Abstract
Phosphorylation of inositol phospholipids plays a key role in cellular regulation via the generation of intracellular second messengers. In addition, it represents a mechanism to regulate interactions of the lipid bilayer with proteins and protein scaffolds involved in vesicle budding, cytoskeletal organization, and signaling. Generation of phosphatidylinositol 4-phosphate [PI(4)P] from phosphatidylinositol (PI) is an important step in this metabolic pathway because PI(4)P is a precursor of other important phosphoinositides and has protein binding properties of its own. We report here that a PI 4-kinase (PI4K) activity previously reported on synaptic vesicles is accounted for by the α isoform of the recently characterized type II PI4K (PI4KII) family. PI4KIIα, which also accounts for the bulk of PI4K activity in brain extracts, is concentrated at synapses and in the region of the Golgi complex in neuronal perikarya. Our results provide new evidence for the occurrence of a cycle of phosphoinositide synthesis and hydrolysis nested within the exo–endocytic cycle of synaptic vesicles and point to PI4KIIα as a critical player in this cycle.
Neurotransmitter-containing synaptic vesicles release their content by exocytosis. After this reaction their membranes are rapidly recaptured by endocytosis and recycled. At least a major fraction of this recycling traffic occurs by clathrin-mediated endocytosis (1, 2). The proper progression of membrane in this pathway implies mechanisms that impart direction and prevent retrograde membrane flow. Studies on a variety of vesicle transport reactions, including the synaptic vesicle cycle, have identified GTPases as critical regulator of vectoriality (3, 4). In addition a role of phosphoinositide synthesis and hydrolysis in this regulation has been proposed (5, 6).
An important first piece of evidence supporting a function of phosphoinositides in the recycling of synaptic vesicles was the identification of the polyphosphoinositide phosphatase synaptojanin 1 as a protein neighbor of dynamin (7). Dynamin, a GTPase, plays a critical role in the fission of endocytic vesicles from the plasma membrane (8). Investigations of synaptojanin in various species provided functional and genetic evidence for its function in the endocytic pathway at the synapse (9–12). These studies have been convergent with the identification and characterization of binding sites for phosphoinositides, primarily phosphatidylinositol (PI) 4,5-bisphosphate [PI(4,5)P2], in endocytic clathrin adaptors and in their accessory factors (13–16). PI(4,5)P2 is selectively concentrated in the plasma membrane (17), possibly explaining why the assembly of clathrin coats on synaptic vesicle membranes occurs only after exocytosis. Conversely, synaptojanin may contribute to clathrin adaptors shedding immediately after the dynamin-dependent step, i.e., the fission reaction of endocytosis. Disruption of synaptojanin function results in delayed uncoating of clathrin-coated vesicles and in delayed reentry of newly internalized synaptic vesicle membranes into a functional synaptic vesicle pool (9, 12). The degradation of PI(4,5)P2 in the endocytic pathway must be compensated by PI(4,5)P2 generation on the plasma membrane. PIPKIγ, i.e., a PI(4)P 5-kinase that may account for this compensatory synthesis, is concentrated in nerve terminals and is thought to act selectively on the plasma membrane or on plasma membrane-derived membrane compartments from which clathrin-coated vesicles originate (18).
An open question is the site of synthesis of the PI(4)P that functions as substrate for PI(4)P 5-kinase action as well as the identity of the corresponding PI 4-kinase(s). PI 4-kinase activity had been detected on synaptic vesicles (19) but its molecular identity has remained unknown. Because of its tight membrane association, this kinase is unlikely to be accounted for by the homologues of Saccharomyces cerevisiae Stt4 and Pik1 (20), the only two classes of PI 4-kinases (collectively referred as type III PI 4-kinases) molecularly characterized until recently (21–24). In 2001, a protein family that is responsible for an important fraction of PI 4-kinase activity in a variety of tissues, and in particular for membrane-bound activity, was identified (25, 26). This family has now been shown to comprise two closely related proteins in mammals, type IIα and type IIβ PI 4-kinases (PI4KIIα and PI4KIIβ), respectively (25–28). [An activity originally identified as a type I PI 4-kinase was subsequently identified as PI 3-kinase (24).]
Here we show that PI4KIIα accounts for the majority of PI 4-kinase activity in brain and that this enzyme is concentrated both at the synapse and in the region of the Golgi complex in neuronal perikarya. A pool of PI4KIIα copurifies with synaptic vesicles and is responsible for the PI 4-kinase activity present on synaptic vesicle membranes. We suggest that generation of PI(4)P by this enzyme on synaptic vesicles may be the first step in the resynthesis of PI(4,5)P2 after the action of synaptojanin.
Materials and Methods
PI Kinase Assay.
In vitro lipid phosphorylation assays on cytosol, immunoprecipitated kinases, or recombinant purified kinases were performed as described with minor modifications (18). In a typical assay, the reaction mixture (50 μl) contained 30 mM Hepes (pH 7.4), 100 mM KCl, 2 mM MgCl2, 1 mM EDTA, 10–20 μg of substrate lipid [synthetic short chain phosphoinositides (Echelon, Salt Lake City) or a brain-derived phosphoinositide mixture (Sigma P-6023)], and 0.2% Triton X-100. The reaction was initiated by addition of 40 μM ATP (10 μCi of [γ-32P]ATP per assay; 1 μCi = 37 kBq) and performed for 10 min at 37°C. Reaction products were extracted and subjected to TLC on silica gel 60 plates and developed by using chloroform/acetone/methanol/acetic acid/water (64:30:24:32:14) as a solvent. 32P-labeled phosphoinositides were visualized by autoradiography. Deacylation of phosphoinositides and HPLC were performed as described (18). Although the presence of Triton X-100 drastically stimulated PI4KIIα activity, it inhibited PI(4)P 5-kinase activity. Unless otherwise indicated, all essays were performed in the presence of Triton X-100.
Antibodies.
Polyclonal Abs directed against rat PI4KIIα were obtained by immunization of rabbits with purified Escherichia coli-expressed full-length rat GST-PI4KIIα. Polyclonal Abs directed against mouse PI4KIIβ were obtained with a synthetic peptide corresponding to residues 14–35 of mouse PI4KIIβ as the immunogen. This sequence is unique to PI4KIIβ and is conserved in human and mouse (81.8% identity). Abs directed against synaptobrevin 2 (mouse monoclonal Cl 69.1) were a kind gift of R. Jahn (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany). mAbs to amphiphysin 1 and synaptojanin 1 and polyclonal Abs to synaptophysin and PIPKIγ were from our laboratory. Antiactin Abs and monoclonal anti-Myc Ab were purchased from Sigma, whereas polyclonal anti-Myc Ab was purchased from Upstate Biotechnology (Lake Placid, NY).
Immunoisolation and Electron Microscopy.
Monoclonal synaptobrevin 2 Abs Cl 69.1 were conjugated to Eupergit C1Z methacrylate microbeads (1-μm mean diameter; Röhm Pharmaceuticals, Darmstadt, Germany) as described (29). LP2, a crude synaptic vesicle fraction (30), was incubated with beads for 2 h at 4°C with constant rotation. After incubation, the beads were sedimented by centrifugation at 9,500 × g for 1 min and washed three times with PBS. For electron microscopy, washing steps were omitted and beads pellets were directly fixed and processed as described (31).
Miscellaneous Procedures.
GST fusion PI4KIIα was subcloned from Myc-tag rat PI4KIIα, which was a generous gift from J. Albanesi (University of Texas, Southwestern Medical Center, Dallas), expressed and purified from E. coli. Cell culture, transfection, tissue homogenization, SDS/PAGE, immunoblotting, Triton X-100 extractions, and immunoprecipitations were performed by standard procedures. Subcellular fractionations of rat brain were performed as reported by Huttner et al. (30). Immunostaining was performed on rat brain (fixed by transcardial perfusion) frozen sections as described (32).
Results
The goal of this study was to determine the identity of a PI 4-kinase activity present on synaptic vesicles. PI 4-kinases fall into two classes, defined as type II and type III. Type III PI 4-kinases are inhibited by wortmannin (IC50 ≈ 50–100 nM) and high (>1 mM) concentrations of adenosine, whereas type II PI 4-kinases are sensitive to low doses of adenosine and are insensitive to wortmannin. Both classes of kinases are more active in the presence of Triton X-100 (24, 33). We examined the properties of PI 4-kinase activity in a crude synaptic vesicle fraction, the LP2 fraction described by Huttner et al. (30), in the presence of Triton X-100. The kinase activity was insensitive to wortmannin (10 μM) and strongly inhibited by 0.5 mM adenosine, consistent with a type II activity (data not shown).
PI4KIIα and PI4KIIβ have similar primary structures but differ in tissue and subcellular distribution. PI4KIIα is exclusively membrane-associated, whereas PI4KIIβ is found in both particulate and soluble fractions (27, 28). Based on Northern blot analysis (26, 27), the isoform predominantly expressed in brain is PI4KIIα. Thus, we concentrated our analysis on PI4KIIα.
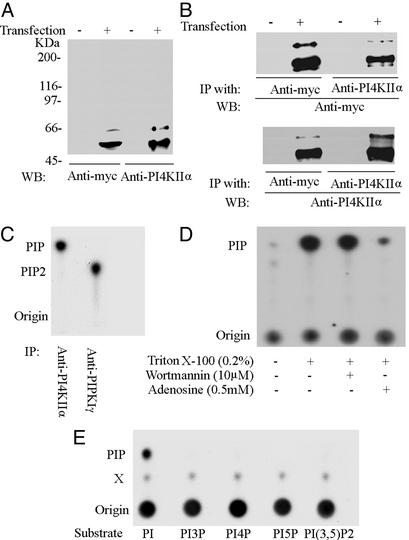
Rat PI4KIIα was expressed in E. coli as a GST fusion protein and purified from Triton X-100 lysates of bacteria by glutathione-affinity purification. The purified protein, which had a typical type II PI 4-kinase activity (data not shown), was injected into rabbits to obtain polyclonal Abs. The affinity-purified anti-PI4KIIα Abs recognized primarily a major band at ≈55 kDa in Western blots of HEK293 cells transfected with Myc-tagged rat PI4KIIα, in agreement with the expected molecular mass of the kinase (Fig. 1A). A minor slower migrating band at ≈66 kDa was also labeled by the Abs, although neither band was detected in untransfected cells. The same two bands were detected by an anti-Myc Ab (Fig. 1A). Furthermore, anti-Myc and anti-PI4KIIα Western blots of immunoprecipitates generated from transfected HEK293 cells by anti-Myc or anti-PI4KIIα Abs, respectively, revealed the same two bands (Fig. 1B). These results confirm the reactivity and specificity of anti-PI4KIIα Abs. The minor band at ≈66 kDa may reflect a posttranslational modification of the protein, which was not further addressed in this study.
Figure 1.
Characterization of anti-PI4KIIα Abs. (A) HEK293 cells were transiently transfected with myc-tagged PI4KIIα and analyzed by Western blotting. Both anti-myc and anti-PI4KIIα Abs recognized a major band at ≈55 kDa and a minor band at ≈66 kDa in transfected cells. (B) Triton X-100 extracts of transfected and mock-transfected cells were immunoprecipitated (IP) with either anti-myc or anti-PI4KIIα rabbit polyclonal Abs. Immunoprecipitates were then subjected to Western blotting (WB) with either mouse monoclonal anti-myc Abs or biotin-labeled anti-PI4KIIα Abs followed by streptavidin. (C) A Triton X-100 extract of rat brain was immunoprecipitated with anti-PI4KIIα or anti-PIPKIγ Abs. The immunoprecipitates (IP) were subjected to a PI kinase assay, and the products were analyzed by TLC and autoradiography. (D) Anti-PI4KIIα immunoprecipitates generated from rat brain Triton X-100 extract were analyzed by the PI kinase assay in the presence of the indicated compounds. (E) TLC analysis of reaction products generated by anti-PI4KIIα immunoprecipitates from Triton X-100 brain extracts in the presence of several synthetic PI substrates. X denotes an unidentified spot.
We next investigated whether the affinity-purified Abs could immunoprecipitate endogenous PI 4-kinase activity from a Triton X-100 extract of rat brain postnuclear supernatant. Anti-PI4KIIα immunoprecipitates, and anti-PIPKIγ immunoprecipitates as a controls, were incubated with liposomes composed of a brain-derived phosphoinositide mixture and [γ-32P]ATP. The resulting reaction products were analyzed by TLC. Anti-PI4KIIα Abs pulled down a PI kinase activity, whereas anti-PIPKIγ immunoprecipitated a PIP kinase activity as expected (Fig. 1C). Furthermore, the activity of immunoprecipitated PI 4-kinase activity was strongly stimulated by the presence of Triton X-100 in the reaction, inhibited by 0.5 mM adenosine, and insensitive to 10 μM wortmannin (Fig. 1D), as typically expected for a type II activity (24). Incubation of the immunoprecipitates with synthetic inositol phospholipids and [γ-32P]ATP further confirmed the high selectivity of this kinase for the conversion of PI to PIP (Fig. 1E).
Western blot analysis of several mouse tissues with anti-PI4KIIα Abs revealed prominent immunoreactivity at the expected molecular mass in brain, liver, and testis and at much lower levels in other tissues (Fig. 2A). An abundant faster-migrating band (≈45 kDa) present in muscle was not further characterized. Another Ab raised to an amino acid sequence specific of PI4KIIβ, which selectively recognized PI4KIIβ, labeled bands at a similar molecular mass in kidney, liver, and testis but not in brain (Fig. 2A). Although it remains to be established whether our anti-PI4KIIα Ab, which was raised against the full-length protein, also recognizes PI4KIIβ, and may account for some of the immunoreactivity seen in nonneuronal tissues, the absence of PI4KIIβ-specific immunoreactivity in brain strongly suggests that PI4KIIα is the main type II PI 4-kinase present in neurons. When immunoprecipitates from Triton X-100 extracts of brain postnuclear supernatants were generated with excess anti-PI4KIIα Abs, analysis of the supernatants revealed a dramatic reduction of PI kinase activity, which correlated with the depletion of PI4KIIα (Fig. 2B). The level in the supernatant of a control protein, amphiphysin, was not affected by the depletion of PI4KIIα. This result demonstrates that the predominant PI kinase activity in brain is a PI 4-kinase activity and, more specifically a type II activity. It remains possible that type III PI 4-kinases may have an important role in brain but require appropriate stimulation. For example, there is evidence that PI 4-kinase type IIIβ, the brain homologue of yeast Pik1, is stimulated by frequenin (34–37).
Figure 2.
PI4KIIα accounts for the majority of PI(4)P-synthesizing activity in brain Triton X-100-soluble extracts. (A) Western blot analysis of total homogenates from mouse tissues with Abs directed against the proteins indicated. PI4KIIα is by far the predominant PI4KII expressed in brain. (B) Immunodepletion of PI4PKIIα from rat brain Triton X-100 extracts results in a dramatic reduction of PI 4-kinase activity. (Upper) Western blots for PI4KIIα and for the control protein amphiphysin 1 (Amph) in the starting material (SM), as well as in the corresponding extracts after immunoprecipitation with anti-PI4KIIα Ab, or with control IgGs. (Lower) The immunodepleted extract has lost nearly all PI kinase activity as revealed by TLC. Under our assay conditions, the bulk of PIP generated by the starting extract was found to be PI(4)P.
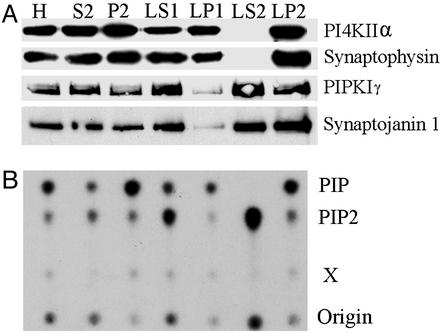
We next investigated the subcellular distribution of PI4KIIα immunoreactivity in brain, with emphasis on its potential localization on synaptic vesicles. The distribution of the kinase in rat brain subcellular fractions (30) roughly paralleled the distribution of the synaptic vesicle marker synaptophysin (38). It was slightly enriched in P2 (synaptosomal fraction) over the homogenate, absent from LS2 (the soluble fraction of synaptosomes), and enriched in LP2, the crude synaptic vesicle fraction (Fig. 3A). This distribution, which agrees with the reported 100% membrane association of PI4KIIα in nonneuronal cells, was quite different from that of synaptojanin and PIPKIγ, which were also present in the soluble fraction LS2. The subcellular fractionation profile of PI4KIIα immunoreactivity was in good agreement with the property of the various fractions to generate labeled PIP during an incubation with a brain phosphoinositide mixture and [γ-32P]ATP (Fig. 3B). The PIP spot generated by LP2 (Fig. 3B) was further analyzed by deacylation followed by HPLC and shown to be represented by PI(4)P. In all fractions, the generation of the PIP spot was stimulated by Triton X-100 and was insensitive to 10 μM wortmannin (data not shown), further confirming that PI4KIIα is the dominant PI 4-kinase activity in brain. In contrast, the greatest accumulation of PIP2 was observed in the LS2 fraction, which did not accumulate PIP (Fig. 3B). It has been shown that PIP2 production in this fraction is accounted for primarily by PIPKIγ and that the interaction of PIPKIγ with membranes is subjected to regulation, including regulation by guanyl nucleotides (M. Krauss, M. Kinuta, M.R.W., P.D.C., K. Takei, and V. Haucke, unpublished observations).
Figure 3.
Subcellular distribution of PI4KIIα and lipid kinase activities. (A) Western blot analysis of subcellular fractions from a rat brain homogenate (H) with Abs directed against PI4KIIα and other neuronal proteins. Fractions were prepared according to Huttner et al. (30). The P2 fraction represents crude synaptosomes, which after lysis yield quickly and slowly sedimenting membrane fractions denoted LP1 and LP2, respectively. The LP2 fraction, which is highly enriched in synaptic vesicles (crude synaptic vesicle fraction), also contains a large pool of PI4KIIα. Note the absence of synaptophysin (a synaptic vesicle membrane marker) and PI4KIIα in the LS2 fraction, which contains only soluble proteins. (B) TLC analysis of 32P incorporation into PIP and PIP2 after incubation of the fractions with a brain phosphoinositide mixture, [32P]ATP, and Triton X-100. X denotes an unidentified spot.
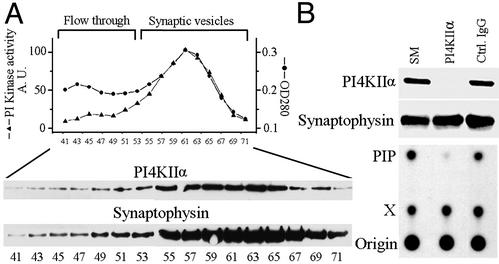
We further investigated the distribution of PI4KIIα in subfractions of LP2. LP2 was fractionated on a sucrose gradient, and a pooled synaptic vesicle-enriched fraction obtained from the gradient was subjected to controlled-pore glass chromatography (CPG). CPG yields two major protein peaks, the second peak being represented by small homogeneously sized vesicles, primarily synaptic vesicles, whereas the first peak (flow-through) is heterogeneous and contains larger membrane fragments (30). Western blotting of the CPG fractions revealed a coenrichment of PI4KIIα with the synaptic vesicle marker synaptophysin (38) in the second protein peak (Fig. 4A), which also coincided with the major peak of PI kinase activity. This activity was wortmannin-insensitive (maximum concentration tested = 10 μM) and adenosine-sensitive (0.5 mM), and its product was determined to be PI(4)P by deacylation followed by HPLC analysis (data not shown). Furthermore, immunodepletion of PI4KIIα from a Triton X-100 extract of the pooled synaptic vesicle fractions resulted in a nearly complete depletion of PI 4-kinase activity (Fig. 4B). These results indicate that PI4KIIα accounts for the bulk of the PI 4-kinase activity present on synaptic vesicles. The presence of a low amount of PI4KIIα, together with a corresponding small peak of PI kinase activity, in the flow-through fractions indicates that this kinase is not restricted to synaptic vesicles, consistent with its presence in the Golgi complex area (see next section and ref. 28) and in other membranes of the endocytic pathway (27, 28). This activity as well could be immunodepleted by anti-PI4KIIα Abs.
Figure 4.
PI4KIIα accounts for the great majority of the PI 4-kinase activity present on synaptic vesicles. (A) CPG leading to a highly purified synaptic vesicle fraction (30). (Upper) Protein content (A280 readings) and the PI kinase activity (TLC analysis) of the fractions in arbitrary units. (Lower) Levels of PI4KIIα and synaptophysin in the same fractions (Western blotting). (B) Synaptic vesicle-containing fractions were pooled, solubilized in Triton X-100 (SM, starting material), and subjected to immunoprecipitation with anti-PI4KIIα Abs or control IgGs. (Upper) Depletion of PI4KIIα, but not of the control protein synaptophysin, from the extract as revealed by Western blotting. (Lower) Drastic decrease in PI kinase activity in the PI4KIIα-depleted extract. X denotes an unidentified spot.
To complement these results, we performed organelle immunoisolation experiments. LP2 fractions were used as starting material. In addition to synaptic vesicles, LP2 fractions contain other small vesicles and medium-sized vesicular structures such as endosomes and plasma membrane fragments. Methacrylate beads coated with Ab directed against the synaptic protein synaptobrevin 2/VAMP2 (39) nearly completely depleted the starting fraction of synaptobrevin 2 (data not shown) and synaptophysin and were heavily decorated by synaptic vesicles when observed by electron microscopy. These beads also pelleted a large pool of PI4KIIα (Fig. 5 A and B). A fraction of synaptobrevin 2, synaptophysin, and PI4KIIα was recovered in the pelleted fraction of control beads as well (Fig. 5B). However, the surfaces of these beads were devoid of associated vesicles (Fig. 5A). The partial pelleting synaptic proteins with control beads could be explained by some membrane aggregation and spontaneous fusion of vesicles into larger membrane structures (unpublished observations) during immunoisolation, thus leading their nonspecific sedimentation (data not shown). Collectively, these results prove the existence of a PI 4-kinase activity tightly associated with synaptic vesicles and strongly suggest that PI4KIIα accounts for this activity.
Figure 5.
Recovery of PI4KIIα and PI 4-kinase activity on synaptobrevin/VAMP2-positive organelles by immunoisolation. Anti-synaptobrevin 2/VAMP2 Abs or control IgGs were conjugated to methacrylate beads and used for organelle immunoisolation from an LP2 fraction. (A) Electron microscopy of the material bound to control beads (Left) and to the anti-synaptobrevin 2/VAMP2 beads (Right). Arrows point to synaptic vesicles, which are not present on the control beads. (B Left) A Western blot analysis of the starting material (LP2) and of the material bound (B) and unbound (U) to the beads. Note that the nearly complete recovery of synaptophysin on the beads correlates with a similar recovery of PI4KIIα. (Right) Similar results were obtained for the PI kinase activity. The partial recovery of synaptic vesicle antigens and PI4KIIα in control IgG bead pellets is due to nonspecific sedimentation of larger membrane fragments present in LP2.
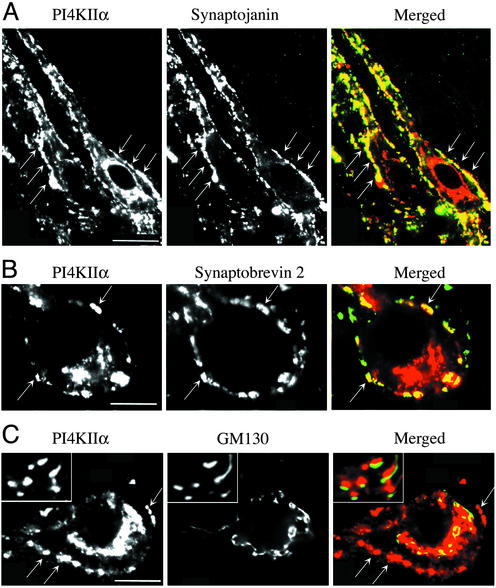
Finally, the localization of PI4KIIα in brain was investigated by immunofluorescence on rat brain frozen sections. In all brain regions examined two major patterns of immunoreactivity were observed. One pattern was coincident with the fluorescence produced by Abs directed against nerve terminal markers, such as synaptobrevin 2/VAMP2 and synaptojanin 1 (Fig. 6 A and B), consistent with a concentration of PI4KIIα on synaptic vesicle membranes. The other predominant pattern was represented by a Golgi complex-like staining (Fig. 6 A and B). Double immunofluorescence for the cis-Golgi marker GM130 (40) revealed that PI4KIIα immunoreactivity was not precisely overlapping with this marker, yet it was always tightly apposed to GM130-positive structures (Fig. 6C and Insets).
Figure 6.
Concentration of PI4KIIα at synapses and in the Golgi complex area. Rat brainstem frozen sections were stained by double immunofluorescence. (A and B) PI4KIIα colocalizes with synaptojanin 1 and synaptobrevin 2/VAMP2 at synapses, which appear as bright fluorescence puncta outlining perikarya and dendrites. (C) Bright perinuclear PI4KIIα immunofluorescence is located in close apposition (see Insets) to elements immunoreactive for the cis-Golgi complex marker GM130. Small arrows point to synapses. In the “merged” images, PI4KIIα immunoreactivity appears in red. (Bar = 30 μm in A and 10 μm in B and C.)
Discussion
Our results confirm the existence of a PI 4-kinase activity on synaptic vesicles (19) and identify the enzyme responsible for this activity as PI4KIIα. We further show that this enzyme accounts for the bulk of the PI 4-kinase activity in a brain extract and is present at high concentration at synapses. Although it remains possible that the predominance of this activity in an extract may be accounted for by the assay conditions that favor type II kinase activity and by the dependence of other PI 4-kinases on activation mechanisms disrupted by homogenization, our findings are consistent with a major role of PI4KII, and more specifically of PI4KIIα, in brain phosphoinositide metabolism.
Brain PI4KIIα is not selectively localized on synaptic vesicles, as demonstrated, for example, by the presence of a pool of this enzyme in the flow-through of the CPG. The material present in this fraction is expected to contain heterogeneous structures such as endosomal membranes, fragments of the plasma membrane, and possibly also of other intracellular organelles such as Golgi complex elements (30). In addition, PI4KIIα was clearly detectable in the region of the Golgi complex by immunofluorescence. Thus, PI4KIIα may have pleiotropic functions in neurons. The localization in the Golgi complex area and in synaptic vesicles is consistent with previous studies of this kinase in nonneuronal cells where PI4KIIα was detected in the Golgi complex, in the plasma membrane, and in membranes of the endosomal system (27, 28, 41). Synaptic vesicles, specialized recycling organelles, share functional similarities with endosomal membranes (31). The concentration of PI4KIIα at one side only of GM130-positive elements in the Golgi complex (40) is likely to reflect its predominant localization in the “trans” side of this organelle, where exit sites form the Golgi complex interface with recycling membranes of the endosomal system.
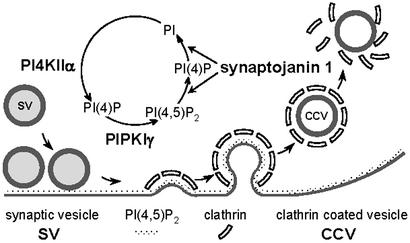
We suggest that PI4KIIα is a key component of an enzymatic machinery that mediates synthesis and metabolism of a pool of PI(4,5)P2 involved in the regulation of the synaptic vesicle cycle. PI4KIIα may act in concert with a plasma membrane PI(4)P 5-kinase, primarily PIPKIγ (18), to regenerate the PI(4,5)P2 pool hydrolyzed by synaptojanin at each round of endocytosis (9, 12). By means of its two distinct inositol-phosphatase domains (42), synaptojanin can remove both phosphates of PI(4,5)P2, thus implying generation of PI(4)P from PI as the first step in PI(4,5)P2 resynthesis. Although PI4KIIα lacks a transmembrane region, it is tightly associated with membranes (25, 26, 28). Thus, in contrast to PIPKIγ and synaptojanin 1, which are soluble enzymes recruited to membranes in a regulated fashion only at specific stages of the vesicle cycle (7, 18, 43), PI4KIIα is expected to be a permanent component of the vesicle membrane. In fact, we also found it in brain clathrin-coated vesicle fractions (data not shown). It will be of interest to determine whether its activity is controlled by excitatory or inhibitory factors at specific stations of the cycle. We speculate that a major site of action of the kinase is the newly reformed synaptic vesicle and that the PI(4)P pool present on synaptic vesicles may be rapidly phosphorylated to PI(4,5)P2 by the type I PIP kinases, primarily PIPKIγ, after exocytosis (18) (Fig. 7). If PI(4)P is generated on synaptic vesicles, it may play a critical role in some aspect of synaptic vesicle function, for example, in the recruitment of synaptic vesicle-associated proteins.
Figure 7.
Putative sites of action of PI-metabolizing enzymes in the synaptic vesicle cycle. PI4KIIα, which is tightly membrane-associated and therefore expected to be present on vesicles throughout the cycle, is proposed to function in the resynthesis of PI(4)P from PI after synaptojanin action on endocytic membranes. The figure is modified from ref. 18.
We have discussed here the potential involvement of PI4KIIα in the regulation of synaptic vesicle traffic. The major role of PI4KIIα in the generation of PI(4)P in brain strongly suggests that this enzyme may have a more general function in phosphoinositide metabolism in this tissue and may generate a significant fraction of the PI(4)P that functions as precursor of PI(4,5)P2 and indirectly of PI(3,4,5)P3 (45, 46). Thus, this enzyme is likely to also have a major role in intracellular signaling. An important avenue for future research is the elucidation of the interplay of phosphoinositide pools involved in the regulation of membrane traffic, actin dynamics, and signaling.
Acknowledgments
We thank Laurie Daniell for technical assistance, and Drs. Michele Solimena and Hassan Mziaut for discussion and communication of unpublished results. This work was supported in part by National Institutes of Health Grants NS36251 and DK54913 (to P.D.C.) and by grants from the Ministero dell'Istruzione, dell'Università e della Ricerca (Cofin 2001/2002) and Telethon-Italy (to F.B.).
Abbreviations
- PI
phosphatidylinositol
- PIP
PI phosphate
- PI(4)P
PI 4-phosphate
- PI(4,5)P2
PI 4,5-bisphosphate
- PIPKIγ
a PI(4)P 5-kinase
- PI4KIIα and PI4KIIβ
type IIα and type IIβ PI 4-kinases
- CPG
controlled-pore glass chromatography
References
- 1.Heuser J E, Reese T S. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Camilli P, Slepnev V I, Shupliakov O, Brodin L. In: Synapses. Cowan W M, Sudhof T C, Stevens C F, editors. Baltimore: Johns Hopkins Univ. Press; 2001. pp. 217–274. [Google Scholar]
- 3.Springer S, Spang A, Schekman R. Cell. 1999;97:145–148. doi: 10.1016/s0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- 4.Rothman J E. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 5.Cremona O, De Camilli P. J Cell Sci. 2001;114:1041–1052. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- 6.Martin T F. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- 7.McPherson P S, Garcia E P, Slepnev V I, David C, Zhang X, Grabs D, Sossin W S, Bauerfeind R, Nemoto Y, De Camilli P. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- 8.Hinshaw J E. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cremona O, Di Paolo G, Wenk M R, Luthi A, Kim W T, Takei K, Daniell L, Nemoto Y, Shears S B, Flavell R A, et al. Cell. 1999;15:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 10.Harris T W, Hartwieg E, Horvitz H R, Jorgensen E M. J Cell Biol. 2000;150:589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luthi A, Di Paolo G, Cremona O, Daniell L, De Camilli P, McCormick D A. J Neurosci. 2001;21:9101–9111. doi: 10.1523/JNEUROSCI.21-23-09101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim W T, Chang S, Daniell L, Cremona O, Di Paolo G, De Camilli P. Proc Natl Acad Sci USA. 2002;99:17143–17148. doi: 10.1073/pnas.222657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao W, Tan Z, Prasad K, Reddy K K, Chen J, Prestwich G D, Falck J R, Shears S B, Lafer E M. J Biol Chem. 1997;272:6393–6398. doi: 10.1074/jbc.272.10.6393. [DOI] [PubMed] [Google Scholar]
- 14.Gaidarov I, Keen J H. J Cell Biol. 1999;146:755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 16.Ford M G, Mills I G, Peter B J, Vallis Y, Praefcke G J, Evans P R, McMahon H T. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 17.Balla T, Bondeva T, Varnai P. Trends Pharmacol Sci. 2000;21:238–241. doi: 10.1016/s0165-6147(00)01500-5. [DOI] [PubMed] [Google Scholar]
- 18.Wenk M R, Pellegrini L, Klenchin V A, Di Paolo G, Chang S, Daniell L, Arioka M, Martin T F, De Camilli P. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 19.Wiedemann C, Schafer T, Burger M M, Sihra T S. J Neurosci. 1998;18:5594–5602. doi: 10.1523/JNEUROSCI.18-15-05594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Audhya A, Foti M, Emr S D. Mol Biol Cell. 2000;11:2673–2689. doi: 10.1091/mbc.11.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa T, Goto K, Kondo H. J Biol Chem. 1996;271:12088–12094. doi: 10.1074/jbc.271.20.12088. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Goto K, Kondo H. Biochem J. 1996;320:643–649. doi: 10.1042/bj3200643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyers R, Cantley L C. J Biol Chem. 1997;272:4384–4390. doi: 10.1074/jbc.272.7.4384. [DOI] [PubMed] [Google Scholar]
- 24.Balla T. Biochim Biophys Acta. 1998;1436:69–85. doi: 10.1016/s0005-2760(98)00134-9. [DOI] [PubMed] [Google Scholar]
- 25.Barylko B, Gerber S H, Binns D D, Grichine N, Khvotchev M, Sudhof T C, Albanesi J P. J Biol Chem. 2001;276:7705–7708. doi: 10.1074/jbc.C000861200. [DOI] [PubMed] [Google Scholar]
- 26.Minogue S, Anderson J S, Waugh M G, dos Santos M, Corless S, Cramer R, Hsuan J J. J Biol Chem. 2001;276:16635–16640. doi: 10.1074/jbc.M100982200. [DOI] [PubMed] [Google Scholar]
- 27.Balla A, Tuymetova G, Barshishat M, Geiszt M, Balla T. J Biol Chem. 2002;277:20041–20050. doi: 10.1074/jbc.M111807200. [DOI] [PubMed] [Google Scholar]
- 28.Wei Y J, Sun H Q, Yamamoto M, Wlodarski P, Kunii K, Martinez M, Barylko B, Albanesi J P, Yin H L. J Biol Chem. 2002;277:46586–46593. doi: 10.1074/jbc.M206860200. [DOI] [PubMed] [Google Scholar]
- 29.Burger P M, Mehl E, Cameron P L, Maycox P R, Baumert M, Lottspeich F, De Camilli P, Jahn R. Neuron. 1989;3:715–720. doi: 10.1016/0896-6273(89)90240-7. [DOI] [PubMed] [Google Scholar]
- 30.Huttner W B, Schiebler W, Greengard P, De Camilli P. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron P L, Sudhof T C, Jahn R, De Camilli P. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Camilli P, Cameron R, Greengard P. J Cell Biol. 1983;96:1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fruman D A, Meyers R E, Cantley L C. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 34.Hendricks K B, Wang B Q, Schnieders E A. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Varnai P, Tuymetova G, Balla A, Toth Z E, Oker-Blom C, Roder J, Jeromin A, Balla T. J Biol Chem. 2001;276:40183–40189. doi: 10.1074/jbc.M104048200. [DOI] [PubMed] [Google Scholar]
- 36. Rajebhosale, M., Greenwood, S., Vidugiriene, J., Jeromin, A. & Hilfiker, S. (2002) J. Biol. Chem., 10.1074/jbc.M204702200. [DOI] [PubMed]
- 37.Taverna E, Francolini M, Jeromin A, Hilfiker S, Roder J, Rosa P. J Cell Sci. 2002;115:3909–3922. doi: 10.1242/jcs.00072. [DOI] [PubMed] [Google Scholar]
- 38.Wiedenmann B, Franke W W. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- 39.Baumert M, Maycox P R, Navone F, De Camilli P, Jahn R. EMBO J. 1989;8:379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis T E, Warren G. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han G S, Audhya A, Markley D J, Emr S D, Carman G M. J Biol Chem. 2002;277:47709–47718. doi: 10.1074/jbc.M207996200. [DOI] [PubMed] [Google Scholar]
- 42.Guo S, Stolz L E, Lemrow S M, York J D. J Biol Chem. 1999;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- 43.Haffner C, Takei K, Chen H, Ringstad N, Hudson A, Butler M H, Salcini A E, Di Fiore P P, De Camilli P. FEBS Lett. 1997;419:175–180. doi: 10.1016/s0014-5793(97)01451-8. [DOI] [PubMed] [Google Scholar]
- 44.Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris A J, Frohman M A, Kanaho Y. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Majerus P W. Semin Cell Dev Biol. 1998;9:153–160. doi: 10.1006/scdb.1997.0220. [DOI] [PubMed] [Google Scholar]
- 46.Cantley L C. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]