Abstract
Our originally designed dansylamidoethylcyclen 4 as a biomimetic Zn2+-selective fluorophore has been demonstrated to be a good detector of the apoptosis (induced by an anticancer agent, etoposide, and H2O2) in cancer cells such as HeLa and HL60 cells. The macrocyclic Zn2+ ligand 4 (mostly as a deprotonated form) is cell-permeable to show weak fluorescence (emission at 550 nm), which forms a strong fluorescent 1:1 Zn2+ complex 5 (emission at 530 nm) when Zn2+ is incorporated into the cells by a zinc(II) ionophore pyrithione. Thus formed, Zn2+ complex 5 is cell-impermeable and remains intact over a few hours. When apoptosis in HeLa or HL60 cells is artificially induced, 4 selectively and strongly stains apoptotic cells only at early stages, which was verified by using the conventional apoptosis detection probe annexin V-Cy3. Detection of the apoptotic cells by 4 was perhaps due to significantly increased free Zn2+ flux at early stages of apoptosis. Apoptotic detection by 4 has been compared with a presently available Zn2+ fluorophore, Zinquin 1. We present that 4 has advantages in detection of apoptosis over annexin V-Cy3 and Zinquin 1.
Apoptosis is a highly programmed cell death mechanism for removal of unwanted cells from tissues and is an important event in tissue development and homeostasis (1–4). Many anticancer agents such as cisplatin (5, 6) and etoposide (7, 8) act by inducing tumor cells to apoptose. Apoptosis is distinguished from necrosis (passive cell death) by a unique series of morphological changes, including a decease in cell volume and budding of the cell contents into membrane-enclosed vesicles. Fragmentation of DNA in apoptotic cells appears on gel electrophoresis (1, 8). Apoptosis is accompanied by a loss of membrane phospholipid asymmetry, resulting in the exposure of phosphatidylserine at the cell surface, which is a principle to be currently used for detection of apoptosis by an annexin V-Cy3 kit (9). Annexin V has a high affinity for phosphatidylserine, whereby the covalently attached fluorescein Cy3 becomes fluorescent (10).
Zinc(II) is now well recognized as an important cofactor in the regulation of apoptosis (11–18). For instance, removal of Zn2+ enhances apoptosis, whereas supplementation of Zn2+, whose mechanism is not yet understood, suppresses it (13). Furthermore, intracellular labile zinc(II) flux was observed during apoptosis by using a fluorescent Zn2+ probe, Zinquin 1 (for structures of Zn2+ ligands, see Fig. 1) (14–18). Zinquin 1 (L1) is a bidentate ligand, and its hydrolyzed form 2 (L2) gives 1:1 [Zn(H–1L2)] and 2:1 [Zn(H–1L2)2] complexes 3 with Zn2+ in physiological media with a respective stepwise Kd of 3.7 × 10–7 and 8.5 × 10–8 M (14). Metalloproteins have much higher affinities for Zn2+, with Kd typically ranging from 10–12 to 10–13 M, than does Zinquin 1. Cellular Zinquin 1 fluorescence could thus be increased to the same extent as in apoptotic cells if there were a substantial release of Zn2+ from metalloproteins. It is not clear yet whether the changes in free Zn2+ are a cause or a consequence of apoptosis. Zalewski et al. (15) hypothesized that the release of Zn2+ from the endonuclease early in apoptosis may facilitate DNA fragmentation; i.e., the changes in intracellular Zn2+ may be a cause of apoptosis.
Figure 1.
The structures of zinc(II) fluorophores and zinc(II) chelators used in this study.
A few years ago, while pursuing a model study of carbonic anhydrase, we synthesized a highly efficient macrocyclic Zn2+-selective fluorescent probe 4 (L3) (18–25). The structure was originally designed as a biomimetic model of the catalytic Zn2+ center of carbonic anhydrase that strongly and selectively binds to dansylamide. Characteristic of our probe dansylamidoethylcyclen 4 was its affinity to Zn2+ (Kd = 1.4 × 10–10 M at pH 7.0 and 5.5 × 10–13 M at pH 7.8), which was much higher than that of Zinquin 1 (19). The Kd values for the 1:1 Zn2+–4 complex, 5 [Zn(H–1L3)] significantly vary at near neutral pH because of the competing protonation. Interestingly, the Kd value of 5.5 × 10–13 M at a pH of 7.8 is comparable to the Kd values for zinc(II) enzymes and the Kd value of 5.7 × 10–12 M at pH 7–7.8 for zinc(II) finger peptides (26, 27). The dansylamide deprotonation in the Zn2+ complex 5 at pH 7.8 increased the emission intensity by 4.8-fold at 540 nm and by 10-fold at 490 nm, whereas the nonmetallated dansylamide deprotonation of L to H–1L3 without Zn2+ at high pH (>12) brought about only a 20% increase in the fluorescence emission intensity (19). The second-order reaction rate of 4 with Zn2+ at neutral pH is 1.7 × 102 M–1⋅s–1 (19), which is not as fast as 1. Until now, there have been few cell-permeable Zn2+ fluorophores in the literature (28–31). Zinpyr-1 6, for example, was reported by Lippard and coworkers (28, 29); however, its applications may be limited due to its only two to three times increase in quantum yield on Zn2+ binding.
In the present study, we have investigated whether this type of Zn2+ fluorophore 4 is biologically useful, i.e., cell-permeable, and, if so, what are its special properties in comparison to currently available but weakly Zn2+-binding agents such as Zinquin 1 in detecting intracellular Zn2+ flux. We have discovered that among those Zn2+ probes, only 4 was a selective and efficient sensor of apoptosis in cancer cells. For the present study, we also have compared 4 with homologous Zn2+ fluorophores 7 (19) and 8 (31). Comparison has also been made with Zinquin 1 and commercial apoptosis detection kit annexin V-Cy3 that adopts an entirely different mechanistic principle.
Materials and Methods
All aqueous solutions were prepared by using trace Zn2+-free deionized and redistilled water. A 4 like L3⋅(HCl)5, either synthesized (19) or purchased from Dojin Kagaku (Kumamoto, Japan), was dissolved in an aqueous buffer solution. Zinquin 1 was a kind gift from P. D. Zalewski (University of Adelaide, Adelaide, Australia), which was stored at 5 mM in dimethyl sulfoxide at −20°C. An N-dansyl-N,N-dimethylethylenediamine 7 (19) and a dansylamidoethyl-[12]aneN3 8 (31) were prepared as described. N,N,N′N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) 9 was purchased from Sigma.
Preparation of Cultured Cells.
Cultured HeLa and HL60 cells were obtained from Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Japan) and were maintained in DMEM (Nissui Seiyaku, Tokyo) supplemented with 10% FBS (GIBCO/BRL)/100 μg/ml streptomycin/100 units/ml penicillin. Cell viability was monitored by trypan blue or propidium iodide (PI) exclusion and phase-contrast microscopy. PI and trypan blue were added to final concentrations of 30 μM and 0.1% (wt/vol), respectively.
Treatment of Cells with Zn2+ Ionophores.
Cells (5 × 106 per ml) were treated for indicated periods of time at 37°C with 25 μM ZnSO4 (approximate concentration of Zn2+ in human plasma is 15 μM) and Zn2+ ionophore sodium pyrithione (14–16) in Hanks' balanced salt solution (HBSS). Cells were then washed three times with HBSS to remove extracellular Zn2+ before labeling with Zn2+ fluorophores 1, 4, 7, and 8.
Cell Staining.
Cells (105/100 μl in complete medium) were placed into each well of a 96-well plate. After 24 h, the medium was exchanged for serum-free medium containing a dye, which was then incubated for 30 min. After washing the cells with PBS, observations were made with an Olympus (New Hyde Park, NY) fluorescence microscope. Excitation light was provided by a 150-W Xe arc lamp transmitted through a 400-nm emission filter (Olympus U-MWU cube). Intracellular acidic compartments were visualized by staining with the acridine orange. Excitation light transmitted through a 460- to 490-nm excitation filter and fluorescence was observed after passage through a 500-nm emission filter (Olympus CX-DMB-2 cube).
Fluorescence Microscopy.
For measurements of intracellular fluorescence wavelength of Zn2+ fluorophores, 1, 4, 7, and 8, suspended HeLa cells (107 cells per ml in medium) or HL60 cells (106 cells per ml in medium) were incubated with 1, 4, 7, or 8 (50 μM) for 30 min at 37°C, and cells were washed three times with PBS buffer, then suspended in PBS in cuvettes. For measurement of intracellular Zn2+ complex of 1 or 4, cells incubated with 1 or 4 were treated with 25 μM ZnSO4 and 20 μM pyrithione for 10 min at 37°C in medium and washed three times with PBS buffer. Fluorescence of cell suspension was measured at 25 ± 0.1°C in a Hitachi (Tokyo) 4500 fluorescence spectrophotometer at excitation wavelength of 330 nm.
Results and Discussion
Intracellular Staining with 4.
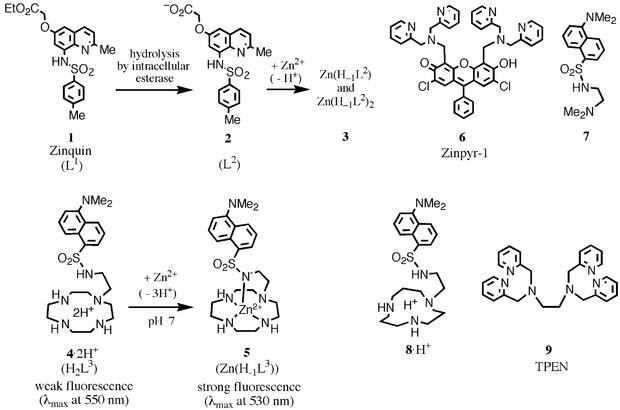
Incubation of cells with 50 μM 4 for 1 h produced all bright punctate staining patterns similar to observations made with Zinquin 1 (14, 15) and Zinpyr-1 6 (28, 29). The brightly fluorescing cells by 4 were seen in cultured cell populations tested, including human cervix epitheloid carcinoma HeLa cells and human promyelocytic leukemia HL60 cells. It is to be noted that 4 (100 μM) by itself induced apoptosis on exposure over 48 h. However, a short exposure (e.g., 1 h) did not cause significant damage to HeLa cells. Fig. 2A shows intensely fluorescent HeLa cells stained with 50 μM 4. A significant enhancement of the blue-shifted fluorescence in the puncta could be observed when the cells were exogeneously treated for 15 min with a mixture of Zn2+ (25 μM) and zinc(II) ionophore pyrithione (2-mercaptopyridine N-oxide, 20 μM) (Fig. 2B). Acridine orange tends to be trapped (as red fluorescence) in acidic compartments of cells as a membrane-impermeable protonated species, due to its weak acidity (pKa = 9.8). 4 also stained the same acidic compartments of cells, due to similar weak acidities (pKa = 11.8, 10.8, 9.37, and 4.03). Namely, a less protonated 4 permeates the cellular membrane, but on more protonations in the acidic cell compartment, 4 would be impermeable and remain emitting weak fluorescence (at 550 nm). The initial fluorescence of Zn2+–4 complex 5 was not readily diminished by addition of a far stronger Zn2+-chelator, TPEN 9 [Kd for Zn2+-TPEN is 6.3 × 10−16 M at pH 7.6 (32)], probably due to kinetic inertness of the macrocyclic Zn2+ complex 5. The strong intracellular fluorescence from 5 remained intact over several hours. Separately, we found that the fluorescence of 5 hardly disappeared by addition of TPEN in a test tube. For comparison, Zinquin 1 by itself (25 μM, the predetermined optimal concentration) stained HeLa cells only a little, but it emitted strong blue-fluorescence when contacted with zinc(II)-pyrithione. However, the intracellular fluorescence by the Zn2+–Zinquin complexes 3 did not last so long as originally reported of chronic lymphocytic leukemia cells by Zalewski et al. (15); i.e., within 10–20 sec, the fluorescence from 3 in HeLa cells became quenched. It is to be emphasized that unlike Zinquin complexes 3, the Zn2+– 4 complex 5 was much more stable kinetically, thermodynamically, and toward UV light. In comparison to cell-permeable Zinpyr-1 6 reported by Lippard et al., whose fluorescence was strongly enhanced not only by Zn2+ but also by protonation (especially at neutral pH), our 4 obviously has more advantage. Another advantage was that metal-free 4 having weak emission maximum at 550 nm is distinguished from the Zn2+–bound 4 having blue-shifted fluorescence at 530 nm. It is now concluded that the free ligand 4 as a protonated form (H2L3) is outer-membrane-permeable at neutral pH and forms a thermodynamically and kinetically stable 1:1 Zn2+ complex 5 emitting stronger blue-shifted fluorescence. The complex 5 remained impermeable and did not diffuse for a long period. Because the nucleic acid and membrane bleb were devoid of the 4-derived fluorescence, we concluded that 4 would localize only in extranuclei cytoplasm.
Figure 2.
(A) Fluorescence microscopy images (×400) of HeLa cells labeled with 50 μM 4 for 1 h at 37°C, and the fluorescence emission maximum at 550 nm, indicating that fluorescence is not Zn2+-induced. (B) The bright perinuclear punctate staining (emission maxima at 530 nm) increases on addition of the zinc(II) ionophore, Zn2+–pyrithione, indicating the intracellular Zn2+– 4 complexation (to yield 5).
After we learned that our zinc(II) fluorophore 4 appeared useful in detecting intracellular Zn2+ influx, we have tested 4 to see how it may image apoptotic processes in cancer cells that may or may not involve the intracellular zinc(II) flux.
Apoptotic Morphology of HeLa Cells.
The HeLa cells were incubated with an anticancer agent etoposide (100 μM, 48 h, 37°C) to induce apoptosis (7, 8). The earliest morphological change occurred to cell size. At 48 h, a subpopulation of cells demonstrated marked shrinkage (36 ± 6%). At longer time periods, all of the dying cells shrank. As another indication of apoptosis, internucleosomal digestion of DNA occurs at an early event of apoptosis (before cell shrinkage). Etoposide broke DNA in 48 h after incubation, as shown by 180-bp DNA ladders on gel electrophoresis (data not shown). The apoptosis-induced HeLa cells (stained with 4) were examined simultaneously by phase-contrast and UV fluorescence microscopy. Very brightly fluorescent apoptotic cells were discernible from nonfluorescent viable (intact) cells. The morphological features of apoptosis include membrane blebbing, decrease in cell volume, and pyknotic nuclei in the phase-contrast microscopic image. Although shrunken apoptotic cells were brightly fluorescent, there was no simple correlation between degree of shrinkage and fluorescence intensity. Another apoptosis-inducing agent, H2O2 (0≈1 mM; ref. 33), also increased proportion of Zn2+-rich cells with similar apoptotic morphological changes.
Dual Labeling of Apoptotic HeLa Cells with 4 and Vital Dye PI.
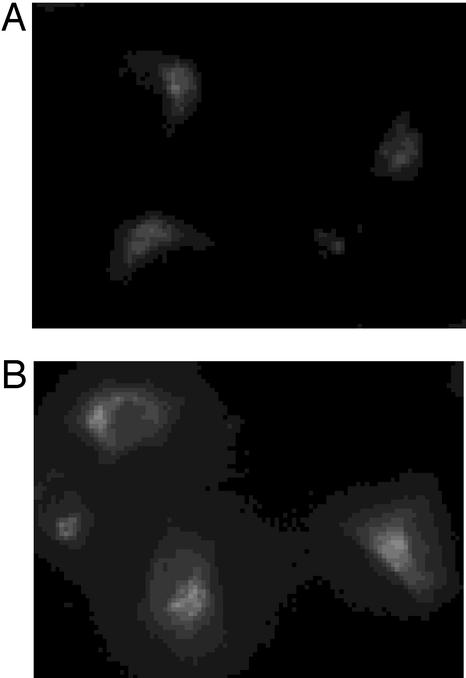
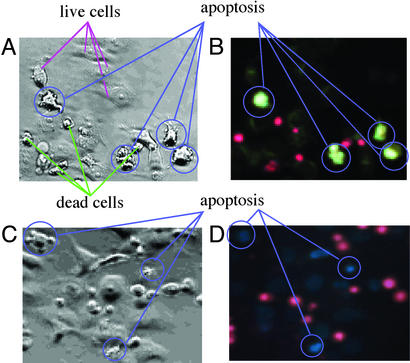
Viable cells seen by phase-contrast microscopic image were devoid of fluorescence. At early stages of apoptosis (for example, treatment with etoposide for at 6 h; Fig. 3 A and B), brightly fluorescent apoptotic cells emerged, but these apoptotic cells still excluded the vital dye PI (Fig. 3 C and D), indicating that an increase in the fluorescence by 4 preceded changes in inner membrane permeability. Later in the etoposide culture (for example, at 48 h), however, the later stages of apoptotic cells permitted the inner membrane permeability of the vital dye PI to allow pink-red-colored fluorescence emission (620 nm) from the nucleus DNA-bound PI (Fig. 3 G–J).
Figure 3.
Morphological changes of HeLa cells (dually labeled with 4 and PI) by simultaneous phase contrast (A, C, E, G, and I) and fluorescence (B, D, F, H, and J) microscopy (×400). (A and B) Live cells and the early stages of apoptotic cells. (C and D) Early stage of apoptosis. (E and F) Middle stages of apoptosis. (G and H) Later stages of apoptosis. (I and J) Dead cells.
By contrast, cell necrosis induced by a high concentration (80 mM) of H2O2 (33) was not accompanied by an increase in fluorescence from 4; rather, the fluorescence diminished to almost none. Furthermore, these dead cells lacked the morphological features of apoptosis. The inner membranes were leaky of the vital dye PI to permit red florescent interaction with DNA and did not contain fragmented DNA (data not shown).
Intracellular Staining with Other Relevant Zn2+ Fluorophores.
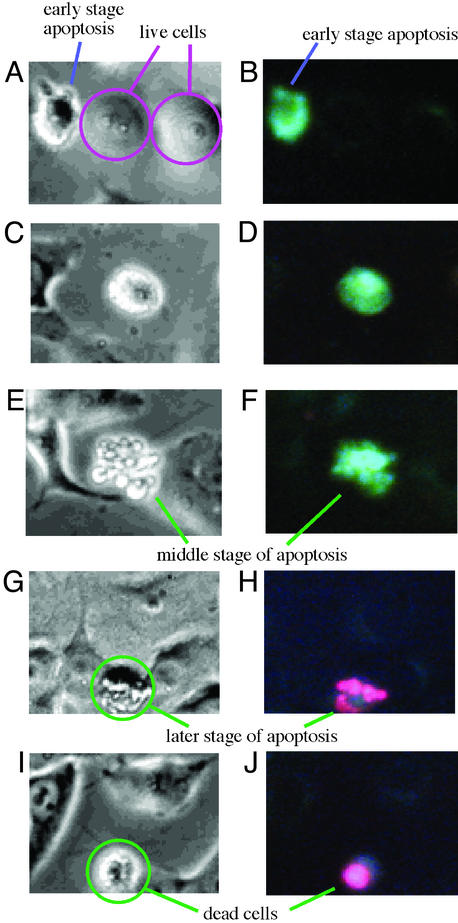
To check that the brighter fluorescence by 4 in HeLa apoptosis was due to increased release in Zn2+, we first tried to stain HeLa cells with homologues 7 or 8 (100 μM, 30 min, 37°C) that were shown to bind with Zn2+ as less strongly as 4 (19, 31). We saw that 7 and 8 similarly permeated the outer membrane to weakly fluoresce with similar perinuclear punctate staining pattern, as seen with 4 (Fig. 4A). However, when 7- (or 8)-permeated cells were treated with various concentrations of Zn2+ (10–100 μM)–pyrithione (8–80 μM), the fluorescence intensity did not change (Fig. 4B), indicating that 7 and 8 could not detect cellular Zn2+. We also observed that on staining apoptotic HeLa cells [with etoposide (100 μM) cultured for 48 h] with 7 or 8, the intracellular fluorescences did not intensify, as observed with 4.
Figure 4.
7 (100 μM)-loaded HeLa cells with UV fluorescence. Images are from before (A) and after (B) increasing intracellular Zn2+ concentration using sodium pyrithione.
To further test that the brighter fluorescence of 4 in apoptotic cells was due to the flux of free Zn2+, we examined the effect of the stronger Zn2+ chelator TPEN 9, which acts as an intracellular Zn2+ ligand (13–18). Apoptosis-induced HeLa cells were treated with TPEN (100 μM) for 2 h after staining with 4 or Zinquin 1. TPEN did not so clearly eliminate the bright fluorescence of 4 as the bright fluorescence of 1. Therefore, it may not be conclusive that the enhanced fluorescence of 4 in apoptosis was due to increased Zn2+. However, we could explain this result by the fact that the intracellular ligand displacement of macrocyclic complex 5 by TPEN was kinetically too slow. Although we tentatively postulated that the brighter fluorescence of 4 in apoptotic cells is due to increased Zn2+ flux, another mechanism, such as altered behaviors of probe 4 to permeate apoptotic cells, may not be completely excluded in accounting for the brighter fluorescence.
Comparison of Fluorescence Microscopic Images of Apoptosis by 4 and Zinquin 1.
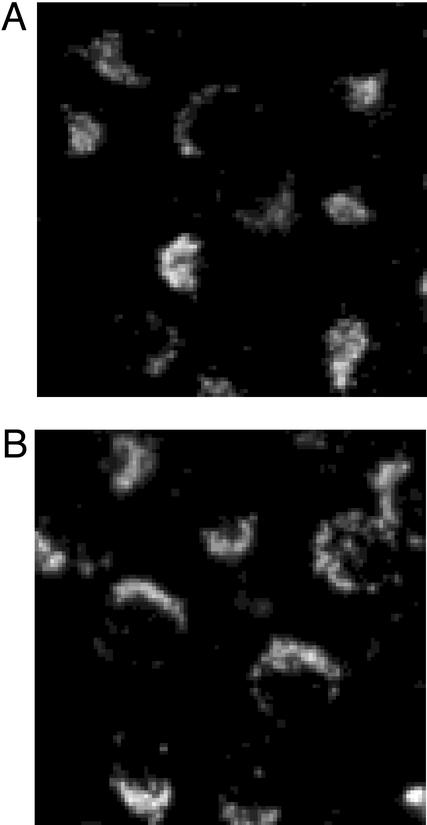
For practical apoptotic detection, we have compared our new probe 4 (100 μM, 1 h) (Fig. 5A) with Zinquin 1 (50 μM, 1 h) (Fig. 5B) on HeLa cells apoptosis in the presence of PI. With either Zn2+ fluorophore, the simultaneous phase contrast and fluorescence microscopic pictures gave similar detection of intact (live) cells by weak fluorescences, apoptotic cells by brighter fluorescences, and the PI-stained necrotic or dead cells. Furthermore, we found that the brighter fluorescence by 4 was stable and sustained more than several hours, whereas the brighter fluorescence by Zinquin 1 disappeared in 10–20 sec. These results demonstrate that our macrocyclic probe 4 is another useful apoptotic detection probe. Statistic determination of apoptotic HeLa cells by 1 or 4 was preliminarily attempted at several concentrations of etoposide (see Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). However, we have failed so far to obtain quantitative results.
Figure 5.
Comparison of microscopic images (×400) of apoptosis by 4 [A (phase contrast) and B (fluorescence)] and by Zinquin 1 [C (phase contrast) and D (fluorescence)]. Etoposide (100 μM, 48 h)-treated HeLa cells were dually stained with 4 (100 μM) or Zinquin 1 (50 μM) and PI (30 μM). Both fluorophores were excited at 330–350 nm.
Fluorescence Microscopic Image of Apoptotic HL60 Cells by 4.
The remarkable increase in fluorescence emission of 4 was also observed with another kind of cancer HL60 cells in apoptosis induced by etoposide (100 μM, 48 h). The phase-contrast and fluorescence microscopic pictures (Fig. 6 A and B) clearly distinguished brighter green-fluorescent apoptotic cells from very weak fluorescent intact (live) cells and nonfluorescent dead cells, a fact that enhances a potential usefulness of 4 as an apoptosis probe. Separately, we confirmed the apoptotic HL60 cells by the after annexin V-Cy3 detector.
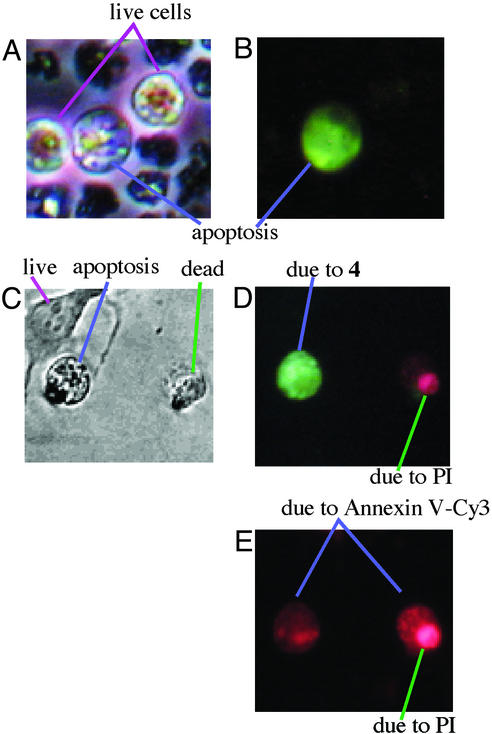
Figure 6.
Phase-contrast (A) and fluorescent (B) images (×400) of apoptotic HL60 cells stained with 4. (C–E) Phase-contrast image (C), fluorescent image [irradiated with UV (330–350 nm), D], and fluorescent image [irradiated with visible light (460–490 nm), E] of apoptotic HeLa cells triply stained with 4, annexin V-Cy3, and PI, showing green-fluorescent apoptotic cells by 4 (D), and red-fluorescent apoptotic cells by annexin V-Cy3 (E) with red-fluorescent dead cells by PI.
Triply Stained Apoptotic HeLa Cells with 4, Annexin V-Cy3, and PI.
The present apoptosis detection by 4 has been compared with a currently available annexin V-Cy3 method (9), which adopts an totally different chemical principle. Entry into apoptosis or necrosis leads to a loss of phospholipid asymmetry with exposure of phosphatidylserine on the outer leaflet. The anticoagulant annexin V binds to the negatively charged phosphatidylserine on the membrane of apoptotic cells as well as dead cells. Fluorescein isothiocynate (Cy3)-labeled annexin V thus stains apoptotic and dead cells. The binding of annexin V correlates with apoptotic nuclear morphology and DNA fragmentation (10). To compare our probe 4 with annexin V for staining apoptotic cells, apoptotic HeLa cells cultured as described above were triply stained with 4, annexin V-Cy3, and PI for 1 h at 37°C. Fig. 6C shows phase-contrast microscopic images of viable (live), apoptotic, and dead cells as morphologically distinguished. Irradiation with UV light (330–350 nm) showed only the apoptotic cells with intensely green fluorescence from 4 (Fig. 6D). The same apoptotic cells also have been stained by annexin V-Cy3 (Fig. 6E), as shown by red fluorescence [irradiated with visible light (460–490 nm)]. These findings are another proof that 3 is as good as the conventional annexin V probe in detecting apoptosis. Moreover, the annexin V-Cy3 method requires the double stain together with PI to distinguish apoptosis from necrosis (9, 10), because annexin V also binds to dead cells. PI distinguishes dead cells from apoptotic cells. As an advantage, our probe 4 acts alone to distinguish apoptosis from necrosis.
Conclusion
First, we found a Zn2+ fluorophore 4 to be a useful biological Zn2+ probe. 4 may be useful for quantification of Zn2+ concentrations in zinc(II)-rich cells including secretory cells or inflammatory cells (34). Secondly, we have demonstrated that 4 may be a new and more practical detector of apoptosis than previously reported Zinquin 1 or annexin V-Cy3 probes. The comparable Zn2+-affinities of 4, Zn2+ enzymes, and Zn2+ finger proteins may be helpful in examining the mechanism of Zn2+ flux in the apoptotic processes.
Supplementary Material
Acknowledgments
S.A. thanks the Uehara Memorial Foundation (Tokyo), the Asahi Glass Foundation (Tokyo), and the Research Foundation for Pharmaceutical Sciences (Tokyo), and S.A. and E. Kikuta thank the Takeda Science Foundation (Osaka). This work was supported by Ministry of Education, Science, and Culture, Japan Grants 08249103 and 12470479 (to E. Kimura), and 12033237, 12771355, and 13557195 (to S.A.).
Abbreviations
- pyrithione
2-mercaptopyridine N-oxide
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
- PI
propidium iodide
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lavin M, Watters D. Programmed Cell Death: The Cellular and Molecular Biology of Apoptosis. Melbourne: Harwood; 1993. [Google Scholar]
- 2.Vaux D J, Korsmeyer S J. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Evans G I, Vousden K H. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z. Chem Biol. 2002;9:1059–1072. doi: 10.1016/s1074-5521(02)00247-8. [DOI] [PubMed] [Google Scholar]
- 5.Barry M A, Behnke C A, Eastman A. Biochem Pharmacol. 1990;40:2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi T, Lippard S J. Proc Natl Acad Sci USA. 1998;95:4219–4223. doi: 10.1073/pnas.95.8.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu T, Kubota M, Tanizawa A, Sano H, Kasai Y, Hashimoto H, Akiyama Y, Mikawa Y. Biochem Biophys Res Commun. 1990;169:1172–1177. doi: 10.1016/0006-291x(90)92019-v. [DOI] [PubMed] [Google Scholar]
- 8.Forbes I J, Zalewski P D, Giannakis C, Cowled P A. Exp Cell Res. 1992;198:367–372. doi: 10.1016/0014-4827(92)90393-m. [DOI] [PubMed] [Google Scholar]
- 9.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 10.Koopman G, Reutelingsperger C P M, Kujiten G A M, Keehnen R M J, Pals S T, van Oers M H J. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 11.Treves S, Trentini P L, Ascanelli M, Bucci G, Di Virglio F. Exp Cell Biol. 1994;211:339–343. doi: 10.1006/excr.1994.1096. [DOI] [PubMed] [Google Scholar]
- 12.Cuajungco M P, Lees G J. Neurobiol Dis. 1997;4:137–169. doi: 10.1006/nbdi.1997.0163. [DOI] [PubMed] [Google Scholar]
- 13.Truong-Tran A Q, Carter J, Ruffin R E, Zalewski P D. BioMetals. 2001;14:315–330. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- 14.Zalewski P D, Forbes I J, Betts W H. Biochem J. 1993;296:403–408. doi: 10.1042/bj2960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zalewski P D, Forbes I J, Seamark R F, Borlinghaus R, Betts W H, Lincoln S F, Ward A D. Chem Biol. 1994;1:153–161. doi: 10.1016/1074-5521(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 16.Zalewski P D, Millard S H, Forbes I J, Kapaniris O, Slavotinek A, Betts W H, Ward A D, Lincoln S F, Mahadevan I. J Histochem Cytochem. 1994;42:877–884. doi: 10.1177/42.7.8014471. [DOI] [PubMed] [Google Scholar]
- 17.Sarwar Nasir M, Fahrni C J, Suhy D A, Kolodsick K J, Singer C P, O'Halloran T V. J Biol Inorg Chem. 1999;4:775–783. doi: 10.1007/s007750050350. [DOI] [PubMed] [Google Scholar]
- 18.Fahrni C J, O'Halloran T V. J Am Chem Soc. 1999;121:11448–11458. [Google Scholar]
- 19.Koike T, Watanabe T, Aoki S, Kimura E, Shiro M. J Am Chem Soc. 1996;118:12696–12703. [Google Scholar]
- 20.Kimura E. S Afr J Chem. 1997;40:240–248. [Google Scholar]
- 21.Kimura E, Koike T. Chem Soc Rev. 1998;27:179–184. [Google Scholar]
- 22. Kimura, E. & Koike, T. (1998) J. Chem. Soc. Chem. Commun. 1495–1500.
- 23.Kimura E, Kikuta E. J Biol Inorg Chem. 2000;5:139–155. doi: 10.1007/s007750050359. [DOI] [PubMed] [Google Scholar]
- 24.Kimura E, Aoki S. BioMetals. 2001;14:191–204. doi: 10.1023/a:1012942511574. [DOI] [PubMed] [Google Scholar]
- 25.Aoki S, Kaido S, Fujioka H, Kimura E. Inorg Chem. 2003;43:1023–1030. doi: 10.1021/ic020545p. [DOI] [PubMed] [Google Scholar]
- 26.Krizek B A, Merkle D L, Berg J M. Inorg Chem. 1993;32:937–940. [Google Scholar]
- 27.Lippard S J, Berg J M. Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books; 1994. [Google Scholar]
- 28.Walkup G K, Burdette S C, Lippard S J, Tsien R Y. J Am Chem Soc. 2000;122:5644–5645. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- 29.Burdette S C, Walkup G K, Springer B, Tsien R Y, Lippard S J. J Am Chem Soc. 2001;123:7831–7841. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- 30.Maruyama S, Kikuchi K, Hirano T, Urano Y, Nagano T. J Am Chem Soc. 2002;124:10650–10651. doi: 10.1021/ja026442n. [DOI] [PubMed] [Google Scholar]
- 31. Koike, T., Abe, T., Takahashi, K., Ohtani, K., Kimura, E. & Shiro, M. (2002) J. Chem. Soc. Dalton Trans. 1764–1768.
- 32.Outten C E, O'Halloran T V. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda K, Kajiwara K, Tanabe E, Tokumaru S, Kishida E, Masuzawa Y, Kojo S. Biochem Pharmacol. 1999;57:1361–1365. doi: 10.1016/s0006-2952(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 34.Frederickson C J. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.