Abstract
Trophic cascades have been a central paradigm in explaining the structure of ecological communities but have been demonstrated mainly through comparative studies or experimental manipulations. In contrast, evidence for shifts in trophic cascades caused by intrinsically driven population dynamics is meager. By using empirical data of a cannibalistic fish population covering a 10-year period and a size-structured population model, we show the occurrence of a dynamic trophic cascade in a lake ecosystem, in which the community over time alternates between two different configurations. The intrinsically driven change in the size structure of the fish population from a dominance of stunted individuals to a dominance of gigantic cannibals among adult individuals is the driving force behind distinct abundance switches observed in zooplankton and phytoplankton. The presence of the phase with gigantic cannibals depends critically on the energy they extract from their victims, allowing strong reproduction for a number of years.
Community-wide trophic cascades, the propagation of indirect mutualism between nonadjacent trophic levels in food webs, have been suggested to occur more frequently in aquatic than in terrestrial systems (1–4). This suggestion is based on the arguments that terrestrial systems have a higher heterogeneity, a higher overall species diversity, and more chemical defenses among primary producers (higher plants vs. algae; ref. 1). Although the validity of all of these arguments has been questioned (2, 4), undoubtedly the empirical evidence for community-wide trophic cascades is, at present, substantially stronger for aquatic than for terrestrial systems. The empirical evidence largely stems from two sources: comparative studies of different systems in which the trophic structure, such as food chain length, differs (5–6) and experimental manipulations of top predators, either intentional or unintentional (species invasions; refs. 2, 3, and 7–9). In contrast, there is hardly any evidence for dynamic trophic cascades, in which major shifts in overall food-web structure are intrinsically driven by population dynamics. Only a few studies on recruitment variation have considered this aspect (10–12).
Cannibalism has been shown to have a number of diverse effects on population dynamics and persistence (13–17). These effects, among others, include a potential for alternative stable states (18, 19) and chaotic dynamics (20). Although many cannibalistic models ignore the energy that cannibals gain from cannibalism and, thus, are essentially “infanticide” models (13, 15, 20), some theoretical studies have shown that the energy extracted by the cannibal may have substantial impact on population persistence and individual life history (14, 16). Empirical evidence also suggests such an effect of energy extraction on population dynamics because of increased growth and thereby increased per-capita fecundity of cannibals (17).
Here we present strong evidence for a whole-lake trophic cascade that is dynamic and intrinsically driven by complex dynamics of a cannibalistic fish population. The trophic cascade involves a major covariation between fish, herbivorous zooplankton, and phytoplankton biomass. We show that a mixture of size-dependent cannibalism and intercohort competition in perch (Perca fluviatilis) leads to shifts between phases in which the population is dominated by stunted cannibals and phases in which it is dominated by recruits and gigantic cannibals. The effects of these shifts propagate through to lower trophic levels. We also show that the mechanisms provided by the empirical analysis of the perch population are upheld in a formal modeling analysis of the cannibalistic population. In particular, we show that energy extraction by cannibals from victims, neglected in the most influential cannibalistic models (15, 20), is of prime importance for the population dynamics. The shifts in the entire biological community as a result of the population dynamics of the cannibal are brought about by only a few giant cannibals that dramatically affect the entire lake system down to the lowest level of phytoplankton.
Methods
Field Data.
The empirical data were derived from a small (9.3-hectare) forest lake of low productivity (17). The population size of perch ≥1 year was estimated by mark-recapture methods (21). Perch were sampled with cylindrical plastic traps. The captured fish were measured (to the nearest millimeter) and weighed (to the nearest gram). One-year-old perch were electrofished from a boat in spring, when they were concentrated along the shore. In years when the numbers of 1-year-old perch were too low (<60 individuals) to allow population estimates based on mark recapture (1992, 1993, 1994, 2000, and 2001), we used population estimates obtained from a regression of estimates based on mark recapture for years when mark recapture was possible on the capture obtained during the spring electrofishing (17). Sampled perch were stomach-flushed, stomach contents were identified to taxa, and the sizes of identified food items were measured. Annual mortality rate of perch was estimated by using age and population estimates, whereas age and growth of individual fish were determined from opercular bones (21).
Zooplankton and phytoplankton were sampled at three pelagic stations seven times during the growth period (May–October) at intervals of 2–3 weeks. Zooplankton was collected with a 100-μm mesh net (diameter, 25 cm) drawn vertically at an approximate speed of 0.5 m/s from the thermocline to the surface. Data used are from sampling occasions after the hatching of young-of-the-year (YOY) perch (July–October; ref. 17). Samples were preserved with Lugol's solution. The zooplankton was classified by species and counted, and the body lengths of 15 individuals (or of all individuals if <15) of each species from each sample were measured with an inverted microscope. Lengths were then transformed to biomass by using length-to-weight regressions.
For estimating phytoplankton biomass, a mixed epilimnetic (including metalimnion) water sample was taken from each station on each sampling date. The depth of the thermocline was determined from temperature profiles taken with a thermistor. Phytoplankton biomass was estimated as chlorophyll a (22). The water samples were filtered through Whatman GF/C filters (1–2.5 liter filtered), and filters were dried and frozen (−25°C) until further analyses. Algae on the filters were extracted in methanol for 24 h, and the absorbance at 665 and 750 nm was used to calculate chlorophyll a. At the first and last sampling dates each year, a subsample of 150 ml of unfiltered water was taken for analyses of total phosphorus, total nitrogen, and micronutrients. The total concentrations of nitrogen and phosphorus were analyzed with an autoanalyzer by using standard analytical methods (22).
Model.
We modeled the cannibalistic dynamics by using a physiologically structured population model, a modeling approach that is designed to handle continuously changing size-dependent processes (16, 23, 24). An important element of these models is that all model assumptions pertain to the individual level, such that parameters can be estimated largely independent of the system under study (16). The structured population model represents a cannibalistic population that feeds on two basic resources, zooplankton and macroinvertebrates, in a size- and age-specific way (16, 23, 25). An extended presentation of the baseline model using only one shared resource (zooplankton) is given in ref. 16. All perch feed on zooplankton, whereas perch ≥2 years old also feed on macroinvertebrates and YOY perch. One-year-old perch are assumed not to cannibalize YOY perch because the former have been shown to be restricted to refuges along the shore, where the risk of cannibalism from larger cannibals is minimized (17). The feeding rates of perch on zooplankton and macroinvertebrates are functions of perch mass and follow a type 2 functional response as a function of resource densities (16, 25). Consumption, metabolism (a power function of mass), and growth are all continuous processes, whereas reproduction takes place once a year (16). Individuals are assumed to mature at a fixed size and reproduce only if they have accumulated sufficient gonad mass. The dynamics of the model were studied with a numerical method tailored for the integration of physiologically structured models (24).
All parameters were set to the default values for perch as used in the original model analysis (16), except for a background mortality rate of 0.004 per day and a cannibalistic voracity of 400 × (perch length)0.6. Macroinvertebrates followed semichemostat regrowth dynamics with a turnover rate of 0.1 per day and a maximum biomass density of 1.0 g/m2. Macroinvertebrates are assumed to live on the bottom in the littoral zone (average water depth 2 m), which is assumed to occupy 20% of the total lake volume (3.0 × 108 liters). The attack rate of perch ≥2 years old on macroinvertebrates equaled 0.1 × (perch weight)0.4. The weight-age relation for YOY perch was prescribed in the model as W(a) = 0.001 × exp(0.08 a), based on empirical data (weight in g biomass; a, age in days). Growth of older perch follows the energy budget model originally developed (16). All individual-level model parameters described above have been estimated in laboratory or pond experiments (16, 26) independent of the perch system studied with the exception of the maximum biomass densities of zooplankton and macroinvertebrates (for obvious reasons) and YOY perch growth.
Results
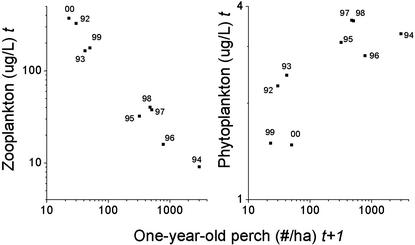
Our data from the lake ecosystem cover a 10-year period and show an alternation in trophic structure mediated via a change in the state of the perch population. In years with high survival of YOY perch, reflected in high numbers of 1-year-old perch the next year, zooplankton biomasses were lower and phytoplankton biomasses higher, whereas the opposite was the case in years with low survival of YOY perch [regressions: zooplankton–1-year-old perch, F1,8 = 234, P < 0.0001; phytoplankton–1-year-old perch, F1,8 = 11.6, P < 0.011, autoregressive-integrated moving average (ARIMA), tests for autocorrelation at lag 1; P = 0.64–0.83 (Fig. 1)]. Furthermore, both zooplankton and phytoplankton data fall into two distinct clusters, suggesting a shift of the lake ecosystem between two different phases.
Figure 1.
(Left) Relationship between zooplankton biomass (average for period of July–August) and the density of 1-year-old perch the next year. (Right) Relationship between phytoplankton biomass (seasonal average) and the density of 1-year-old perch the next year. Years refer to zooplankton/phytoplankton. Densities of 1-year-old perch in year t + 1 are taken as a measure of YOY densities in year t.
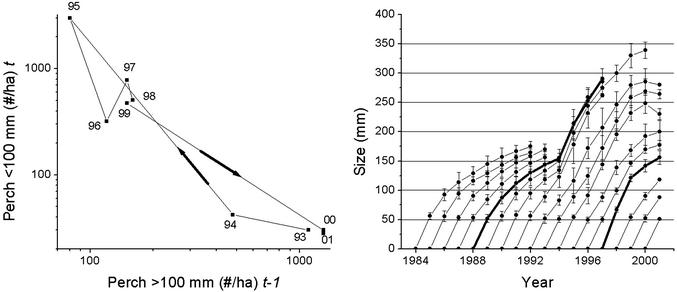
The trophic cascade observed in zooplankton and phytoplankton can clearly be related to the observed population dynamics of perch. At the start of the study in 1992, the perch population was dominated by high numbers of perch of an age of 2 years and older, and particularly by a cohort that hatched in 1988 (75% of the population). Very few 1-year-old perch were present (Fig. 2 Left and ref. 17). This low number of 1-year-old perch was not because of low population fecundity but because of high cannibalistic mortality (almost 100%) imposed by the numerous perch ≥2 years old. The energy extracted from cannibalism was, however, small, and the energy intake by perch consisted mainly of zooplankton and macroinvertebrates. As a result, macroinvertebrate densities were low (17). Energy intake from cannibalism was low because YOY perch were cannibalized early on when they were small and, hence, only represented small energy packages. As a result, perch grew slowly during this phase and only reached a small maximum size (Fig. 2 Right). A decrease in the number of perch ≥2 years old in 1993 and 1994 resulted in a lower mortality of YOY perch during 1994 and, thus, in a strong cohort of 1-year-old perch in 1995 (Fig. 2 Left). The lower mortality of these YOY perch individuals allowed substantial numbers of them to reach sizes at which they represented larger energy packages for the cannibals. Energy extraction from cannibalism was thus high, and the growth rate of the remaining cannibals increased dramatically (Fig. 2 Right). Increased individual growth caused by cannibalistic energy extraction also led to increased per-capita fecundity that more than compensated for the lower numbers of remaining cannibals. As a result, a substantial reproductive outburst took place in 1995. The new YOY cohort depressed the shared zooplankton resource to low levels (Fig. 1 Left), thereby outcompeting the 1-year-old cohort that starved to death (17). The competitive superiority of YOY perch over 1-year-old individuals results from the former's lower metabolic demands and hence lower resource demands (23), a conclusion that is supported by experiments showing a competitive superiority of YOY perch over 1-year-old perch (26).
Figure 2.
(Left) Phase plot of the observed density of perch <100 mm vs. the density of perch >100 mm the previous year. Years refer to perch <100 mm. (Right) Observed growth trajectories (means ± 1 SD) of perch during 1984–2001. Thick growth curves show dominating year classes born in 1988 and 1997. High survival in YOY perch cohorts and giant growth among cannibals was observed during 1994–1998. The negative growth rates observed for year classes >200 mm during 2000–2001 may be caused by either actual negative growth or size-dependent mortality of larger perch or both.
This strong recruitment of 1-year-old perch, followed by their starvation death the same year because of competition with new YOY perch for zooplankton, was repeated for 4 years (1995–1998). Growth rates of the few cannibals remained high, which allowed them to reach gigantic sizes (Fig. 2 Right). In contrast to zooplankton biomasses, macroinvertebrate densities were high in this giant phase because of the low number of adult perch and the ample abundance of energy-rich YOY perch (17). The giant phase ended in 1998 when a cohort of 1-year-old perch with a slightly larger size escaped competition from YOY perch. This cohort totally dominated the perch population (90% of the individuals) the following years and, when increasing in size, through strong cannibalism prevented new YOY perch cohorts to reach an age of 1 year. Because of their high cannibalistic impact early on when YOY perch only represented small energy packages, the growth rate of this cohort was at this time reduced, and they became stunted as the perch population had been before 1994 (Fig. 2 Right). In a phase plane, the overall dynamics results in two clusters: in one cluster the density of individuals ≥2 years old is high and that of 1-year-old perch is low (high cannibalism, low energy extraction, low per-capita fecundity); in the other cluster the density of individuals ≥2 years old is low and that of 1-year-old perch is high (low cannibalism, high energy extraction of cannibalism, high per-capita fecundity; Fig. 2 Left).
We analyzed our data set to see whether the low recruitment in some years could be explained by alternative mechanisms such as intercohort competition affecting YOY perch performance or environmental factors (nutrient levels, temperature). An explanation based on intercohort competition is at odds with the fact that zooplankton (the main food of YOY perch) resource levels were much higher in years with low YOY survival than in years with high survival (Fig. 1 Left). Furthermore, our experimental studies show that small perch can sustain themselves at lower resource levels than large perch, and that small perch do not starve under the resource levels prevailing in the study system (26). Neither nutrients (nitrogen, phosphorus) nor average summer temperature (June–August) showed any relationship with YOY survival [regressions: F1,8 = 0.74–2.02, P = 0.19–0.42, autoregressive-integrated moving average (ARIMA), tests for autocorrelations at lag 1; P = 0.26–0.66].
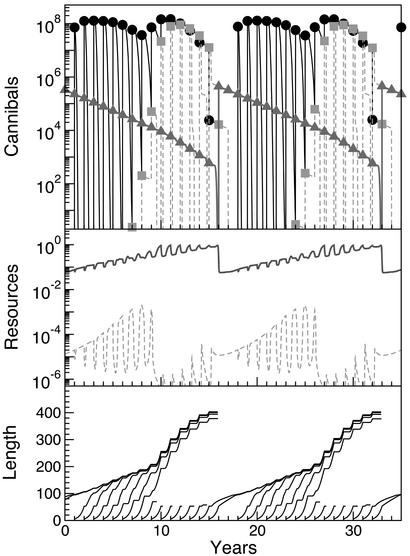
Modeling results strongly support the interpretation of the empirical data, because they also show a shift in dominance of different size cohorts in the cannibalistic populations. In one phase, the density of perch ≥2 years old is high, and the number of perch recruited to an age of 1 year is negligible because of high cannibalism from perch ≥2 years old (t = 1–8; Fig. 3 Top). Although cannibals impose a high mortality on YOY perch in these years, they grow slowly and only reach a small maximum size because of low energy extraction from cannibalism (Fig. 3 Bottom). Zooplankton resource levels are high and macroinvertebrate resource levels low during this phase (Fig. 3 Middle; ref. 13). A decrease over years in the number of perch ≥2 years old because of background mortality causes the cannibals at a certain density threshold to lose control over victims, allowing a strong recruitment of YOY perch to an age of 1 year old (t = 9–10; Fig. 3 Top). This high number of surviving, larger YOY perch leads to gigantic growth in remaining cannibals (Fig. 3 Bottom) and increased per-capita fecundity that compensates for their lower numbers. As a result, substantial reproductive outbursts take place for a number of years. One-year-old cohorts are outcompeted by the new YOY cohorts and starve to death (t = 9–14; Fig. 3 Top). In this phase, the zooplankton resource levels are low and macroinvertebrate resource levels high (Fig. 3 Middle). When the decrease in adult numbers is no longer compensated for by increased per-capita fecundity, and population fecundity hence decreases, the impact of YOY individuals on zooplankton is reduced to a level at which a 1-year-old cohort survives its second year of life (t = 15–16; Fig. 3 Top). When increasing in size, this surviving cohort subsequently controls new offspring cohorts through strong cannibalism, but their growth is retarded (t = 15–24; Fig. 3 Bottom).
Figure 3.
Model predictions including energy extraction from cannibalism. (Top) YOY perch (black circles), 1-year-old perch (gray squares), and perch ≥2 years old (gray triangles) (numbers per lake). (Middle) Predicted biomasses of the macroinvertebrate (solid line; g wet weight/m2) and zooplankton (dotted line; g wet weight/liter) resources. (Bottom) Predicted growth curves (mm) of perch during the stunted and giant phases.
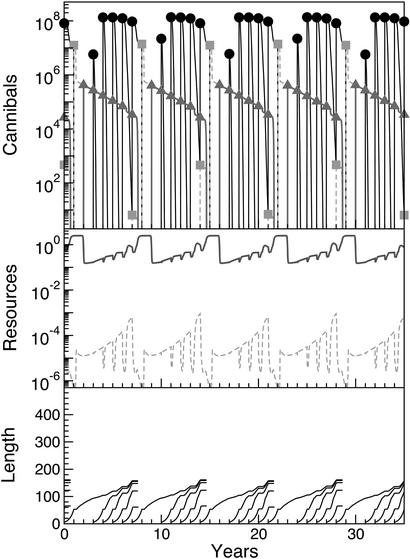
Excluding energy extraction from cannibalism by setting the conversion efficiency for feeding on victims to zero resulted in qualitatively different dynamics. In particular, the strong feedback of energy extraction on cannibal growth, and hence per-capita fecundity, was absent; the first strong 1-year-old cohort recruited did not suffer from intercohort competition from a strong YOY cohort (see the 1-year-old cohort at t = 1, 8, 15, 22, and 29 in Fig. 4 Top and Bottom). Actually, YOY perch were totally absent in these years with a strong 1-year-old cohort, as mature perch had starved to death the previous year because of competition with the YOY perch cohort subsequently forming the strong 1-year-old cohort. Energy extraction from cannibalism is hence not only crucial for growth and high fecundity of cannibals, but also for their survival. The strong 1-year-old cohort became the dominating cohort during the following phase with strong cannibal control (low YOY perch survival). The absence of repeated strong recruitment of YOY perch for several years also leads to the strong cascading effect of YOY perch on zooplankton only being present for 1 year (Fig. 4 Middle).
Figure 4.
Model predictions excluding energy extraction from cannibalism. (Top) YOY perch (black circles), 1-year-old perch (gray squares), and perch ≥2 years old (gray triangles) (numbers per lake). (Middle) Predicted biomasses of the macroinvertebrate (solid line; g wet weight/m2) and zooplankton (dotted line; g wet weight/liter) resources. (Bottom) Predicted growth curves (mm) of perch during the stunted and giant phases.
In conclusion, the results of this model, in all major aspects, confirm and provide strong theoretical support for our interpretation of the data with respect to the mechanisms driving the cannibalistic population dynamics. The modeling analysis excluding energy extraction from cannibalism strongly reinforces our interpretation that energy extraction is an essential part of the cannibalistic population dynamics.
Discussion
Our overall conclusion, based on an interpretation of the empirical data and modeling, is that the population dynamics observed can be explained by intrinsic factors alone. The vital ingredients involved are variable intensities in intercohort cannibalism (older perch vs. YOY perch) and intercohort competition (1-year-old vs. YOY perch). When cannibals control victims, cannibal population density is high, whereas the size structure is stunted with only intermediately sized cannibals. When cannibals do not control victims, the population is characterized by a low abundance and a bimodal size distribution, including giant cannibals. The changes in perch population size structure, in turn, cascade down to phytoplankton through zooplankton (Fig. 1). Cannibalism intensity determines the recruitment success of YOY perch. Energy extraction by cannibals during the giant phase is dynamically important, because their high fecundity is crucial for the strong competitive interactions between the 1-year-old and the numerous YOY perch. These results contrast with present cannibal models in which neither the energy gained by cannibals nor intercohort competition have been included (13, 15, 20). For example, in the experimentally and theoretically heavily elaborated Tribolium system, cannibals only impose a mortality impact on the victims and do not gain any energy from the act, thus leaving out an essential part of this intraspecific predator–prey interaction (15, 20).
Data on other fish populations such as largemouth bass (Micropterus salmoides; ref. 11) and Arctic charr (Salvelinus alpinus; ref. 27) show elements of similar alternations in population size structure over time, pointing to a substantial generality of our results to other cannibalistic systems. This especially concerns the temporary appearance of giants in connection with strong surviving YOY cohorts with cascading effects to zooplankton and phytoplankton. However, at least for the largemouth bass system, in which a larger database is present, the giant phase is very transient and does not exceed 1 year because the main interaction between YOY and 1-year-old individuals is cannibalistic and not an intercohort competition (11, 17).
Our verbal analysis of the mechanisms causing the dynamics of the cannibalistic perch system shows a strong correspondence with the mechanisms provided by the modeling analysis. A relevant question to ask, given the qualitative shift in population size structure of perch over time, is whether the stunted and giant phases represent two alternative but unstable attractors. Alternative stable states were first demonstrated in a cannibalistic model by Botsford (18). His model, however, did not incorporate energy gain, which we have found to be such a prominent element of the dynamics. A continuous time version of our cannibalistic model does indeed show that the stunted and giant phases represent two alternative stable states (19). Still, our analyses of the discrete/continuous model that we have used here have so far only identified the cannibal-driven stunted phase as a model attractor (16). An alternative attractor with giants has not yet been found in this version. It is thus quite possible that the dynamics we observe relates to a single attractor with complex dynamics. Overall, our analysis, together with previous analyses of the cannibalistic system (16, 28), suggests that the cannibal-driven dynamics is robust to substantial variation in parameter values. The appearance of giants has been shown to result from a destabilization of the cannibal-driven dynamics, although the precise mechanism behind this may differ (this article vs. refs. 16 and 28).
In conclusion, our empirical and modeling results have implications beyond the cannibalistic system studied here in at least three major respects. First, our analyses show the importance of considering all aspects of size-dependent processes (intercohort competition, cannibalism) including energy extraction by the cannibals (16). Second, the use of a modeling approach that includes both individual-level and population-level information allows for a deeper mechanistic understanding of the critical processes involved than model approaches that are based only on population-level information. In particular, individual growth information forms a critical element to reveal the mechanisms behind the sustenance of the phase with a dominance of giants among cannibals for several years. Third, the size-structured interactions shown at the population level and intrinsic to the system had far-reaching effects on overall food-web dynamics, implying that food-web dynamics of aquatic systems may only to a limited extent be understood outside the context of these size-structured processes. This conclusion is reinforced by recent studies that show that size-dependent competitive and predatory interactions per se increase the likelihood for coexisting attractors including catastrophic behavior in ecological systems (29, 30).
Acknowledgments
We thank J. Andersson, C. Halvarsson, J. Hjelm, J. Karlsson, E. Lindgren, P. Nilsson, P. Nordin, A. Persson, K. Samuelsson, F. Staffans, and R. Wallin for help with extensive field samplings. N. G. Hairston, Jr., made helpful comments on the manuscript. Two anonymous reviewers gave most useful comments on the manuscript. L.P. was supported by the Swedish Research Council and the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning, and A.M.D.R. was supported by The Netherlands Organization for Scientific Research.
Abbreviation
- YOY
young-of-the-year
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Polis G A, Strong D R. Am Nat. 1996;147:813–846. [Google Scholar]
- 2.Persson L. Oikos. 1999;85:385–397. [Google Scholar]
- 3.Schmitz O J, Hambäck P A, Beckerman A R. Am Nat. 2000;155:141–153. doi: 10.1086/303311. [DOI] [PubMed] [Google Scholar]
- 4.Chase J M. Trends Ecol Evol. 2000;15:408–412. doi: 10.1016/s0169-5347(00)01942-x. [DOI] [PubMed] [Google Scholar]
- 5.Persson L, Diehl S, Johansson L, Andersson G, Hamrin S F. Am Nat. 1992;140:59–84. [Google Scholar]
- 6.Hansson L-A, Lindell M, Tranvik L J. Oikos. 1993;66:101–106. [Google Scholar]
- 7.Carpenter S R, Kitchell J F, editors. The Trophic Cascade in Lakes. Cambridge, U.K.: Cambridge Univ. Press; 1993. [Google Scholar]
- 8.Menge B A. Ecol Monogr. 1995;65:21–74. [Google Scholar]
- 9.Brett M T, Goldman C R. Science. 1997;275:384–386. doi: 10.1126/science.275.5298.384. [DOI] [PubMed] [Google Scholar]
- 10.Post D M, Kitchell J F, Hodgson J R. Can J Fish Aquat Sci. 1998;55:2588–2600. [Google Scholar]
- 11.Post D M, Carpenter S R, Christensen D L, Cottingham K L, Kitchell J F, Schindler D E. Limnol Oceanogr. 1997;42:722–729. [Google Scholar]
- 12.Shiomoto A, Tadokoro K, Nagasawa K, Ishida Y. Mar Ecol Prog Ser. 1997;150:75–85. [Google Scholar]
- 13.Diekmann O, Nisbet R M, Gurney W S C, van den Bosch F. Math Biosci. 1986;78:21–46. [Google Scholar]
- 14.van den Bosch F, De Roos A M, Gabriel W. J Math Biol. 1988;26:619–633. [Google Scholar]
- 15.Hastings A, Costantino R F. Am Nat. 1987;130:36–52. [Google Scholar]
- 16.Claessen D, De Roos A M, Persson L. Am Nat. 2000;155:219–237. doi: 10.1086/303315. [DOI] [PubMed] [Google Scholar]
- 17.Persson L, Byström P, Wahlström E. Ecology. 2000;81:1058–1071. [Google Scholar]
- 18.Botsford L W. Am Nat. 1981;117:38–63. [Google Scholar]
- 19. Claessen, D. & De Roos, A. M. (2003) Theor. Popul. Biol., in press. [DOI] [PubMed]
- 20.Costantino R F, Desharnais R A, Cushing J M, Dennis B. Science. 1997;275:389–391. doi: 10.1126/science.275.5298.389. [DOI] [PubMed] [Google Scholar]
- 21.Bagenal T B, editor. Methods for Assessment of Fish Production in Fresh Waters: IBP Handbook No. 3. Oxford: Blackwell Scientific; 1978. [Google Scholar]
- 22.Grasshoff K, Ehrhardt M, Kremling K. Methods of Seawater Analysis. Weinhem, Germany: Verlag Chemie; 1983. [Google Scholar]
- 23.Persson L, Leonardsson K, Gyllenberg M, De Roos A M, Christensen B. Theor Popul Biol. 1998;54:270–293. doi: 10.1006/tpbi.1998.1380. [DOI] [PubMed] [Google Scholar]
- 24.De Roos A M. In: Structured-Population Models in Marine, Terrestrial, and Freshwater Systems. Tuljapurkar S, Caswell H, editors. New York: Chapman & Hall; 1997. pp. 119–204. [Google Scholar]
- 25.De Roos A M, Leonardsson K, Persson L, Mittelbach G G. Ecol Monogr. 2002;72:271–292. [Google Scholar]
- 26.Byström P, Gàrcia-Berthóu E. Oikos. 1999;86:217–232. [Google Scholar]
- 27.Klemetsen A, Amundsen P-A, Grotnes P E, Knudsen R, Kristoffersen R, Svenning M-A. Environ Biol Fish. 2002;64:39–47. [Google Scholar]
- 28.Claessen D, van Oss C, De Roos A M, Persson L. Ecology. 2002;83:1660–1675. [Google Scholar]
- 29.McCauley E, Nisbet R M, Murdoch W W, De Roos A M, Gurney W S C. Nature. 1999;402:653–656. [Google Scholar]
- 30.De Roos A M, Persson L. Proc Natl Acad Sci USA. 2002;99:12907–12912. doi: 10.1073/pnas.192174199. [DOI] [PMC free article] [PubMed] [Google Scholar]