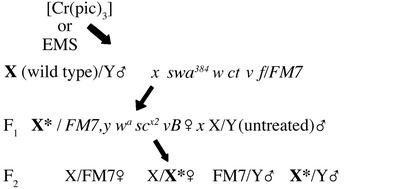Abstract
The nutritional dietary supplement chromium picolinate, [Cr(pic)3], has gained much notoriety as a safe supplement that supposedly promotes fat loss and muscle enhancement in humans. Thus, a significant industry has materialized around the incorporation of [Cr(pic)3] in many sports foods and drinks and a variety of weight loss products. However, in vitro studies have suggested that low levels of [Cr(pic)3] in the presence of biological reducing agents can catalytically generate reactive oxygen species, and recent in vivo studies have detected oxidative damage in rats receiving the supplement. The potential deleterious in vivo effects of this activity were examined by using Drosophila melanogaster. [Cr(pic)3], but not CrCl3, at levels of 260 μg Cr/kg food or less were found to lower the success rate of pupation and eclosion and to arrest development of pupae in a concentration dependent fashion. X-linked lethal analysis indicates that the supplement greatly enhances the rate of appearance of lethal mutations and dominant female sterility.
The element chromium apparently has a role in maintaining proper carbohydrate and lipid metabolism in mammals (1). Because this role probably involves potentiation of insulin signaling, chromium dietary supplementation has been postulated to have effects on body composition, including reducing fat mass and increasing lean body mass. Because the supplement is absorbed better than dietary chromium (2–5% vs. 0.5–2%) (2), most studies have focused on the use of chromium picolinate, [Cr(pic)3]. [Cr(pic)3] has been amazingly popular with the general public since the first reports of effects from the supplement in 1989 (3), with products containing the supplement currently having annual sales of ≈$500 million (4). However, its effectiveness in manifesting body composition changes has been an area of intense debate in the last decade. Recent reviews (5) and metaanalyses (6) of human studies with [Cr(pic)3] indicate that the supplement has no demonstrated effects on body composition of healthy individuals, even when taken in combination with an exercise training program, and no effect on blood plasma insulin and glucose concentrations (1).
Claims have also appeared that the supplement might give rise to deleterious effects. Recent cell culture and in vivo rat studies have indicated that [Cr(pic)3] probably generates oxidative damage of DNA and lipids and is mutagenic, though the significance of these results on humans taking the supplement for prolonged periods of time is unknown and should be a focus for future investigations. In 1995 questions first arose about the safety of [Cr(pic)3] as a dietary supplement as Wetterhahn and coworkers (7) showed that the compound caused clastogenic damage (i.e., cleavage of chromosomes) in Chinese hamster ovary (CHO) cells. When intracellular Cr levels generated by using CrCl3 or Cr nicotinate were comparable to those generated by using [Cr(pic)3], no chromosome aberrations were found. Stohs and coworkers (8) subsequently observed DNA fragmentation in macrophage J774A.1 cells treated with [Cr(pic)3]. Stearns and coworkers in 2002 reported that [Cr(pic)3] is mutagenic at the hypoxanthine phosphoribosyltransferase locus in CHO cells over the concentration range of 0.2–1 mM (9). In related studies, this group has also shown that [Cr(pic)3] also leads to mitochondrial damage and apoptosis in CHO cells (10). Wetterhahn and coworkers have suggested from pharmokinetic modeling studies that taking [Cr(pic)3] supplements for 5 years could result in accumulated Cr concentrations as high as 13 μM in liver tissue (11).
In an in vitro study (12), physiologically relevant concentrations of Cr as [Cr(pic)3] (as low as 120 nM) and of biological reductants, such as ascorbate and thiols, resulted in catalytic production of reactive oxygen species that can cleave DNA. This ability apparently stems from the combination of Cr and picolinate (13); neither the picolinate nor the Cr(III) catalyze this separately. The picolinate ligands shift the redox potential of the chromic center such that it is susceptible to reduction. The reduced chromous species could interact with dioxygen to produce reactive oxygen species. These findings are consistent with earlier results that showed mutagenic forms of Cr(III) possessed chelating ligands containing pyridine-type nitrogens coordinated to the metal and damage required the presence of dioxygen (14).
Recent studies have also shown that [Cr(pic)3] is remarkably stable in buffered aqueous solution (12, 15), similar to the pH of body fluids, and in synthetic gastric fluid and passes unhindered through the jejunum (16). Consequently, when [Cr(pic)3] is taken orally, the supplement probably enters cells intact, i.e., in the potentially harmful form. [Cr(pic)3] possesses a lifetime of <24 h in rats (17, 18). A study of the transport of [Cr(pic)3] in rats during the first 24 h after i.v. injection demonstrated that Cr enters hepatocyte nuclei rapidly, reaching a maximum concentration 1 h after injection; Cr levels decreased rapidly thereafter (D.D.D.H. and J.B.V., unpublished data). Behavior in mitochondria was similar. However, the supplement has little propensity to bind to isolated DNA (18). Kareus et al. (19) demonstrated in vitro that micrososmal hepatocyte enzymes can degrade picolinate from [Cr(pic)3] efficiently. Anderson and coworkers (20) have shown that [Cr(pic)3] is not acutely toxic. Four-week-old rats were fed diets containing up to 100 mg of Cr as [Cr(pic)3] per kg of diet for 24 weeks. No effects were seen from supplementation on body mass, organ mass, or numerous blood variables. Histological evaluation of liver and kidney tissues revealed no effects from the supplement; however, Cr concentrations in the liver and kidney increased linearly with the amount of Cr in the food (20). Isolated incidents of deleterious effects of [Cr(pic)3] supplementation of humans have been reported: weight loss, anemia, thrombocytopenia, liver dysfunction, and renal failure (21, 22); rhaddomyolysis (23); dermatitis (24); acute, short-lasting cognitive, perceptual, and motor changes (25); exanthematous pustulosis (26); and hypoglycemia (27). The significance of these isolated incidents is difficult to ascertain.
Studies have been performed to look for the effects of potential [Cr(pic)3]-generated reactive oxygen species on DNA in vivo. No effect on 5-hydroxymethyl uracil, a product of oxidative DNA damage, levels was observed in a study of 10 obese women given 400 μg [Cr(pic)3] per day for 8 weeks (28). However, studies that observed oxidative damage from the supplement in vivo have appeared recently. i.v. injection of rats with [Cr(pic)3] (5 μg Cr, ≈20 times the amount a human taking commercial supplements receives on a per kg body mass basis) daily for 60 days resulted in significant increases in urinary 8-hydroxydeoxyguanosine (8-OhdG), a product of oxidative DNA damage, in urine starting after 32 days of treatment (18). At the end of the 60 days, 8-OHdG levels were significantly greater in liver and kidney tissue. Additionally, levels of lipid peroxidation in the tissues were significantly increased. In addition to the oxidative damage, this lipid peroxidation can in turn lead to DNA and chromosome damage (29) of the type observed by Stearns and coworkers in 1995.
The genetic model system, Drosophila melanogaster, has been used to evaluate a variety of chromium compounds, and results of these studies parallel those performed in mammals and mammalian cell culture systems. Amrani et al. (30) analyzed the consequences of exposure to K2Cr(VI)O4, K2Cr(VI)2O7, and Cr(III)Cl3 by using the Drosophila wing spot mitotic clone assay for genotoxic effects. This study revealed strong somatic recombinogenic activity in the wings of adults that had been fed Cr(VI) salts as larvae, whereas no significant effects were observed after exposure to CrCl3. Similarly, Katz et al. (31) and Graf et al. (32) found that Cr(VI) and Cr(IV) complexes generated somatic mutations, whereas Cr(III) complexes did not. Other analyses employing the wing spot assay also detected no genotoxic effects from CrCl3 exposure (33, 34). Although these studies clearly exhibit results that mirror the responses of mammalian systems to Cr(III), Cr (IV), and Cr(VI), none of the Drosophila studies included an analysis of [Cr(pic)3]. Because [Cr(pic)3] appears to cause oxidative DNA damage in rats and in mammalian cell culture, it is reasonable to expect comparable genotoxic effects in Drosophila. The study reported here tests this expectation. It does not do so, however, by the standard wing spot test, which, although highly sensitive and simple to perform, limits analysis of biological effects to a single feature-effects on mitotic recombination. Because this compound is a popular dietary supplement, it becomes of particular interest to assess its effects over the entire life span. The current study evaluates the effects of [Cr(pic)3] on development of Drosophila throughout each phase of its life cycle and its effects on populations exposed to the compound over successive generations. Moreover, we have extended the study to assess effects on germ line heredity. In contrast to Cr(III)Cl3, which had no deleterious effects in our studies (in agreement with its lack of effects in the previously cited wing spot assays), the Cr(III) compound [Cr(pic)3] caused developmental delays, lethality during development, and significant levels of germ-line lethal and semilethal mutations.
Materials and Methods
Materials.
[Cr(pic)3] was prepared by the method of Press et al. (35). [Cr(pic)3]⋅H2O prepared by this method has been extensively characterized (15, 36). Analysis calculated (Found) for C18H14N3O7Cr: C, 49.55(49.42); H, 3.23(3.31); N, 9.63(9.68). The complex elutes as a single peak on Shodex-OH Pak HPLC.
Strains.
An isogenized line of the wild-type strain Canton S were used for all tests of development and mutagenicity. Germ-line mutagenesis studies used the marked X-chromosome stock, swa384 w ct v f/FM7, kindly provided by Edwin Stephenson. Both stocks and experimental cultures were maintained in a controlled environment incubator at 25°C and 65% relative humidity. All studies were conducted on standard D. melanogaster media (4.2% agar/10.6% brewers yeast/24.5% cornmeal/56.3% sucrose/0.42% methylparaben), in which chromium solutions or picolinate solutions at the appropriate concentration were substituted for water in tests for toxicity and mutagenicity.
X-Linked Lethal Mutagenesis.
Mutagenesis with ethylmethanesulfonate (EMS) was performed by feeding adult wild-type males that were 24 h posteclosion overnight on filter paper saturated with 25 mM EMS prepared in 1% sucrose. After feeding, the males were transferred to standard medium for 24 h for recovery and then mated to swa384 w ct v f/FM7 B females as described in Fig. 1, a standard mating scheme for the detection of X chromosome mutations. Parents were removed from the cultures after 5 days of egg laying. Single F1 progeny females that were heterozygous for an EMS-exposed X chromosome and the FM7 balancer chromosome (heterozygous Bar eye phenotype) were mated with three wild-type males that had been reared on standard medium. The F2 progeny in each of the four expected genotypic classes were scored until all progeny had eclosed. In control and mutagenized vials, the number of females of the class wild-type X/FM7 and the class wild-type X/EMS-exposed X were approximately equal (data not shown). The number of progeny males of the wild-type X/Y was expected to equal the number of wild-type X/wild-type X females, and results are reported as the percent of these males relative to the number of progeny in the corresponding female class. FM7/Y males typically emerge in significantly reduced numbers because of deleterious effects from some of the hemizygous marker mutations on the FM7 chromosome and this class was disregarded in all calculations. Males exposed to [Cr(pic)3] were tested for the generation of X-linked lethals in precisely the same scheme, the only difference being that these males were fed the Cr compound throughout larval life and were not re-exposed as adults.
Figure 1.
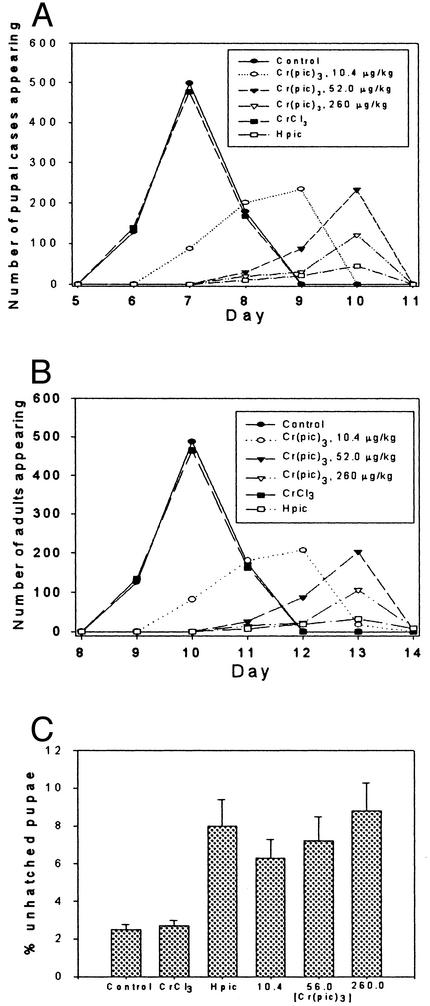
(A) Effects of [Cr(pic)3] on developmental to pupal stage. Bars represent percentage of pupae recovered after [Cr(pic)3] exposure relative to untreated control culture. (B) Unhatched pupae in the control and [Cr(pic)3] groups through four generations. Bars represent the percentage of pupa that failed to eclose. Differences between the [Cr(pic)3] cultures and control for a given generation were considered significant at P ≤ 0.05. Values are means ± SD (*, P < 0.002; ***, P < 0.003; **, P = 0.05).
Results
Exposure to [Cr(pic)3] Over Successive Generations.
Adult Drosophila that had been reared on standard, nonsupplemented Drosophila medium were collected 16–24 h after eclosion. These were placed on vials containing standard Drosophila medium or medium prepared by using a saturated [Cr(pic)3] solution (600 μM) at a density of three females and three males per vial (20 vial for each data set). The adults were removed after 3 days, and the developmental stage of the resulting progeny was assessed every 24 h. The number of pupal cases and the number of progeny adults were scored to assess fertility effects on progeny reared on this Cr compound. Progeny were collected from these vials and used to repeat the culture protocol for an additional three generations. In each generation, the number of progeny reaching the pupal stage of development (normally ≈5 days at 25°C) was diminished by ≈20–30% in the [Cr(pic)3] vials (Fig. 2A). Most of the individuals that survived to pupation were able to complete metamorphosis and emerge as adults with no obvious morphological or behavioral abnormalities. However, pupae that had been exposed to [Cr(pic)3] during larval development exhibited significant reductions in successful eclosion in every generation tested (Fig. 2B). The extent of the effect is similar in each generation. There is no evidence of additive or cumulative effects.
Figure 2.
(A) The appearance of pupal cases (total) in cultures containing media supplemented with chromium picolinate [Cr(pic)3], chromium chloride (CrCl), picolinic acid (Hpic), and standard media as a function of time. (B) Eclosion of adult progeny from vials. (C) Percentage of unhatched pupae from vials. For [Cr(pic)3] supplemented media, the Cr concentrations of the media are given in μg/kg. For Hpic and CrCl3, the picolinate and Cr concentrations, respectively, were equivalent to those of the highest [Cr(pic)3] concentration.
As each generation of progeny was scored, it was observed that the [Cr(pic)3]-treated cultures exhibited developmental delays, with individuals reaching pupation and eclosion at least 24 h later than control cultures. It was also observed that survival of male progeny in the [Cr(pic)3]-treated cultures seemed to be depressed relative to female survival (data not shown). This observation suggested the possibility that [Cr(pic)3] might be inducing recessive mutations that adversely affected survival of males, which possess a single X chromosome, an effect from which females, having two X chromosomes, are protected. These results led to a more detailed examination of developmental delays and to a systematic analysis of accumulation in lethal mutations in the progeny of flies exposed to [Cr(pic)3].
Concentration Dependency and Specificity of [Cr(pic)3] Effects on Viability and Development.
The above experiments were conducted by using the maximum possible dosage of [Cr(pic)3], 260 μg Cr/kg of medium. To determine whether the observed effects on viability and developmental rate of the progeny were specific, untreated adults derived from standard culture were placed on medium supplemented with [Cr(pic)3] ranging from 10.4 to 260 μg of Cr as [Cr(pic)3] per kg medium for a single generation. In addition, CrCl3 and picolinic acid at concentrations equivalent to the maximum Cr and picolinate concentrations if [Cr(pic)3] were completely dissociated were tested. Progeny were scored as described above. It was observed that the numbers of individuals reaching pupation and eclosion were diminished at every concentration of [Cr(pic)3] tested, with the numbers decreasing proportionately to increasing concentration of the supplement (Fig. 2 A and B). Moreover, the number of unhatched pupae increased with increasing [Cr(pic)3] concentrations (Fig. 2C). In agreement with earlier wing spot assays of recombinogenic activity CrCl3 had no observable effects on larval or adult viability. In contrast, picolinic acid produced an even greater depression of larval and adult viability, as scored by numbers of pupae and adults recovered, than did [Cr(pic)3]. Picolinic acid treatment also was associated with an elevation in individuals arrested during pupation that was very similar to the results of [Cr(pic)3] treatment.
In these experiments the progress of the cultures through each developmental stage was monitored daily, and the number of individuals reaching pupation and eclosion each day was scored. Even at the lowest [Cr(pic)3] concentration of 10.4 μg Cr per kg of medium, both developmental stages were reached on average ≈2 full days after control cultures. Elevation of the concentration to 52 μg of Cr per kg of medium resulted in an additional 24-h delay. Cultures exposed to CrCl3 exhibited development rates indistinguishable from control cultures, whereas individuals exposed to picolinic acid exhibited rates of development identical to flies exposed to the maximum concentration of [Cr(pic)3].
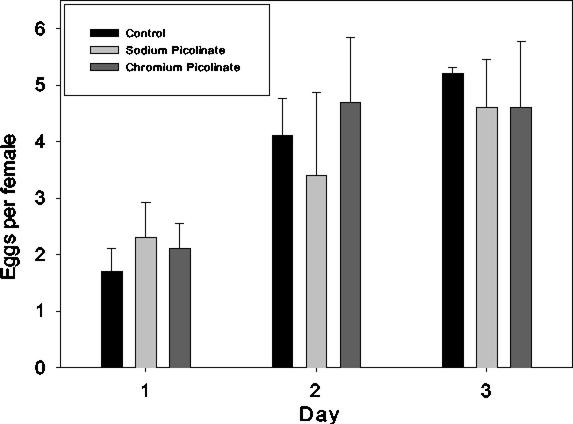
There are three possible explanations for these results: (i) apparent developmental delays could be observed if females on the [Cr(pic)3] or picolinic acid- supplemented medium delayed egg deposition after fertilization; (ii) [Cr(pic)3] or picolinic acid could adversely affect the general state of adult health resulting in fewer fertilized eggs or abnormal embryonic development; and (iii) the progeny developing on these supplements could be directly affected. It appears that neither the first nor the second explanation applies to these results. We have monitored adults after exposure to [Cr(pic)3] and find no significant differences from the control adults in either adult survival or behavior during the 3-day interval in which parents were maintained on test media (data not shown). Therefore, the adults seem to be in reasonable health and exhibiting no adverse effects on viability. To address the possibility of delays in egg deposition or diminution of egg numbers, adults at 24 h after eclosion were placed on a hard agar egg laying medium prepared with either water, [Cr(pic)3], or picolinic acid, and the number of eggs deposited each day for 3 days was determined. The results shown in Fig. 3 show that the number of eggs laid per day per female on [Cr(pic)3] or picolinic acid medium is not significantly different from the number laid on control medium. In addition, there is no evidence for delays in egg deposition. Therefore, we conclude that the most likely explanation for the adverse effects of these compounds is that they are acting directly on the immature progeny, resulting in progeny lethality and slowed development of survivors.
Figure 3.
Number of eggs per female laid on standard media and media supplemented with [Cr(pic)3] (260 μg/kg) or an equivalent amount of picolinate as sodium picolinate.
Evaluation of Germ-Line Mutagenicity of [Cr(pic)3].
Preliminary observations of enhanced deleterious effects of [Cr(pic)3] on the survival of males relative to females, combined with previous studies demonstrating oxidative damage of DNA by [Cr(pic)3], suggested the possibility that the compound is capable of inducing germ-line mutations. Accordingly, wild-type males reared on [Cr(pic)3] at dietary concentrations equivalent to those in human Cr supplementation were subjected to a standard mating scheme for the detection of X-linked lethal mutagenesis (Fig. 4). The results were compared with those obtained after mutagenesis with the DNA alkylating agent EMS. This mutagen was selected for comparison because it is a common laboratory mutagen that efficiently generates germ-line mutations. Potential mutagenic action by [Cr(pic)3] would almost certainly be distinct from the chemical effects of EMS, as would effective concentrations. The design of the experiment cannot reveal underlying mutagenic mechanisms but does provide a sensitive means of detecting even low efficiency germ-line mutagenicity. In these experiments, the male parents were subjected to exposure to the chemicals; all other individuals and generations were maintained on nonsupplemented standard Drosophila medium. In an examination of 270 X chromosomes through the matings of control F1 females, male viability was normal in 99% of the vials scored (Table 1). In contrast, only 2% of the F1 female progeny of males exposed to EMS produced F2 male progeny that survived at wild-type levels. A total of 98% of the EMS-exposed X chromosomes were associated with deleterious effects on F2 male survival, with 19% of the X chromosomes sustaining lethal mutations. The remaining 79% of the X chromosomes exhibited semilethal mutations that resulted in male viability ranging from 10–80% of control survival. [Cr(pic)3] at nutritional supplement concentrations was not as strongly mutagenic as EMS. However, the results clearly demonstrate that [Cr(pic)3] has significant germ-line mutagenic activity that dramatically parallels the mutagenic activity of EMS. Eight percent of the X chromosomes tested exhibited lethal mutations and another 81% are associated with mutations that reduce F2 male survival to degrees ranging from 10–80% of the survival of control males. Only 11% of the females carrying an X chromosome exposed to [Cr(pic)3] in the previous generation produced male offspring that survived in normal proportions (Table 1).
Figure 4.
Mating scheme for detection of X-linked lethal mutations. Wild-type males were treated with [Cr(pic)3] or EMS and subsequently mated to produce heterozygous F1 female progeny carrying one X chromosome that had been exposed to either agent and an unexposed X chromosome marked with the dominant eye-shape marker, Bar(B). The F1 females were mated to males from the parental wild-type culture, and the F2 progeny of all classes were counted. Males carrying a wild-type chromosome (X/Y) are expected in approximately equal proportions with females carrying two wild-type chromosomes (X/X). The FM7 chromosome adversely affects male viability and, therefore, progeny survival of both males and females with an FM7 balancer chromosome was not considered in this analysis.
Table 1.
X-linked mutation
| Treatment | Total no. of fertile F1 females | % Expected male F2 progeny | % Vial in each F2 male viability class |
|---|---|---|---|
| Control | 270 | 80–100 | 99 |
| 60–79 | 1 | ||
| 40–59 | |||
| 20–39 | |||
| 10–19 | |||
| 0–9 | |||
| [Cr(pic)3] | 132 | 80–100 | 11 |
| 60–79 | 22 | ||
| 40–59 | 20 | ||
| 20–39 | 27 | ||
| 10–19 | 12 | ||
| 0–9 | 8 | ||
| EMS | 98 | 80–100 | 2 |
| 60–79 | 8 | ||
| 40–59 | 29 | ||
| 20–39 | 30 | ||
| 10–19 | 12 | ||
| 0–9 | 19 |
Proportion of surviving X*/Y males relative to X*/X sibling females. As indicated by ratios of surviving F2 progeny in control crosses, the number of sibling males and females should be equal.
Effects of exposure to either EMS or [Cr(pic)3] are also apparent in the F1 females, which carry only one set of treated chromosomes, because their homologs were derived from the untreated female parent. In the EMS experiment, 16% of the F1 females were sterile, whereas 12% of the [Cr(pic)3] females failed to produce any offspring (Table 2). Thus, both treatments resulted in significant levels of female sterility, and the proportion of sterile females in the [Cr(pic)3] is quite comparable to those in the EMS treated set of females. Because these females contain a complete complement of chromosomes derived from an untreated parent, the female sterile effects are necessarily dominant.
Table 2.
Dominant female sterility
| Treatment | Total no. of F1 X/X* female parents | % Sterile F1 females |
|---|---|---|
| Control | 270 | 0 |
| [Cr(pic)3] | 150 | 12 |
| EMS | 116 | 16 |
X chromosome exposed to [Cr(pic)3] or EMS treatment.
Discussion
Epidemiologic analysis of humans exposed to chromate and studies in animal and cell culture models demonstrate that exposure to chromium compounds is strongly correlated with DNA damage in somatic cells and to elevation of cancer rates in humans (31). These studies indicate that Cr6+,Cr5+, and Cr4+ are particularly strong in inducing genotoxic effects, whereas trivalent chromium appears to be substantially more innocuous. Nevertheless, evidence has accumulated in studies of CHO cells and in rats over recent years that the trivalent [Cr(pic)3] is potentially capable of producing oxidative and other damage to DNA (7–10, 12, 13, 18). These studies are of substantial concern given the popularity of [Cr(pic)3 ] as a dietary supplement, particularly in light of the potential for long-term ingestion of the supplement. The present study employs the genetic model organism, D. melanogaster, to assess the potential hazards of [Cr(pic)3] ingestion.
The wing spot test of Drosophila has become an efficient and sensitive means of detecting damage to DNA in somatic cells. In this test, larvae are fed an agent to be tested for periods ranging from 2 days to a few hours, and effects on DNA are then monitored in adults (30–34). During the larval phase of the life cycle, the imaginal disks, the precursors to adult structures that develop during the pupal stage, are undergoing active mitosis. Few larval cells in Drosophila actively divide; most undergo endoreplication of DNA and an associated enlargement of cells (and ultimately the larva itself). Imaginal disks are among the few tissues in which cells are dividing, and they continue to do so until the initiation of pupation when they begin to differentiate into adult structures. Normally, mitotic cells have very low recombinogenic activity, but damage to chromosomes can result in an elevation of mitotic recombination between homologous chromatids as damaged DNA strands are repaired. Because imaginal cells have no function in larval life and few other cells divide, the larvae can sustain quite significant levels of DNA damage, with little effect on viability. Thus, generation of homozygous mutant cells via elevation of mitotic recombination rates provides a simple and efficient means of assessing genotoxic activity. Such assays have been performed for numerous chromium complexes, and the results of such analyses strongly parallel those of studies in mammalian cells. These studies show that hexavalent and tetravalent chromium species exhibit high levels of recombinogenic activity, whereas the trivalent CrCl3 is inactive (30–34). None of these studies, however, address the effect of [Cr(pic)3] on dividing somatic cells. Thus, there is no evidence available that allows a comparison of the effects of this compound on Drosophila and mammalian cells. The activity of the compound in mammalian systems leads us to expect recombinogenic activity, a possibility that we have not yet tested.
The wing spot assay is restricted in the type of biological effects that can be monitored. Exposure to an agent is short-term, and effects are restricted solely to mitotic cells (where the damage induced by chromium compounds is caused by chromosome breakage and subsequent recombination and point mutations; ref. 31). In this study, in light of concerns over the effects of long-term supplementation and possible effects in subsequent generations, we used a somewhat different approach that allows the assessment of the effects of exposure to juveniles on developmental processes and to possible mutagenic activity in the germ line. We observe significant elevation of lethality during development, developmental delays in those animals that survive to complete each developmental phase, and a striking elevation of germ-line mutation that compares quite well in effectiveness with EMS.
Drosophila in this study were fed a media containing 10.4 to 260 μg of Cr per kg of media with observable effects on development, sterility, and mutagenicity at all concentrations examined. In comparison, rat diets containing <100 μg Cr/kg diet are considered Cr deficient (37). Anderson and Kozlovsky have determined that the Cr content of a composite human diet sample was ≈100 μg/kg diet; mean Cr intake per 1,000 calories was ≈15 μg such that adult males intake ≈33 μg per day and females intake 28 μg per day (38). Most [Cr(pic)3] dietary supplements provide 200–600 μg of Cr per day if the suggested dosage is followed. Consequently, the fruit flies are feeding on media with a concentration of [Cr(pic)3] well below that of a supplemented human diet.
Adult Drosophila appear to be quite tolerant to [Cr(pic)3] and are able to ingest very high concentrations without overt effects on viability, fertility, or behavior. It is only when earlier stages in the life cycle or effects on reproductive cells are examined that evidence of deleterious effects are apparent. In all instances, larva that are exposed to the compound exhibit diminished ability to pupate, reduced viability of pupae, and dose-dependent delays in reaching the developmental milestones of pupal formation and eclosion. Effects on larval viability, although always apparent, were somewhat variable from experiment to experiment. We suggest that ability of the larvae to survive exposure to [Cr(pic)3] may be sensitive to minor environmental variation. It is not clear whether inviability and slowed development are associated with DNA damage or to interference with other physiological processes, but we anticipated that the deleterious effects must, at least in part, be associated with genetic damage. In light of this expectation, we were somewhat surprised to observe that continuous maintenance of cultures on [Cr(pic)3], for multiple generations, did not lead to additive effects which might be expected if mutations were accumulating. There are a number of possible explanations for this result. First, [Cr(pic)3] might not induce genetic damage in Drosophila, but solely interfere with metabolic or physiological processes. The extent of interference and subsequent effects on the organism would not, in this case, vary from generation to generation. However, our subsequent analysis of mutagenesis by [Cr(pic)3] rules out this possibility. Thus, a genetic explanation for the lack of additive effects may lie in the nature and number of mutations generated by [Cr(pic)3]. The X-linked lethal mutagenesis analysis demonstrates that, although the compound clearly shows mutagenic activity, the bulk of the damage is recessive in nature. This can be seen by comparing the survival of the heterozygous females [X(Cr)/X(untreated)] with survival of males of the genotype X(Cr)/Y. The hemizygous males exhibit striking reductions in their ability to survive to the adult stage, where they are scored, relative to the female classes. Survival of the two female classes are not significantly different (data not shown), suggesting that most of the genetic effects are rescued by the nonmutated homologs in the female. That there are dominant effects, however, may be seen by the low level of sterility observed in the heterozygous females (Table 2). The failure to observe cumulative effects during multigeneration exposure is likely to be caused by relatively low rates of mutagenesis and low rates of homozygosis of mutant DNA during the relatively short term of the multiple generation analysis (only four generations). Randomized mating of progeny in each generation diminishes the rate of inbreeding, which would also contribute to apparent lack of cumulative effects.
EMS, the alkylating agent used for comparison to [Cr(pic)3] in the mutagenesis study, was used at a concentration expected to yield one mutation per chromosome per individual (39). EMS mutagenesis was included simply to provide a scale for severity of mutagenic effects. Lethal mutations on the X chromosome were expected to be in the range of 20–30%, a figure within experimental error of the number of lethal mutations recovered in these experiments. It should be noted that for both the EMS and [Cr(pic)3] mutagenesis tests, the progeny actually tested for mutagenic effects are two generations removed from direct exposure of the chromosomes to either agent. None of these effects then can be attributed to disturbances in metabolism or physiology of the animal during exposure, but rather to hereditary damage. [Cr(pic)3], at nutrition supplement levels is clearly not as efficient in generating damage as EMS under the experimental conditions. Nonetheless, the evidence for genetic damage follows the same patterns as in the EMS experiment, with few chromosomes tested yielding males that survive at wild-type levels (only 2% in the case of EMS and 11% in the case of [Cr(pic)3]). This result demonstrates that significant levels of genetic change have occurred during exposure of the organism to [Cr(pic)3]. Aside from the fact that it can be concluded that the bulk of the damage is recessive in resulting phenotype, nothing can be inferred about the nature of the damage. Mutagenesis of single gene targets and subsequent sequence and cytogenetic analysis will be used to address this issue.
In the present study, comparable concentrations of [Cr(pic)3] and picolinic acid were found to result in comparable changes in development. This is most curious, as the transport and distribution of the supplement and the free acid are unlikely to be similar, based on the results of studies on the distribution of the supplement in rats (17). Picolinic acid is rapidly cleared from the body, whereas [Cr(pic)3] passes intact from the bloodstream to the kidneys and then urine and also passes into cells where it is degraded (ref. 17 and D.D.D.H. and J.B.V., unpublished data). For the effects from the supplement to be derived solely from picolinate generated by dissociation of the complex, both the free ligand and complex would need to be absorbed, transported through the bloodstream, absorbed by tissues, and distributed within cells similarly, whereas decomposition of [Cr(pic)3] would need to be extremely rapid. Consequently, the similar effects are probably serendipitous, although the possibility that in Drosophila [Cr(pic)3] damage results from free picolinate acid generated from the dissociation of the complex cannot strictly be ruled out.
Acknowledgments
This research was funded by the American Diabetes Association (J.B.V.) and by National Institutes of Health Grant GM 62879 (to J.O.). S.B. was supported by a McNair Scholars Foundation grant to the University of Alabama.
Abbreviations
- [Cr(pic)3]
chromium picolinate
- CHO
Chinese hamster ovary
- EMS
ethylmethanesulfonate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vincent J B. Polyhedron. 2001;20:1–26. [Google Scholar]
- 2.Anderson R A, Bryden N A, Polansky M M, Gautschi K. J Trace Elem Exp Med. 1996;9:11–25. [Google Scholar]
- 3.Evans G W. Int J Biosocial Med Res. 1989;11:163–180. [Google Scholar]
- 4. Mirasol, F. (2000) Chem. Market Rep.257, www.findarticles.com/cf_0/m0FVP/7_257/59680267/print.jhtml.
- 5. Vincent, J. B. (2003) Sports Med., in press. [DOI] [PubMed]
- 6.Althius M D, Jordan N E, Ludington E A, Wittes J T. Am J Clin Nutr. 2002;76:148–155. doi: 10.1093/ajcn/76.1.148. [DOI] [PubMed] [Google Scholar]
- 7.Stearns D M, Wise J P, Sr, Patierno S R, Wetterhahn K E. FASEB J. 1995;9:1643–1648. [PubMed] [Google Scholar]
- 8.Bagchi D, Bagchi M, Balmoori J, Ye X, Stohs S J. Res Commun Mol Pathol Pharmacol. 1997;97:335–346. [PubMed] [Google Scholar]
- 9.Stearns D M, Silveira S M, Wolf K K, Luice A M. Mutat Res. 2002;513:135–142. doi: 10.1016/s1383-5718(01)00301-1. [DOI] [PubMed] [Google Scholar]
- 10.Manygoats K R, Yazzie M, Stearns D M. J Biol Inorg Chem. 2002;7:791–798. doi: 10.1007/s00775-002-0357-z. [DOI] [PubMed] [Google Scholar]
- 11.Stearns D M, Belbruno J J, Wetterhahn K E. FASEB J. 1995;9:1650–1657. doi: 10.1096/fasebj.9.15.8529846. [DOI] [PubMed] [Google Scholar]
- 12.Speetjens J K, Collins R A, Vincent J B, Woski S A. Chem Res Toxicol. 1999;12:483–487. doi: 10.1021/tx9900167. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Ramirez J, Woski S A, Vincent J B. J Biol Inorg Chem. 2000;5:129–136. doi: 10.1007/s007750050016. [DOI] [PubMed] [Google Scholar]
- 14.Sugden K D, Geer R D, Rogers S G. Biochemistry. 1992;31:11626–11631. doi: 10.1021/bi00161a049. [DOI] [PubMed] [Google Scholar]
- 15.Chakov N E, Collins R A, Vincent J B. Polyhedron. 1999;18:2891–2897. [Google Scholar]
- 16.Gammelgaard B, Jensen K, Steffansen B. J Trace Elem Med Biol. 1999;13:82–88. doi: 10.1016/S0946-672X(99)80028-5. [DOI] [PubMed] [Google Scholar]
- 17.Hepburn D D D, Vincent J B. Chem Res Toxicol. 2002;15:93–100. doi: 10.1021/tx010091t. [DOI] [PubMed] [Google Scholar]
- 18.Hepburn D D D, Burney J M, Woski S A, Vincent J B. Polyhedron. 2003;22:455–463. [Google Scholar]
- 19.Kareus S A, Kelley C, Walton H S, Sinclair P R. J Hazardous Mat B. 2001;84:163–174. doi: 10.1016/s0304-3894(01)00199-6. [DOI] [PubMed] [Google Scholar]
- 20.Anderson R A, Bryden N A, Polansky M M. J Am Coll Nutr. 1997;16:273–279. doi: 10.1080/07315724.1997.10718685. [DOI] [PubMed] [Google Scholar]
- 21.Cerulli J, Grabe D W, Gauthier I, Malone M D. Ann Pharmacother. 1998;32:428–431. doi: 10.1345/aph.17327. [DOI] [PubMed] [Google Scholar]
- 22.Wasser W G, Feldman N S, D'Agati V D. Ann Int Med. 1997;126:410. doi: 10.7326/0003-4819-126-5-199703010-00019. (lett.). [DOI] [PubMed] [Google Scholar]
- 23.Martin W R, Fuller R E. Pharmacotherapy. 1998;18:860–862. [PubMed] [Google Scholar]
- 24.Fowler J F., Jr Cutis. 2000;65:116. [PubMed] [Google Scholar]
- 25.Huszonek J. Am J Pyschiatry. 1993;150:1560–1561. doi: 10.1176/ajp.150.10.1560b. [DOI] [PubMed] [Google Scholar]
- 26.Young P C, Turiansky G W, Bonner M W, Benson P M. J Am Acad Dermatol. 1999;41:820–823. doi: 10.1016/s0190-9622(99)70333-6. [DOI] [PubMed] [Google Scholar]
- 27.Bunner S P, McGinnis R. Psychosomatics. 1998;39:298–299. doi: 10.1016/S0033-3182(98)71351-9. [DOI] [PubMed] [Google Scholar]
- 28.Kato I, Vogelman J H, Dilman V, Karkoszka J, Frenkel K, Durr N P, Orentreich N, Toniolo P. Eur J Epidemol. 1998;14:621–626. doi: 10.1023/a:1007442203258. [DOI] [PubMed] [Google Scholar]
- 29.Ringden D, Lee S H, Nakajima M, Blair I A. Chem Res Toxicol. 2000;13:846–852. doi: 10.1021/tx0000771. [DOI] [PubMed] [Google Scholar]
- 30.Amrani S, Rizki M, Creus A, Marcos R. Environ Mol Mutagen. 1999;34:47–51. doi: 10.1002/(sici)1098-2280(1999)34:1<47::aid-em7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Katz A J, Chiu A, Beaubier J, Shi X. Mol Cell Biochem. 2001;222:61–68. doi: 10.1023/a:1017959222379. [DOI] [PubMed] [Google Scholar]
- 32.Yesilada E. Bull Environ Contam Toxicol. 2001;66:464–469. doi: 10.1007/s001280029. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa H I, Shibahara T, Iwata H, Okada T, Tsuruta S, Kakimoto K, Sakata K, Kato Y, Ryo H, Itoh T, Jujikawa K. Mutat Res. 1994;320:133–140. doi: 10.1016/0165-1218(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 34.Graf U, Heo O S, Ramirez O O. Mutat Res. 1992;266:197–203. doi: 10.1016/0027-5107(92)90187-7. [DOI] [PubMed] [Google Scholar]
- 35.Press R I, Geller J, Evans G W. West J Med. 1990;152:41–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Kingry K F, Royer A C, Vincent J B. J Inorg Biochem. 1998;72:79–88. [Google Scholar]
- 37.Striffler J S, Law J S, Polansky M M, Bhathena S J, Anderson R A. Metabolism. 1995;44:1314–1320. doi: 10.1016/0026-0495(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 38.Anderson R A, Kozlovsky A S. Am J Clin Nutr. 1985;41:1177–1183. doi: 10.1093/ajcn/41.6.1177. [DOI] [PubMed] [Google Scholar]
- 39.Ashburner M. Drosophila: A Laboratory Handbook. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. pp. 345–349. [Google Scholar]