Abstract
From the metal ions and metal compounds that are known to bind to DNA, many anticancer Pt(II) and Ru(II)/Ru(III) compounds are known to have ligand-exchange kinetics in the same order of magnitude as the division of tumor cells. The present article discusses this process in detail with special attention to cisplatin and related compounds and the cellular binding sites and processes of such compounds. Detailed platinated DNA structures are presented and discussed in light of the mechanistic studies of metal antitumor compounds. It is now known that platinum antitumor drugs eventually end up on the DNA. However, it remains a challenge to understand how (fast) they reach the DNA and how they are removed. The kinetics of ligand exchange around platinum appear to play a crucial role, and the possible role of other ligands as intermediates, especially those with S-donor sites, is of great interest. New types of Pt compounds with additional functionalities influencing DNA binding and kinetics are discussed in the context of steric and H-bonding properties. A comparison is made with more sterically crowded Ru complexes. The effects on activity and correlations with structural and kinetic properties are clues in understanding the biological activities of these classes of compounds.
Metal ions and metal coordination compounds are known to affect cellular processes in a dramatic way (1). This metal effect influences not only natural processes, such as cell division and gene expression, but also non-natural processes, such as toxicity, carcinogenicity, and antitumor chemistry (2). This article deals with a special aspect of metal biochemistry, namely the reactivity and kinetics of metal coordination complexes in living systems, with a focus on heavy metals like Pt and Ru and their antitumor action (3).
In chemotherapy the key issue is killing the tumor cells, without causing too much harm to healthy cells. Most anticancer drugs act on DNA in one way or another, and the interaction pathways of Pt and Ru compounds with nucleic acids form the core of this article.
The successful development of metal-containing anticancer drugs clearly starts with cis-[PtCl2(NH3)2] (see Fig. 1) (4), often referred to as cisplatin. Although the compound was first described in 1845, its anticancer properties were not discovered until 1964, when Rosenberg et al. (5) investigated the effects of electric fields on bacterial growth. By growing Escherichia coli in a solution of NH4Cl and Pt electrodes in sunlight, strong filamentous growth and cell division arrest were observed. They realized that the electric field was not responsible for the cell division arrest, but rather the presence of small amounts of certain platinum compounds, like cis-[Pt(NH3)2Cl2] and cis-[Pt(NH3)2Cl4] caused arrest. Later experiments (6) showed that such compounds form by the slow reaction of the platinum electrodes with the NH4Cl solution in the presence of light and an electrical current (7).
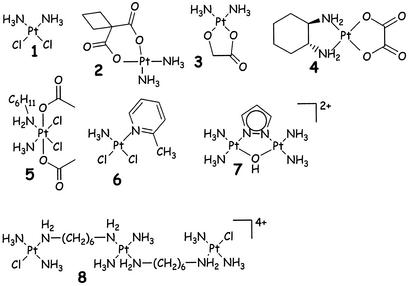
Figure 1.
Structures of cisplatin (1) and some first-generation drugs: Carboplatin (2); Nedaplatin (3); Oxaliplatin (4), an orally active drug (5, JM-216) and some recently introduced new mononuclear (6, AMD473); dinuclear (7) and trinuclear (8) Pt-antitumor drugs.
Structure–activity relationships for a class of related compounds confirmed (8) that only those compounds having cis geometry block cell growth. The most active complex, cisplatin (see Fig. 1), was found to exhibit antitumor activity, whereas its trans isomer showed no such activity. Many derivatives of cisplatin also inhibit growth, and these compounds have at least one N-H group, which is responsible for important hydrogen-bond donor properties (9), either in the approach of the biological target or the final structure (9). A schematic illustration of such hydrogen bonding and its effect on purine bases is given in Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org, whereas an example of hydrogen bonding in a crystal structure (amine to phosphate) (10) is depicted in Fig. 2.
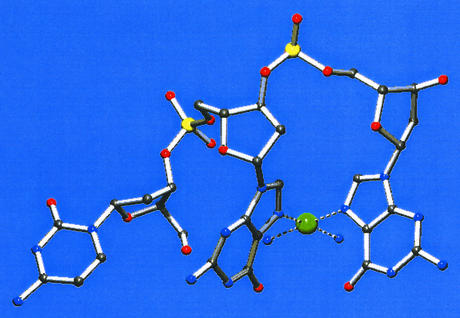
Figure 2.
Amine N-H groups contribute to a macrochelate via hydrogen bonding with the phosphate of GMP in Pt(en)(5′-GMP-N7)2.
After cisplatin-dependent regression of animal tumors was observed, clinical trials on solid tumors in humans followed rapidly. Phase I clinical trials started in 1971 (11), and Food and Drug Administration approval was obtained in 1978 under the name Platinol. Carboplatin followed with Food and Drug Administration approval in 1989 under the name Paraplatin, whereas most recently oxaliplatin (Eloxatin) also was added for routine treatments of colon cancer (www.cancerbacup.org.uk/info) and others are in phase I and phase II clinical trials. Cisplatin is known to be particularly effective against solid tumor types, such as testicular, ovarian, head, and neck cancers, and against small-cell lung cancer (12) with a cure rate as high as 90%. Typical drug prices are ≈$300/g, and sales for carboplatin have been reported to be $480 million in 2001 (www.fda.gov/bbs/topics/NEWS/2002/NEW00825.html).
Most of the well-known platinum anticancer complexes have the general formula (8) cis-[PtX2(NHR2)2], in which R = organic fragment and X = leaving group, such as chloride or (chelating bis)carboxylate. Some representative structures have been included in Fig. 1. Many other active Pt(II) compounds are known now, even with trans geometries, and these will be dealt with below.
The development of cisplatin as a successful antitumor drug is often seen as the prototypical success story. The large number of patients who have been cured after cisplatin treatment of cancer is impressive. However, the fact that the precise mechanism of action remains elusive has resulted in great interest in metal DNA binding generally and cisplatin and its analogs' binding properties particularly. As a consequence, cisplatin chemistry has provided a fertile ground for exciting (bio)inorganic chemistry research (13).
Quite severe side effects of cisplatin treatment (e.g., nausea, ear damage, vomiting) have stimulated research toward developing less toxic derivatives. These side effects limit the dose that can be administered to patients to 100 mg per day for 5 consecutive days. The nephrotoxicity can be reduced by saline (hydration) and diuresis, and special drug-dosing protocols have been developed, making use of chemoprotecting agents, such as sodium dithiocarbamate (14). The second-generation platinum drug carboplatin, [Pt(C6H6O4)(NH3)2], has fewer toxic side effects than cisplatin and is more easily used in combination therapy. Its low reactivity allows a higher dose to be administered (up to 2,000 mg per day). Carboplatin is used more for ovarian cancer treatment, whereas oxaliplatin is known to be most effective in colon cancer treatment (15). In Japan another second-generation derivative, Nedaplatin (see Fig. 1), is recommended for a variety of cancer treatments, including testicular, ovarian, and cervical cancers (16).
More recent developments have shown that spontaneous (intrinsic) drug resistance may develop in certain tumors, which is one of the main limitations when treating patients. Such resistance is easily detected in tumor cell lines, so that new drugs can now be rapidly screened. As a result, a new group of compounds with different amines and lacking the classical cis-diamine structure with two leaving groups has evolved during the last decade. These compounds are often considered the so-called third-generation drugs (15, 17–19).
Similar compounds from other group 10 elements (Ni, Pd) do not yield active compounds. The key factor explaining why Pt is most useful clearly relates to ligand-exchange kinetics, which for platinum and this type of ligand is on the order of a few hours, thereby preventing rapid equilibration reactions (20).
An important property of the platinum coordination compounds is the fact that the Pt–ligand bond, which has the thermodynamic strength of a typical coordination bond (say 100 kJ/mol or below), and are much weaker than (covalent) C—C and C—N or C—O single and double bonds (which are between 250 and 500 kJ/mol) (21). However, the ligand-exchange behavior of Pt compounds is quite slow, which gives them a high kinetic stability and results in ligand-exchange reactions of minutes to days, rather than microseconds to seconds for many other coordination compounds. The same holds true for ruthenium coordination compounds, and this kinetic behavior makes such compounds very useful. Although several ruthenium compounds have been reported to show antitumor behavior in cell-line studies (22–25), only one of them has entered clinical trials so far (26). It should be noted that Ru compounds are intrinsically octahedrally coordinated, and even though a cis chelate is possible, the space that axial ligands require prevent it from forming similar structures with DNA as Pt(II) compounds can (Fig. 3). The compounds with three labile ligands, like Ru(terpy), may in theory also bind trans or in a tridentate manner (25).
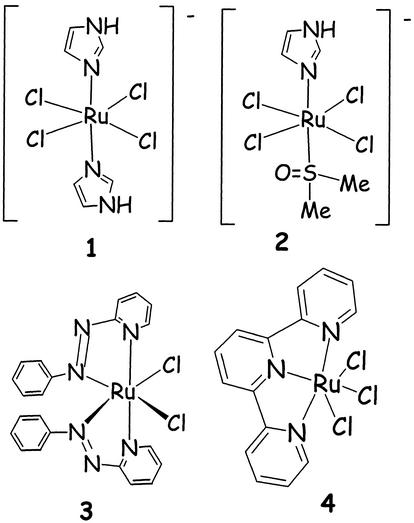
Figure 3.
A selection of anticancer-active ruthenium coordination compounds. The compounds NAMI (1) and NAMI-A (2), each having imidazolium as cation; a Ru-terpy compound (4) and a very active compound (3) of the α isomer of a Ru-azpy compound (azpy = 2-phenylazopyridine). From these, NAMI-A has been in clinical trials since 2000 (26).
Another unusual phenomenon deals with the preferred ligands for Pt and Ru ions. Pt(II), and to a lesser extent, Ru(II) do have a strong thermodynamic preference for binding to S-donor ligands. For that reason one would predict that platinum compounds would perhaps never reach DNA, with many cellular platinophiles (S-donor ligands, such as glutathione, methionine) as competing ligands in the cytosol.
Finally, the so-called kinetic trans effect should be mentioned, which is responsible for ligand-exchange reactions on metals ions. The effect is most pronounced for Pt(II) compounds, where it has been studied in great detail (21). The rule can be quite simply formulated as: ligands located trans to another ligand with a strong trans effect (such as many soft ligands) are more rapidly substituted then ligands in cis positions.
Three factors play an important role in understanding the ligand-exchange processes in Pt antitumor chemistry.
(i) As shown above in compounds of formula cis-Pt(RNH2)2Cl2 (8), the amine N-H group is crucial for the activity of the classical Pt compounds. The ionic/dipolar interaction with nucleic acids, known to be important in discriminating between A and G, is connected with this hydrogen bonding.
(ii) The observation that Pt amine compounds eventually reach purine N7 sites appears to be related to a migration of Pt from an S ligand to purine N7. Therefore, reactions of S-guanosyl-l-homocysteine (SGH) initially yield [Pt(dien)(SGH-S)]2+ (starting from [PtCl(dien)]Cl at 2 < pH < 6.5), but isomerize intramolecularly into [Pt(dien)(SGH-N7)] with Pt coordination at N7 of guanosine (27, 28). Sadler and colleagues (29) found similar results for methionine ligands, suggesting that in vivo Pt species might migrate from S to N donor ligands (30), albeit not for all Pt-S compounds, such as some (dinuclear) stable glutathione adducts (31).
(iii) The fact that the bulk of the Pt compounds probably only stay at the DNA for a short time (<10 h, as shown by observation of fluorophore-labeled Pt compounds). After some time the kinetic lability and thermodynamic instability may lead to loss of Pt from the nuclear DNA, which eventually may result in excretion via the Golgi organelles (32). However, small fractions of Pt–DNA adducts have been detected in patients long after treatment (33, 34).
Recent Mechanistic Insights for Cisplatin
DNA Is the Target.
The activity of cisplatin is closely related to its binding to DNA (35). Highly conclusive evidence for this target was the early observation that cells deficient in DNA repair are hypersensitive to cisplatin (36, 37), suggesting that cisplatin and other drugs bind DNA. Early studies showed that of the four bases the N7 site of guanine is strongly preferred (see also Fig. 6; ref. 20). Many other DNA crosslinking antitumor compounds are known, including cyclophosphamides, nitrogen mustards, nitrosoureas, epoxides, and anthracyclines (38). A drug bound to DNA may interfere with transcription and/or DNA replication mechanisms (39), which may (eventually) trigger processes like apoptosis that lead to cell death (40).
Thus the key elements in the effects of cisplatin (and derivatives) on DNA are its controlled hydrolysis, transport to and within the cell, and binding to DNA; specific binding at adjacent guanine bases; and especially a specific distortion of DNA, changing its interactions with proteins, leading to either repair of the damage, or cell killing by apoptosis (13).
Cellular Uptake of Cisplatin.
Cisplatin is administered to patients i.v. The physiological chloride concentration in blood and extracellular body fluids is 100 mM, which is high enough to suppress cisplatin hydrolysis, so that it can reach the outer surface of cells as a neutral molecule. Early studies have shown that ≈50% of the cisplatin leaves the body through the kidneys within 48 h, and that the remaining 50% spends up to 2 months in the body. However, the precise mechanism of cellular uptake of cisplatin has remained unclear, although evidence has been published showing that the presence of phosphatidyl serine in membranes plays an important role in uptake (41). Although passive diffusion is believed to be the main mechanism, some evidence supports the involvement of active transport mechanisms (42). Very recently strong evidence has been reported showing that a copper transport agent, Ctr1, is mediating cisplatin uptake (43, 44).
Inside cells the chloride concentration is much lower (4 mM) and consequently hydrolysis of cisplatin occurs, albeit slowly. The most important hydrolysis product is the [PtCl(H2O)(NH3)2]+ cation (45), which has a pKa value of 6.5. Above pH 6 it starts to ionize to form [PtCl(OH)- (NH3)2] (46), and the cationic species is known to be much more reactive than cisplatin, so the monoaqua species is most likely to react with DNA and other molecules in the cell. Transport through the nuclear membrane is also poorly understood, and whether or not special nuclear localizing signal peptides play a role remains uncertain (47).
Resistance Development.
An important clinical limitation is resistance to cisplatin. Some types of cancer are known to be intrinsically insensitive to cisplatin treatment, whereas other cancers develop resistance only during chemotherapy. Therefore, the applicability of cisplatin is limited to a relatively narrow range of tumors. The cisplatin-resistance mechanism appears to be multifactorial, with at least three factors identified as potential modulators of cellular resistance (31, 48, 49). Decreased uptake, as in many cisplatin-resistant cell lines, reduced intracellular accumulation (48). Increased intracellular detoxification by glutathione reacting with platinum drugs forms deactivated adducts (31) that are known to be excreted by a glutathione S-conjugate export pump. Finally, enhanced DNA repair has been observed in some cisplatin-resistant cell lines (49). Most recently, decreased apoptosis has been associated with drug resistance (50). Full details on resistance development can be found in specialty literature (51).
Kinetics of Formation and Structures of Metal–DNA Complexes.
A variety of studies have dealt with the DNA binding of Pt drugs. These studies have encompassed single nucleosides to oligonucleotides, to plasmids and genomic DNA. This field has been reviewed by others (15, 35, 52, 53) and myself (13, 20). Guanine sites have strong affinity for Pt amine compounds and two adjacent guanines are preferred, with the first binding occurring most rapidly at the 3′ G. Monofunctional adducts at 5′ G may persist and play a role in protein crosslinking (54). The two most frequent binding sites, GG and AG (55), appear to be valid for several sources of DNA (56), and they are schematically depicted in Fig. 7, which is published as supporting information on the PNAS web site.
Examples of G binding for other metals are Ni(II) and Cu(II) (57). However, in case of sterically crowded Ru(II) and dinuclear Rh(II) compounds, it appeared that certain adenine binding sites can be competitive (58–61). Antitumor activity of other, less soft metals, like Ti(IV) or Sn(IV), results from better binding of phosphate, and such interactions have been excluded from the present discussion.
The work of Taube (62) has been crucial for our current understanding of metal binding (reversibly) kinetically fast or slow. He showed that ligand-exchange processes for the same ligands, but for a variation of metal ions, can vary by at least 14 orders of magnitude. For small ligands, like water, the ligand exchange for metals ions like Pt(II), Ru(II), Ru(III), and low-spin Co(III) is a few hours, similar to the time scale for many cell-division processes.
In the case of Pt(II) the trans effect explains the stereochemically controlled kinetics of ligand exchange. With two cis-oriented amines at a Pt(II) ion, the other two ligands are more easily substituted, which results in two amines remaining initially coordinated when the Pt compound is bound to a biological target. If the target is a soft ligand, in a subsequent reaction, the amine ligand may become labile, especially when monodentates like ammonia or primary amines are used.
Structures of Pt and Ru compounds coordinated to DNA fragments are numerous and have been reviewed (13). A simple case for ruthenium, showing the hydrogen-bonding importance, is depicted in Fig. 8, which is published as supporting information on the PNAS web site (63). The x-ray structure of one of the adducts of Pt(II) with d(CGG) fragments is redrawn (64) as a simple projection in Fig. 4. The chelation of two N7 sites in cis orientation does not allow the two guanines to be parallel, resulting in a kink of the DNA when double-stranded. Many more structures of GG adducts in single-stranded and double-stranded are known (see refs. 54 and 65 and references therein).
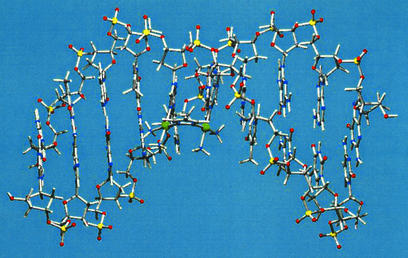
Figure 4.
X-ray structure of d(CGG) chelated to a cisplatin unit, drawn after Admiraal et al. (64).
The Recognition of the Cisplatin GG Adduct and Subsequent Protein Binding.
The structure of the GG-Pt chelated species appears to be quite generally observed, as shown in Fig. 4 and Fig. 9, which is published as supporting information on the PNAS web site. In the next mechanistic step it is assumed that the Pt-GG adduct is recognized by proteins, followed by either stabilization of the distorted DNA structure or removal of the lesion through repair (35, 66). Such Pt-GG chelates are basically the same (65) in many cases, and even when a protein is bound to it, this kinked DNA structure is retained (67). The biological consequences of the protein binding at GG-platinated sites are the next challenge to understand (68).
New Types of Drugs
Based on mechanistic findings, coordination chemists are designing and synthesizing new compounds. A novel DNA-binding metal compound with antitumor activity and clinical efficacy must fulfill the following key requirements: (i) good intrinsic properties, including saline solubility and enough stability to arrive intact at the cellular target; (ii) efficient transport properties in blood and through membranes; (iii) efficient DNA-binding properties but slow reactivity with proteins; (iv) the ability to differentiate between cancerous and normal cells; and (v) activity against tumors that are, or have become, resistant to cisplatin and derivatives. This latter requirement usually implies a structure that is distinct from cisplatin-type species.
It is impossible to discuss or describe all recently studied new compounds, and only a few will be given below based mainly on my lab's work. The first two groups are based on the classical cisplatin derivatives. The second category deals with quite different drugs and DNA binders.
Pt(IV) Compounds and Prodrugs.
The so-called JM-216, a Pt(IV) compound (Fig. 1; refs. 69 and 70), has been in routine clinical use as an orally administered drug. An important question is whether such compounds are reduced before entering the cell, inside the cell, or perhaps not at all. A study by Novakova et al. (71) has shown that in the case of binding of cis,cis,trans-PtCl2(NH3)2(OH)2 the isolated DNA adducts differ from those formed with cisplatin under the same conditions, and that no external (added) reducing agent is needed for their formation. Whether or not the adducts formed contain Pt(II) or Pt(IV) is not completely clear, although most evidence points toward slowly formed Pt(IV) adducts (71). Work with model bases (72) has made it clear that reduction can occur, albeit slowly. Recent work with a variety of different amines showed that the Pt redox potential is influenced by the coordinated amine (73).
Given the toxicity and side effects of cisplatin, much activity has been generated on slow-release drugs, such as through use of a polymer. Such a biodegradable polymer can bind cisplatin analogs that are released gradually (74). A different approach toward prodrugs has been the formation of membrane-encapsulated cisplatin, which can be formed only by repeated freeze-thaw cycles (75). The resulting products are highly soluble and appear to result in slow releases and high activity (76).
Pt-GG Crosslinkers Bearing a Second DNA-Binding Function.
In the amine part of the cisplatin derivatives, substitutions allow the introduction of DNA binding or repelling side arms, so that the kinetics of the DNA binding can be influenced by charge, hydrogen bonding, or steric effects. The stabilization after Pt binding at guanines can be influenced in this way (Fig. 9). In the case of the peptide ligands, application of solid-phase synthesis allows combinatorial variations (77–79).
Flexible Dinuclear and Oligonuclear Pt Compounds.
Going from mononuclear Pt compounds to dinuclear (and oligonuclear) Pt compounds might seem logical, but it took quite some time before the dinuclear compounds of the type given in Fig. 1 were introduced (80, 81). The dinuclear compounds of general formula [ClPt(NH3)2(H2N-(CH2)nNH2Pt(NH3)2Cl](anion)2] were found to be active and able to chelate at two guanines at N7 (82), forming a hairpin structure (17) with double-stranded DNA. Unfortunately, these compounds were too toxic for clinical trials.
A trinuclear compound (8 in Fig. 1) was shown to have unique DNA binding properties (19, 83, 84). The prototype, also called BBR3464, is active against melanoma, lung cancer, and pancreatic cancers and retains active in cisplatin-resistant cell lines. It is active at 10-fold lower concentrations than cisplatin and makes long-range interstrand and intrastrand crosslinks.
Rigid Dinuclear Pt Compounds.
Komeda et al. (85) followed a different approach toward creating dinuclear drugs. They looked for an intrastrand crosslink that caused only minor distortion of the double helix, which reduces the chance of recognition by repair enzymes. Simple modeling made clear that a fixed azole bridge (pyrazole, triazole) would generate a base-to-base contact of 350 pm. The prototypical compound is listed as 7 in Fig. 1. When reacted with the model DNA base 9-ethylguanine (9-Etgua), the bis adduct (Fig. 10, which is published as supporting information on the PNAS web site) beautifully shows the parallel orientation of the two bases, suggesting that in double-stranded DNA such parallel orientation would be possible.
High activity against a variety of different tumor cell lines was found in the meantime (86), and detailed binding studies of these compounds with DNA fragments were undertaken. In one case an exciting ligand isomerization was found upon DNA binding (87). A reaction with double-stranded oligonucleotides having GG sequences showed that the strand is hardly distorted (refs. 88 and 89; S. Komeda, J. Kozelka, and J.R., unpublished work). A projection of an NMR structure is given in Fig. 5, which illustrates the nonkinked behavior (86, 88).
Figure 5.
A double-stranded DNA remains almost unkinked when the dinuclear azole-bridged Pt amine species is chelating to two neighboring guanines (88). Pt, green; P, yellow.
Ruthenium and Transamine Pt Compounds.
Although ruthenium antitumor chemistry is more recent than cisplatin and derivatives, the compounds given in Fig. 3 have generated tremendous synthetic activity. My lab has recently modified the azapyridine compounds and made them water soluble (91), which results in compounds with a high antitumor activity. Sadler and colleagues (92) have shown that even organometallic half-sandwich Ru compounds show activity and interesting DNA-binding properties.
Finally, it should be mentioned that many trans-Pt compounds show activity, at least with bulky amines. The interest in transamine Pt compounds is very rapidly growing, and the reader is referred to a review (66). Binding to DNA is prominent, and both interstrand and intrastrand crosslinking have been reported. An important property for the trans compounds appears to be their activity against tumor cell lines that are not sensitive for cisplatin.
Kinetics and H-Bonding Properties
From the previous sections it has become clear that kinetics are crucial to all properties related to antitumor activity and DNA binding. If a potentially active compound with the right geometric and electronic properties does not stay coordinated to the DNA for long enough, no activity will result. Fine-tuning of the compounds with substituents that have steric, electronic, and/or H-bonding implications for DNA binding provides great variety for new compounds.
Recent examples of this principle have appeared in the literature (78, 93), and a very active compound has already resulted from this kinetic approach, as shown in Fig. 1, structure 6 (94). This very simple Pt compound has a structure where the axial attack of incoming ligand at one side of the Pt-ligand plane is significantly slowed down. As a consequence of this geometry, thio ligands bind more slowly; indeed, this is a beautiful example of a simple steric control of ligand exchange on simple square planar Pt(II) compounds. This kinetic effect has now also been studied theoretically, including competition between protein and DNA (95).
A Labeling Application of the Kinetics of Pt-DNA Binding
The kinetically controlled reactions of Pt compounds to DNA have also resulted in an interesting high-potential labeling application (96). By applying the unit Pt(ethylenediamine) attached to an oligonucleotide, and carrying a fluorescent label, in combination with hybridization to a complementary strand to form double-stranded DNA, it appeared possible to detect specific sequences of DNA in biological samples. Crucial is the fact that the Pt remains coordinated long enough, ensuring that the fluorescent label would only be at the specific DNA sequence. A schematic structure is given in Fig. 11, which is published as supporting information on the PNAS web site.
The moderate stability of the coordination bond between platinum and the guanine-N7, coupled with appropriately slow ligand-exchange kinetics, appears to be a key feature. New applications of this promising methodology have been reported (97).
Perspective and Future of Metal-Containing Drugs
Coordination chemistry in living systems is more than just a matter of metal–ligand bond formation and metal–ligand stability. Terminology using the adjective supramolecular, although originally meant for other classes of chemistry also applies here.
Control of metal binding to DNA, by simultaneous coordination and hydrogen bonding has been crucial to my lab's research and has been a focus of this perspective. Starting with a simple working hypothesis in 1980, we have seen several recent examples to appreciate this approach as useful. The studies have clearly led to the acceptance of the view that:
(i) In metal-DNA binding the kinetics of the metal–ligand binding are more important than the thermodynamic binding.
(ii) The role of additional H-bonding interactions, both in the kinetics of the process, and in the stabilization of the adduct structure, is very important.
It needs no discussion that the above-presented highlights and outlook provide fascinating new possibilities for research in the coming decade. New techniques like specialized MS and NMR including the powerful [1H,15N] method (heteronuclear sequential quantum correlation), which allows following the reaction of Pt amines and nucleic acids and proteins, will allow the detection of otherwise invisible intermediate products (90).
In summary, it is generally appreciated that enormous progress has been made in the understanding of the mode of action of cisplatin, for which kinetics plays such an important role. Application of this knowledge in drug design is close, and it is generally expected that in the next decade improved antitumor drugs will be developed based on the knowledge of the Pt–DNA interactions (and their repair) and on the kinetics of binding of Pt and Ru compounds to proteins and DNA. Kinetic understanding is of great importance for controlling the toxic side effects of such compounds. Although questions have been raised about whether the intrinsically weak metal–ligand coordination bond will ever lead to new drug applications (www.callerio.org/forum), the kinetic control of stability is likely to overcome this.
The next stage in drug design is likely to be the development of dedicated drugs that comprise the transport (through the membranes), survival in the cell, binding to the DNA, and eventually excretion from the body with minimum side effects. In my perception of this process, both (kinetically controlled) metal coordination and hydrogen bonding will be key factors at the molecular level.
Supplementary Material
Acknowledgments
All work done in Leiden and mentioned above was obtained thanks to the dedication, inspiration, quality, and hard work of undergraduates, graduate students, postdocs, visiting scientists, and collaborators over the last two decades. Their names appear as coauthors in the references from our laboratory. Support from Leiden University and the Councils of Chemical Research and Applied Sciences of The Netherlands Organization for Research is gratefully acknowledged, as are the highly appreciated loan of schemes for K2PtCl4 and RuCl3 from Johnson & Matthey (Reading, U.K.). European help for postdocs and research trainees from COST and Framework programs 3, 4, and 5 since 1992 is thankfully mentioned, allowing regular research exchanges, including short visits of students, to partner laboratories inside European Union countries.
References
- 1.Reedijk J, Bouwman E. Bioinorganic Catalysis. New York: Dekker; 1999. [Google Scholar]
- 2.Lippard S J. Science. 1993;261:699–700. doi: 10.1126/science.8342037. [DOI] [PubMed] [Google Scholar]
- 3.Reedijk J. Curr Opin Chem Biol. 1999;3:236–240. doi: 10.1016/s1367-5931(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 4.Peyrone M. Ann Chemie Pharm. 1845;51:1–29. [Google Scholar]
- 5.Rosenberg B, Van Camp L, Krigas T. Nature. 1965;205:698–700. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg B, Van Camp L, Trosko J E, Mansour V H. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 7.Lippert B. Cisplatin, Chemistry and Biochemistry of a Leading Anticancer Drug. Weinheim, Germany: Wiley; 1999. [Google Scholar]
- 8.van Kralingen C G, Reedijk J, Spek A L. Inorg Chem. 1980;19:1481–1485. [Google Scholar]
- 9.van Kralingen C G, Reedijk J. Ciencia Biol. 1980;5:159–161. [Google Scholar]
- 10.Barnham K J, Bauer C J, Djuran M I, Mazid M A, Rau T, Sadler P J. Inorg Chem. 1995;34:2826–2832. [Google Scholar]
- 11.Higby D J, Wallace H J, Albert D J, Holland J F. Cancer. 1974;33:1219–1228. doi: 10.1002/1097-0142(197405)33:5<1219::aid-cncr2820330505>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Giaccone G. Drugs. 2000;59, Suppl.:4–9. doi: 10.2165/00003495-200059004-00002. [DOI] [PubMed] [Google Scholar]
- 13. Reedijk, J. (1996) Chem. Commun., 801–806.
- 14.Reedijk J, Teuben J M. In: Cisplatin, Chemistry and Biochemistry of a Leading Anticancer Drug. Lippert B, editor. Weinheim, Germany: Wiley; 1999. pp. 339–362. [Google Scholar]
- 15.Wong E, Giandomenico C M. Chem Rev. 1999;99:2451–2466. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 16.Desoize B, Madoulet C. Crit Rev Oncol Hematol. 2002;42:317–325. doi: 10.1016/s1040-8428(01)00219-0. [DOI] [PubMed] [Google Scholar]
- 17.Yang D, van Boom S S G E, Reedijk J, van Boom J H, Farrell N, Wang A H-J. Nat Struct Biol. 1995;2:577–586. doi: 10.1038/nsb0795-577. [DOI] [PubMed] [Google Scholar]
- 18.Farrell N, Kelland L R, Roberts J D, Vanbeusichem M. Cancer Res. 1992;52:5065–5072. [PubMed] [Google Scholar]
- 19.Brabec V, Kasparkova J, Vrana O, Novakova O, Cox J W, Qu Y, Farrell N. Biochemistry. 1999;38:6781–6790. doi: 10.1021/bi990124s. [DOI] [PubMed] [Google Scholar]
- 20.Reedijk J. Inorg Chim Acta. 1992;198–200:873–881. [Google Scholar]
- 21.Housecroft C E, Sharpe A G. Inorganic Chemistry. Englewood Cliffs, NJ: Prentice–Hall; 2001. [Google Scholar]
- 22.Clarke M J, Zhu F, Frasca D R. Chem Rev. 1999;99:2511–2533. doi: 10.1021/cr9804238. [DOI] [PubMed] [Google Scholar]
- 23.Chen H M, Parkinson J A, Parsons S, Coxall R A, Gould R O, Sadler P J. J Am Chem Soc. 2002;124:3064–3082. doi: 10.1021/ja017482e. [DOI] [PubMed] [Google Scholar]
- 24.Velders A H, Kooijman H, Spek A L, Haasnoot J G, de Vos D, Reedijk J. Inorg Chem. 2000;39:2966–2967. doi: 10.1021/ic000167t. [DOI] [PubMed] [Google Scholar]
- 25.van Vliet P M, Toekimin S M S, Haasnoot J G, Reedijk J, Novakova O, Vrana O, Brabec V. Inorg Chim Acta. 1995;231:57–64. [Google Scholar]
- 26.Sava G, Bergamo A, Zorzet S, Gava B, Casarsa C, Cocchietto M, Furlani A, Scarcia V, Serli B, Iengo E, et al. Eur J Cancer. 2002;38:427–435. doi: 10.1016/s0959-8049(01)00389-6. [DOI] [PubMed] [Google Scholar]
- 27. van Boom, S. S. G. E. & Reedijk, J. (1993) J. Chem. Soc. Chem. Commun., 1397–1398.
- 28.van Boom S S G E, Chen B W, Teuben J M, Reedijk J. Inorg Chem. 1999;38:1450–1455. [Google Scholar]
- 29. Chen, Y., Guo, Z. J., Murdoch, P. D., Zang, E. L. & Sadler, P. J. (1998) J. Chem. Soc. Dalton Trans., 1503–1508.
- 30.Reedijk J. Chem Rev. 1999;99:2499–2510. doi: 10.1021/cr980422f. [DOI] [PubMed] [Google Scholar]
- 31.Murdoch P D, Kratochwil N A, Parkinson J A, Patriarca M, Sadler P J. Angew Chem Int Ed. 1999;38:2949–2951. doi: 10.1002/(sici)1521-3773(19991004)38:19<2949::aid-anie2949>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Molenaar C, Teuben J-M, Heetebrij R J, Tanke H J, Reedijk J. J Biol Inorg Chem. 2000;5:655–665. doi: 10.1007/s007750000153. [DOI] [PubMed] [Google Scholar]
- 33.Reed E. Cytotechnology. 1998;27:187–201. doi: 10.1023/A:1008016922425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed E. Cancer Treat Rev. 1998;24:331–344. doi: 10.1016/s0305-7372(98)90056-1. [DOI] [PubMed] [Google Scholar]
- 35.Jamieson E R, Lippard S J. Chem Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 36.Brouwer J, van de Putte P, Fichtinger-Schepman A M J, Reedijk J. Proc Natl Acad Sci USA. 1981;78:7010–7014. doi: 10.1073/pnas.78.11.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popoff S, Beck D J, Rupp W D. Mutat Res. 1987;1987:129–145. doi: 10.1016/0167-8817(87)90055-1. [DOI] [PubMed] [Google Scholar]
- 38.Rajski S R, Williams R M. Chem Rev. 1998;98:2723–2740. doi: 10.1021/cr9800199. [DOI] [PubMed] [Google Scholar]
- 39.Mello J A, Lippard S J, Essigmann J M. Biochemistry. 1995;34:14783–14792. doi: 10.1021/bi00045a020. [DOI] [PubMed] [Google Scholar]
- 40.Barry M A, Behnke C A, Eastman A. Biochem Pharmacol. 1990;40:2353–2360. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 41.Speelmans G, Staffhorst R W H M, Versluis K, Reedijk J, de Kruijff B. Biochemistry. 1997;36:10545–10550. doi: 10.1021/bi9703047. [DOI] [PubMed] [Google Scholar]
- 42.Gately D P, Howell S B. Br J Cancer. 1993;67:1171–1177. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishida S, Lee J, Thiele D J, Herskowitz I. Proc Natl Acad Sci USA. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin X J, Okuda T, Holzer A, Howell S B. Mol Pharmacol. 2002;62:1154–1159. doi: 10.1124/mol.62.5.1154. [DOI] [PubMed] [Google Scholar]
- 45.Miller S E, House D A. Inorg Chim Acta. 1989;166:189–192. [Google Scholar]
- 46. Berners-Price, S. J., Frenkiel, T. A., Frey, U., Ranford, J. D. & Sadler, P. J. (1992) J. Chem. Soc. Chem. Commun., 789–791.
- 47.Nitiss J L. Proc Natl Acad Sci USA. 2002;99:13963–13965. doi: 10.1073/pnas.232574299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hospers G A P, Mulder N H, de Vries E G E. Med Oncol Tumor Pharmacother. 1988;5:145–150. doi: 10.1007/BF02986437. [DOI] [PubMed] [Google Scholar]
- 49.Chaney S G, Sancar A. J Natl Cancer Inst. 1996;88:1346–1350. doi: 10.1093/jnci/88.19.1346. [DOI] [PubMed] [Google Scholar]
- 50.Borst P, Borst J, Smets L A. Drug Resist Updates. 2001;4:129–131. doi: 10.1054/drup.2001.0187. [DOI] [PubMed] [Google Scholar]
- 51.Brabec V, Kasparkova J. Drug Resist Updates. 2002;5:147–161. doi: 10.1016/s1368-7646(02)00047-x. [DOI] [PubMed] [Google Scholar]
- 52.Lippert B. Coord Chem Rev. 1999;182:263–295. [Google Scholar]
- 53.Hall M D, Hambley T W. Coord Chem Rev. 2002;232:49–67. [Google Scholar]
- 54.Reeder F, Guo Z J, Murdoch P D, Corazza A, Hambley T W, Berners-Price S J, Chottard J C, Sadler P J. Eur J Biochem. 1997;249:370–382. doi: 10.1111/j.1432-1033.1997.00370.x. [DOI] [PubMed] [Google Scholar]
- 55.Fichtinger-Schepman A M J, van der Veer J L, den Hartog J H J, Lohman P H M, Reedijk J. Biochemistry. 1985;24:707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- 56.Eastman A, Schulte N. Biochemistry. 1988;27:4730–4734. doi: 10.1021/bi00413a022. [DOI] [PubMed] [Google Scholar]
- 57.Song B, Zhao J, Griesser R, Meiser C, Sigel H, Lippert B. Chem Eur J. 1999;5:2374–2387. [Google Scholar]
- 58.Dunbar K R. Inorg Chem. 1997;36:9999–10001. [Google Scholar]
- 59. Velders, A. H., van der Geest, B., Kooijman, H., Spek, A. L., Haasnoot, J. G. & Reedijk, J. (2001) Eur. J. Inorg. Chem., 369–372. [DOI] [PubMed]
- 60. Hotze, A. C. G., Broekhuisen, M. E. T., Velders, A. H., van der Schilden, K., Haasnoot, J. G. & Reedijk, J. (2002) Eur. J. Inorg. Chem., 369–376.
- 61.Asara J M, Hess J S, Lozada E, Dunbar K R, Allison J. J Am Chem Soc. 2000;122:8–13. [Google Scholar]
- 62.Taube H. Chem Rev. 1952;50:69–78. [Google Scholar]
- 63. Velders, A. H., Ugozzoli, F., Biagini-Cingi, M., Manotti-Lanfredi, A. M., Haasnoot, J. G. & Reedijk, J. (1999) Eur. J. Inorg. Chem., 213–215. [DOI] [PubMed]
- 64.Admiraal G, van der Veer J L, de Graaff R A G, den Hartog J H J, Reedijk J. J Am Chem Soc. 1987;109:592–594. [Google Scholar]
- 65.Marzilli L G, Saad J S, Kuklenyik Z, Keating K A, Xu Y H. J Am Chem Soc. 2001;123:2764–2770. doi: 10.1021/ja0007915. [DOI] [PubMed] [Google Scholar]
- 66.Brabec V. Progr Nucleic Acid Res Mol Biol. 2002;71:1–68. doi: 10.1016/s0079-6603(02)71040-4. [DOI] [PubMed] [Google Scholar]
- 67.Ohndorf U M, Rould M A, He Q, Pabo C O, Lippard S J. Nature. 1999;399:708–712. doi: 10.1038/21460. [DOI] [PubMed] [Google Scholar]
- 68.Zamble D B, Mikata Y, Eng C H, Sandman K E, Lippard S J. J Inorg Biochem. 2002;91:451–462. doi: 10.1016/s0162-0134(02)00472-5. [DOI] [PubMed] [Google Scholar]
- 69.Neidle S, Snook C F, Murrer B A, Barnard C F J. Acta Crystallogr C. 1995;51:822–824. doi: 10.1107/s0108270194013600. [DOI] [PubMed] [Google Scholar]
- 70.Berners-Price S J, Sadler P J. Coord Chem Rev. 1996;151:1–40. [Google Scholar]
- 71.Novakova O, Vrana O, Kiseleva V, Brabec V. Eur J Biochem. 1995;228:616–622. doi: 10.1111/j.1432-1033.1995.tb20301.x. [DOI] [PubMed] [Google Scholar]
- 72.Talman E G, Brüning W, Reedijk J. J Inorg Biochem. 1995;59:138. [Google Scholar]
- 73.Choi S, Delaney S, Orbai L, Padgett E J, Hakemian A S. Inorg Chem. 2001;40:5481–5486. doi: 10.1021/ic015549t. [DOI] [PubMed] [Google Scholar]
- 74.Bouma M, Nuijen B, Stewart D R, Rice J R, Jansen B A J, Reedijk J, Bult A, Beijen J H. Anticancer Drugs. 2002;13:915–924. doi: 10.1097/00001813-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 75. De Kruijff, B., Speelmans, G., Staffhorst, R., Willibrordus, H. M. & Reedijk, J. (1998) Int. Appl. WO9824424 A119980611.
- 76.Burger K N J, Staffhorst R W H M, de Vijlder H C, Velinova M J, Bomans P H, Frederik P M, de Kruijff B. Nat Med. 2002;8:81–84. doi: 10.1038/nm0102-81. [DOI] [PubMed] [Google Scholar]
- 77.Robillard M S, Valentijn A P M, Meeuwenoord N J, van der Marel G A, van Boom J H, Reedijk J. Angew Chem Int Ed. 2000;39:3096–3099. doi: 10.1002/1521-3773(20000901)39:17<3096::aid-anie3096>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 78.Robillard M S, Galanski M, Zimmermann W, Keppler B K, Reedijk J. J Inorg Biochem. 2002;88:254–259. doi: 10.1016/s0162-0134(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 79.Robillard M S, Jansen B A J, Lochner M, Geneste H, Brouwer J, Hesse M, Reedijk J. Helv Chim Acta. 2001;84:3023–3030. [Google Scholar]
- 80.Stetsenko A I, Yakovlev K I, Rozhkova N D, Pogareva V G, Kazakov S A. Russ J Coord Chem. 1990;16:560–565. [Google Scholar]
- 81.Farrell N. Commun Inorg Chem. 1995;16:373–389. [Google Scholar]
- 82. Bloemink, M. J., Reedijk, J., Farrell, N., Qu, Y. & Stetsenko, A. I. (1992) J. Chem. Soc. Chem. Commun., 1002–1003.
- 83.McGregor T D, Balcarova Z, Qu Y, Tran M C, Zaludova R, Brabec V, Farrell N. J Inorg Biochem. 1999;77:43–46. doi: 10.1016/s0162-0134(99)00136-1. [DOI] [PubMed] [Google Scholar]
- 84.Zehnulova J, Kasparkova J, Farrell N, Brabec V. J Biol Chem. 2001;276:22191–22199. doi: 10.1074/jbc.M103118200. [DOI] [PubMed] [Google Scholar]
- 85. Komeda, S., Ohishi, H., Yamane, H., Harikawa, M., Sakaguchi, K. & Chikuma, M. (1999) J. Chem. Soc. Dalton Trans., 2959–2962.
- 86.Komeda S, Lutz M, Spek A L, Chikuma M, Reedijk J. Inorg Chem. 2000;39:4230–4236. doi: 10.1021/ic000273v. [DOI] [PubMed] [Google Scholar]
- 87.Komeda S, Lutz M, Spek A L, Yamanaka Y, Sato T, Chikuma M, Reedijk J. J Am Chem Soc. 2002;124:4738–4746. doi: 10.1021/ja0168559. [DOI] [PubMed] [Google Scholar]
- 88.Komeda S. Ph.D. thesis. Leiden, The Netherlands: Leiden University; 2002. [Google Scholar]
- 89.Teuben J M. Ph.D. thesis. Leiden, The Netherlands: Leiden University; 2000. [Google Scholar]
- 90.Barton S J, Barnham K J, Habtemariam A, Sue R E, Sadler P J. Inorg Chim Acta. 1998;273:8–13. [Google Scholar]
- 91. Hotze, A. C. G., Bacac, M., Velders, A. H., Jansen, B. A. J., Kooijman, H., Spek, A. L., Haasnoot, J. G. & Reedijk, J. (2002) J. Med. Chem., in press. [DOI] [PubMed]
- 92.Morris R E, Aird R E, Murdoch P D, Chen H M, Cummings J, Hughes N D, Parsons S, Parkin A, Boyd G, Jodrell D I, Sadler P J. J Med Chem. 2001;44:3616–3621. doi: 10.1021/jm010051m. [DOI] [PubMed] [Google Scholar]
- 93.Zenker A, Galanski M, Bereuter T L, Keppler B K, Lindner W. J Chromatogr B. 2000;745:211–219. doi: 10.1016/s0378-4347(00)00096-7. [DOI] [PubMed] [Google Scholar]
- 94. Hotze, A. C. G., Chen, Y., Hambley, T. W., Parsons, S., Kratochwil, N. A., Parkinson, J. A., Munk, V. P. & Sadler, P. J. (2002) Eur. J. Inorg. Chem., 1035–1039.
- 95.Deubel D V. J Am Chem Soc. 2002;124:5834–5842. doi: 10.1021/ja012221q. [DOI] [PubMed] [Google Scholar]
- 96. Houthoff, H. J., Reedijk, J., Jelsma, T., Heetebrij, R. J. & Volkers, H. H. (1998) Patent Cooperation Treaty (PCT) Int. Appl. WO9815564 A119980416.
- 97.van Gijlswijk R P M, Talman E G, Peekel I, Bloem J, van Velzen M A, Heetebrij R J, Tanke H J. Clin Chem. 2002;48:1352–1359. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.