol Inorg Chem. 2003;8:176–184. doi: 10.1007/s00775-002-0403-x. [DOI] [PubMed] [Google Scholar]
- 105.Vallee B. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 37–70. [Google Scholar]
- 106.Hutchings E, Bonner J. Eng Sci. 1977;40:22–23. [Google Scholar]
- 107.Logan D T, Mulliez E, Larsson K-M, Bodevin S, Atta M, Garnaud P E, Sjöberg B-M, Fontecave M. Proc Natl Acad Sci USA. 2003;100:3826–3831. doi: 10.1073/pnas.0736456100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sigel A, Sigel H, editors. Metal Ions in Biological Systems. New York: Dekker; 1994. [Google Scholar]
- 109.Sjöberg B-M. Struct Bonding. 1997;88:139–173. [Google Scholar]
- 110.Stubbe J, van der Donk W A. Chem Rev. 1998;98:705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- 111.Di Bilio A J, Crane B R, Wehbi W A, Kiser C N, Abu-Omar M M, Carlos R M, Richards J H, Winkler J R, Gray H B. J Am Chem Soc. 2001;123:3181–3182. doi: 10.1021/ja0043183. [DOI] [PubMed] [Google Scholar]
- 112.Dove J E, Klinman J P. Adv Protein Chem. 2001;58:141–174. doi: 10.1016/s0065-3233(01)58004-3. [DOI] [PubMed] [Google Scholar]
- 113.Gräslund A. Methods Enzymol. 2002;354:399–414. doi: 10.1016/s0076-6879(02)54031-3. [DOI] [PubMed] [Google Scholar]
- 114.Yun D, Krebs C, Gupta G P, Iwig D F, Huynh B H, Bollinger J M. Biochemistry. 2002;41:981–990. doi: 10.1021/bi011797p. [DOI] [PubMed] [Google Scholar]
- 115.Zouni A, Witt H-T, Kern J, Fromme P, Krauss N, Saenger W, Orth P. Nature. 2001;409:739–743. doi: 10.1038/35055589. [DOI] [PubMed] [Google Scholar]
- 116.Tommos C, Babcock G T. Acc Chem Res. 1998;31:18–25. [Google Scholar]
- 117.Yocum C F, Pecoraro V L. Curr Opin Chem Biol. 1999;3:182–187. doi: 10.1016/S1367-5931(99)80031-3. [DOI] [PubMed] [Google Scholar]
- 118.Sigel A, Sigel H, editors. Metal Ions in Biological Systems. New York: Dekker; 2000. [Google Scholar]
- 119.Siegbahn P E M. Curr Opin Chem Biol. 2002;6:227–235. doi: 10.1016/s1367-5931(02)00312-5. [DOI] [PubMed] [Google Scholar]
- 120.Maneiro M, Ruettinger W F, Bourles E, McLendon G L, Dismukes G C. Proc Natl Acad Sci USA. 2003;100:3707–3712. doi: 10.1073/pnas.0637229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abeles R H. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 245–260. [Google Scholar]
- 122.Buckingham D A. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 141–196. [Google Scholar]
- 123.Pennella M A, Shokes J E, Cosper N J, Scott R A, Giedroc D P. Proc Natl Acad Sci USA. 2003;100:3713–3718. doi: 10.1073/pnas.0636943100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ragsdale S W. Curr Opin Chem Biol. 1998;2:208–215. doi: 10.1016/s1367-5931(98)80062-8. [DOI] [PubMed] [Google Scholar]
- 125.Maroney M J. Curr Opin Chem Biol. 1999;3:188–199. doi: 10.1016/S1367-5931(99)80032-5. [DOI] [PubMed] [Google Scholar]
- 126.McMaster J, Enemark J H. Curr Opin Chem Biol. 1998;2:201–207. doi: 10.1016/s1367-5931(98)80061-6. [DOI] [PubMed] [Google Scholar]
- 127.Sigel A, Sigel H, editors. Metal Ions in Biological Systems. New York: Dekker; 2002. [Google Scholar]
- 128.Joshi H K, Cooney J J A, Inscore F E, Gruhn N E, Lichtenberger D L, Enemark J H. Proc Natl Acad Sci USA. 2003;100:3719–3724. doi: 10.1073/pnas.0636832100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sigel H, Sigel A, editors. Metal Ions in Biological Systems. New York: Dekker; 1995. [Google Scholar]
- 130.Butler A. Curr Opin Chem Biol. 1998;2:279–285. doi: 10.1016/s1367-5931(98)80070-7. [DOI] [PubMed] [Google Scholar]
- 131.Hepburn D D D, Xiao J, Bindom S, Vincent J B, O'Donnell J. Proc Natl Acad Sci USA. 2003;100:3766–3771. doi: 10.1073/pnas.0636646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dunitz J D, Dobler M. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 113–140. [Google Scholar]
- 133.Evenas J, Malmendal A, Forsen S. Curr Opin Chem Biol. 1998;2:293–302. doi: 10.1016/s1367-5931(98)80072-0. [DOI] [PubMed] [Google Scholar]
- 134.Carafoli E, Brini M. Curr Opin Chem Biol. 2000;4:152–161. doi: 10.1016/s1367-5931(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 135.Miller C. Curr Opin Chem Biol. 2000;4:148–151. doi: 10.1016/s1367-5931(99)00068-x. [DOI] [PubMed] [Google Scholar]
- 136.Hanna R, Doudna J A. Curr Opin Chem Biol. 2002;4:166–170. doi: 10.1016/s1367-5931(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 137.Burdette S C, Lippard S J. Proc Natl Acad Sci USA. 2003;100:3605–3610. doi: 10.1073/pnas.0637711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Welch J T, Kearney W R, Franklin S J. Proc Natl Acad Sci USA. 2003;100:3725–3730. doi: 10.1073/pnas.0536562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kimura E, Aoki S, Kikuta E, Koike T. Proc Natl Acad Sci USA. 2003;100:3731–3736. doi: 10.1073/pnas.0637275100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Junicke H, Hart J R, Kisko J, Glebov O, Kirsch I R, Barton J K. Proc Natl Acad Sci USA. 2003;100:3737–3742. doi: 10.1073/pnas.0537194100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo H, Tullius T D. Proc Natl Acad Sci USA. 2003;100:3743–3747. doi: 10.1073/pnas.0737062100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Freisinger E, Rother I B, Lüth M S, Lippert B. Proc Natl Acad Sci USA. 2003;100:3748–3753. doi: 10.1073/pnas.0436700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reedijk J. Proc Natl Acad Sci USA. 2003;100:3611–3616. doi: 10.1073/pnas.0737293100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schibli R, Schubiger P A. Eur J Nucl Med Mol Imag. 2002;29:1529–1542. doi: 10.1007/s00259-002-0900-8. [DOI] [PubMed] [Google Scholar]
- 145.Li W, Parigi G, Fragai M, Luchinat C, Meade T J. Inorg Chem. 2002;41:4018–4024. doi: 10.1021/ic0200390. [DOI] [PubMed] [Google Scholar]
- 146.Gray H B, Winkler J R. Annu Rev Biochem. 1996;65:537–561. doi: 10.1146/annurev.bi.65.070196.002541. [DOI] [PubMed] [Google Scholar]
- 147.Winkler J R, Di Bilio A J, Farrow N A, Richards J H, Gray H B. Pure Appl Chem. 1999;71:1753–1764. [Google Scholar]
- 148.Gray H B, Winkler J R. In: Electron Transfer in Chemistry. Balzani V, editor. Vol. 3. Weinheim, Germany: Wiley; 2001. pp. 3–23. [Google Scholar]
- 149.Dunn A R, Dmochowski I J, Bilwes A M, Gray H B, Crane B R. Proc Natl Acad Sci USA. 2001;98:12420–12425. doi: 10.1073/pnas.221297998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Crane B R, Di Bilio A J, Winkler J R, Gray H B. J Am Chem Soc. 2001;123:11623–11631. doi: 10.1021/ja0115870. [DOI] [PubMed] [Google Scholar]
- 151.Tezcan F A, Crane B R, Winkler J R, Gray H B. Proc Natl Acad Sci USA. 2001;98:5002–5006. doi: 10.1073/pnas.081072898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wherland S, Gray H B. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 289–368. [Google Scholar]
- 153.Williams R J P. J Inorg Biochem. 2002;88:241–250. doi: 10.1016/s0162-0134(01)00350-6. [DOI] [PubMed] [Google Scholar]
- 154.Martinez J S, Carter-Franklin J N, Mann E L, Martin J D, Haygood M G, Butler A. Proc Natl Acad Sci USA. 2003;100:3754–3759. doi: 10.1073/pnas.0637444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wood J M, Lipscomb J D, Que L, Stephens R S, Orme-Johnson W H, Munck E, Ridley W P, Dizikes L, Cheh A, Francia M, F, et al. In: Biological Aspects of Inorganic Chemistry. Addison A W, Cullen W R, Dolphin D, James B R, editors. New York: Wiley; 1977. pp. 261–288. [Google Scholar]
- 156.Godwin H A. Curr Opin Chem Biol. 2001;5:223–227. doi: 10.1016/s1367-5931(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 157.Sigel A, Sigel H, editors. Metal Ions in Biological Systems. New York: Dekker; 1997. [Google Scholar]
- 158.Winkler J R, Gray H B. Acc Chem Res. 1998;31:98. [Google Scholar]
- 159.Telford J R, Wittung-Stafshede P, Gray H B, Winkler J R. Acc Chem Res. 1998;31:755–763. [Google Scholar]
- 160.Farrer B T, Pecoraro V L. Proc Natl Acad Sci USA. 2003;100:3760–3765. doi: 10.1073/pnas.0336055100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chang I-J, Lee J C, Winkler J R, Gray H B. Proc Natl Acad Sci USA. 2003;100:3838–3840. doi: 10.1073/pnas.0637283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tezcan F A, Findley W M, Ross S A, Lyubovitsky J G, Gray H B, Winkler J R. Proc Natl Acad Sci USA. 2002;99:8626–8630. doi: 10.1073/pnas.132254499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dobson C M. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 164.Dobson C M. Philos Trans R Soc B. 2001;356:133–145. doi: 10.1098/rstb.2000.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Valentine J S, Hart P J. Proc Natl Acad Sci USA. 2003;100:3617–3622. doi: 10.1073/pnas.0730423100. [DOI] [PMC free article] [PubMed] [Google Scholar]