Abstract
Determination and differentiation of cells in the skeletal muscle lineage is positively regulated by cell–cell contact. Cell-surface proteins proposed to mediate this effect include both classical cadherins and Ig superfamily members; potential interactions between the promyogenic activities of these classes of protein, however, are unknown. We show here that CDO and BOC, two promyogenic Ig superfamily members that bind to each other in a cis fashion, form complexes with N- and M-cadherin. These complexes contain β-catenin and are enriched at sites of cell–cell contact between myoblasts. In transient expression assays, the ectodomains and intracellular regions of CDO, BOC, and N-cadherin each interact independently, suggesting that the interactions occur in a cis fashion; consistent with this conclusion, cadherin-mediated cell adhesion is not required for them to occur. Stable expression in myoblasts of a CDO deletion mutant deficient in its ability to associate with N-cadherin interferes with differentiation as assessed by biochemical, morphological, and reporter gene assays, suggesting that this interaction is functionally important in myogenesis. Thus, some of the cell–cell contact-mediated activities that are required for myogenesis seem to be based on interdependent activities of promyogenic classical cadherins and Ig superfamily members.
Many types of cell–cell communication require the formation of intimate intercellular contacts (1). Cadherins play a key role in mediating such phenomena, because they are centrally involved in establishing cell–cell adhesive structures. Furthermore, cadherin-based adhesion can activate signaling pathways via two nonmutually exclusive mechanisms: (i) control of the ability of juxtacrine signaling ligands and receptors to make intercellular connections and (ii) direct regulation of signaling processes (1, 2). Among the numerous cellular activities that are regulated by cadherins is cell differentiation (1).
Skeletal myogenesis is viewed as a paradigm for understanding the molecular events that link cell-lineage determination, cell differentiation, and tissue-specific gene expression (3, 4). Interestingly, determination and differentiation of cells in the skeletal muscle lineage is positively regulated by cell–cell contact (5–7). Several cadherins have been implicated in this process including N-, M-, and R-cadherin. N-cadherin has been studied most extensively; several lines of evidence indicate that it plays a positive role in skeletal myogenesis. Expression of a dominant-negative form of N-cadherin in early Xenopus laevis embryos suppresses expression of the myogenic transcription factor, MyoD, in muscle progenitor cells (8). Furthermore, several different function-perturbing antibodies to N-cadherin inhibit myogenic differentiation in vitro of chick primitive streak epiblast cells (9), primary chicken embryo myoblasts (10), and C2C12 murine myoblasts (11). Conversely, incubation of C2C12 and other myoblast cell lines with beads coated with recombinant N-cadherin extracellular domains enhances both biochemical and morphological aspects of differentiation (12). Antisense and ectopic expression studies also implicate M- and R-cadherin as positive regulators of myogenesis (13, 14).
We have been studying the roles of CDO and BOC, two recently identified cell-surface receptors of the Ig/fibronectin type III repeat family, in myogenic differentiation. CDO and BOC are closely related in their ectodomains, but each contains a long cytoplasmic tail that does not resemble other known proteins, including the tail of the other (15, 16). CDO and BOC are coexpressed in muscle precursor cells during mouse development and form complexes with each other in a cis fashion (16–19). Each protein positively regulates differentiation of myoblast cell lines in vitro and participates in a positive feedback loop with MyoD (16, 17). The intracellular region of BOC is dispensable for its promyogenic effects, whereas that of CDO is required; furthermore, the activity of BOC depends on CDO, suggesting that CDO plays a role in signaling (16).
CDO, BOC, and promyogenic cadherins are coexpressed during murine myogenesis (13, 16–21), raising the possibility that their promyogenic activities may be related in a mechanistic fashion. It is reported here that CDO and BOC form complexes with promyogenic cadherins at sites of cell–cell contact and that expression of a CDO deletion mutant deficient in its ability to associate with N-cadherin interferes with myogenesis in vitro. These data suggest that formation of a higher-order complex between classical cadherins and Ig superfamily members is of functional importance in mediating the promyogenic effects of cell–cell contact.
Materials and Methods
Cell Culture.
C2C12 myoblasts were cultured as described (16, 17). Differentiation was induced by switching cultures from DMEM/15% FBS to DMEM/2% horse serum. 293T cells were cultured in DMEM/10% FBS as described (16, 17). RD human rhabdomyosarcoma cells were obtained from S. Aaronson (Mount Sinai School of Medicine) and cultured in DMEM/10% FBS.
Ectopic Expression of N-Cadherin, CDO, BOC, and Deletion Mutants.
Stable expression of CDO and a CDO deletion mutant that lacks the first fibronectin type III repeat [CDO(ΔFN1)] was via retroviral transduction with the pBabePuro vector as described (16, 17). CDO(ΔFN1) was constructed by a combination of conventional cloning and PCR techniques and verified by sequencing; details are available on request. Transient transfections of 293T cells were performed with the calcium-phosphate technique.
Protein Analysis.
Immunoblot analyses were performed as described by Kang et al. (15). Antibodies used were anti-CDO (Zymed), anti-pan cadherin (Sigma), anti-β-catenin (Becton Dickinson Transduction Laboratories, Lexington, KY), anti-N-cadherin (Zymed), anti-M-cadherin (Becton Dickinson Transduction Laboratories), anti-BOC (affinity-purified rabbit antisera against the intracellular region of human BOC, developed in the Krauss Laboratory), anti-MHC (MF20, Development Studies Hybridoma Bank, Iowa City, IA), anti-MyoD (Santa Cruz Biotechnology), antimyogenin (F5D, Santa Cruz Biotechnology), anti-Flag (Sigma), anti-human Fc (Jackson ImmunoResearch), and anti-myc (9E10, Mount Sinai School of Medicine Hybridoma Core Facility). Immunostaining for MHC was performed as described by Kang et al. (17).
To study CDO–BOC–cadherin complex formation, C2C12 and 293T cells were lysed in 50 mM Tris⋅HCl, pH 7.5/100 mM NaCl/1% Triton-X containing 50 mM NaF, 1 mM sodium orthovanadate, and proteinase inhibitor mixture (Roche, Indianapolis) and subjected to coimmunoprecipitation techniques by using the above-listed antibodies. Immunocomplexes then were precipitated with either protein A-Sepharose or rabbit anti-mouse IgG-conjugated protein A-Sepharose followed by immunoblot analysis with various antibodies.
Confocal Microscopy.
Cells grown on coverslips were fixed in 3% paraformaldehyde in PBS, pH 7.4, for 15 min and permeabilized in 0.1% Triton X-100 in PBS for 1 min followed by quenching aldehyde-induced fluorescence with NH4Cl (50 mM) for 1 min. Samples were blocked with 5% (vol/vol) goat serum (in PBS) and then incubated with primary followed by secondary antibodies with extensive washing between incubations. Primary antibodies are as described above; the secondary antibodies used were anti-mouse conjugated to Alexa 488, anti-mouse Alexa 568, and anti-rabbit Alexa 488. Coverslips were mounted with antifade agent (n-propyl-gallate) and viewed with a Leica TCS-SP (UV) confocal microscope. Images were assembled for presentation in PHOTOSHOP (Adobe Systems, Mountain View, CA).
Luciferase Assays.
4Rtk-Luc was cotransfected into C2C12 cells with either pBabePuro or pBabePuro/CDO(ΔFN1) with the FuGene6 reagent (Roche), and reporter activity was assayed 2 days later with a luciferase assay system according to manufacturer instructions (Promega). To control for transfection efficiency, luciferase activity was normalized to β-galactosidase activity driven by a cotransfected pCMV-lacZ vector.
Results
Coimmunoprecipitation of CDO, BOC, and Cadherins.
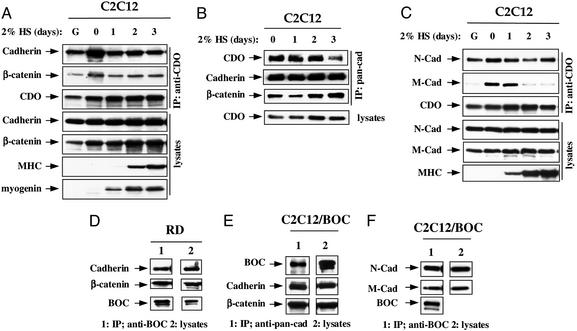
To test for an interaction between CDO and cadherins, lysates from C2C12 cells that were proliferating or differentiating over a 3-day time course were immunoprecipitated with antibodies to CDO and immunoblotted with an antibody that recognizes several classical cadherins; direct immunoblotting of lysates was performed as a control and to monitor progression of differentiation. A 125- to 130-kDa cadherin was observed in CDO immunoprecipitates from both proliferating and differentiating cells (Fig. 1A). The cadherin-associated protein, β-catenin, also coimmunoprecipitated with CDO (Fig. 1A). Moreover, coimmunoprecipitation was observed with a second CDO antibody directed against a different epitope (data not shown). A reciprocal experiment in which antibodies to cadherin were used further demonstrated that CDO coimmunoprecipitates with cadherin (Fig. 1B). To determine what cadherin(s) might be recognized by the pan-cadherin antibody, antibodies to specific cadherins were used. N- and M-cadherin were each expressed in proliferating and differentiating cultures of C2C12 cells as determined by immunoblotting with specific antibodies (Fig. 1C). Immunoblotting of CDO immunoprecipitates with these antibodies revealed that N-cadherin was associated with CDO under all conditions; in contrast, M-cadherin associated with CDO transiently, early in the differentiation process (Fig. 1C). R-cadherin levels were relatively low in these cells, and coimmunoprecipitation experiments were ambiguous (data not shown).
Figure 1.
Association of endogenous cadherins with CDO and BOC. (A and B) Association of CDO, cadherin, and β-catenin in C2C12 cells. Lysates from proliferating, low-density C2C12 cells cultured in growth medium (G) or confluent cultures in differentiation medium [2% horse serum (HS)] for the indicated times were immunoprecipitated with antibodies to CDO (A) or cadherin (pancadherin antibody) (B), fractionated by SDS/PAGE, and immunoblotted with the indicated antibodies. Straight lysates were also probed as indicated. Immunoblotting for myogenin and MHC indicates the progression of cell differentiation. (C) Association of N- and M-cadherin with CDO. (D) Association of BOC, cadherin, and β-catenin in RD cells. (E) Association of cadherin and β-catenin with stably expressed BOC(flag) in C2C12 cells. (F) Association of N- and M-cadherin with stably expressed BOC(flag) in C2C12 cells. For C–F, immunoprecipitation and immunoblotting are as described for A and B.
Because the existing antibodies to BOC are human-specific, assessment of an interaction between endogenous BOC and cadherins was performed by coimmunoprecipitation from lysates of the human myogenic cell line, RD. As was seen with CDO, cadherin and β-catenin coimmunoprecipitated with BOC (Fig. 1D). To test for a cadherin–BOC interaction in C2C12 cells, a derivative of this line that stably expresses a flag epitope-tagged form of human BOC [BOC(flag)] (16) was analyzed. As was observed with CDO, BOC was present in cadherin immunoprecipitates of lysates of these cells (Fig. 1E). Similarly, N- and M-cadherin each coimmunoprecipitated with BOC(flag) (Fig. 1F). Taken together, these data indicate that CDO and BOC form complexes with two promyogenic classical cadherins.
Colocalization of CDO and BOC with Cadherins in C2C12 Myoblasts.
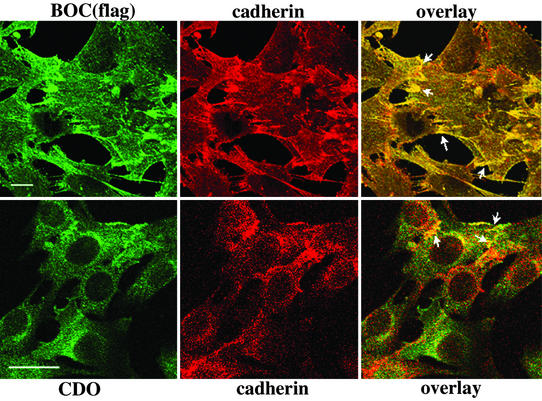
Colocalization of BOC and CDO with cadherins was assessed by confocal microscopy of C2C12 cell derivatives that stably express either BOC(flag) or an ≈2-fold excess of CDO, respectively (refs. 16 and 17; Fig. 2). Colocalization of BOC and cadherins is extensive, and sites of cell–cell contact and overlap display a strong enrichment for this colocalization. Overall colocalization of CDO and cadherins is not as pronounced as that of BOC and cadherins, but similar to BOC, CDO colocalized with cadherins at sites of cell–cell contact.
Figure 2.
Colocalization of CDO and BOC with cadherin at sites of cell–cell contact and overlap in C2C12 myoblasts. C2C12 cells were double-stained with anti-flag and anti-pan-cadherin antibodies (Upper) or anti-CDO and anti-pan-cadherin antibodies (Lower). The arrows indicate sites of cell–cell contact and overlap that display enriched colocalization of the indicated proteins. (Scale bar, 25 μm.)
Interactions Among CDO, BOC, and N-Cadherin Occur in cis.
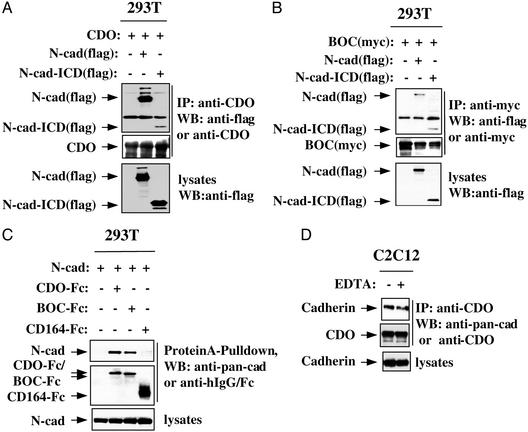
To investigate complex formation among CDO, BOC, and cadherins in more detail, transient transfections in 293T cells were performed. As expected, a flag-tagged form of N-cadherin coimmunoprecipitated with CDO and, independently, with a myc epitope-tagged form of BOC (Fig. 3 A and B). Similarly, an N-cadherin deletion mutant consisting only of its transmembrane and intracellular domains [N-cad-ICD(flag)] coimmunoprecipitated with CDO and BOC (Fig. 3 A and B). An N-cadherin deletion mutant containing only the extracellular and transmembrane domains also coimmunoprecipitated with both CDO and BOC (data not shown), but this deletion mutant was subject to degradation in transient transfections, and thus extracellular domain interactions were studied further with soluble ectodomain fusion proteins of CDO and BOC (refs. 16 and 17; Fig. 3C). Full-length N-cadherin was coexpressed with CDO-Fc, BOC-Fc, or (as a control) CD164-Fc, a fusion protein harboring the ectodomain of the promyogenic cell-surface sialomucin, CD164 (22). Cell lysates then were precipitated with protein A-Sepharose to bring down the Fc fusion proteins and immunoblotted with antibodies to cadherin. Full-length N-cadherin efficiently coprecipitated with CDO-Fc and BOC-Fc but not with CD164-Fc. The transient transfection data indicate that, as is seen for the interactions between CDO and BOC themselves (16), both the ectodomains and intracellular regions of N-cadherin associate with the respective regions of CDO and BOC. These results suggest that the interactions occur in a cis fashion and are consistent with the ability of N-cadherin to coimmunoprecipitate with CDO in low-density, proliferating cultures of C2C12 cells (Fig. 1A). Furthermore, coimmunoprecipitation of cadherins and CDO was not diminished when C2C12 cells were collected as a single-cell suspension in the presence of EDTA, conditions that preclude cadherin-based cell adhesion (Fig. 3D). Taken together, these data indicate that the interactions among CDO, BOC, and cadherins occur in cis.
Figure 3.
Association of N-cadherin with CDO and BOC via ectodomains and intracellular domains. (A and B) Association of full-length N-cadherin and the intracellular region of N-cadherin with CDO and BOC. 293T cells were transiently cotransfected with expression vectors for CDO (A), BOC(myc) (B), N-cadherin(flag) (A and B), or N-cadherin-intracellular domain [N-cad-ICD(flag)] (A and B). Immunoprecipitation and immunoblotting are as described for Fig. 1. (C) Association of N-cadherin with the ectodomains of CDO and BOC. 293T cells were transiently cotransfected with expression vectors for N-cadherin and CDO-Fc, BOC-Fc, or CD164-Fc. Cell lysates were precipitated with protein A-Sepharose, fractionated by SDS/PAGE, and immunoblotted with the indicated antibodies. Straight lysates were also probed with anti-cadherin antibodies. (D) Cadherin-based adhesion is not required for association of cadherins with CDO. C2C12 cells were cultured on plastic dishes or as a single-cell suspension in the presence of EDTA. Immunoprecipitation and immunoblotting are as described for Fig. 1.
A CDO Deletion Mutant Deficient in Its Ability to Associate with N-Cadherin Interferes with C2C12 Cell Differentiation.
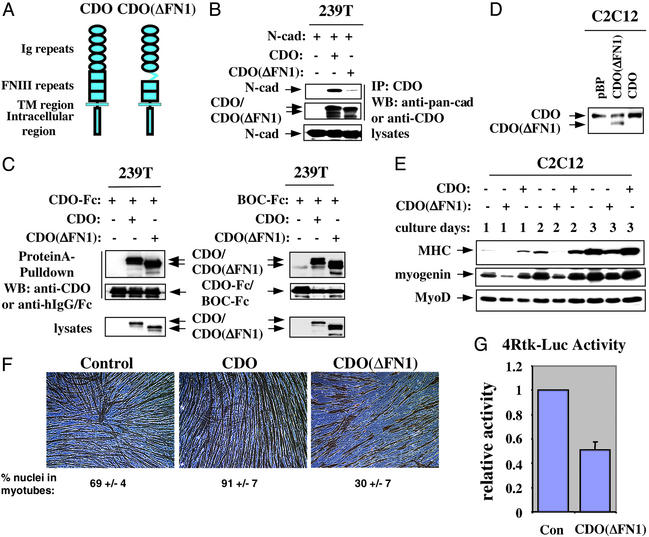
It has been shown that overexpression of CDO or BOC in C2C12 cells accelerates and enhances differentiation, and that CDO is a likely signaling component of a CDO/BOC receptor (16, 17). In addition to forming heteromeric complexes with each other (16), CDO and BOC each form complexes with themselves (J.-S. K., unpublished observations). To determine whether the interaction between cadherins and CDO plays a functional role in myogenesis, we sought to identify a CDO deletion mutant that is selectively deficient in its ability to associate with cadherins. A series of CDO deletion mutants that lack each individual Ig and fibronectin type III repeat from the CDO ectodomain were constructed and tested for their ability, relative to full-length CDO, to coimmunoprecipitate with N-cadherin, CDO, or BOC. The deletion mutant that lacks the first fibronectin type III repeat [CDO(ΔFN1); Fig. 4A] displayed the desired property. Precipitation of lysates of transiently transfected 293T cells with antibodies to the intracellular region of CDO revealed that CDO(ΔFN1) brought down significantly less N-cadherin than did full-length CDO (Fig. 4B). In contrast, when coexpressed with CDO-Fc or BOC-Fc, equivalent levels of full-length CDO and CDO(ΔFN1) were brought down by protein A-Sepharose (Fig. 4C). Thus, CDO(ΔFN1) is deficient in interacting with N-cadherin but not in interacting with CDO or BOC. Whether CDO(ΔFN1) is also deficient in interacting with M-cadherin remains to be established, although this seems likely. CDO(ΔFN1) then was stably expressed in C2C12 myoblasts, and the cells [C2C12/CDO(ΔFN1)] were compared with control (C2C12/pBabePuro) and CDO-overexpressing (C2C12/CDO) cells for their ability to differentiate.
Figure 4.
A CDO deletion mutant deficient in associating with N-cadherin interferes with differentiation of C2C12 cells. (A) Schematic diagram of CDO and CDO(ΔFN1). TM, transmembrane. (B) CDO(ΔFN1) is deficient in association with N-cadherin. 293T cells were transiently cotransfected with expression vectors for N-cadherin and CDO or CDO(ΔFN1). Immunoprecipitation (IP) and immunoblotting (WB) are as described for Fig. 1. (C) CDO(ΔFN1) associates normally with CDO-Fc and BOC-Fc. 293T cells were transiently cotransfected with expression vectors for CDO(ΔFN1) and CDO-Fc or BOC-Fc. Cell lysates were precipitated with protein A-Sepharose, fractionated by SDS/PAGE, and immunoblotted with the indicated antibodies. Straight lysates were also probed with anti-CDO antibodies. (D) Stable expression of full-length CDO and CDO(ΔFN1) in C2C12 cells. Western blot (WB) analysis of C2C12 cells infected with pBabePuro-based retroviruses. pBP, cells infected with control retrovirus lacking a cDNA insert; CDO(ΔFN1) and CDO, cells infected with retroviruses harboring the respective mutant and full-length Cdo cDNAs. The blot was probed with antibodies to the CDO intracellular region. (E) Western blot analysis of muscle-specific proteins expressed by the different C2C12 cell infectants cultured under differentiation-inducing conditions for the indicated times. Blots were probed with antibodies to the indicated proteins. (F) Photomicrographs of C2C12 cell infectants cultured in differentiation medium for 3 days, fixed, and stained with an antibody to MHC. The percentage of nuclei in MHC-positive myotubes is indicated. Values are means of triplicate determinations ± 1 SD performed three times. (G) Muscle-specific reporter gene activity. C2C12 cells were transfected in growth medium with the 4Rtk-Luc reporter construct (which is driven by a tetramerized myogenic E-box) and either a control (con) or CDO(ΔFN1)-containing expression vector and then assessed for normalized luciferase activity after 24 h in differentiation medium. Values are means of independent triplicate determinations performed four times.
Western blot analysis showed that C2C12/CDO(ΔFN1) cells had approximately equivalent levels of endogenous CDO and CDO(ΔFN1), whereas the C2C12/CDO cells produced ≈2-fold more CDO than the control cells (Fig. 4D). Expression of CDO(ΔFN1) had no discernable effect on the morphology or proliferation of C2C12 cells cultured in growth medium (data not shown). However, when cultured under differentiation-inducing conditions, expression of the differentiation markers myogenin and MHC was delayed and decreased in C2C12/CDO(ΔFN1) cells relative to control C2C12/pBabePuro cells (Fig. 4E). As reported (17), C2C12/CDO cells displayed accelerated expression of these markers (Fig. 4E). Furthermore, C2C12/CDO(ΔFN1) cells formed shorter, irregularly shaped myotubes with fewer nuclei when compared with control cells; again, as reported (17), the C2C12/CDO cells showed an exaggerated differentiation response, forming larger myotubes with more nuclei than control cells (Fig. 4F). Finally, expression of a transiently transfected, myogenic basic-helix–loop–helix factor-dependent reporter construct (4Rtk-luc) was reduced by ≈50% in CDO(ΔFN1)-expressing C2C12 cells relative to control cells (Fig. 4G). Therefore, as assessed by biochemical, morphological, and transient reporter gene assays, expression of CDO(ΔFN1) interfered with myogenic differentiation. These results suggest that the interaction between CDO and cadherins is important for the promyogenic properties of CDO, although one cannot exclude the possibility that the CDO(ΔFN1) mutant interferes with myogenesis through an inability to interact with an additional, noncadherin, class of protein that is also functionally involved in this process. It should be noted, however, that a different CDO mutant, CDO(ΔIg5), that lacks the fifth Ig domain is active in promoting differentiation of C2C12 cells (data not shown). Because Ig5 is the largest single repeat in the CDO ectodomain (15), this result indicates that CDO can be “shortened” by at least one extracellular repeat domain without significant effect. Thus, CDO(ΔFN1) does not inhibit myogenesis simply by depriving CDO of sufficient “length” to reach other putative binding proteins.
Discussion
The results presented here provide evidence for a functional interaction between two major classes of cell-surface protein implicated in positive regulation of myogenesis via cell–cell contact; i.e., the Ig superfamily members CDO and BOC form higher-order structures with cadherin–β-catenin complexes at sites of cell–cell contact. Our previous work implicated CDO and BOC as directly interacting components of a receptor complex, with BOC dependent on CDO for its promyogenic activity, and suggested that CDO plays a role in signaling (16). We show here that expression of a deletion mutant of CDO that is unable to associate efficiently with N-cadherin interferes with myogenic differentiation, suggesting that interaction with cadherins may be required for CDO to stimulate myogenesis. One possible explanation for this is that interaction with cadherins brings CDO/BOC receptors to sites of adhesive cell contact so they can interact with putative downstream targets that may also be found at these sites. CDO and BOC do not have demonstrable adhesive activity, and thus their association with cadherins provides a mechanism for localization of their signaling function at sites of cell–cell adhesion. Furthermore, because myoblast contact and recognition precede myoblast fusion, a process stimulated by CDO and BOC (16, 17), it is tempting to speculate that signaling activities from such complexes will be involved in the changes in cytoskeletal dynamics that must occur during morphological differentiation. An alternative but not mutually exclusive mechanism is that association with CDO and BOC might alter the adhesive properties of cadherins in a fashion that enhances their promyogenic potential. Although this cannot be ruled out, it seems less likely, because CDO and BOC stimulate myogenesis without obvious changes in the ability of myoblast cell lines to adhere or aggregate with each other (J.-S.K. and R.S.K., unpublished observations).
Genetic proof of a role in vertebrate muscle development for the cadherins has been elusive most likely because of redundant or compensatory functions between the several different cadherins expressed during myogenesis (23, 24). In contrast, mice and primary myoblasts that lack CDO via targeted mutagenesis display delayed myogenesis (F. Cole and R.S.K, unpublished data). The current studies strongly suggest that some of the promyogenic effect of CDO depends on an interaction with cadherins. Because CDO and BOC can interact with both N- and M- (and perhaps other) cadherins, it is possible that some of the apparent redundancy among cadherins in myogenesis could be explained by a shared ability to interact with CDO and BOC. The observation that CDO associated with N-cadherin in proliferating myoblasts and throughout differentiation while it associated with M-cadherin only transiently during differentiation suggests that functional distinctions between these interactions may also exist. Finally, it is worth noting that N-cadherin, CDO, and BOC are coexpressed in additional cell types during development such as neural precursors and precartilaginous, condensing mesenchymal cells (18, 19, 25, 26). The interactions described here may play a developmental role in these cell types as well.
Acknowledgments
We thank M. Frasch, D. Coletti, and L. Ossowski for critiquing the manuscript and J. Hemperly (Becton Dickinson Technologies, Research Triangle Park, NC) and R. Hazan (Mount Sinai School of Medicine) for full-length and mutant N-cadherin expression vectors. This work was supported by National Institutes of Health Grants AR46207 and CA59474 (to R.S.K.). J.-S.K. was supported by the T. J. Martell Foundation. Confocal microscopy was performed at the Mount Sinai School of Medicine–Microscopy Shared Resource Facility, supported by NIH/National Cancer Institute Shared Resources Grant 1R24CA095823-01, National Science Foundation Major Research Instrumentation Grant DBI-9724504, and NIH Shared Instrumentation Grant 1S10RR09145-01.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fagotto F, Gumbiner B M. Dev Biol. 1996;180:445–454. doi: 10.1006/dbio.1996.0318. [DOI] [PubMed] [Google Scholar]
- 2.Yap A S, Kovacs E M. J Cell Biol. 2003;160:11–16. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludolph D C, Konieczny S F. FASEB J. 1995;9:1595–1604. doi: 10.1096/fasebj.9.15.8529839. [DOI] [PubMed] [Google Scholar]
- 4.Molkentin J D, Olson E N. Proc Natl Acad Sci USA. 1996;93:9366–9373. doi: 10.1073/pnas.93.18.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cossu G, Kelly R, Di Donna S, Vivarelli E, Buckingham M. Proc Natl Acad Sci USA. 1995;92:2254–2258. doi: 10.1073/pnas.92.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurdon J B, Tiller E, Roberts J, Kato K. Curr Biol. 1993;3:1–11. doi: 10.1016/0960-9822(93)90139-f. [DOI] [PubMed] [Google Scholar]
- 7.Skerjanc I S, Slack R S, McBurney M W. Mol Cell Biol. 1994;14:8541–8549. doi: 10.1128/mcb.14.12.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holt C E, Lemaire P, Gurdon J B. Proc Natl Acad Sci USA. 1994;91:10844–10848. doi: 10.1073/pnas.91.23.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George-Weinstein M, Gerhart J, Blitz J, Simak E, Knudsen K A. Dev Biol. 1997;185:14–24. doi: 10.1006/dbio.1997.8542. [DOI] [PubMed] [Google Scholar]
- 10.Knudsen K A, Myers L, McElwee S A. Exp Cell Res. 1990;188:175–184. doi: 10.1016/0014-4827(90)90157-6. [DOI] [PubMed] [Google Scholar]
- 11.Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. J Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goichberg P, Geiger B. Mol Biol Cell. 1998;9:3119–3131. doi: 10.1091/mbc.9.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg P, Esni F, Sjodin A, Larue L, Carlsson L, Gullberg D, Takeichi M, Kemler R, Semb H. Dev Biol. 1997;187:55–70. doi: 10.1006/dbio.1997.8602. [DOI] [PubMed] [Google Scholar]
- 14.Zeschnigk M, Kozian D, Kuch C, Schmoll M, Starzinski-Powitz A. J Cell Sci. 1995;108:2973–2981. doi: 10.1242/jcs.108.9.2973. [DOI] [PubMed] [Google Scholar]
- 15.Kang J S, Gao M, Feinleib J L, Cotter P D, Guadagno S N, Krauss R S. J Cell Biol. 1997;138:203–213. doi: 10.1083/jcb.138.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang J-S, Mulieri P J, Hu Y, Taliana L, Krauss R S. EMBO J. 2002;21:114–124. doi: 10.1093/emboj/21.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang J-S, Mulieri P J, Miller C, Sassoon D A, Krauss R S. J Cell Biol. 1998;143:403–413. doi: 10.1083/jcb.143.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulieri P J, Okada A, Sassoon D A, McConnell S K, Krauss R S. Dev Dyn. 2000;219:40–49. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1032>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Mulieri P M, Kang J-S, Sassoon D A, Krauss R S. Dev Dyn. 2002;223:379–388. doi: 10.1002/dvdy.10063. [DOI] [PubMed] [Google Scholar]
- 20.Radice G L, Rayburn H, Matsunami H, Knudsen K A, Takeichi M, Hynes R O. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 21.Moore R, Walsh F S. Development (Cambridge, UK) 1993;117:1409–1420. doi: 10.1242/dev.117.4.1409. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y-N, Kang J-S, Krauss R S. Mol Cell Biol. 2001;21:7696–7706. doi: 10.1128/MCB.21.22.7696-7706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlton C A, Mohler W A, Radice G L, Hynes R O, Blau H M. J Cell Biol. 1997;138:331–336. doi: 10.1083/jcb.138.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollnagel A, Grund C, Franke W W, Arnold H-H. Mol Cell Biol. 2002;22:4760–4770. doi: 10.1128/MCB.22.13.4760-4770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberlander S A, Tuan R S. Development (Cambridge, UK) 1994;120:177–187. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 26.Redies C, Takeichi M. Dev Dyn. 1993;197:26–39. doi: 10.1002/aja.1001970104. [DOI] [PubMed] [Google Scholar]