Abstract
The O2-reaction chemistry of 1:1 mixtures of (F8)FeII (1; F8 = tetrakis(2,6-diflurorophenyl)porphyrinate) and [(LMe2N)CuI]+ (2; LMe2N = N,N-bis{2-[2-(N′,N′-4-dimethylamino)pyridyl]ethyl}methylamine) is described, to model aspects of the chemistry occurring in cytochrome c oxidase. Spectroscopic investigations, along with stopped-flow kinetics, reveal that low-temperature oxygenation of 1/2 leads to rapid formation of a heme-superoxo species (F8)FeIII-(O ) (3), whether or not 2 is present. Complex 3 subsequently reacts with 2 to form [(F8)FeIII–(O
) (3), whether or not 2 is present. Complex 3 subsequently reacts with 2 to form [(F8)FeIII–(O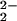 )–CuII(LMe2N)]+ (4), which thermally converts to [(F8)FeIII–(O)–CuII(LMe2N)]+ (5), which has an unusually bent (Fe–O–Cu) bond moiety. Tridentate chelation, compared with tetradentate, is shown to dramatically lower the ν(O–O) values observed in 4 and give rise to the novel structural features in 5.
)–CuII(LMe2N)]+ (4), which thermally converts to [(F8)FeIII–(O)–CuII(LMe2N)]+ (5), which has an unusually bent (Fe–O–Cu) bond moiety. Tridentate chelation, compared with tetradentate, is shown to dramatically lower the ν(O–O) values observed in 4 and give rise to the novel structural features in 5.
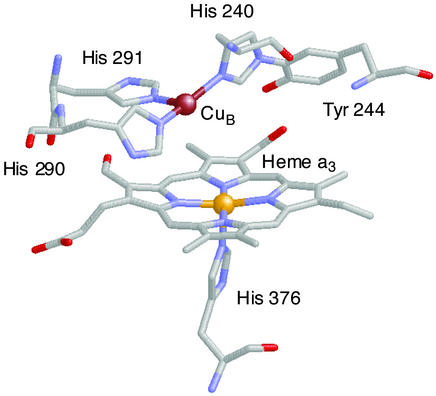
Dioxygen binding to metal ion centers such as copper and iron is of great importance and interest, for fundamental and practical reasons. In nature, copper and iron proteins serve to process O2 in a variety of functions, such as in transport of dioxygen in blood, mono- or dioxygenation involving oxygen atom incorporation, and substrate oxidation (dehydrogenation or removal of electrons) with concomitant reduction of O2 to hydrogen peroxide or water. Cytochrome c oxidases mediate the four-electron four-proton reduction of O2 to water, coupling this exergonic reaction to membrane proton translocation, which drives ATP synthesis (1, 2). Protein x-ray structures reveal that O2 binding and reduction occur at a binuclear active site consisting of a high-spin heme group (with proximal histidine), with a tris-histidine-ligated copper ion (CuB) situated on the distal side (see Fig. 1) (3–6). Spectroscopic–mechanistic investigations suggest that after a possible initial O2 interaction with CuB, a myoglobin-like Fe–O2 adduct forms (best described as a FeIII–O superoxo species), subsequently leading to a FeIV⩵O (ferryl-oxo) species (7–10), although a prior peroxo-bridged FeIII–(O
superoxo species), subsequently leading to a FeIV⩵O (ferryl-oxo) species (7–10), although a prior peroxo-bridged FeIII–(O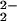 )-CuII intermediate has not been ruled out. The tyrosine-crossed-linked CuB site (Fig. 1) is thought to be critical in electron transfer (from CuI and the phenol group) leading to reductive O–O cleavage and proton-translocation chemistry (11–13).
)-CuII intermediate has not been ruled out. The tyrosine-crossed-linked CuB site (Fig. 1) is thought to be critical in electron transfer (from CuI and the phenol group) leading to reductive O–O cleavage and proton-translocation chemistry (11–13).
Figure 1.
Structure of the fully reduced (FeIII⋅⋅⋅CuII) bovine cytochrome c oxidase structure, Cu⋅⋅⋅Fe = 5.1 Å. The diagram was assembled by using PDB ID 1OCR coordinates and the program RASMOL.
Our research program is focused on elucidating the fundamental aspects of O2 interactions with heme and copper centers. Thus, we have reported several examples where reduced heme–FeII/CuI complexes react with O2, giving μ-peroxo FeIII–(O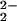 )–CuII species (14–18). Most of these studies have used tris[2-pyridylmethyl]amine (TMPA) as the Cu ligand. Extensive studies on copper (only) dioxygen systems have shown that even subtle changes in ligand structure/denticity can dramatically alter the nature of the (ligand)CuI/O2 product [giving Cun–O2 (n = 1 or 2) or Cun–(O)2, n = 2 or 3] (19–21). Tetradentate TMPA induces formation of an end-on ligated (CuII–O–O–CuII) peroxo-dicopper(II) structure upon O2 reaction with [CuI(TMPA)(MeCN)]+ (22), whereas tridentate ligands generate side-on μ-η2:η2-peroxo-dicopper(II) species, which can be in equilibrium with bis-μ-oxo-dicopper(III) isomers (21, 23, 24).
)–CuII species (14–18). Most of these studies have used tris[2-pyridylmethyl]amine (TMPA) as the Cu ligand. Extensive studies on copper (only) dioxygen systems have shown that even subtle changes in ligand structure/denticity can dramatically alter the nature of the (ligand)CuI/O2 product [giving Cun–O2 (n = 1 or 2) or Cun–(O)2, n = 2 or 3] (19–21). Tetradentate TMPA induces formation of an end-on ligated (CuII–O–O–CuII) peroxo-dicopper(II) structure upon O2 reaction with [CuI(TMPA)(MeCN)]+ (22), whereas tridentate ligands generate side-on μ-η2:η2-peroxo-dicopper(II) species, which can be in equilibrium with bis-μ-oxo-dicopper(III) isomers (21, 23, 24).
Thus, with the known tridentate chelation for CuB in cytochrome c oxidases, we are intensifying efforts to study such situations in model systems, to see whether O2 intermediates form with FeII/CuI precursors, and to understand the detailed role of the copper ligand (tridentate versus tetradentate) in heme-copper/O2 chemistry. Here we describe the oxygenation chemistry of 1:1 mixtures of (F8)FeII (1) and [(LMe2N)CuI]+ (2). ![]()
A very rich chemistry ensues (Scheme S1), wherein there is an initial rapid formation of a heme-superoxo complex (solvent)(F8)FeIII–(O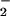 ) (3) [referred to subsequently as (F8)FeIII-(O
) (3) [referred to subsequently as (F8)FeIII-(O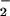 ) (3)], as elucidated from stopped-flow kinetic studies. Superoxo species 3 subsequently reacts with 2, forming the heterobinuclear μ-peroxo complex [(F8)FeIII-(O
) (3)], as elucidated from stopped-flow kinetic studies. Superoxo species 3 subsequently reacts with 2, forming the heterobinuclear μ-peroxo complex [(F8)FeIII-(O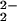 )–CuII(LMe2N)]+ (4), which has been characterized by ultraviolet–visible (UV-Vis), nuclear magnetic resonance (NMR), and resonance Raman (RR) spectroscopies. Notably, the tridentate versus tetradentate Cu chelation leads to striking differences in the nature of the peroxo moiety (i.e., the O–O stretching frequency) formed in 4 versus that in [(F8)FeIII–(O
)–CuII(LMe2N)]+ (4), which has been characterized by ultraviolet–visible (UV-Vis), nuclear magnetic resonance (NMR), and resonance Raman (RR) spectroscopies. Notably, the tridentate versus tetradentate Cu chelation leads to striking differences in the nature of the peroxo moiety (i.e., the O–O stretching frequency) formed in 4 versus that in [(F8)FeIII–(O )–CuII(TMPA)]+ (6). The μ-peroxo complex 4 thermally transforms to the μ-oxo complex [(F8)FeIII-(O)–CuII(LMe2N)]+ (5), whose x-ray structure is described, and found to be dramatically different from that found in [(F8)FeIII–(O)-CuII(TMPA)]+ (7).
)–CuII(TMPA)]+ (6). The μ-peroxo complex 4 thermally transforms to the μ-oxo complex [(F8)FeIII-(O)–CuII(LMe2N)]+ (5), whose x-ray structure is described, and found to be dramatically different from that found in [(F8)FeIII–(O)-CuII(TMPA)]+ (7).
Scheme 1.
Materials and Methods
Compounds (F8)FeII (1) (17, 25), (F8)FeII-d8 (1-d8) (17), and [(LMe2N)CuI]B(C6F5)4 (2) (25) have been previously described. The dioxygen adduct, μ-peroxo complex [(F8)FeIII–(O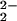 )–CuII(LMe2N)]+ (4), was generated by bubbling O2 through 178–193 K solutions of 1 and 2 that were prepared in an inert atmosphere glove box. The μ-oxo complex 5 was isolated by generation of 4 in CH2Cl2/10% CH3CN with subsequent thermal transformation (193 K to room temperature, 1 h) to the product. Precipitation with heptane gave microcrystalline [(F8)FeIII–(O)–CuII(LMe2N)]+ (5) in 58% yield. See Experimental Methods in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org, for further details concerning syntheses and spectroscopic methods.
)–CuII(LMe2N)]+ (4), was generated by bubbling O2 through 178–193 K solutions of 1 and 2 that were prepared in an inert atmosphere glove box. The μ-oxo complex 5 was isolated by generation of 4 in CH2Cl2/10% CH3CN with subsequent thermal transformation (193 K to room temperature, 1 h) to the product. Precipitation with heptane gave microcrystalline [(F8)FeIII–(O)–CuII(LMe2N)]+ (5) in 58% yield. See Experimental Methods in Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org, for further details concerning syntheses and spectroscopic methods.
Results and Discussion
(F8)FeII (1)/[(LMe2N)CuI]+ (2)/O2 Reaction: Benchtop UV-Vis Spectroscopy.
Investigations of the independent chemistry for (F8)FeII (1)/O2 (26) and [(LMe2N)CuI]+ (2)/O2 (24) have been described. The O2 adduct observed from low-temperature reactions of 1 with O2 is solvent dependent (Fe/O2 = 1:1 or 2:1). In coordinating solvents [e.g., tetrahydrofuran (THF) and propionitrile (EtCN)], oxygenation of complex 1 yields a heme-superoxo complex (Eq. 1), whereas only the peroxo-bridged homobinuclear diiron complex is observed in noncoordinating solvents (CH2Cl2, toluene; Eq. 2). [(LMe2N)CuI]+ (2) reacts with O2, yielding a bis-μ-oxo dicopper(III) and side-on peroxo dicopper(II) equilibrium mixture at low temperature (Eq. 3) (24), yet we observe that the
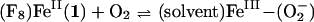 |
1 |
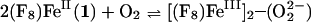 |
2 |
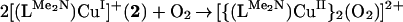 |
3 |
presence of a nitrile strongly inhibits CuI/O2 reactivity. With this background, we have designed a solvent system, CH2Cl2/6% (vol/vol) EtCN, that should force a reaction of an initially formed iron–superoxide species with our copper(I) complex, in close analogy to one of the key steps of the O2-reduction cycle in cytochrome c oxidase (see above). In CH2Cl2/6% EtCN, the EtCN (i) serves as an axial base heme ligand promoting iron–superoxide formation (Eq. 1) (26) and (ii) causes the [(LMe2N)CuI]+ (2)/O2 reaction to become insignificant.** Yet, for the 1/2/O2 mixture in CH2Cl2/6% EtCN, an iron–superoxo/copper reaction occurs, leading to the μ-peroxo heterobinuclear species [(F8)FeIII–(O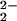 )–CuII(LMe2N)]+ (4). Such heme-peroxo-copper species were not observed from the reactions of 3 with analogous CuI complexes ([(LR)CuI]+, where L = N,N-bis[2-(2-pyridyl)ethyl]methylamine and R = 4-pyridyl substituents Cl, H, or OMe); the electron-donating dimethylamino groups in the LMe2N increase reactivity of CuI with 3 leading to formation of 4.
)–CuII(LMe2N)]+ (4). Such heme-peroxo-copper species were not observed from the reactions of 3 with analogous CuI complexes ([(LR)CuI]+, where L = N,N-bis[2-(2-pyridyl)ethyl]methylamine and R = 4-pyridyl substituents Cl, H, or OMe); the electron-donating dimethylamino groups in the LMe2N increase reactivity of CuI with 3 leading to formation of 4.
Benchtop UV-Vis spectroscopic changes of the oxygenation reaction of an equimolar mixture of (F8)FeII (1) and [(LMe2N)CuI]+ (2) are shown in Fig. 2. The reduced mixture has absorptions at 415, 425, and 527 nm at 193 K in CH2Cl2/6% EtCN solution. Bubbling with dioxygen produces a stable dioxygen adduct with new spectral features at 420, 540, and 567 nm. We formulate this as a peroxo complex (probably as two isomeric forms), [(F8)FeIII–(O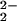 )–CuII(LMe2N)]+ (4), based on RR and 1H and 2H NMR spectroscopies (see below). Complex 4 is not stable at higher temperatures (≥203 K), primarily leading to the formation of μ-oxo complex 5, with its characteristic red-shifted Soret band (449 nm) and broad 558-nm absorption. Trace amounts of water, always present in the solvent, result in some hydrolysis of the μ-oxo bridge, especially in the low complex concentrations (≈10−5 M) of the UV-Vis experiment. Thus, mixtures of [(F8)FeIII–O–CuII(LMe2N)]+ (5) (449 and 558 nm) and (F8)FeIII–OH (407 and 571 nm) (26) are observed, as shown in Fig. 2.
)–CuII(LMe2N)]+ (4), based on RR and 1H and 2H NMR spectroscopies (see below). Complex 4 is not stable at higher temperatures (≥203 K), primarily leading to the formation of μ-oxo complex 5, with its characteristic red-shifted Soret band (449 nm) and broad 558-nm absorption. Trace amounts of water, always present in the solvent, result in some hydrolysis of the μ-oxo bridge, especially in the low complex concentrations (≈10−5 M) of the UV-Vis experiment. Thus, mixtures of [(F8)FeIII–O–CuII(LMe2N)]+ (5) (449 and 558 nm) and (F8)FeIII–OH (407 and 571 nm) (26) are observed, as shown in Fig. 2.
Figure 2.
UV-Vis spectra of the oxygenation reaction of (F8)FeII (1)/[(LMe2N)CuI]+ (2) in CH2Cl2/6% EtCN at 193 K. Shown are an equimolar mixture of 1 and 2 (black); [(F8)FeIII–(O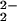 )–CuII(LMe2N)]+ (4) (red); a product mixture obtained after warming 4, which includes [(F8)FeIII–O–CuII(LMe2N)]+ (5) and (F8)FeIII–OH (green).
)–CuII(LMe2N)]+ (4) (red); a product mixture obtained after warming 4, which includes [(F8)FeIII–O–CuII(LMe2N)]+ (5) and (F8)FeIII–OH (green).
NMR Spectroscopy.
The oxygenation reaction of an equimolar mixture of (F8)FeII (1) and [(LMe2N)CuI]+ (2) was followed by low-temperature NMR spectroscopy. To unambiguously identify pyrrole resonances, which aid iron oxidation and spin-state assignments (27), complementary 2H NMR spectroscopic investigations were also carried out employing a deuterated (β-pyrrolic hydrogens) analog of 1 (1-d8). In CD2Cl2/6% EtCN-d5 at 178 K, 1H NMR spectra of the reduced 1/2 mixture exhibit only diamagnetic resonances (i.e., 1–10 ppm, Fig. 3A), indicating the presence of a low-spin six-coordinate ferrous heme (d6, S = 0; including two EtCN axial base ligands) and a CuI complex (d10, S = 0). Bubbling dry O2 into a precooled NMR tube containing 1 and 2 leads to the formation of [(F8)FeIII–(O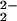 )–CuII(LMe2N)]+ (4), with downfield-shifted pyrrole resonances at ≈110 ppm (Fig. 3B), verified by 2H NMR spectroscopy (Fig. 7, which is published as supporting information on the PNAS web site). This observed pyrrole chemical shift position excludes the possibility that 4 is (i) an iron–superoxide, i.e., (F8)FeIII–(O
)–CuII(LMe2N)]+ (4), with downfield-shifted pyrrole resonances at ≈110 ppm (Fig. 3B), verified by 2H NMR spectroscopy (Fig. 7, which is published as supporting information on the PNAS web site). This observed pyrrole chemical shift position excludes the possibility that 4 is (i) an iron–superoxide, i.e., (F8)FeIII–(O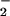 ) (3) (8.9 ppm, 193 K), (ii) the μ-peroxo complex [(F8)FeIII]2–(O
) (3) (8.9 ppm, 193 K), (ii) the μ-peroxo complex [(F8)FeIII]2–(O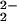 ) (17.5 ppm, 193 K), (iii) the ferryl-oxo species (F8)FeIV⩵O (3.5 ppm, 193 K), or (iv) (F8)FeIII–OH (≈135 ppm, 178 K) (26). The 110 ppm pyrrole resonance in 4 is in the range for high-spin Fe(III), yet it has a significantly diminished downfield shift compared with that seen for S = 5/2 complexes such as (F8)FeIII–X (X = OH− or Cl−; ≈135 ppm at 178 K). This observation is consistent with the presence of strong antiferromagnetic coupling between high-spin FeIII (d5, S = 5/2) and CuII (d9, S = 1/2) in [(F8)FeIII–(O
) (17.5 ppm, 193 K), (iii) the ferryl-oxo species (F8)FeIV⩵O (3.5 ppm, 193 K), or (iv) (F8)FeIII–OH (≈135 ppm, 178 K) (26). The 110 ppm pyrrole resonance in 4 is in the range for high-spin Fe(III), yet it has a significantly diminished downfield shift compared with that seen for S = 5/2 complexes such as (F8)FeIII–X (X = OH− or Cl−; ≈135 ppm at 178 K). This observation is consistent with the presence of strong antiferromagnetic coupling between high-spin FeIII (d5, S = 5/2) and CuII (d9, S = 1/2) in [(F8)FeIII–(O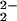 )–CuII(LMe2N)]+ (4). Cu-ligand hydrogen resonances are observed here for the LMe2N moiety in 4 (25, −24, −32, and −46 ppm, Fig. 4B). The position of the pyrrole resonances and the observable copper ligand hydrogen signals are characteristic features of (porphyrinate)FeIII–X–CuII (X = O
)–CuII(LMe2N)]+ (4). Cu-ligand hydrogen resonances are observed here for the LMe2N moiety in 4 (25, −24, −32, and −46 ppm, Fig. 4B). The position of the pyrrole resonances and the observable copper ligand hydrogen signals are characteristic features of (porphyrinate)FeIII–X–CuII (X = O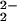 or O2−) S = 2 systems, as previously described (14, 15, 17, 28).
or O2−) S = 2 systems, as previously described (14, 15, 17, 28).
Figure 3.
1H NMR spectra of the oxygenation reaction of (F8)FeII (1)/[(LMe2N)CuI]+ (2) in CD2Cl2/6% EtCN-d5 at 178 K. (A) Equimolar mixture of 1 and 2. (B) [(F8)FeIII–(O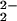 )–CuII(LMe2N)]+ (4) (red). (C) A product mixture obtained after warming 4, containing [(F8)FeIII–O–CuII(LMe2N)]+ (5) (green) and (F8)FeIII–OH (pink).
)–CuII(LMe2N)]+ (4) (red). (C) A product mixture obtained after warming 4, containing [(F8)FeIII–O–CuII(LMe2N)]+ (5) (green) and (F8)FeIII–OH (pink).
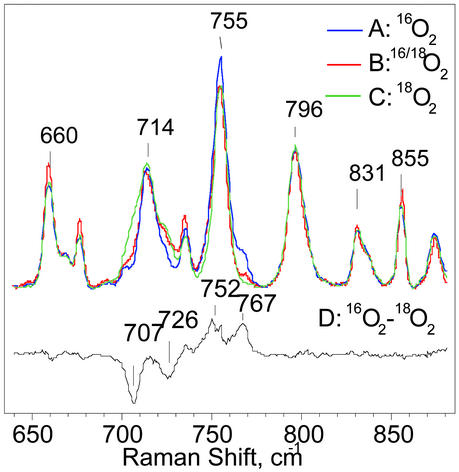
Figure 4.
RR spectra of [(F8)FeIII–(O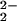 )–CuII(LMe2N)]+ (4) formed by oxygenation with 16O2 (trace A), a scrambled 16O/18O gas containing 25% 16O2, 50% 16O18O, and 25% 18O2 (trace B), or 18O2 (trace C). The difference spectrum of A minus C is shown as trace D. All spectra were obtained at ≈90 K with excitation at 413 nm.
)–CuII(LMe2N)]+ (4) formed by oxygenation with 16O2 (trace A), a scrambled 16O/18O gas containing 25% 16O2, 50% 16O18O, and 25% 18O2 (trace B), or 18O2 (trace C). The difference spectrum of A minus C is shown as trace D. All spectra were obtained at ≈90 K with excitation at 413 nm.
RR Spectroscopy.
Soret excitation of [(F8)FeIII–(O )–CuII(LMe2N)]+ (4) at 413 nm results in RR spectra dominated by strong porphyrin skeletal modes and weak iron-ligand vibrations (29, 30). Porphyrin skeletal modes in the high-frequency region of the RR spectra are consistent with a five-coordinate high-spin ferric heme species as previously observed in [(F8)FeIII–(O
)–CuII(LMe2N)]+ (4) at 413 nm results in RR spectra dominated by strong porphyrin skeletal modes and weak iron-ligand vibrations (29, 30). Porphyrin skeletal modes in the high-frequency region of the RR spectra are consistent with a five-coordinate high-spin ferric heme species as previously observed in [(F8)FeIII–(O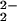 )–CuII(TMPA)]+ (6) (14, 30). Comparison of low-temperature RR spectra of 4 generated in CH2Cl2/6% EtCN with 16O2 or 18O2 reveals two isotopic shifts of ≥−40 cm−1 that identify two bands at 767 and 752 cm−1 as putative ν(O–O) peroxo stretching vibrations (Fig. 4). The latter assignment was confirmed by complementary experiments with scrambled isotope gas mixtures composed of 25% 16O2, 50% 16O18O, and 25% 18O2. Differential RR spectra, i.e., compared with spectra with pure 16O2, are expected to present additional intensity at intermediate frequency with isotope shifts of ≈−20 cm−1 for νas(16O–18O) vibrations. Large porphyrin vibrations hinder direct visualization of additional bands originating from 16O–18O peroxo species in Fig. 4, but their presence becomes apparent in difference spectra (see Fig. 8, which is published as supporting information on the PNAS web site). In trace B (Fig. 4 trace B), the relative intensity of the isotope-sensitive band at 767 cm−1 corresponds to 25% of that observed in a sample formed with pure 16O2 gas (Fig. 4 trace A). Similarly, the intensity of the 707-cm−1 band obtained from the scrambled gas samples represents 25% of the intensity observed with the pure 18O2 gas (Fig. 4 trace C). We can therefore assign these signals to peroxo ν(O–O), rather than a ν(FeIV⩵O) stretch or a ν(FeIII–O–CuII), where the dioxygen bond has been cleaved and 50% intensity ratios are expected.
)–CuII(TMPA)]+ (6) (14, 30). Comparison of low-temperature RR spectra of 4 generated in CH2Cl2/6% EtCN with 16O2 or 18O2 reveals two isotopic shifts of ≥−40 cm−1 that identify two bands at 767 and 752 cm−1 as putative ν(O–O) peroxo stretching vibrations (Fig. 4). The latter assignment was confirmed by complementary experiments with scrambled isotope gas mixtures composed of 25% 16O2, 50% 16O18O, and 25% 18O2. Differential RR spectra, i.e., compared with spectra with pure 16O2, are expected to present additional intensity at intermediate frequency with isotope shifts of ≈−20 cm−1 for νas(16O–18O) vibrations. Large porphyrin vibrations hinder direct visualization of additional bands originating from 16O–18O peroxo species in Fig. 4, but their presence becomes apparent in difference spectra (see Fig. 8, which is published as supporting information on the PNAS web site). In trace B (Fig. 4 trace B), the relative intensity of the isotope-sensitive band at 767 cm−1 corresponds to 25% of that observed in a sample formed with pure 16O2 gas (Fig. 4 trace A). Similarly, the intensity of the 707-cm−1 band obtained from the scrambled gas samples represents 25% of the intensity observed with the pure 18O2 gas (Fig. 4 trace C). We can therefore assign these signals to peroxo ν(O–O), rather than a ν(FeIV⩵O) stretch or a ν(FeIII–O–CuII), where the dioxygen bond has been cleaved and 50% intensity ratios are expected.
Assignment of the O–O stretching vibrations to a homonuclear peroxo–dicopper complex, [{(LMe2N)CuII}2(O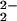 )]2+, was ruled out in a control experiment where the oxygenation reaction was carried out with a 5-fold excess of [(LMe2N)CuI]+ (2) over (F8)FeII (1). No significant difference could be observed in the RR spectra, thus affirming that the reaction of 2 with O2 is not of consequence in the presence of nitrile. {Note: independent RR studies on [{(LMe2N)CuII}2(O
)]2+, was ruled out in a control experiment where the oxygenation reaction was carried out with a 5-fold excess of [(LMe2N)CuI]+ (2) over (F8)FeII (1). No significant difference could be observed in the RR spectra, thus affirming that the reaction of 2 with O2 is not of consequence in the presence of nitrile. {Note: independent RR studies on [{(LMe2N)CuII}2(O )]2+ formed in solvents with no EtCN present show a ν(O–O) vibration at 729 cm−1, Δ18O2 = −40 cm−1, in acetone (31).}
)]2+ formed in solvents with no EtCN present show a ν(O–O) vibration at 729 cm−1, Δ18O2 = −40 cm−1, in acetone (31).}
While anionic mononuclear heme–peroxo species [(F8)FeIII-(O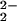 )]− might conceivably form, as is known with other porphyrinate ligands, they display ν(O–O) at significantly higher frequencies, i.e., >800 cm−1 (32, 33). Peroxo stretching vibrations from homonuclear diheme–peroxo complexes [(P)FeIII]2-(O
)]− might conceivably form, as is known with other porphyrinate ligands, they display ν(O–O) at significantly higher frequencies, i.e., >800 cm−1 (32, 33). Peroxo stretching vibrations from homonuclear diheme–peroxo complexes [(P)FeIII]2-(O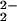 ) (P = porphyrinate) have not been observed with RR spectroscopy, but low-temperature NMR spectroscopy in any case rules out such an assignment (see above). The RR results clearly establish that two very similar iron-porphyrinate peroxo species with ν(O–O) at 767 and 752 cm−1 are present in [(F8)FeIII–(O
) (P = porphyrinate) have not been observed with RR spectroscopy, but low-temperature NMR spectroscopy in any case rules out such an assignment (see above). The RR results clearly establish that two very similar iron-porphyrinate peroxo species with ν(O–O) at 767 and 752 cm−1 are present in [(F8)FeIII–(O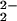 )–CuII(LMe2N)]+ (4) and strongly suggest a heme–peroxo–copper complex that adopts two closely related isomer forms. These are not distinguishable by UV-Vis or NMR spectroscopy.
)–CuII(LMe2N)]+ (4) and strongly suggest a heme–peroxo–copper complex that adopts two closely related isomer forms. These are not distinguishable by UV-Vis or NMR spectroscopy.
From the work of Collman, Naruta, and ourselves, there are now several examples of heme–peroxo–copper complexes that are characterized by RR spectroscopy (Table 1). Our previous investigations of two closely related systems, “untethered” [(F8)FeIII–(O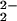 )–CuII(TMPA)]+ (6) and an analogue where the TMPA moiety is covalently attached to the heme, “tethered” [(6L)FeIII–(O
)–CuII(TMPA)]+ (6) and an analogue where the TMPA moiety is covalently attached to the heme, “tethered” [(6L)FeIII–(O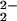 )–CuII]+, show a significant 20-cm−1 difference in ν(O–O) stretching vibrations (Table 1). This observation suggests that ligand constraints in the tethered complexes can strongly influence peroxo O–O stretching vibrations. Systems with nonheme diferric μ-1,2 peroxo groups, where differences in bond angles influence coupling of ν(O–O) with ν(Fe–O) vibrations, lead to large variations in observed frequencies (34–36). The differences in ν(O–O) observed in this series of heme–peroxo–copper complexes (i.e., 6 vs. [(6L)FeIII–(O
)–CuII]+, show a significant 20-cm−1 difference in ν(O–O) stretching vibrations (Table 1). This observation suggests that ligand constraints in the tethered complexes can strongly influence peroxo O–O stretching vibrations. Systems with nonheme diferric μ-1,2 peroxo groups, where differences in bond angles influence coupling of ν(O–O) with ν(Fe–O) vibrations, lead to large variations in observed frequencies (34–36). The differences in ν(O–O) observed in this series of heme–peroxo–copper complexes (i.e., 6 vs. [(6L)FeIII–(O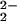 )–CuII]+ and 4) likely relate to changes in Fe–O–O angles and possibly a transition from μ-1,2 to μ-η2:η2 bridging geometry (see below). In rather similar complexes, Naruta and coworkers (37) reported that the incorporation of electron-donating copper-ligand substituents results in a weaker O–O bond. (Compare [(PTPA)FeIIICuII–(O
)–CuII]+ and 4) likely relate to changes in Fe–O–O angles and possibly a transition from μ-1,2 to μ-η2:η2 bridging geometry (see below). In rather similar complexes, Naruta and coworkers (37) reported that the incorporation of electron-donating copper-ligand substituents results in a weaker O–O bond. (Compare [(PTPA)FeIIICuII–(O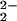 )]+ and [(P5-MeTPA)FeIIICuII–(O
)]+ and [(P5-MeTPA)FeIIICuII–(O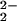 )]+; Table 1.)
)]+; Table 1.)
Table 1.
RR data of heme–peroxo–Cu complexes
| Complex | ν(16O2) (Δ(18O2)), cm−1 | Ref. |
|---|---|---|
[(F8)FeIII–(O )–CuII(LMe2N)]+ (4) )–CuII(LMe2N)]+ (4) |
767 (41), 752 (45) | This work |
[(F8)FeIII–(O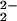 )–CuII(TMPA)]+ (6) )–CuII(TMPA)]+ (6) |
808 (46) | 14 |
[(6L)FeIII–(O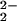 )–CuII]+ )–CuII]+
|
788 (44) | 15 |
[(PTPA)FeIIICuII–(O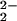 )]+ )]+
|
803 (44) | 37 |
[(P5-MeTPA)FeIII–(O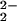 )–CuII]+ )–CuII]+
|
793 (42) | 37 |
[(PTACN)FeIII–(O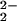 )–CuII]+ )–CuII]+
|
758 (18) | 38 |
PTPA, tris(2-picolylamine)- or P5-MeTPA, tris(2-(5methyl)pyridylmethyl)amine-linked tetraphenylporphyrinate; PTACN, triazacyclononane-capped tetraarylporphyrin.
The present studies reveal that tridentate versus tetradentate copper chelation plays a key role in determining heme–peroxo–copper complex stability and peroxo ν(O–O) stretching vibration. [(F8)FeIII–(O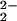 )–CuII(LMe2N)]+ (4) is not as thermally stable as the tetradentate containing compounds. For example, 4 decomposes above ≈213 K, whereas [(F8)FeIII–(O
)–CuII(LMe2N)]+ (4) is not as thermally stable as the tetradentate containing compounds. For example, 4 decomposes above ≈213 K, whereas [(F8)FeIII–(O )– CuII(TMPA)]+ (6) has room temperature stability in MeCN solution. From a more quantitative perspective, the RR studies (see above) show that complex 4 exhibits significantly lower values in ν(O–O) vibrations (752 and 767 cm−1) than does 6 (808 cm−1), a decrease in frequency of ≥40 cm−1. The ν(O–O) vibrations in 4 are comparable with the 758-cm−1 value (Table 1) seen by Collman et al. (38) in the [(PTACN)FeIIICuII–(O
)– CuII(TMPA)]+ (6) has room temperature stability in MeCN solution. From a more quantitative perspective, the RR studies (see above) show that complex 4 exhibits significantly lower values in ν(O–O) vibrations (752 and 767 cm−1) than does 6 (808 cm−1), a decrease in frequency of ≥40 cm−1. The ν(O–O) vibrations in 4 are comparable with the 758-cm−1 value (Table 1) seen by Collman et al. (38) in the [(PTACN)FeIIICuII–(O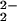 )]+ complex, where a tridentate copper ligand triazacyclononane (TACN) as well as heme imidazole axial base ligand was used.
)]+ complex, where a tridentate copper ligand triazacyclononane (TACN) as well as heme imidazole axial base ligand was used.
The origin of the instability and O–O bond weakening are not yet fully resolved, but in comparison to the tetradentate Cu ligation in [(F8)FeIII–(O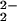 )–CuII(TMPA)]+ (6) or other complexes, tridentate Cu ligation in [(F8)FeIII–(O
)–CuII(TMPA)]+ (6) or other complexes, tridentate Cu ligation in [(F8)FeIII–(O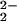 )–CuII(LMe2N)]+ (4) provides an additional open coordination site. As is now well known in copper(I)/dioxygen chemistry, this could result in μ-η2:η2 side-on versus μ-1,2-peroxo end-on ligation, which considerably reduces the ν(O–O) value (805–830 cm−1 going down to 710–760 cm−1) (20, 39, 40) and poises the Cu2(O2) peroxo-dicopper(II) moiety for O–O bond cleavage (41). Thus, along with the knowledge that side-on (as well as end-on) (42) ligation for heme-peroxo species exists, we suggest that a side-on heme-peroxo-copper intermediate structure (left structure in diagram below) is a strong possibility for 4.
)–CuII(LMe2N)]+ (4) provides an additional open coordination site. As is now well known in copper(I)/dioxygen chemistry, this could result in μ-η2:η2 side-on versus μ-1,2-peroxo end-on ligation, which considerably reduces the ν(O–O) value (805–830 cm−1 going down to 710–760 cm−1) (20, 39, 40) and poises the Cu2(O2) peroxo-dicopper(II) moiety for O–O bond cleavage (41). Thus, along with the knowledge that side-on (as well as end-on) (42) ligation for heme-peroxo species exists, we suggest that a side-on heme-peroxo-copper intermediate structure (left structure in diagram below) is a strong possibility for 4. ![]()
The observation (from RR spectroscopy) of two isomers in [(F8)FeIII–(O )–CuII(LMe2N)]+ (4) may be a result of different conformers caused by different copper ligand orientations. If the copper center in 4 has a square-pyramidal geometry, as is well known for five-coordinate complexes containing LR (R = H, MeO, or Me2N) (43), there are two reasonable possibilities for the positioning of the LMe2N ligand–copper complex in [(F8)FeIII–(O
)–CuII(LMe2N)]+ (4) may be a result of different conformers caused by different copper ligand orientations. If the copper center in 4 has a square-pyramidal geometry, as is well known for five-coordinate complexes containing LR (R = H, MeO, or Me2N) (43), there are two reasonable possibilities for the positioning of the LMe2N ligand–copper complex in [(F8)FeIII–(O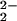 )–CuII(LMe2N)]+ (4) in the basal plane: (i) one pyridyl and one alkylamino group are trans to the O atoms of the peroxo bridging ligand or (ii) two pyridyl groups are trans to the O atoms.
)–CuII(LMe2N)]+ (4) in the basal plane: (i) one pyridyl and one alkylamino group are trans to the O atoms of the peroxo bridging ligand or (ii) two pyridyl groups are trans to the O atoms.
Kinetics of the Reaction of (F8)FeII (1)/[(LMe2N)CuI]+ (2)/O2 and 1/O2.
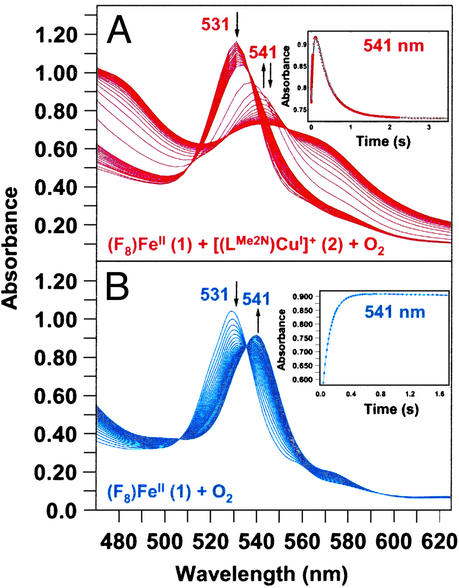
The reaction of 1 and 2 with O2 was investigated by stopped-flow kinetics in CH2Cl2/6% EtCN. Fig. 5A shows spectroscopic monitoring in the heme α/β region (470 nm to 625 nm) over the time period of 3.5 s. A band at 531 nm (reduced complex 1) decays and an intermediate with 541-nm absorption, ascribed to (F8)FeIII–(O ) (3) (see below), grows in during the first ≈0.25 s (Fig. 5A). This then decays over 2–3 s to more complex spectra with broad maxima at ≈540 nm and ≈565 nm, which we ascribe to the μ-peroxo species [(F8)FeIII–(O
) (3) (see below), grows in during the first ≈0.25 s (Fig. 5A). This then decays over 2–3 s to more complex spectra with broad maxima at ≈540 nm and ≈565 nm, which we ascribe to the μ-peroxo species [(F8)FeIII–(O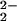 )–CuII(LMe2N)]+ (4). (Small differences in λmax values seen in the kinetics studies here, compared with benchtop UV-Vis monitoring, are ascribed to the use of different spectrometers at different temperatures.)
)–CuII(LMe2N)]+ (4). (Small differences in λmax values seen in the kinetics studies here, compared with benchtop UV-Vis monitoring, are ascribed to the use of different spectrometers at different temperatures.)
Figure 5.
(A) Reaction of (F8)FeII (1) (3.43 ⋅ 10−4 M) and [(LMe2N)CuI]+ (2) (3.69 ⋅ 10−4 M) with O2 (2.20 ⋅ 10−3 M) at 168 K in CH2Cl2/6% EtCN (50 out of a total of 172 spectra shown). (Inset) Absorbance–time traces at 541 nm [formation and decay of (F8)FeIII–(O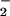 ) (3) over ≈3.5 s]. (B) Reaction of 1 (2.67 ⋅ 10−4 M) with O2 (2.20 ⋅ 10−3 M) at 168 K in CH2Cl2/6% EtCN (50 out of a total of 150 spectra shown). (Inset) Absorbance–time trace at 541 nm (over ≈1.7 s) for formation of 3.
) (3) over ≈3.5 s]. (B) Reaction of 1 (2.67 ⋅ 10−4 M) with O2 (2.20 ⋅ 10−3 M) at 168 K in CH2Cl2/6% EtCN (50 out of a total of 150 spectra shown). (Inset) Absorbance–time trace at 541 nm (over ≈1.7 s) for formation of 3.
Fig. 5B shows the UV-Vis spectra for the oxygenation of a solution containing only 1 in CH2Cl2/6% EtCN. As in the (F8)FeII (1)/[(LMe2N)CuI]+ (2) mixture, 1 has an absorbance maximum near 531 nm, which rapidly decreases upon reaction with dioxygen. Concomitant generation of (F8)FeIII–(O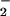 ) (3) (λmax = 541 nm) occurs and its formation is complete within ≈0.5 s (Fig. 5B Inset). This species is quite stable at low temperatures. RR characterization of 3 formed in THF unambiguously confirms this to be a low-spin six-coordinate superoxo species, with ν(O–O) = 1178 cm−1 [Δ(18O2) = −64 cm−1] and ν(Fe–O) = 568 cm−1 [Δ(18O2) = −24 cm−1] (see Fig. 9, which is published as supporting information on the PNAS web site), in line with previous chemical and other spectroscopic studies (26).
) (3) (λmax = 541 nm) occurs and its formation is complete within ≈0.5 s (Fig. 5B Inset). This species is quite stable at low temperatures. RR characterization of 3 formed in THF unambiguously confirms this to be a low-spin six-coordinate superoxo species, with ν(O–O) = 1178 cm−1 [Δ(18O2) = −64 cm−1] and ν(Fe–O) = 568 cm−1 [Δ(18O2) = −24 cm−1] (see Fig. 9, which is published as supporting information on the PNAS web site), in line with previous chemical and other spectroscopic studies (26).
The data obtained for the reaction of (F8)FeII (1) with O2 as well as for (F8)FeII (1)/[(LMe2N)CuI]+ (2) with O2 in CH2Cl2/6% EtCN and in THF/6% EtCN are presented in Table 2. They allow us to compare formation of (F8)FeIII–(O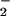 ) (3) in the presence or absence of copper. In fact, the results show that copper complex 2 has essentially no effect on the formation of 3 as seen from the rate constants and activation parameters obtained (Table 2).
) (3) in the presence or absence of copper. In fact, the results show that copper complex 2 has essentially no effect on the formation of 3 as seen from the rate constants and activation parameters obtained (Table 2).
Table 2.
Kinetic parameters for the formation of (F8)FeIII–(O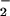 ) (3)
) (3)
| Parameter | (F8)FeII (1)/[(LMe2N)CuI]+ (2) + O2
|
(F8)FeII (1) + O2
|
|||
|---|---|---|---|---|---|
| CH2Cl2/6% EtCN*† | THF/6% EtCN* | CH2Cl2/6% EtCN*† | THF/6% EtCN* | EtCN§ | |
| ΔH‡, kJ/mol | NA | 20 ± 1 | 41.2 ± 0.6 | 21.0 ± 0.6 | 38.6 ± 0.4 |
| ΔS‡, J/mol·K | NA | −25 ± 6 | 74 ± 4 | −19 ± 4 | 42 ± 2 |
| k1, M−1·s−1 168 K | (6 ± 2) · 103 | (8.5 ± 0.2) · 104 | (4.00 ± 0.16) · 103 | (1.23 ± 0.04) · 105 | (5.5 ± 0.2) · 102 |
| k1, M−1·s−1 183 K | (6 ± 2) · 104 | (3.05 ± 0.14) · 105 | (4.86 ± 0.10) · 104 | (4.21 ± 0.08) · 105 | (5.57 ± 0.04) · 103 |
| k1, M−1·s−1 198 K | (5 ± 1) · 105 | (9.0 ± 0.8) · 105 | (4.07 ± 0.16) · 105 | (1.29 ± 0.06) · 106 | (4.19 ± 0.06) · 104 |
Data are presented as best estimates with twice their standard errors. NA, not applicable. Sources are as follows:
, this work;
, preliminary data;
, ref. 26.
The kinetics of formation of (F8)FeII (1) in the various solvents used indicate a high degree of similarity, but there are certain differences in detail. Below 200 K, the formation of (F8)FeIII–(O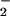 ) (3) in THF/6% EtCN is faster compared with the same reaction in CH2Cl2/6% EtCN, primarily because of an effect of activation enthalpy (Table 2). Because in CH2Cl2/6% EtCN, EtCN binds to 1 as an axial base ligand (low-spin six- coordinate; see above), the enhanced rate of O2 reaction with 1 in THF/6% EtCN therefore must be due to an effect of THF. Factors such as THF versus EtCN binding strengths, lability, electron-donating ability, and iron(II) spin state need to be considered. We note that in CH2Cl2/6% EtCN, ΔS‡ = 74 ± 4 J/mol⋅K is suggestive of dissociative loss of EtCN during O2 binding, whereas in THF/6% EtCN a negative value (ΔS‡ = −19 ± 4 J/mol⋅K) perhaps indicates an associative binding of O2 to a pentacoordinate (THF)FeII species. (Note that in THF, 1 is high-spin at all temperatures.) Comparing formation of (F8)FeIII–(O
) (3) in THF/6% EtCN is faster compared with the same reaction in CH2Cl2/6% EtCN, primarily because of an effect of activation enthalpy (Table 2). Because in CH2Cl2/6% EtCN, EtCN binds to 1 as an axial base ligand (low-spin six- coordinate; see above), the enhanced rate of O2 reaction with 1 in THF/6% EtCN therefore must be due to an effect of THF. Factors such as THF versus EtCN binding strengths, lability, electron-donating ability, and iron(II) spin state need to be considered. We note that in CH2Cl2/6% EtCN, ΔS‡ = 74 ± 4 J/mol⋅K is suggestive of dissociative loss of EtCN during O2 binding, whereas in THF/6% EtCN a negative value (ΔS‡ = −19 ± 4 J/mol⋅K) perhaps indicates an associative binding of O2 to a pentacoordinate (THF)FeII species. (Note that in THF, 1 is high-spin at all temperatures.) Comparing formation of (F8)FeIII–(O ) (3) in EtCN and CH2Cl2/6% EtCN, the activation enthalpies are nearly identical (Table 2); the activation entropy varies because the equilibrium for binding of EtCN to (F8)FeII (1) lies further to the right in pure EtCN.
) (3) in EtCN and CH2Cl2/6% EtCN, the activation enthalpies are nearly identical (Table 2); the activation entropy varies because the equilibrium for binding of EtCN to (F8)FeII (1) lies further to the right in pure EtCN.
In both CH2Cl2/6% EtCN and THF/6% EtCN, the decay of the band at 541 nm shows that (F8)FeIII–(O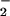 ) (3) is short-lived in the presence of copper complex 2, because of further reaction to give μ-peroxo species [(F8)FeIII–(O
) (3) is short-lived in the presence of copper complex 2, because of further reaction to give μ-peroxo species [(F8)FeIII–(O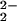 )–CuII(LMe2N)]+ (4). As mentioned above, the reaction of [(LMe2N)CuI]+ (2) with O2 in CH2Cl2/6% EtCN is inconsequential, but 2 does react with 3, i.e., a heme-bound O2 species. In CH2Cl2/6% EtCN (Fig. 5A), the half-life for decay of 3 to form 4 at 168 K is t1/2 = 0.40 s ([Fe] = 3.43 ⋅ 10−4 M, [Cu] = 3.69 ⋅ 10−4 M, [O2] = 2.21 ⋅ 10−3 M). A preliminary data analysis reveals that this transformation contains at least two relaxations. Because the RR data for solutions of 4 suggest the presence of two μ-peroxo species (see above), parallel reactions (leading to two different products) have to be considered as a mechanistic possibility. For a full understanding of the reaction of 1/2/O2, including the decay of μ-peroxo complex 4 to [(F8)FeIII–(O)–CuII(LMe2N)]+ (5), a complete kinetic analysis is required and is forthcoming.
)–CuII(LMe2N)]+ (4). As mentioned above, the reaction of [(LMe2N)CuI]+ (2) with O2 in CH2Cl2/6% EtCN is inconsequential, but 2 does react with 3, i.e., a heme-bound O2 species. In CH2Cl2/6% EtCN (Fig. 5A), the half-life for decay of 3 to form 4 at 168 K is t1/2 = 0.40 s ([Fe] = 3.43 ⋅ 10−4 M, [Cu] = 3.69 ⋅ 10−4 M, [O2] = 2.21 ⋅ 10−3 M). A preliminary data analysis reveals that this transformation contains at least two relaxations. Because the RR data for solutions of 4 suggest the presence of two μ-peroxo species (see above), parallel reactions (leading to two different products) have to be considered as a mechanistic possibility. For a full understanding of the reaction of 1/2/O2, including the decay of μ-peroxo complex 4 to [(F8)FeIII–(O)–CuII(LMe2N)]+ (5), a complete kinetic analysis is required and is forthcoming.
X-Ray Structure and Properties of [(F8)FeIII–O–CuII(LMe2N)]+ (5).
Warming the μ-peroxo complex 4 to room temperature leads to formation of 5; by analogy to the process shown to occur with the TMPA ligand–Cu system (14), it is presumed that this occurs by a disproportionation reaction, i.e., 2[(F8)FeIII–(O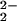 )–CuII(LMe2N)]+ (4) → 2[(F8)FeIII–O–CuII(LMe2N)]B(C6F5)4 (5) + O2. The product 5 is highly moisture sensitive, but is stable at room temperature under inert atmosphere. Complex 5 (Fig. 6) possesses an oxide bridge between a square-pyramidal iron(III) porphyrinate and a highly distorted tetracoordinate copper(II) ion. Notably, a FeIII–(X)–CuII moiety, with three copper–N donors and a bridging ligand (X = O
)–CuII(LMe2N)]+ (4) → 2[(F8)FeIII–O–CuII(LMe2N)]B(C6F5)4 (5) + O2. The product 5 is highly moisture sensitive, but is stable at room temperature under inert atmosphere. Complex 5 (Fig. 6) possesses an oxide bridge between a square-pyramidal iron(III) porphyrinate and a highly distorted tetracoordinate copper(II) ion. Notably, a FeIII–(X)–CuII moiety, with three copper–N donors and a bridging ligand (X = O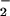 , OH−, or H2O) is observed in the active-site structure of the ba3-type cytochrome c oxidase (5). The Fe–N bond distances in 5 and the large displacement of the iron atom from the porphyrinate ligand (0.55 Å) are very similar to those of five-coordinate high-spin Fe(III) porphyrinates (44). The Cu–N bond distances are typical for Cu(II) with alkyl and/or pyridylamine ligation (Fig. 6). Both pyridyl groups on the copper ligand are placed “above” and between the four difluorophenyl meso-substituents of the heme (Fig. 6).
, OH−, or H2O) is observed in the active-site structure of the ba3-type cytochrome c oxidase (5). The Fe–N bond distances in 5 and the large displacement of the iron atom from the porphyrinate ligand (0.55 Å) are very similar to those of five-coordinate high-spin Fe(III) porphyrinates (44). The Cu–N bond distances are typical for Cu(II) with alkyl and/or pyridylamine ligation (Fig. 6). Both pyridyl groups on the copper ligand are placed “above” and between the four difluorophenyl meso-substituents of the heme (Fig. 6).
Figure 6.
ORTEP representation showing the cationic portion of [(F8)FeIII–O–CuII(LMe2N)]B(C6F5)4 (5). Fe⋅⋅⋅Cu, 3.417 Å; Cu1–O1–Fe1, 143.44(12)°. See Tables 4–8 and an expanded version of this figure with further details, which are published as supporting information on the PNAS web site.
The most significant structural feature of [(F8)FeIII–O–CuII(LMe2N)]+ (5) is the Fe–O–Cu core. Table 3 shows comparisons of the metal–oxygen bond distances and the (Fe–O–Cu) bond angle for 5 with other (porphyrinate)FeIII–O–CuII complexes. The metal–oxygen bond distances are all similar and characteristically short, as has been described previously (45). The Fe–O bond length in 5 is similar to those of μ-oxo-diiron(porphyrinates) (48), and the short Cu–O bond distance [1.852(2) Å] is not far off from values observed for bis-μ-oxodicopper(III) complexes (Cu–O ≈ 1.8 Å) (21). Most notably, there is a large difference in the (Fe–O–Cu) bond angle in 5 compared with complexes with a similar chemical framework. Almost all (porphyrinate)FeIII–(O2−)–CuII complexes with tetradentate copper ligands have near-linear core structures, but the Fe–O–Cu bridge in 5 is severely bent to an angle of 143.4° (Table 3). A similar small angle (≈140 ± 3°) was deduced from an x-ray absorption study on [(5L)Fe–(O)–Cu]+, where tethering a TMPA chelate to the porphyrin imposes severe ligand constraints affecting structural, chemical, and physical properties (47).
Table 3.
Comparison of the core structures of [(P)FeIII–(O2−)–CuII]+ complexes
| Complex | Distance, Å
|
Angle, °
|
Ref. | |
|---|---|---|---|---|
| Fe–O | Cu–O | Fe–O–Cu | ||
| 5 | 1.747 (2) | 1.852 (2) | 143.4 (2) | This work |
| 7 | 1.740 (5) | 1.856 (5) | 178.2 (4) | 45 |
| [(6L)Fe–O–Cu]+ | 1.750 (4) | 1.848 (4) | 171.1 (3) | 16 |
| [(OEP)Fe–O–Cu(tren′)]+ | 1.747 (6) | 1.827 (6) | 176.0 (7) | 46 |
| (F8)Fe–O–Fe(F8) | 1.760 (2) | 178.5 (8) | 45 | |
| [(5L)Fe–O–Cu]+* | 1.76 | 1.84 | 141 ± 6 | 47 |
OEP, dianion of octaethylporphyrin; tren′, tris[(N,N-dimethylamino)ethyl]amine.
X-ray absorption spectroscopic data.
Despite the difference in the Fe–O–Cu core structures, there is a strong magnetic coupling between iron (d5, S = 5/2) and copper (d9, S = 1/2) in both [(F8)FeIII–O–CuII(LMe2N)]+ (5) and [(F8)Fe–O–Cu(TMPA)]+ (7). A low-temperature 1H NMR spectrum of 5 (Fig. 3C) exhibits an upfield and downfield pattern of signals similar to the μ-peroxo species 4 (Fig. 3B). [A fully assigned room-temperature spectrum of 5 (Fig. 10) and a Curie plot (δ vs. 1/T; Fig. 11) are published as supporting information on the PNAS web site.] Thus, 5 is also an S = 2 spin system, supported by solution Evans method magnetic moment determination (μeff = 4.9 ± 0.2 μB at room temperature). Strong magnetic coupling is a general feature of μ-oxo dimetal complexes, over a wide range of M–O–M′ angles (48).
Summary/Conclusions.
In continuing studies of heme-copper/dioxygen chemistry, we have used a novel tridentate ligand for copper(I), in complex [(LMe2N)CuI]+ (2), whose reaction with (F8)FeII (1) and O2 leads first to the superoxo-iron(III) complex (F8)FeIII–(O ) (3), with subsequent formation of the μ-peroxo species [(F8)FeIII–(O
) (3), with subsequent formation of the μ-peroxo species [(F8)FeIII–(O )–CuII(LMe2N)]+ (4). In a slower reaction, 4 transforms to a μ-oxo complex [(F8)FeIII–(O)–CuII(LMe2N)]+ (5). From studies in a number of solvents and solvent mixtures, we find that low temperatures (i.e., <205 K) are required to observe the fast formation of 3. Reaction rates are enhanced by the presence of THF, and the kinetics of formation of 3 are independent of the presence of copper complex 2. [(F8)FeIII–(O
)–CuII(LMe2N)]+ (4). In a slower reaction, 4 transforms to a μ-oxo complex [(F8)FeIII–(O)–CuII(LMe2N)]+ (5). From studies in a number of solvents and solvent mixtures, we find that low temperatures (i.e., <205 K) are required to observe the fast formation of 3. Reaction rates are enhanced by the presence of THF, and the kinetics of formation of 3 are independent of the presence of copper complex 2. [(F8)FeIII–(O )–CuII(LMe2N)]+ (4) has characteristic UV-Vis and 1H NMR spectroscopic features, which compare well with other FeIII–O
)–CuII(LMe2N)]+ (4) has characteristic UV-Vis and 1H NMR spectroscopic features, which compare well with other FeIII–O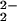 –CuII species employing tetradentate Cu ligands; they are all S = 2 systems with strong magnetic coupling between the iron and copper centers. RR spectroscopy clearly identifies 4 as having a peroxo moiety; actually two such species are present in solution. The strongly reduced ν(O–O) values observed are ascribed to the tridentate Cu–LMe2N ligation; A μ-η2:η2 side-on peroxo structure for 4 is a reasonable possibility. Complex [(F8)FeIII–(O)–CuII(LMe2N)]+ (5) is also a strongly coupled S = 2 system. The x-ray structure of 5 reveals a severely bent FeIII-O2−-CuII core, in contrast to near-linear analogs that possess tetradentate copper chelates. This study adds significantly to ongoing investigations focused on how heme-copper centers react with dioxygen, with particular consideration of the important influence of the denticity (tetra- vs. tridentate) of the copper ligand.
–CuII species employing tetradentate Cu ligands; they are all S = 2 systems with strong magnetic coupling between the iron and copper centers. RR spectroscopy clearly identifies 4 as having a peroxo moiety; actually two such species are present in solution. The strongly reduced ν(O–O) values observed are ascribed to the tridentate Cu–LMe2N ligation; A μ-η2:η2 side-on peroxo structure for 4 is a reasonable possibility. Complex [(F8)FeIII–(O)–CuII(LMe2N)]+ (5) is also a strongly coupled S = 2 system. The x-ray structure of 5 reveals a severely bent FeIII-O2−-CuII core, in contrast to near-linear analogs that possess tetradentate copper chelates. This study adds significantly to ongoing investigations focused on how heme-copper centers react with dioxygen, with particular consideration of the important influence of the denticity (tetra- vs. tridentate) of the copper ligand.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants GM60353 (to K.D.K.), GM18865 (to P.M.-L.) and GM20805 (to M.E.H.), and financial support from the Swiss National Science Foundation (to A.D.Z.).
Abbreviations
- TMPA
tris[2-pyridylmethyl]amine
- F8
tetrakis(2,6-diflurorophenyl)porphyrinate
- LMe2NN,N-bis{2-[2-(N′,N′-4-dimethylamino)pyridyl]ethyl}methylamine
UV-Vis, ultraviolet–visible
- RR
resonance Raman
- THF
tetrahydrofuran
- EtCN
propionitrile
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The x-ray data have been deposited in the Cambridge Crystallographic Data Centre, www.ccdc,cam.ac.uk (CCDC 206505).
A minor 2/O2 reaction is observed in CH2Cl2/6% EtCN, but it does not affect the 1/2/O2 kinetics described in this report.
References
- 1.Ferguson-Miller S, Babcock G T. Chem Rev. 1996;96:2889–2907. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 2.Michel H, Behr J, Harrenga A, Kannt A. Annu Rev Biophys Biomol Struct. 1998;27:329–356. doi: 10.1146/annurev.biophys.27.1.329. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Jei-Fei M, Libeu C P, Mizushima T, et al. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 4.Ostermeier C, Harrenga A, Ermler U, Michel H. Proc Natl Acad Sci USA. 1997;94:10547–10553. doi: 10.1073/pnas.94.20.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soulimane T, Buse G, Bourenkov G P, Bartunik H D, Huber R, Than M E. EMBO J. 2000;19:1766–1776. doi: 10.1093/emboj/19.8.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svensson-Ek M, Abramson J, Larsson G, Tornroth S, Brzezinski P, Iwata S. J Mol Biol. 2002;321:329–339. doi: 10.1016/s0022-2836(02)00619-8. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa T. J Inorg Biochem. 2000;82:9–18. doi: 10.1016/s0162-0134(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 8.Babcock G T. Proc Natl Acad Sci USA. 1999;96:12971–12973. doi: 10.1073/pnas.96.23.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blomberg M R A, Siegbahn P E M, Babcock G T, Wikström M. J Am Chem Soc. 2000;122:12848–12858. [Google Scholar]
- 10.Fabian M, Wong W W, Gennis R B, Palmer G. Proc Natl Acad Sci USA. 1999;96:13114–13117. doi: 10.1073/pnas.96.23.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gennis R B. Biochim Biophys Acta. 1998;1365:241–248. doi: 10.1016/s0005-2728(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 12.Proshlyakov D A, Pressler M A, DeMaso C, Leykam J F, DeWitt D L, Babcock G T. Science. 2000;290:1588–1591. doi: 10.1126/science.290.5496.1588. [DOI] [PubMed] [Google Scholar]
- 13.MacMillan F, Kannt A, Behr J, Prisner T, Michel H. Biochemistry. 1999;38:9179–9184. doi: 10.1021/bi9911987. [DOI] [PubMed] [Google Scholar]
- 14.Ghiladi R A, Hatwell K R, Karlin K D, Huang H-w, Moënne-Loccoz P, Krebs C, Huynh B H, Marzilli L A, Cotter R J, Kaderli S, Zuberbühler A D. J Am Chem Soc. 2001;123:6183–6184. doi: 10.1021/ja010602y. [DOI] [PubMed] [Google Scholar]
- 15.Ghiladi R A, Ju T D, Lee D-H, Moënne-Loccoz P, Kaderli S, Neuhold Y-M, Zuberbühler A D, Woods A S, Cotter R J, Karlin K D. J Am Chem Soc. 1999;121:9885–9886. [Google Scholar]
- 16.Ju T D, Ghiladi R A, Lee D-H, van Strijdonck G P F, Woods A S, Cotter R J, Young J, V G, Karlin K D. Inorg Chem. 1999;38:2244–2245. [Google Scholar]
- 17.Kopf M A, Neuhold Y M, Zuberbühler A D, Karlin K D. Inorg Chem. 1999;38:3093–3102. [Google Scholar]
- 18.Kopf M A, Karlin K D. Inorg Chem. 1999;38:4922–4923. doi: 10.1021/ic990546q. [DOI] [PubMed] [Google Scholar]
- 19.Karlin K D, Kaderli S, Zuberbühler A D. Acc Chem Res. 1997;30:139–147. [Google Scholar]
- 20.Blackman A G, Tolman W B. Struct Bonding (Berlin) 2000;97:179–211. [Google Scholar]
- 21.Que L, Jr, Tolman W B. Angew Chem Int Ed Engl. 2002;41:1114–1137. doi: 10.1002/1521-3773(20020402)41:7<1114::aid-anie1114>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Tyeklár Z, Jacobson R R, Wei N, Murthy N N, Zubieta J, Karlin K D. J Am Chem Soc. 1993;115:2677–2689. [Google Scholar]
- 23.Liang H-C, Zhang C X, Henson M J, Sommer R D, Hatwell K R, Kaderli S, Zuberbühler A D, Rheingold A L, Solomon E I, Karlin K D. J Am Chem Soc. 2002;124:4170–4171. doi: 10.1021/ja0125265. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C X, Liang H-C, Kim E-i, Shearer J, Helton M E, Kim E, Kaderli S, Incarvito C D, Zuberbühler A D, Rheingold A L, Karlin K D. J Am Chem Soc. 2003;125:634–635. doi: 10.1021/ja028779v. [DOI] [PubMed] [Google Scholar]
- 25. Nanthakumar, A., Fox, S. & Karlin, K. D. (1995) J. Chem. Soc. Chem. Commun., 499–501.
- 26.Ghiladi R A, Kretzer R M, Guzei I, Rheingold A L, Neuhold Y-M, Hatwell K R, Zuberbühler A D, Karlin K D. Inorg Chem. 2001;40:5754–5767. doi: 10.1021/ic0105866. [DOI] [PubMed] [Google Scholar]
- 27.Walker F A. In: NMR and EPR, The Porphyrin Handbook. Kadish K M, Smith K M, Guilard R, editors. Vol. 5. San Diego: Academic; 2000. pp. 81–184. [Google Scholar]
- 28.Nanthakumar A, Fox S, Murthy N N, Karlin K D. J Am Chem Soc. 1997;119:3898–3906. [Google Scholar]
- 29.Spiro T G, Li Y-Y. In: Resonance Raman Spectroscopy of Metalloporphyrins. Spiro T G, editor. Vol. 3. New York: Wiley Interscience; 1988. pp. 1–38. [Google Scholar]
- 30.Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. New York: Wiley Interscience; 1997. [Google Scholar]
- 31. Henson, M., J., Vance, M. A., Zhang, C. X., Liang, H.-C., Karlin, K. D. & Solomon, E. I. (2003) J. Am. Chem. Soc. 125, in press. [DOI] [PubMed]
- 32.Burstyn J N, Roe J A, Miksztal A R, Shaevitz B A, Lang G, Valentine J S. J Am Chem Soc. 1988;110:1382–1388. [Google Scholar]
- 33.Selke M, Sisemore M F, Valentine J S. J Am Chem Soc. 1996;118:2008–2012. [Google Scholar]
- 34.Brunold T C, Tamura N, Kitajima N, Moro-Oka Y, Solomon E I. J Am Chem Soc. 1998;120:5674–5690. [Google Scholar]
- 35.Moënne-Loccoz P, Baldwin J, Ley B A, Loehr T M, Bollinger J M., Jr Biochemistry. 1998;37:14659–14663. doi: 10.1021/bi981838q. [DOI] [PubMed] [Google Scholar]
- 36.Moënne-Loccoz P, Krebs C, Herlihy K, Edmondson D E, Theil E C, Huynh B H, Loehr T M. Biochemistry. 1999;38:5290–5295. doi: 10.1021/bi990095l. [DOI] [PubMed] [Google Scholar]
- 37.Naruta Y, Sasaki T, Tani F, Tachi Y, Kawato N, Nakamura N. J Inorg Biochem. 2001;83:239–246. doi: 10.1016/s0162-0134(00)00170-7. [DOI] [PubMed] [Google Scholar]
- 38.Collman J P, Herrmann P C, Boitrel B, Zhang X, Eberspacher T A, Fu L, Wang J, Rousseau D L, Williams E R. J Am Chem Soc. 1994;116:9783–9784. [Google Scholar]
- 39.Baldwin M J, Ross P K, Pate J E, Tyeklár Z, Karlin K D, Solomon E I. J Am Chem Soc. 1991;113:8671–8679. [Google Scholar]
- 40.Pidcock E, Obias H V, Abe M, Liang H-C, Karlin K D, Solomon E I. J Am Chem Soc. 1999;121:1299–1308. [Google Scholar]
- 41.Henson M J, Mukherjee P, Root D E, Stack T D P, Solomon E I. J Am Chem Soc. 1999;121:10332–10345. [Google Scholar]
- 42.Balch A L. Inorg Chim Acta. 1992;198–200:297–307. [Google Scholar]
- 43.Obias H V, Lin Y, Murthy N N, Pidcock E, Solomon E I, Ralle M, Blackburn N J, Neuhold Y-M, Zuberbühler A D, Karlin K D. J Am Chem Soc. 1998;120:12960–12961. [Google Scholar]
- 44.Scheidt W R, Reed C A. Chem Rev. 1981;81:543–555. [Google Scholar]
- 45.Karlin K D, Nanthakumar A, Fox S, Murthy N N, Ravi N, Huynh B H, Orosz R D, Day E P. J Am Chem Soc. 1994;116:4753–4763. [Google Scholar]
- 46.Lee S C, Holm R H. J Am Chem Soc. 1993;115:11789–11798. [Google Scholar]
- 47.Obias H V, van Strijdonck G P F, Lee D-H, Ralle M, Blackburn N J, Karlin K D. J Am Chem Soc. 1998;120:9696–9697. [Google Scholar]
- 48.Kurtz D M., Jr Chem Rev. 1990;90:585–606. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.