Abstract
A recombinant virus assay was used to characterize in detail neutralizing antibody responses directed at circulating autologous HIV in plasma. Examining serial plasma specimens in a matrix format, most patients with primary HIV infection rapidly generated significant neutralizing antibody responses to early (0–39 months) autologous viruses, whereas responses to laboratory and heterologous primary strains were often lower and delayed. Plasma virus continually and rapidly evolved to escape neutralization, indicating that neutralizing antibody exerts a level of selective pressure that has been underappreciated based on earlier, less comprehensive characterizations. These data argue that neutralizing antibody responses account for the extensive variation in the envelope gene that is observed in the early months after primary HIV infection.
Neutralizing antibody responses after natural infection or vaccination comprise a major component of protection from virus infection (1). The majority of antibodies directed against the viral envelope glycoprotein (Env) recognizes nonneutralizing epitopes of glycoprotein monomers and is ineffectual (2, 3). Characterizing the neutralizing antibody response to HIV-1 has been limited by technical challenges. The measurement of serial responses to autologous virus has generally required isolation of primary viruses from peripheral blood mononuclear cells, preparation of virus stocks, and titration of these stocks from sequential blood specimens. Neutralizing antibody responses to heterologous primary isolates and to laboratory strains are easier to characterize but seem to develop slowly after infection and to relatively low titers (2, 4, 5).
Neutralization escape mutants of the animal lentiviruses such as equine infectious anemia virus, visna virus, and simian immunodeficiency virus evolve in infected horses, sheep, and rhesus monkeys, respectively (6–8). Neutralizing antibody responses against autologous HIV-1 were reported first by Weiss in 1986 (9), and several later studies have suggested that its appearance is slow to develop and of low titer (2, 4, 5). Neutralization escape of HIV has been reported in limited cases (10–15); however, many studies of autologous neutralizing antibody after primary HIV infection stress the low or absent responses with only infrequent examples of escape (5, 16–18). We report here that in most patients, potent neutralizing antibody responses are generated early after infection, at first to the autologous infecting HIV variant and then to subsequent variants. The antibody responses to these variants exert a selective pressure that drives continuous evolution of neutralization escape mutants.
Materials and Methods
Study Subjects.
Study subjects were recruited with a diagnosis of primary (recent) HIV infection as part of the San Diego Acute and Early Infectious Disease Research Program. Serial blood specimens were collected, separated by centrifugation into plasma and cells, and frozen at −70°C. All subjects signed informed consents to protocols approved by the University of California Human Subjects Committee (La Jolla).
Neutralization Assay.
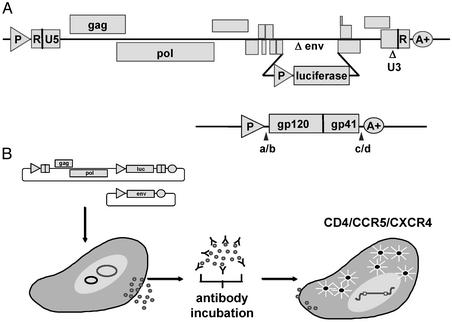
A recombinant virus assay initially developed to measure antiretroviral drug resistance during a single round of virus replication was adapted to measure virus-antibody neutralization (19). HIV genomic RNA was isolated from virus stocks or plasma by using oligo(dT) magnetic beads. First-strand cDNA was synthesized in a standard reverse transcription reaction by using an oligo(dT) primer. Env DNA (gp160) was amplified by PCR using forward and reverse primers located immediately upstream and downstream of the env initiation and termination codons, respectively. The forward and reverse primers contain recognition sites for PinAI and MluI, respectively. Env PCR products were digested with PinAI and MluI and ligated to compatible ends in the pCXAS expression vector, which uses the cytomegalovirus immediate-early promoter enhancer to drive env insert expression in transfected cells (Fig. 1A). Ligation products were introduced into competent Escherichia coli (Invitrogen) by transformation, and pCXAS-env plasmid DNA was purified from bacterial cultures (Qiagen, Valencia, CA). An aliquot of each transformation was plated onto agar, and colony counts were used to estimate the number of envelope sequences represented in each pCXAS-env library (generally 500–5,000 clones). Sequence analysis of individual pCXAS-env clones (10–20) was used to verify the heterogeneous composition (i.e., quasispecies) of pCXAS-env libraries. Virus particles containing patient virus envelope proteins were produced by cotransfecting HEK293 cells with pCXAS-env libraries plus an HIV genomic vector that contains a firefly luciferase indicator gene (Fig. 1A). pCXAS-env plasmid preparation and HEK293 cell-transfection conditions have been optimized to ensure consistent virus particle production. Recombinant viruses pseudotyped with patient virus envelope proteins were harvested 48 h posttransfection and incubated for 1 h at 37°C with serial 4-fold dilutions of heat-inactivated patient plasma samples (antibody) (Fig. 1B). U87 cells that express CD4 plus the CCR5 and CXCR4 coreceptors were inoculated with virus-plasma (antibody) dilutions in the absence of added cations. Virus infectivity was determined 72 h postinoculation by measuring the amount of luciferase activity expressed in infected cells. Neutralizing activity is displayed as the percent inhibition of viral replication (luciferase activity) at each antibody dilution compared with an antibody-negative control: % inhibition = {1 − [luciferase + Ab/luciferase − Ab]} × 100. Titers were calculated as the reciprocal of the plasma dilution conferring 50% inhibition (IC50), which is demarcated as a dashed vertical line in Fig. 2. A series of experiments using diluted virus stocks (1:2, 1:5, 1:10, or 1:20) has demonstrated that luciferase activity correlates with virus inoculum, but that antibody neutralization titers are not significantly affected (data not shown).
Figure 1.
(A) Diagrams of the expression vectors used to generate the pseudovirions used in the neutralization assay. The envelope-defective, luciferase-expressing vector is above the vector that expresses the full-length envelopes amplified from patient plasmas. (B) Schema of the generation of pseudovirions by cotransfection of the two vectors depicted in A. These pseudovirions then are incubated for 1 h with serial 4-fold dilutions of plasma or antibody solutions before infection of the U87-derived target cells to generate luciferase activity.
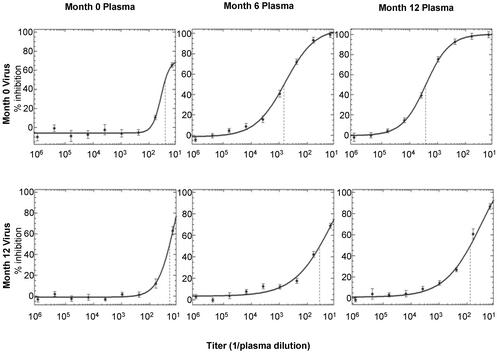
Figure 2.
Neutralization of autologous HIV. The neutralizing activity of plasmas obtained from patient 1 at months 0, 6, and 12 after presentation with primary infection is assayed against virus from months 0 and 12. The titer is defined as the reciprocal of the dilution of plasma that produces 50% inhibition of virus replication (dashed lines). The error at each dilution reflects the standard error of duplicate wells.
Results
Measuring the Autologous Neutralizing Antibody Response.
We began our investigation by studying 14 subjects who presented to the San Diego Acute and Early Infection Disease Research Program 30–65 days after their estimated date of HIV infection and elected to defer or delay antiretroviral therapy. Plasma samples (3–13 per patient) were obtained at presentation to the clinic and at regular intervals for 6–39 months of follow-up. Neutralization activity was measured by using a cell-based infectivity assay that greatly facilitates the characterization of antibodies and virus envelope proteins derived from the same plasma sample (i.e., autologous envelope–antibody pairs). Infectivity is measured by using recombinant viruses that carry a luciferase reporter gene and are pseudotyped with patient HIV envelope proteins (Fig. 1). Fig. 2 demonstrates the ability of this assay to detect the emergence of autologous neutralization activity directed against the virus present at presentation of primary HIV infection (month 0) in serial plasma samples (0, 6, and 12 months).
This assay consistently generates neutralization curves similar in shape and slope and with little variability in duplicate assay wells (Fig. 2). As a result, IC50 titers (1/dilution that confers 50% neutralization) were typically 5-fold higher than the IC80 values and 10-fold higher than the IC90 values. The IC50 titers are reported because they can be most precisely derived from the linear portion of the sigmoid curve. In contrast to most published assays, plasmas with IC50 titers >100 in this assay have less than a 1% nonneutralized fraction (i.e., inhibition curves typically plateau at 100% neutralization). To monitor the amount of neutralization activity that is not mediated by antibodies directed against HIV-1 env proteins, each plasma sample was also tested against a recombinant virus stock that was pseudotyped with amphotropic murine leukemia virus envelope proteins (gp70SU and p15TM). Typically, the IC50 values of amphotropic murine leukemia virus controls were <50.
Autologous Neutralizing Antibody Response in Patients with Primary HIV Infection.
The neutralization activities of sequential plasma samples against sequential virus envelope proteins from the same patient (autologous responses) or against two reference viruses (heterologous responses) are displayed in Tables 1–3. For 6 of the 14 patients, peak neutralizing antibody titers reached >1,000 as exemplified in patient TN-1. For two patients, negligible neutralizing antibody titers (<100) to autologous viruses were generated as exemplified in patient TN-2. For the remaining six patients, peak titers to autologous virus ranged between 100 and 1,000 as exemplified in patient TN-3; however, for three of these patients the period of follow-up was <12 months, and antibody neutralization titers may not have peaked yet.
Table 1.
Antibody neutralization titers (subject TN-1, treatment naive)
| Virus, months | Plasma, months
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 25 | |
| 0 | 26 | 219 | 675 | 1403 | 2670 | 2089 | 2190 | 2363 | 2411 |
| 3 | 29 | 179 | 1024 | 2151 | 3733 | 3152 | 2808 | 2953 | 3086 |
| 6 | 27 | 35 | 78 | 358 | 1769 | 1939 | 2247 | 3112 | 4345 |
| 9 | 36 | 67 | 82 | 200 | 795 | 1078 | 1371 | 2208 | 3375 |
| 12 | 19 | 48 | 36 | 64 | 76 | 166 | 556 | 937 | 1407 |
| 15 | 29 | 43 | 64 | 76 | 90 | 119 | 374 | 721 | 1234 |
| 18 | 42 | 65 | 61 | 152 | 117 | 134 | 122 | 289 | 526 |
| 21 | 41 | 66 | 82 | 84 | 85 | 113 | 78 | 107 | 296 |
| 25 | 42 | 62 | 56 | 62 | 85 | 77 | 55 | 61 | 95 |
| Controls | |||||||||
| NL43 | 17 | 138 | 294 | 956 | 1172 | 953 | 1584 | 1868 | 2143 |
| JRCSF | 24 | 37 | 35 | 60 | 87 | 97 | 105 | 152 | 209 |
| AMPHO | <10 | 32 | 14 | 13 | 14 | 13 | <10 | <10 | 31 |
Neutralizing HIV antibody titers of sequential plasma specimens against autologous virus. Serial plasmas were obtained from three untreated patients presenting with primary HIV infection. The titer of each plasma against its concurrent virus specimen is in bold type. Control viruses include an amphotropic murine leukemia virus (AMPHO), a neutralization-sensitive X4-tropic virus (NL4-3), and a relatively neutralization-resistant R5-tropic virus (JR-CSF).
Table 3.
Antibody neutralization titers (subject TN-3, treatment naive)
| Virus, months | Plasma, months
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 10 | 14 | 19 | 22 | 30 | 35 | 39 | |
| 0 | 39 | 67 | 103 | 102 | 152 | 303 | 376 | 403 | 362 | 449 |
| 3 | 47 | 69 | 142 | 231 | 261 | 547 | 488 | 419 | 392 | 464 |
| 6 | 37 | 50 | 81 | 91 | 172 | 340 | 308 | 360 | 386 | 363 |
| 10 | 32 | 34 | 47 | 75 | 117 | 295 | 321 | 336 | 400 | 406 |
| 14 | 34 | 43 | 50 | 45 | 69 | 164 | 142 | 235 | 236 | 245 |
| 19 | 29 | 39 | 54 | 51 | 50 | 67 | 62 | 188 | 235 | 223 |
| 22 | 37 | 37 | 45 | 51 | 44 | 41 | 55 | 185 | 311 | 221 |
| 30 | 24 | 29 | 43 | 48 | 34 | 33 | 79 | 44 | 56 | 90 |
| 35 | 27 | 30 | 34 | 32 | 29 | 31 | 29 | 41 | 33 | 41 |
| 39 | 40 | 36 | 53 | 59 | 40 | 49 | 27 | 45 | 36 | 40 |
| Controls | ||||||||||
| NL43 | 29 | 63 | 104 | 197 | 261 | 733 | 509 | 610 | 662 | 744 |
| JRCSF | 23 | 23 | 28 | 26 | 32 | 75 | 65 | 72 | 67 | 70 |
| AMPHO | 35 | 23 | 27 | 29 | NA | 39 | 49 | 45 | 45 | 20 |
See Table 1 legend for details.
Time of Appearance of the Autologous Antibody Response.
To address more precisely the time of appearance of measurable neutralizing antibody responses, more frequent serial plasmas were examined from three patients shortly after the onset of symptoms of primary HIV infection. In patient TN-1 for example (Table 4), neutralizing activity could be discerned 4–8 weeks after presentation, characteristic of those patients with neutralizing antibody responses. The neutralizing responses to a heterologous primary isolate (JR-CSF) and laboratory strain (NL4-3) were delayed and of modest magnitude consistent with the published literature (2, 4, 5). The detection of this initial response required a sensitive and accurate assay using early autologous virus and antibody. The true timing of emerging neutralizing antibody responses may be masked by the extensive levels of virus replication (≈1010 virions generated daily during chronic infection (20) and 100 times that during acute infection (21). Therefore, much of the neutralizing antibody that is generated early in infection may be bound to virions in lymphoid germinal centers and elsewhere and thus undetectable in plasma.
Table 4.
Initial detection of antibody neutralization activity for subject TN-1
| Virus, week | Plasma, week
|
|||||
|---|---|---|---|---|---|---|
| 0 | 2 | 3 | 4 | 8 | 12 | |
| 0 | 36 | 38 | 42 | 58 | 184 | 319 |
| 2 | 41 | 43 | 37 | 54 | 200 | 437 |
| 3 | 26 | 42 | 38 | 55 | 236 | 490 |
| 4 | 40 | 50 | 52 | 68 | 277 | 518 |
| 8 | 30 | 46 | 49 | 64 | 246 | 465 |
| 12 | 36 | 45 | 37 | 59 | 183 | 296 |
| AMPHO | 22 | 20 | <15 | 15 | 26 | 19 |
The time course of development of neutralizing antibody in frequently obtained plasmas from patient 1 early after infection is shown. Sequential plasmas were obtained at the indicated weeks after presentation against sequential autologous viruses. The values of concurrent assays are in bold type. AMPHO, amphotropic murine leukemia virus.
Investigation of Poor Autologous Neutralizing Antibody Responses.
The failure of 2 of 14 patients to generate a significant neutralizing antibody response (Table 2) and the varying levels and timing of peak antibody titers among the untreated patients did not seem to correlate with levels of plasma HIV RNA or CD4 lymphocyte counts during the period of follow-up (data not shown). To address whether a generalized or inherent neutralization susceptibility of the patients' viruses accounted for this variability, viruses derived from two subjects who did not generate neutralization responses (TN-2 and TN-4) and two subjects who did generate neutralization responses (TN-1 and TN-3) were tested against three well characterized, broadly neutralizing monoclonal antibodies (b12, 2F5, and 2G12) (Table 5; refs. 22–24). Monoclonal antibody neutralization patterns did not correlate with the presence or absence of an autologous neutralizing antibody response. Viruses derived from all time points from each subject were susceptible to at least one monoclonal antibody. Thus viruses are not inherently resistant to neutralization. Notably, for subject TN-1 the appearance of a 2G12 neutralization-sensitive virus at month 6 and the disappearance of an IgG1b12 neutralization-sensitive virus at month 21 exemplifies the continual evolution of virus envelope sequence in response to neutralizing antibody. In contrast, the two patients who failed to develop measurable neutralizing antibody responses did not evolve changes in response to these monoclonal antibodies. Preliminary sequencing analysis suggests that neutralization escape involves multiple variations throughout env that included missense mutations, insertions, deletions, and glycosylation site mutations, often as mixtures of clones or in combinations on clones (data not shown). This complexity of env sequence evolution defies a single simple explanation for evolution of neutralization escape between time points.
Table 2.
Antibody neutralization titers (subject TN-2, treatment naive)
| Virus, months | Plasma, months
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | 17 | 20 | 24 | 27 | 29 | 32 | 36 | |
| 0 | 51 | 53 | 53 | 72 | 56 | 87 | 80 | 66 | 69 | 76 | 57 |
| 2 | 45 | 48 | 46 | 62 | 59 | 77 | 65 | 56 | 54 | 64 | 63 |
| 5 | 46 | 51 | 42 | 57 | 38 | 54 | 52 | 43 | 49 | 60 | 55 |
| 10 | 52 | 57 | 37 | 58 | 50 | 73 | 81 | 67 | 58 | 59 | 46 |
| 17 | 44 | 41 | <10 | 61 | 38 | 61 | 55 | 70 | 83 | 64 | 41 |
| 20 | 62 | 50 | <10 | 119 | 69 | 86 | 94 | 122 | 75 | 104 | 54 |
| 24 | 66 | 79 | 66 | 78 | 59 | 115 | 166 | 78 | 88 | 100 | 72 |
| 27 | 50 | 96 | 49 | 101 | 56 | 84 | 95 | 97 | 61 | 116 | 82 |
| 29 | 71 | 63 | <10 | 114 | 59 | 88 | 80 | 56 | 61 | 111 | 53 |
| 32 | 65 | 48 | 159 | 118 | 53 | 72 | 70 | 67 | 46 | 44 | 44 |
| 36 | 51 | 83 | <10 | 85 | 59 | 116 | 82 | 93 | 75 | 40 | NT |
| Controls | |||||||||||
| NL43 | 46 | 69 | 90 | 129 | 123 | 212 | 221 | 181 | 172 | 138 | 207 |
| JRCSF | 34 | 39 | 28 | 39 | 31 | 39 | 44 | 32 | 31 | 28 | 30 |
| AMPHO | <10 | 25 | 16 | 28 | 17 | NT | 32 | NT | 22 | 20 | 33 |
See Table 1 legend for details.
Table 5.
Fifty percent neutralization titers (μg/ml) by monoclonal antibodies
| Patient no. | Virus, month | b12 | 2F5 | 2G12 |
|---|---|---|---|---|
| TN-1 | 0 | 2.3 | 10.5 | >50 |
| 3 | 3.1 | 10.2 | >50 | |
| 6 | 2.4 | 3.7 | 1.2 | |
| 9 | 0.4 | 2.1 | 2.1 | |
| 12 | 2.4 | 3.1 | 1.3 | |
| 15 | 3.7 | 2.5 | 0.8 | |
| 18 | 6.6 | 1.8 | 0.4 | |
| 21 | >25 | 2.9 | 0.9 | |
| 25 | >25 | 4.7 | 4.3 | |
| TN-2 | 0 | >25 | 1.9 | 8.0 |
| 3 | >25 | 1.8 | 9.6 | |
| 9 | >25 | 1.9 | 8.2 | |
| 15 | >25 | 1.5 | 5.7 | |
| 23 | >25 | 2.1 | 4.4 | |
| 28 | >25 | 2.4 | 3.8 | |
| 35 | >25 | 2.7 | 6.2 | |
| TN-3 | 0 | 12.1 | 9.4 | 1.5 |
| 7 | >25 | 4.8 | 0.7 | |
| 15 | >25 | 3.7 | 0.5 | |
| 24 | >25 | 7.0 | >50 | |
| 37 | >25 | 13.4 | >50 | |
| 41 | >25 | 12.1 | >50 | |
| TN-4 | 0 | >25 | 19.0 | >50 |
| 3 | >25 | 17.9 | >50 | |
| 6 | >25 | 9.4 | >50 |
Susceptibility of sequential virus isolates from patients 1–4 to neutralization by three broadly reactive monoclonal antibodies is shown. The values are the concentration of antibody (in μg/ml) that produces 50% inhibition of virus replication.
Crossreactivity of Neutralizing Responses to Heterologous Viruses.
To address further whether the observed variability in neutralization responses was attributable to variability in antibody response or in virus susceptibility to neutralization, cross-neutralization assays were performed with 13 of the month-0 isolates and several plasma specimens from each of the corresponding patients (Table 6). Compared with autologous viruses, neutralization of heterologous viruses was absent or at best negligible during the first year of HIV infection. The possibility that plasma samples from patients with poor neutralizing responses contained blocking antibodies or other inhibitors of neutralization was investigated by mixing plasma samples from the two patients with poor responses with neutralization-positive plasmas to look for reduced titers against neutralization-sensitive viruses. No suggestion of such interference was observed (data not shown).
Table 6.
Antibody neutralization titers against heterologous viruses
| Virus, month 0 | Plasma
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TN-1, month
|
TN-2, month
|
TN-5, month
|
TN-6, month
|
TN-7, month
|
TN-9, month
|
|||||||||||||
| 0 | 6 | 12 | 0 | 7 | 11 | 0 | 6 | 11 | 0 | 6 | 12 | 0 | 6 | 12 | 0 | 6 | 12 | |
| TN-1 | 54 | 1236 | 3677 | 70 | 66 | 52 | 34 | 38 | 40 | 35 | 45 | 79 | 41 | 40 | 109 | 83 | 40 | 27 |
| TN-2 | 27 | 42 | 67 | 44 | 78 | 73 | 17 | <15 | 21 | 44 | 22 | 30 | 22 | 27 | 89 | 66 | 32 | 28 |
| TN-5 | <15 | 22 | 36 | 37 | 25 | 22 | 54 | 3020 | 1435 | <15 | 16 | 23 | <15 | <15 | 33 | 37 | <15 | <15 |
| TN-6 | 45 | 56 | 59 | 44 | 53 | 49 | 20 | 27 | 26 | 62 | 355 | 1097 | 28 | 47 | 126 | 99 | 51 | 33 |
| TN-7 | 47 | 55 | 67 | 57 | 70 | 54 | 25 | 23 | 33 | 39 | 54 | 81 | 41 | 2915 | 3741 | 90 | 53 | 51 |
| TN-9 | 50 | 48 | 43 | 62 | 71 | 60 | 41 | 36 | 30 | 39 | 66 | 72 | 23 | 24 | 91 | 70 | 374 | 991 |
| AMPHO | 20 | 22 | 19 | 43 | 29 | 22 | <15 | <15 | <15 | <15 | 17 | 22 | 23 | 16 | 80 | 85 | <15 | <15 |
Cross neutralization among plasmas and viruses from patients with primary HIV infection. The month-0 viruses from 13 patients were assayed for neutralization activity against serial plasmas from 13 patients, of which six representative results are displayed. The autologous reactions are in bold type. AMPHO, amphotropic murine leukemia virus.
Impact of Potent Antiretroviral Therapy of Neutralizing Antibody Responses.
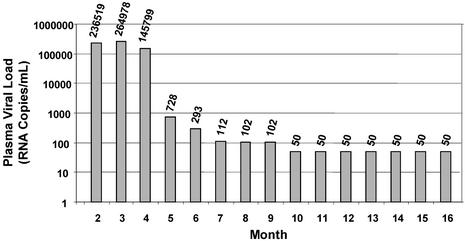
Using a second group of subjects with recent HIV infection, we investigated the impact of the administration of potent antiretroviral therapy on the neutralizing antibody response. To conduct these studies, a genomic HIV vector was constructed by using a pol gene derived from a patient virus that was highly resistant to protease and reverse-transcriptase inhibitors. This vector, in conjunction with patient virus envelope expression vectors can be used to measure neutralizing antibody accurately despite the presence of inhibitory drugs in plasma of treated patients that confound standard neutralization assays (data not shown). Autologous antibody neutralization activities were measured in longitudinal plasma samples collected from five patients who were administered antiretroviral drugs shortly after presentation and sustained suppression of plasma HIV RNA below 50 copies per ml. In all five subjects, antibody titers plateaued at relatively low titers (<1:500), and their spectrum of activity evolved very little. This pattern is exemplified by patient TE-1 (Table 7 and Fig. 3).
Table 7.
Antibody neutralization titers for Subject TE-1 (treatment experienced)
| Virus, months | Plasma, months
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 8 | 11 | 14 | 17 | 19 | |
| 0 | 107 | 193 | 292 | 264 | 505 | 504 | 519 | 440 |
| 2 | 113 | 62 | 160 | 191 | 370 | 435 | 475 | 335 |
| 5 | 85 | 52 | 119 | 165 | 255 | 248 | 388 | 279 |
| Controls | ||||||||
| NL43 | 76 | 108 | 153 | 149 | 145 | 85 | 134 | 69 |
| JRCSF | 88 | 57 | 134 | 166 | 155 | 100 | 152 | 71 |
| AMPHO | 59 | 34 | 90 | 130 | 140 | 106 | 113 | 57 |
The neutralizing antibody titers are depicted over time against three viruses that could be tested before plasma virus became undetectable. AMPHO, amphotropic murine leukemia virus. The values of concurrent assays are in bold type.
Figure 3.
Plasma HIV viral load in patient TE-1, who initiated potent antiretroviral therapy 16 weeks after presentation (see Table 7). The plasma HIV RNA values over time are shown.
Individual Variability of Neutralizing Antibody Responses.
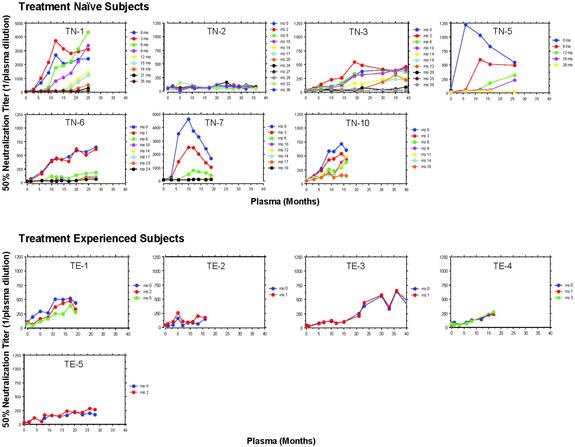
The impact of antiretroviral treatment on the emergence and evolution of neutralization responses can be appreciated by comparing the patterns of individual responses among seven patients who declined treatment and five patients who successfully suppressed plasma HIV RNA with antiretroviral therapy (Fig. 4). Fig. 4 also depicts the considerable intersubject variation in the time to peak titer and the potency of neutralizing antibody responses directed at viruses that emerged later in infection. In 9 of the 12 untreated patients with detectable neutralizing antibody, the highest measured neutralization titer was directed against the baseline virus (month 0) whereas in three others higher titers of neutralizing antibody developed against viruses that emerged later in infection.
Figure 4.
Variable individual autologous neutralizing responses. The autologous neutralizing antibody responses are displayed for seven primary HIV infection patients who declined antiretroviral therapy and for five patients who initiated potent suppressive therapy within 3 months of seroconversion.
Discussion
The role of neutralizing antibody in modulating the natural course of infection or as a vaccine strategy has received limited attention for several reasons. Neutralizing antibody responses, especially to autologous viruses, have been difficult to measure because of the technical challenges associated with the preparation of autologous virus stocks that are typically obtained from peripheral blood mononuclear cells. Furthermore, cell-derived virus does not accurately reflect the actively replicating population present in plasma; the detection of drug-resistance mutations in lymphocytes lags >1 month behind those detectable in plasma virus (25–27). To date, immunizations with envelope proteins (or expression vectors) have proved disappointing, generating low levels of neutralizing antibody or antibody restricted to the autologous strain and laboratory-adapted strains but lacking activity against most primary isolates (2, 3). In addition, the interest in neutralizing antibody has also been overshadowed by studies that implicate cell-mediated immunity in the control of HIV/simian immunodeficiency virus infection. Partial control of HIV replication in vivo has been temporally associated with the appearance of cytotoxic CD8 T cell responses (28). In simian immunodeficiency virus infection, the elimination of CD8 lymphocytes significantly releases simian immunodeficiency virus replication from partial immune control (29, 30).
The rate of antibody neutralization escape and evolution in recently infected, untreated patients described in this report exceeds the relatively rapid rates of change that are characteristic of the emergence of drug resistance during suboptimal antiretroviral therapy. This observation indicates that the potency of the selective pressure exerted by neutralizing antibodies can account for the extensive variability of env in comparison to other HIV genes (31). The question then arises why such a strong selective pressure fails to appreciably impact levels of virus replication as does chemotherapy. During the course of HIV evolution, the envelope protein has acquired the ability to retain function (i.e., bind receptors) while tolerating multiple and repeated changes in several highly variable regions containing numerous glycosylation sites (32). Although drug-resistance mutations confer much greater fitness in the presence of antiretroviral drugs, they typically do not exist as common polymorphisms in untreated patients because they impair the replication of wild-type viruses. In contrast, during the natural course of early HIV infection, fully functional envelope variants continuously emerge and compete for outgrowth in the presence of a rapidly evolving neutralizing antibody response.
The lack of cross-neutralizing antibody responses against heterologous primary isolates during the early stages of HIV infection adds to existing concerns about the difficulty of identifying immunogens capable of inducing broadly protective responses. It will be of interest to determine whether more broadly reactive antibody responses evolve over a longer course of HIV infection (i.e., >39 months). Nevertheless, an optimist might argue that neutralizing antibody confers such potent selective pressure that antibody targeted against a broad range of circulating viruses could contribute to an effective HIV vaccine. Moreover, in contrast to the selection for escape by a narrowly focused, potent neutralizing response that is reactive to remarkably high levels of virus replication, the prophylactic use of such potent activity against a relatively modest inoculum might confer significant levels of protection and is consistent with the efficacy of passive prophylaxis with antibody to autologous virus in the macaque model (33–37).
Acknowledgments
We gratefully acknowledge the invaluable nursing support of Joanne Santangelo and Paula Potter and the technical support of Nancy Keating, Sherri Rostami, Heather Overton, Melissa Moore, Wei Huang, Jeannette Whitcomb, Signe Fransen, and Jeff Beauchaine. This work was supported by the Veterans Affairs San Diego Research Center for AIDS and HIV Infection, the National Institutes of Health Acute and Early Infectious Disease Research Program, the University of California Center for AIDS Research (San Diego), National Institutes of Health Drug Resistance Grant AI 29164, and Small Business Innovation Research Grant R43-AI 48890. Monoclonal antibodies 2G12 and 2F5 were generously provided by Hermann Katinger through the AIDS Research and Reference Reagent Program, and b12 was generously provided by Dennis Burton (The Scripps Research Institute, La Jolla, CA).
Abbreviation
- Env
viral envelope glycoprotein
References
- 1.Parren P W, Burton D R. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton D R. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 4.Parren P W, Moore J P, Burton D R, Sattentau Q J. AIDS. 1999;13, Suppl. A:S137–S162. [PubMed] [Google Scholar]
- 5.Moore J P, Cao Y, Ho D D, Koup R A. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kono Y, Kobayashi K, Fukunaga Y. Arch Gesamte Virusforsch. 1973;41:1–10. doi: 10.1007/BF01249923. [DOI] [PubMed] [Google Scholar]
- 7.Narayan O, Griffin D E, Chase J. Science. 1977;197:376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- 8.Burns D P, Collignon C, Desrosiers R C. J Virol. 1993;67:4104–4113. doi: 10.1128/jvi.67.7.4104-4113.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss R A, Clapham P R, Weber J N, Dalgleish A G, Lasky L A, Berman P W. Nature. 1986;324:572–575. doi: 10.1038/324572a0. [DOI] [PubMed] [Google Scholar]
- 10.Albert J, Abrahamsson B, Nagy K, Aurelius E, Gaines H, Nystrom G, Fenyo E M. AIDS. 1990;4:107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Tremblay M, Wainberg M A. J Infect Dis. 1990;162:735–737. doi: 10.1093/infdis/162.3.735. [DOI] [PubMed] [Google Scholar]
- 12.Arendrup M, Nielsen C, Hansen J E, Pedersen C, Mathiesen L, Nielsen J O. J Acquired Immune Defic Syndr. 1992;5:303–307. [PubMed] [Google Scholar]
- 13.Montefiori D C, Zhou I Y, Barnes B, Lake D, Hersh E M, Masuho Y, Lefkowitz L B., Jr Virology. 1991;182:635–643. doi: 10.1016/0042-6822(91)90604-a. [DOI] [PubMed] [Google Scholar]
- 14.Wrin T, Crawford L, Sawyer L, Weber P, Sheppard H W, Hanson C V. J Acquired Immune Defic Syndr. 1994;7:211–219. [PubMed] [Google Scholar]
- 15.Bradney A P, Scheer S, Crawford J M, Buchbinder S P, Montefiori D C. J Infect Dis. 1999;179:1264–1267. doi: 10.1086/314711. [DOI] [PubMed] [Google Scholar]
- 16.Homsy J, Meyer M, Levy J A. J Virol. 1990;64:1437–1440. doi: 10.1128/jvi.64.4.1437-1440.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ariyoshi K, Harwood E, Chiengsong-Popov R, Weber J. Lancet. 1992;340:1257–1258. doi: 10.1016/0140-6736(92)92953-d. [DOI] [PubMed] [Google Scholar]
- 18.Connick E, Marr D G, Zhang X-Q, Clark S J, Saag M S, Schooley R T, Curiel T J. AIDS Res Hum Retroviruses. 1996;12:1129–1140. doi: 10.1089/aid.1996.12.1129. [DOI] [PubMed] [Google Scholar]
- 19.Petropoulos C J, Parkin N T, Limoli K L, Lie Y S, Wrin T, Huang W, Tian H, Smith D, Winslow G A, Capon D J, et al. Antimicrob Agents Chemother. 2000;44:920–928. doi: 10.1128/aac.44.4.920-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 21.Little S J, McLean A R, Spina C A, Richman D D, Havlir D V. J Exp Med. 1999;190:841–850. doi: 10.1084/jem.190.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Souza M P, Livnat D, Bradac J A, Bridges S H. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 23.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas C F, III, Burton D R, Ho D D. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessler J A, McKenna P M, Emini E A, Chan C P, Patel M D, Gupta S K, Mark G E, III, Barbas C F, III, Burton D R, Conley A J. AIDS Res Hum Retroviruses. 1997;13:575–582. doi: 10.1089/aid.1997.13.575. [DOI] [PubMed] [Google Scholar]
- 25.Kozal M J, Shafer R W, Winters M A, Katzenstein D A, Merigan T C. J Infect Dis. 1993;167:526–532. doi: 10.1093/infdis/167.3.526. [DOI] [PubMed] [Google Scholar]
- 26.Wei X, Ghosh S K, Taylor M E, Jonson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, et al. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 27.Havlir D V, Gamst A, Eastman S, Richman D D. J Virol. 1996;70:7894–7899. doi: 10.1128/jvi.70.11.7894-7899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, et al. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 30.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, et al. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, Detours V. Br Med Bull. 2001;58:19–42. doi: 10.1093/bmb/58.1.19. [DOI] [PubMed] [Google Scholar]
- 32.Kwong P D, Doyle M L, Casper D J, Cicala C, Leavitt S A, Majeed S, Steenbeke T D, Venturi M, Chaiken I, Fung M, et al. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 33.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 34.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, et al. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann-Lehmann R, Vlasak J, Rasmussen R A, Smith B A, Baba T W, Liska V, Ferrantelli F, Montefiori D C, McClure H M, Anderson D C, et al. J Virol. 2001;75:7470–7480. doi: 10.1128/JVI.75.16.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parren P W, Marx P A, Hessell A J, Luckay A, Harouse J, Cheng-Mayer C, Moore J P, Burton D R. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura Y, Igarashi T, Haigwood N, Sadjadpour R, Plishka R J, Buckler-White A, Shibata R, Martin M A. J Virol. 2002;76:2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]