Abstract
This article is an account of the birth and evolution of surface science as an interdisciplinary research area. Surface science emanated from the confluence of concepts and tools in physics and chemistry with technological innovations that made it possible to determine the structure and properties of surfaces and interfaces and the dynamics of chemical reactions at surfaces. The combination in the 1960s and 1970s of ultra-high-vacuum (i.e., P < 10−7 Pascal or 10−9 Torr) technology with the recognition that electrons in the energy range from 50 to 500 eV exhibited inelastic collision mean free paths of the order of a few angstroms fostered an explosion of activity. The results were a reformulation of the theory of electron solid scattering, the nearly universal use of electron spectroscopies for surface characterization, the rise of surface science as an independent interdisciplinary research area, and the emergence of the American Vacuum Society (AVS) as a major international scientific society. The rise of microelectronics in the 1970s and 1980s resulted in huge increases in computational power. These increases enabled more complex experiments and the utilization of density functional theory for the quantitative prediction of surface structure and dynamics. Development of scanning-probe microscopies in the 1990s led to atomic-resolution images of macroscopic surfaces and interfaces as well as videos of atoms moving about on surfaces during growth and diffusion. Scanning probes have since brought solid–liquid interfaces into the realm of atomic-level surface science, expanding its scope to more complex systems, including fragile biological materials and processes.
In 1965, although vacuum systems were available for stabilizing surfaces for hours, neither the composition nor the structure of a solid surface could be determined experimentally as may be ascertained by inspection of the preeminent surface science text of the day, Semiconductor Surfaces (ref. 1; for an expanded reference list, see Supporting References, which is published as supporting information on the PNAS web site, www.pnas.org). By the mid-1980s both the atomic composition and structure could be determined quantitatively for both clean and adsorbate-covered single crystal surfaces of elements and simple compounds (2). Today, the morphology of such surfaces and the dynamics of chemical reactions on them (including growth) are readily observed and predicted (3). Moreover, the atomic-level characterization of surfaces has moved from the realm of “vacuum”–solid surfaces to liquid–solid interfaces (3). This article is an account of how this remarkable transformation, a scientific revolution in the sense of Thomas Kuhn (4, 5), came to be. It is the story of the birth of surface science as an interdisciplinary area of research and its continuing evolution caused by the interplay between concepts in condensed-matter physics and evolving technologies like semiconductor microelectronics, vacuum processing, and the construction of ever more flexible and robust scanning probe microscopes. In addition, many of the process technologies that are used to fabricate modern microelectronic and electrooptic devices emanated from semiconductor surface science during the decades of the 1970s through 1990s. Thus, this article also is the story of surface science as a wellspring of the semiconductor processing innovations that have created the device hardware of the digital age.
Surface science is the child of the union of science and technology. Essentially all of the concepts and theoretical tools used in the field emanate from condensed-matter physics and physical chemistry. Examples include electron scattering and emission for surface characterization, electron tunneling for surface imaging, and the use of density functional theory for the prediction of surface structures and reaction dynamics. The productive application of these to surface science has been enabled by four waves of rapidly improving experimentation. The first wave came in the 1960s when the combination of reliable metal ultra high vacuum (UHV, i.e., P < 10−7 Pascal or 10−9 Torr) systems and the use of electron spectroscopy for surface characterization gave birth to the field. The second wave began in the 1980s when the rise of semiconductor microelectronics generated electronics of sufficient reliability that multiple sophisticated experimental probes of surfaces could be used simultaneously in multiport UHV systems on a routine basis. Beginning in the early 1970s it became customary to have a variety of sample preparation and analysis experiments in the same vacuum chamber. Unfortunately, all of the equipment needed for this diversity of measurements rarely was functional at the same time. By the mid-1980s much of this problem had disappeared, and the goal of having many sample preparation and characterization techniques operating simultaneously in the same vacuum system became a practical reality. This same microelectronics revolution, based on the inexorable doubling of cost/performance figures of merit of computing power roughly every 2 years, transformed theoretical surface science from the use of illustrative simple models into a quantitative predictor of surface structures and properties. The third wave, initiated by the invention of the scanning tunneling microscope in 1982 and its coming to maturity in the 1990s, led to the age of imaging in surface science. Atomic-resolution images of highly complex macroscopic surfaces and videos of the dynamics of their evolution generated by scanning probe microscopies have become routine, thereby revolutionizing our understanding of deposition, growth, etching, and chemical reactions at surfaces. The fourth wave, the application of surface science techniques to examine more complex systems, including liquid–solid interfaces and fragile biological samples, is only now beginning, although its consequences may well dwarf those of its predecessors. Each of these four waves is discussed in turn, indicating the interplay between the attendant scientific advances and their technological enablers to provide insight into the origins of surface science as an interdisciplinary field of research.
The Birth of Surface Science
The first chapter of this story occurred between 1964 and 1973 because of the confluence of three factors: UHV technology, the availability of single crystal samples, and discoveries in the physics of electron–solid interactions. For many years the technology of producing and measuring low pressures (“vacuum”) under controlled conditions had been advancing steadily, driven by industries such as vacuum-tube electronics and television (6). By the mid-1960s, it had progressed to the point that apparatus for generating UHV was readily available and could be combined in commercial instrumentation with electron, ion, and photon sources, versatile sample manipulators, and detectors for the measurement of scattered electron, photon, and ion beams (7). A UHV environment is essential for surface science experimentation because UHV pressures are required for a surface to remain stable for the time required (i.e., hours) to characterize its composition and structure. The development of commercial metal UHV vacuum technology was important because before that time a good glass shop was required to do UHV experiments. With the advent of metal UHV systems graduate students could assemble surface science instrumentation for themselves from commercially available parts. Moreover, single crystals became commercially available in this time period, so samples that were useful for high-precision scientific studies could be purchased. The commercial availability of vacuum components, instrumentation for electron spectroscopy, and single crystal samples greatly broadened the base of surface science research in the mid-1960s and extended its reach beyond the few industrial and university laboratories in which it previously had been practiced (7).
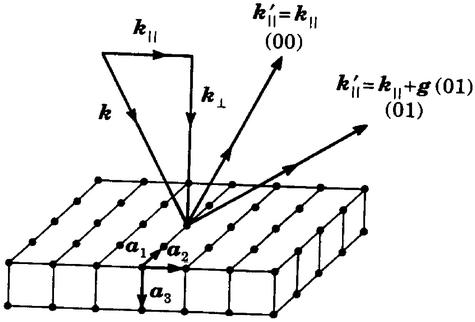
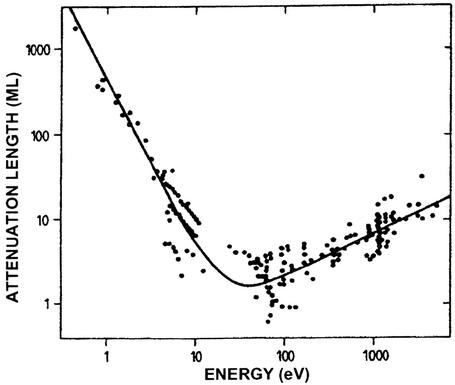
This arena of endeavor was the province of the American Vacuum Society, now known by its initials AVS. Paul Redhead (8) has recounted its history. The condensed-matter physics connection arose from solving a mystery. The “low-energy” electron diffraction (LEED) of back-scattered electrons in the 50- to 500-eV energy range was widely used in the 1950s and 1960s to determine the state of perfection of a crystal surface by virtue of measuring the back scattering of these electrons into beams associated with the translational symmetry of the crystal parallel to its surface as indicated in Fig. 1 (9, 10). It was known that the directions of the back-scattered beams are sensitive to the surface translational symmetry and that this symmetry could be different from that of the bulk, due e.g., to the reconstruction of the surface to form a new chemical compound at the surface (11). By the early 1970s it had been recognized that the inelastic scattering of such electrons via the creation of collective excitations of valence electrons was a vital element in their interactions with solids (12–16). Moreover, as indicated in Fig. 2 (17, 18) in this low-energy range the inelastic-collision mean free paths for electrons are only a few angstroms, leading to the vastly important conclusion that electrons being elastically scattered by or emitted from the solid must have come from the top few atomic layers. This combination of readily available technology for electron scattering and emission experiments in UHV, single crystal samples, and the insight that these experiments probe the surface rather than the bulk of a solid set off an explosion of activity beginning in the late 1960s that defined surface science research as we know it today.
Figure 1.
Schematic illustration of an incident electron or position beam of wave vector k = k⊥ + k‡, scattered elastically from a single crystal into a state characterized by the wave vector k′ = k′∥ + k′⊥; k′‡ = k∥ + g(hk); g(hk) = 2π (hb1 + kb2), b1 = a2 × a3/[a1 ⋅ a2 × a3], etc. The magnitude of k is related to the energy E of the incident electrons via E =ℏ2 k2/2m, in which ℏ is Planck's constant and m is the mass of the electron. The construction of the reciprocal lattice associated with the single crystal surface also is shown. The vectors g(hk) designate the reciprocal-lattice vectors associated with the lowest-symmetry Bravais net parallel to the surface. [After Duke (20), with permission.]
Figure 2.
Attenuation lengths of electrons in solids as a function of their energy. An early compilation (17) of a variety of experimental data are given by the dots. An interpolation formula is shown by the solid line. At the time that these data were compiled, the attenuation lengths were regarded as identical to the inelastic collision mean free paths, although later it was realized (18) that these quantities could differ by roughly 50%. [Adapted from Seah and Dench (17) with permission.]
This explosion had, moreover, institutional and personal as well as scientific and technological consequences. The journals Surface Science and the Journal of Vacuum Science and Technology were founded in 1964. Reminiscences of this era have been published by Harry Gatos (19) and myself (20), the initial editors of the journal Surface Science from its founding in 1964 through 2001. AVS was the leading technical society of the day that recognized the emergence of surface science. It featured and nurtured surface science via the programs sponsored by the surface science division (21) at its meetings, while providing surface scientists with an essential base of vacuum technology needed for the development of cutting-edge instrumentation. It founded the leading international meeting on surface science (22). The combination of the ready availability of UHV technology and the discovery of the inelastic-collision-induced surface sensitivity of electron probes of solids transformed the intellectual landscape of electron–solid collision theory, the practical landscape of surface characterization, and the institutional landscape of the AVS. A symbol of this transformation was the publication in August 1972 of a special issue of Physics Today entitled Special Report: Vacuum (23) in which articles on the generation and measurement of vacuum, and its newfound use as the basis for surface science, were the topics of feature articles.
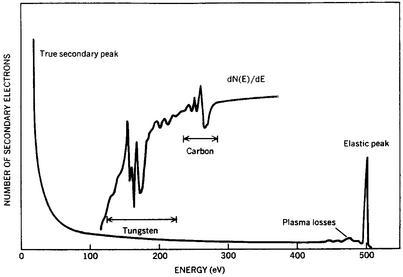
Electron scattering and emission are used to characterize surfaces by measuring the energies and intensities of the elastically scattered electrons to extract information on the atomic geometry (15, 24–30) and those of either core-level photoemission (x-ray photemission spectroscopy) or Auger emission (Auger electron spectroscopy, the detection of the emitted electron from a pair of electrons, one of which fills a hole in a core electron state) to determine the atomic composition (16, 31). In the latter case the emission can be stimulated either by the absorption of incident radiation (typically x-rays or synchrotron radiation) or the inelastic collision with an incident electron as indicated in Fig. 3 (32). Although a few early observations of Auger features in the secondary electron spectrum from surface contaminants had been reported in the 1950s (31), the surface sensitivity of Auger electron spectroscopy began to be recognized more broadly in the mid-1960s (31) and was established directly by Paul Palmberg and Thor Rhodin (33) in 1968. As shown in Fig. 3, typically a derivative of the secondary spectrum must be taken to observe the small Auger electron features. By 1974 Auger electron spectroscopy had become a routine tool for assessing the atomic composition of solid surfaces (16, 24). X-ray photoemission spectroscopy already was a known technique for materials analysis (34), the surface sensitivity of which came to be recognized during this time period and subsequently exploited for surface analysis by both its originator (35) and many others (16).
Figure 3.
Secondary electron spectrum of 500 eV electrons incident on a tungsten surface. The elastic peak is associated with LEED and the plasma loss peaks are associated with the creation by the incident electron of collective excitations of the valence electrons. These also emerge from the solid in inelastic LEED beams. The spectrum shown is taken with a large area detector and hence incorporates results from several diffracted beams. The Auger peaks associated with the tungsten itself and a carbon contaminant on the surface are shown in the derivative spectrum (Inset). [After Duke and Park (32) with permission.]
By measuring the directions of the emergent beams the symmetry of the crystal lattice parallel is ascertained by LEED, as indicated in Fig. 1. By measuring the intensities of the elastically scattered electrons relative to their energies the atomic geometry of the surface may be determined. A detailed understanding of the combined elastic and inelastic scattering of electrons by the solid is required for the calculation of the intensities of the elastically and inelastically diffracted beams. Incident energetic electrons interact strongly with both the valence electrons and the ion cores of a solid, generating strong elastic scattering from the ion cores (leading, e.g., to the energy band structure of solids), inelastic scattering from the ion cores [leading to the excitation of lattice vibrations (i.e., phonons) and core electrons], and inelastic scattering from the valence electrons in the solid (leading to the excitation of the valence electron collective modes). The effects of all of these processes must be sorted out in a systematic way to calculate the elastic and inelastic diffracted intensities. The condensed-matter physics notions characteristic of many-body theory were brought to bear on this problem in the early 1970s (36, 37) to generate a renormalizable quantum field theory of the electron scattering processes. In the ensuing few years this theory was converted into phenomenological models to calculate the intensities of the elastic and inelastic diffracted beams (24). Although in 1965 only the lattice symmetry was able to be determined (1), by 1974 reasonably complete characterizations of simple surfaces were achievable (24). Many additional techniques have been added to the arsenal in recent years (2, 3, 38, 39), so that today both the atomic composition and the atomic geometries of single crystal solid surfaces (and of ordered adsorbed overlayers thereupon) can be determined routinely by surface-sensitive electron spectroscopy in UHV environments, the roots of which may be found in the period 1968–1973 when modern surface science was born.
Impact of the Semiconductor Microelectronics Revolution
Surface science grew up with semiconductor microelectronics. The amazing story of the evolution of the discovery of the transistor in 1947 into the ultra large-scale integration of today's microelectronics has been told repeatedly. It recently has been captured in two special volumes (40, 41) on the occasion of the 50th anniversary of this discovery. In 1968, the year that the surface sensitivity of low-energy electrons was recognized (13, 33), Intel was founded (42) to produce what already was fifth- and sixth-generation (40, 41, 43) microelectronic circuits. The semiconductor microelectronics revolution was well on its way, enabled by the materials science of growing large pure samples of silicon, zone-refining them into single crystals, and doping them by diffusion, and the surface science of oxide growth, epitaxial growth of device quality silicon on silicon substrates, metallization via physical vapor deposition, the use of oxides and resists for pattern wise deposition and etching, and the use of surface analysis for device and process characterization.
In a prescient paper (ref. 44; www.intel.com/research/silicon/moorespaper.pdf) in 1965, Gordon Moore, then director of research and development at Fairchild Semiconductor, coined the term “integrated electronics” to describe the integration of active and passive electronic components on a semiconductor substrate to form functional devices and projected that the packing density of these components would grow exponentially, doubling roughly every year. Three years later, he left Fairchild to cofound Intel [an abbreviation of integrated electronics (42)] with Robert Noyce, inventor of the planar integrated circuit, and thereby to make his 1965 prediction a self-fulfilling prophecy. In fact, for both memory and processor chips, during the 30 years between 1970 and 2000 the number of transistors per chip has increased by a factor of ≈10,000 so the packing density has doubled roughly every 2 years (45), with Intel being one of the leading firms whose products satisfy this “law” (www.intel.com/research/silicon/mooreslaw.htm).
The design and manufacture of semiconductor microelectronics have become big business globally. The total value of semiconductor electronics exceeded $200 billion in 2000 (45). The start-up cost of a wafer fabrication plant is increasing by an order of magnitude every 10 years and now exceeds $2 billion (45). Thus, huge financial investments are being made to maintain this trend to ever smaller, ever cheaper on a per-unit basis. Two aspects of this trend are of particular interest to us here. First, with the inexorable shrinking of feature size on chips the roles of vacuum-based (“dry”) processing and surface chemical reactions have increased, and hence so has the impact of surface science on the semiconductor industry. Second, the exponential scaling of packing density implies a similar scaling of functions per unit cost (45) with the consequence that accessible computing power to surface science researchers also has been growing exponentially. This, in turn, has led to an explosive increase in the quantitative predictive capability of theories of surface phenomena as well as to a continual improvement in the complexity and reliability of surface processing and experimentation. Thus, surface science's growth and advance are being supported by the multibillion-dollar annual investments made by the semiconductor microelectronics industry.
In addition to basic surface science and vacuum technology, the science and instrumentation of semiconductor processing is the province of AVS. AVS serves as a locus of scientific studies of the process steps used in semiconductor microelectronics manufacturing. It is a source of technology for processes like metallization, ion implantation, and plasma etching as well as for device characterization via electron-, ion- and photon-based spectroscopies (46). Its thin film division was formed in 1964 with programs emphasizing the fundamentals involved in semiconductor processing technologies like metallization (8). Throughout the 1970s and 1980s the programs at the AVS annual international symposium reflected technical papers at the cutting edge of semiconductor microelectronics fabrication, metrology, and processing. The AVS electronic materials and processing division was founded in 1979 (8, 46). The fusion technology division of the AVS was founded in 1980 with a programmatic emphasis on the science of plasma–surface interactions and the vacuum technology associated with controlled fusion research. It was transformed into the plasma science and technology division in 1987 to focus on plasma processing of microelectronics. The conferences sponsored by this division and the associated articles published in the Journal of Vacuum Science and Technology became and remain to this day the dominant professional forum for the science and technology of plasma deposition and plasma etching techniques.
In addition to its international symposium and activities initiated by its divisions, AVS sponsors its flagship journal, Journal of Vacuum Science and Technology, and a variety of topical conferences that have proved indispensable to the evolution of semiconductor microelectronics. In 1983 AVS introduced a new part B of Journal of Vacuum Science and Technology entitled Microelectronics: Processing and Phenomena. By 1994 it was larger than the original journal, now part A. Three important AVS-sponsored annual conferences where semiconductor process technologists, device fabricators, and surface scientists meet are the conference on the Physics and Chemistry of Semiconductor Interfaces (PCSI); the conference on Ion, Photon, and Electron Beams (“three beams”); and, since 2000, the International Conference on Microelectronic and Interfaces. A review (47) of some of the highlights of the PCSI conferences during their first 20 years of existence revealed the profound effect that the microelectronics revolution had on the practice of semiconductor surface science during 1974–1993.
During the 1970s and 1980s, AVS was one of the pioneering technical societies mediating the birth of semiconductor optoelectronics: the use of semiconductor lasers and other optoelectronic devices to use light (“photonics”) rather than electronics for communications and signal processing. These devices typically are based on compound semiconductors like GaAs and AlAs and its alloys rather than silicon. The major fabrication process for these devices, molecular beam epitaxy, emerged from semiconductor surface science in the 1970s and found a friendly home at AVS meetings and topical conferences (48). The combination of semiconductor microelectronics and optoelectronics is providing the physical hardware technology for a global communications revolution, whose impact is expected to dwarf that of the telephone, television, and computer (49). Thus, AVS helped to establish semiconductor surface science as one the greatest wellsprings of technical and social change that the world has ever seen.
To illustrate the profound consequences of the microelectronics revolution on the practice of surface science, it is instructive to compare the contents of two major overviews of the field prepared roughly a decade apart. The first, Surface Science: The First Thirty Years (2), contains articles written in 1992–1993 describing the history and scope of the field by the practitioners who created it. The second, Frontiers in Surface and Interface Science (3), consists of articles written in 2001 by leaders in the field, describing not only its current state, but also its prospects for the new millennium. During the intervening 8 or 9 years, the computer power available to these researchers at constant cost doubled three times.
Surface Science: The First Thirty Years (2) describes a field in which the basic techniques for the determination of surface structure and composition have been validated on simple systems such as clean low-index single crystal surfaces and monolayers of adsorbates thereon. Surface-phase diagrams of adsorbate systems were being mapped and the dynamics of surface diffusion were followed by field ion microscopy. Initial results on more complex systems such as steps on surfaces, buried interfaces, and the dynamics of simple surface chemical reactions were beginning to be reported. Although the scanning tunneling microscope (STM) had been invented, its application for spectroscopy was in its infancy. The extension of scanning probe notions to atomic force microscopy (AFM) was in a highly exploratory state. All of these results were light years ahead of the situation a mere 2 decades earlier when neither the composition nor the structure nor the atomistic dynamics of surfaces could be determined with confidence. This remarkable advance was attributable in part to the impact of vastly increased experimental reliability and computational power caused by the 1,000-fold increase in local processor power generated by the first 2½ decades of the electronics revolution (15, 47). The development of theory had generally lagged the experimental progress, with papers on semiempirical models and calculational techniques dominating the agenda, with the exception of three papers on density functional theory applied to both ground-state properties of metal surfaces and the electronic excitation spectra of a few well chosen semiconductor surfaces. Those papers and those on STM and AFM were harbingers of the future.
Frontiers in Surface and Interface Science (3) describes a transformed field. The ability to characterize the atomic geometry, surface morphology, atomic composition, and dynamics of the evolution of simple diffusion, growth, and chemical reactions is taken as given. STM, AFM, and their progeny have transformed the field into one characterized by digital images, at atomic resolution if need be, of surfaces in vacuum, solid–liquid interfaces, and surfaces embodying biological samples. The atomic motions in growth and surface diffusion are shown in digital videos of either experimental measurements, made via, e.g., STM, or simulations. Thanks to the extra three doublings of computer power at constant cost since 1993, density functional theory is now applied in individual articles to define a virtual chemistry laboratory, delineate the mechanisms of catalysis and corrosion, and study the reactions of enzymes. The real-time dynamics of some relatively simple reactions are mapped in detail both experimentally and theoretically. The focus of the entire field had shifted from the development of characterization techniques, both experimental and theoretical, to the characterization of vastly more complex samples, including electrochemical and biological systems. All of this is possible only because of the advances in experimental technique, data acquisition and analysis, and theoretical prediction and simulation enabled by the 4 orders of magnitude improvements in cost performance of semiconductor processors between 1970 and 2000. The semiconductor microelectronics revolution formed the technological basis for a second wave of advances that lifted surface science to a new plateau of capability beyond that of the 1980s into the new millennium.
Scanning Probe Microscopy and Spectroscopy
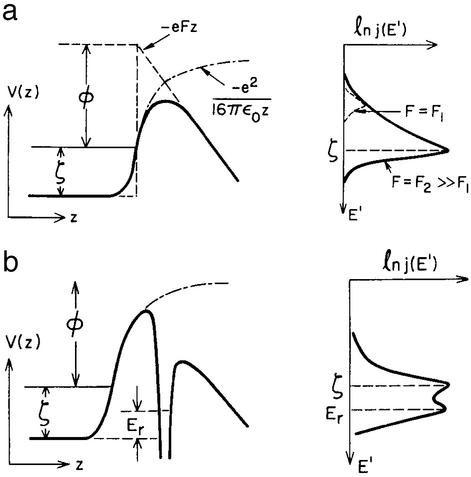
Another important concept from condensed-matter physics that proved indispensable to the evolution of surface science is the notion of electron tunneling. In the earliest days of experiments carried out in glass vacuum containers, the tunneling of the valence electrons out of a solid cathode in a strong electric field was indispensable to the operation of electron tubes. Eugen Merzbacher (50) has given a delightful history of the theoretical description of this effect in the late 1920s. Both Oppenheimer and a collaborative work by Fowler and Nordheim attacked this problem, with the latter solution being the one that survives in textbooks today (for review see refs. 51 and 52). The next big step in the application of tunneling concepts to surface science came in response to a puzzle: the adsorption of nitrogen on tungsten was known to increase the work function (and hence increase the field emission barrier as shown in Fig. 4a) yet the emission current increased rather than decreased, in contradiction to the predictions of the Fowler–Nordheim model. This observation led Michael Alferieff and myself (53) to propose in 1967 a model of resonant elastic tunneling through electronic states of the adsorbed nitrogen as shown in Fig. 4b. At that time there was no confirmation of such a notion, although 3 years later Ward Plummer and Russ Young (54) confirmed the existence of such resonances by measuring the field emission energy distributions (FEED) of alkaline earth elements on W and Mo. In time FEED became an accepted probe of the electronic states of adsorbed species (55). During this same period the concept of the measurement of the characteristics of resonant electronic states in adsorbed species was transferred to photoemission energy distributions, the measurement of which became a major occupation of surface scientists in the mid-1970s (56). The high-impact application of tunneling to surface science came, however, with the invention of the STM by Gerd Binnig and Heine Rohrer (57, 58).
Figure 4.
Schematic diagrams of the potential energy and field emission energy distributions of a clean metal surface (a) and an adsorbate on that surface (b) in a strong electric field. [After Duke (20) with permission.]
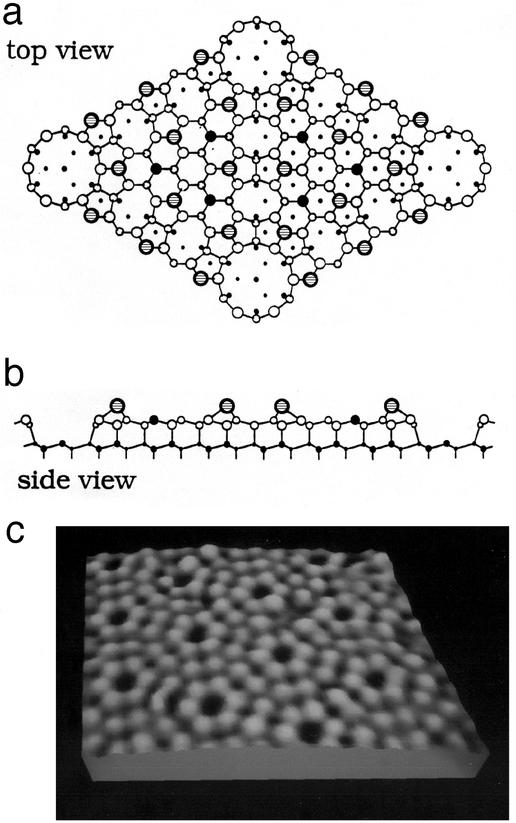
The resonant tunneling idea described above is readily applied to the tunneling between an adatom, e.g., on a tip, and nearby solid surface (51). In 1971, using an instrument that was the forerunner of the STM, Russ Young and collaborators (59) demonstrated tunneling through the vacuum between a tip and a nearby solid surface. Until Binnig and Rohrer, however, no one had been able to design a tip moving system with sufficient stability to scan a tip over the surface to generate an image based on measuring the tunneling current directly between the tip and the surface. The two key problems are to construct a (tip) geometry in which the tunneling from a single atom predominates and to measure the height of the tip above the surface while stabilizing it from crashing into the surface. An indication of Binnig and Rohrer's notion of the tip geometry that they fabricated is shown in Fig. 5, illustrating their recognition of the fact that tunneling through an individual atom is required for atomic resolution. Through a combination of good design, persistence, and the use of feedback control, they achieved this objective, ultimately obtaining atomic resolution on (2 × 1) structure of the (110) surface of clean Au and on a famous semiconductor surface structure: the (7 × 7) structure on the (111) surface of silicon. The (hkl) notation signifies the crystallographic direction of the normal to the surface and the (axb) notation signifies the symmetry of the surface structure relative that of the bulk unit cell in the surface layer. Although the existence of the Si(111)–(7 × 7) structure had been discovered in 1959 by LEED, the determination of its atomic geometry and the origin thereof had been one of the main objectives in semiconductor surface science for decades (11). Binnig and Rohrer's STM image of the Si(111)–(7 × 7) structure was published in 1983 (60) and presented at the Physics and Chemistry of Semiconductor Interfaces conference that year (47). It provided important clues to the atomic geometry of this structure, which ultimately was determined (61) and refined (11) by electron diffraction techniques. Of more importance, it captured the attention and imagination of the surface science community and led to a rapid emulation of the STM technique in other laboratories (57, 58, 62). A schematic diagram of Si(111)–(7 × 7) structure is shown in Fig. 6 together with a modern STM micrograph of its atomic geometry. Although the STM gives a vivid picture of the adatoms in the top atomic layer of the structure, the reconstructed surface structure penetrates multiple layers into the sample. It took many years and the application of multiple techniques to determine the detailed atomic geometry of this structure (11).
Figure 5.
Schematic diagram of a STM tip. The termination of both a primary and a secondary tip by single atoms at the apex is indicated. Such single-atom termination is regarded as being necessary to generate atomic resolution from a STM. [After Rohrer (58) with permission.]
Figure 6.
The (7 × 7) structure of the (111) surface of silicon. (a) Schematic top view. (b) Schematic side plan view. (c) STM micrograph. The side view is a plan view of the nearest neighbor bonding in a plane normal to the surface containing the long diagonal of the surface unit cell. In the top view (a) the large shaded circles designate the adatoms in the top layer of the structure. It is evident from c that the STM images only these atomic species. The large solid circles designate second-layer “rest atoms” that are not bonded to an adatom. Large open circles designate triply bonded atoms in this layer. Small open circles designated 4-fold-bonded atoms in the bilayer beneath. Smaller solid circles designate atoms in the fourth and fifth bilayers beneath the surface. The size of circles is proportional to the distance from the surface. In the side view (b) smaller circles indicate atoms out of the plane of this diagonal. [(a and b) adapted from Takayanagi et al. (61) with permission; (c) after Joel Kubby, personal communication.]
The invention of the STM ushered in the age of images in surface science. A mere 4 years after the invention of the STM, the same Zurich group plus Cal Quate of Stanford University (Stanford, CA) invented the AFM, based on an STM-like tip mounted on a tiny cantilever whose pointwise deflection gave a measure of the surface topography (63). Unlike the STM, it could be used on the surfaces of insulating materials and hence opened up all surfaces to local probe measurements. The technology of designing and manufacturing these instruments advanced rapidly in the early 1990s, including major improvements in tip fabrication, control of tip motion, and of course, digital data acquisition and processing enabled by the semiconductor microelectronics revolution (62, 63). A particularly important advance in technique was the development of the capability to scan the voltage on an STM tip while it was in one position, thus giving rise to scanning tunneling spectroscopy (64). The interpretation of either STM or AFM images requires considerable care since both reflect the imaging conditions, and in the case of STM the electronic structure of the surface and tip atoms, as well as the topographical structure of the sample (62, 64). Nevertheless, it is hard to find an experimental surface science paper that does not offer at least one STM or AFM image of the surface being studied. As noted above for the Si(111)–(7 × 7) structure, these images do not define the atomic geometries of the surfaces involved. Other surface structure spectroscopies are required for that. But they give a striking sense of the overall topography of the surface, the presence of defects and multiple surface structures, and by taking snapshots at different times, the time dependence of atomic motions across the surface. By identifying the local structure over macroscopic areas of “real” surfaces, they have enabled vast improvements in sample preparation. Moreover they can be obtained at solid–liquid interfaces, opening up new vistas in electrochemical (65) and biomedical (66) research.
Toward More Complex Systems
As the new millennium dawns, surface science finds itself at a tipping point. Born of the union of vacuum technology and condensed-matter physics, matured in the shadow of the microelectronics revolution, and entering the age of imagery via local probe microscopy, surface science is on the verge of disappearing into the infrastructure of the many interdisciplinary research areas that it services. In the keynote paper in Frontiers in Surface and Interface Science (3) Ward Plummer and his coauthors (67) identify complexity as the overarching theme of the surface science of the future: complexity in spatial scale from nuclei to galaxies, complexity in time scale from femtoseconds to eons, and complexity in function from the hydrogen atom to life forms. All of these dimensions of surface science are described in individual articles in that volume.
Although it can and will be improved, the toolkit is full. A wide variety of surface characterization tools have been developed, validated, and deployed in laboratories across the world (2, 3). Moreover, surfaces, as entities unto themselves, are no longer interesting objects of study. What is of interest is what they reveal, e.g., about the nature of inhomogeneous multiphase nanostructures (67); what they do, e.g., catalyze highly complex biological reactions at room temperature (68); and what they can be used for, e.g., as templates for a new generation of computers (69) or the basis for a complex technologies like semiconductor microelectronics fabrication (70) or xerography (71). Unlike the first three generations, fourth-generation surface science is likely to become increasingly invisible, part of the substrate on which new generations of interdisciplinary research and manufacturing technology from the micro to the nano scale are built. Like the first three generations, however, it will remain as one of the most prolific scientific wellsprings of technological and social advance that the world has ever seen as it extends its reach from the hardware of the digital age to the processes of life.
Supplementary Material
Acknowledgments
Surface science is a community effort, and I am privileged to have been a member of this community. I am indebted to my many coauthors and colleagues over the years for their generous contributions to my education and our work together. I thank Fred Dylla, Peter Feibelman, Joel Kubby, Max Lagally, Rudy Ludeke, and Paul Redhead for critical readings of the manuscript and helpful corrections and additions to its contents.
Abbreviations
- AVS
American Vacuum Society
- UHV
ultra high vacuum
- LEED
low-energy electron diffraction
- STM
scanning tunneling microscope
- AFM
atomic force microscopy
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 1, 2001.
References
- 1.Many A, Goldstein Y, Grover N B. Semiconductor Surfaces. Amsterdam: North–Holland; 1965. [Google Scholar]
- 2.Duke C B, editor. Surf Sci. 1994;299/300:1–1054. [Google Scholar]
- 3.Duke C B, Plummer E W, editors. Surf Sci. 2002;500:1–1053. [Google Scholar]
- 4.Kuhn T S. The Structure of Scientific Revolutions. Chicago: Univ. of Chicago; 1962. [Google Scholar]
- 5.Duke C B. J Vac Sci Technol A. 1984;2:139–143. [Google Scholar]
- 6.Redhead P A. In: Vacuum Science and Technology: Pioneers of the 20th Century. Redhead P A, editor. New York: Am. Institute of Physics; 1995. pp. 133–143. [Google Scholar]
- 7.Redhead P A, editor. Vacuum Science and Technology: Pioneers of the 20th Century. New York: Am. Institute of Physics; 1993. [Google Scholar]
- 8.Redhead P A. A History of the American Vacuum Society. New York: Am. Vacuum Soc.; 1995. [Google Scholar]
- 9.Lander J J. Prog Solid-State Chem. 1965;2:26–116. [Google Scholar]
- 10.Estrup P J, McRae E G. Surf Sci. 1971;25:1–52. [Google Scholar]
- 11.Duke C B. Chem Rev. 1996;96:1237–1259. doi: 10.1021/cr950212s. [DOI] [PubMed] [Google Scholar]
- 12.Quinn J J. Phys Rev. 1962;126:1453–1457. [Google Scholar]
- 13.Duke C B, Tucker C W., Jr Surf Sci. 1969;15:231–256. [Google Scholar]
- 14.Jones R O, Strozier J A., Jr Phys Rev Lett. 1969;22:1186–1188. [Google Scholar]
- 15.Duke C B. Surf Sci. 1994;299/300:24–33. [Google Scholar]
- 16.Powell C J. Surf Sci. 1994;299/300:34–48. [Google Scholar]
- 17.Seah M P, Dench W A. Surf Inter Anal. 1979;1:2–11. [Google Scholar]
- 18.Jablonski A, Powell C J. Surf Sci Rep. 2002;47:33–92. [Google Scholar]
- 19.Gatos H C. Surf Sci. 1994;299/300:1–23. [Google Scholar]
- 20.Duke C B. J Vac Sci Technol. 1978;15:157–169. [Google Scholar]
- 21.Hobson P J, Czanderna A W. AVS Newsletter. 1999. July/August, 4–5. [Google Scholar]
- 22.Duke C B. AVS Newsletter. 2001. Spring, 11. [Google Scholar]
- 23.Davis H L, editor. Phys Today. 1972;25(8):23–58. [Google Scholar]
- 24.Duke C B. Adv Chem Phys. 1974;27:1–210. [Google Scholar]
- 25.Tong S Y. Surf Sci. 1994;299/300:358–374. [Google Scholar]
- 26.Pendry J B. Surf Sci. 1994;299/300:375–390. [Google Scholar]
- 27.Heinz K. Surf Sci. 1994;299/300:433–446. [Google Scholar]
- 28.Marcus P M. Surf Sci. 1994;299/300:447–453. [Google Scholar]
- 29.Kahn A. Surf Sci. 1994;299/300:469–486. [Google Scholar]
- 30.Van Hove M A, Somorjai G A. Surf Sci. 1994;299/300:487–501. [Google Scholar]
- 31.Harris L A. J Vac Sci Technol. 1974;11:23–28. [Google Scholar]
- 32.Duke C B, Park R L. Phys Today. 1972;25(8):23–30. [Google Scholar]
- 33.Palmberg P W, Rhodin T N. J Appl Phys. 1968;39:2425–2432. [Google Scholar]
- 34.Siegbahn K, Nordling C, Fahlman A, Nordberg R, Hamrin K, Hedman J, Johansson G, Berkmark T, Karlsson S, Lindgren I, Lindberg B. Atomic, Molecular, and Solid-State Structure Studied by Means of Electron Spectroscopy. Uppsala, Sweden: Almqvist and Wiksells; 1967. [Google Scholar]
- 35.Siegbahn K. In: Nobel Lectures in Physics 1981–1990. Frängsmyr T, Ekspong G, editors. Singapore: World Scientific; 1993. pp. 63–92. [Google Scholar]
- 36.Duke C B, Laramore G E. Phys Rev B Condens Matter. 1970;2:4765–4782. [Google Scholar]
- 37.Duke C B, Laramore G E. Phys Rev B Condens Matter. 1971;3:3183–3197. [Google Scholar]
- 38.Duke C B. In: Positron Spectroscopy of Solids: Proceedings of the International School of Physics Enrico Fermi, Course CXXV. Dupasquier A, Mills A P Jr, editors. Amsterdam: IOS; 1995. pp. 317–359. [Google Scholar]
- 39.Woodruff D P. Surf Sci. 2002;500:147–171. [Google Scholar]
- 40.Bondyopadhyay P K, Chatterjee P K, Chakrabarti U, editors. Proc IEEE. 1998;86:3–217. [Google Scholar]
- 41.Brinkman W F, Troutman W F, editors. Bell Labs Technol J. 1997;2:3–168. [Google Scholar]
- 42.Jackson T. Inside Intel. New York: Penguin Putnam; 1997. [Google Scholar]
- 43.Runyan W R, Bean K E. Semiconductor Integrated Circuit Processing. Reading, MA: Addison–Wesley; 1990. pp. 2–16. [Google Scholar]
- 44.Moore G E. Electronics. 1965;38:114–117. [Google Scholar]
- 45.Quirk M, Serda J. Semiconductor Manufacturing Technology. Upper Saddle River, NJ: Prentice–Hall; 2001. [Google Scholar]
- 46.Duke C B. J Vac Sci Technol. 1980;17:1–8. [Google Scholar]
- 47.Duke C B. J Vac Sci Technol B. 1993;11:1336–1346. [Google Scholar]
- 48.Arthur J R. Surf Sci. 2002;500:189–217. [Google Scholar]
- 49.Cairncross F. The Death of Distance. Boston: Harvard Business School Press; 1997. [Google Scholar]
- 50.Merzbacher E. Phys Today. 2002;55(8):44–49. [Google Scholar]
- 51.Duke C B. Tunneling in Solids. New York: Academic; 1969. [Google Scholar]
- 52.Gomer R. Surf Sci. 1994;299/300:129–152. [Google Scholar]
- 53.Duke C B, Alferieff M E. J Chem Phys. 1967;46:923–937. [Google Scholar]
- 54.Plummer E W, Young R D. Phys Rev B Condens Matter. 1970;1:2088–2109. [Google Scholar]
- 55.Gadzuk J W, Plummer E W. Rev Mod Phys. 1973;45:487–548. [Google Scholar]
- 56.Gomer R. Adv Chem Phys. 1974;27:211–264. [Google Scholar]
- 57.Binnig G, Rohrer H. In: Nobel Lectures in Physics 1981–1990. Frängsmyr T, Ekspong G, editors. Singapore: World Scientific; 1993. pp. 389–409. [Google Scholar]
- 58.Rohrer H. Surf Sci. 1994;299/300:965–964. [Google Scholar]
- 59.Young R, Ward J, Scire F. Phys Rev Lett. 1971;27:922–924. [Google Scholar]
- 60.Binnig G, Rohrer H, Gerber Ch, Weibel E. Phys Rev Lett. 1983;50:120–123. [Google Scholar]
- 61.Takayanagi K, Tanishiro Y, Takahashi S, Takahashi M. Surf Sci. 1985;164:367–392. [Google Scholar]
- 62.Kubby J A, Boland J J. Surf Sci Rep. 1996;26:61–204. [Google Scholar]
- 63.Quate C F. Surf Sci. 1994;299/300:980–995. [Google Scholar]
- 64.Feenstra R M. Surf Sci. 1994;299/300:965–979. [Google Scholar]
- 65.Kolb D M. Surf Sci. 2002;500:722–740. [Google Scholar]
- 66.Castner D G, Ratner B D. Surf Sci. 2002;500:28–60. [Google Scholar]
- 67.Plummer E W, Ismail, Matzdorf R, Melechko A V, Pierce J P, Zhang J. Surf Sci. 2002;500:1–27. [Google Scholar]
- 68.Rod T H, Norskov J K. Surf Sci. 2002;500:678–698. [Google Scholar]
- 69.Gillmor S D, Rugheimer P P, Lagally M G. Surf Sci. 2002;500:699–721. [Google Scholar]
- 70.Weldon M K, Queeney K T, Eng J, Jr, Raghavachari K, Chabal Y J. Surf Sci. 2002;500:859–878. [Google Scholar]
- 71.Duke C B, Noolandi J, Thieret T. Surf Sci. 2002;500:1005–1023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.