Abstract
Innate host defenses at mucosal surfaces are critical in the early stages of many bacterial infections. In addition to cells of the traditional innate immune system, epithelial cells can also produce inflammatory mediators during an infection. However, the role of the epithelium in innate host defense in vivo is unclear. Recent studies have shown that lipopolysaccharide (LPS) recognition is critical for bladder epithelial cells to recognize and respond to Escherichia coli. Moreover, the LPS-nonresponsive mouse strain C3H/HeJ, which has a mutation in the primary LPS receptor, Toll-like receptor 4 (TLR4), is extremely susceptible to infection with uropathogenic strains of E. coli. In this study, a bone marrow transplant approach was used to investigate the specific contributions of the bladder epithelium (and other stromal cells) in the TLR4-mediated innate immune response to the invading E. coli pathogen. Mice expressing the mutant TLR4 in the epithelial/stromal compartment were not able to mount a protective inflammatory response to control the early infection even when their hematopoietic cells expressed wild-type TLR4. However, the presence of TLR4+ epithelial/stromal cells was not sufficient to activate an acute inflammatory response unless the hematopoietic cells were also TLR4+. These results demonstrated that bladder epithelial cells play a critical role in TLR4-mediated innate immunity in vivo during a mucosal bacterial infection.
The early recognition of pathogens by cells of the innate immune system is critical to the survival of the host. To facilitate this process, host organisms have evolved a series of germ line-encoded pathogen-pattern recognition receptors capable of recognizing a broad spectrum of pathogen-associated molecular patterns. The discovery of the Toll-like receptor (TLR) family has significantly enhanced our understanding of this process. TLRs recognize a variety of microbial products including lipopolysaccharide (LPS), lipoprotein, peptidoglycan, and bacterial DNA, and stimulation of these receptors leads to the induction of acute inflammatory responses and enhances the capacity of professional antigen-presenting cells to stimulate T cells (1, 2). Thus, TLRs may play a critical role in both the local control of pathogens early in infection and also the induction of an adaptive immune response.
The traditional innate immune system consists of cells from the hematopoietic lineage. With respect to the role of TLRs in innate host defense, macrophages and dendritic cells (DCs) are the hematopoietic cells that have been the focus of most investigations. However, it has been demonstrated, primarily by using cell lines in vitro, that a variety of other cell types, including epithelial cells, also express TLRs (3–6). The epithelial surfaces that line mucosal compartments traditionally have been considered barriers to pathogen entry, not contributors to active innate host defenses. However, several studies, primarily in vitro, have demonstrated that epithelial cells can produce cytokines, chemokines, and antimicrobial peptides after bacterial stimulation (7), suggesting that these cells have the capacity to influence acute inflammatory responses. Despite evidence that epithelial cells can be activated by bacterial pathogens, the role of these cells in innate host defense during in vivo infections is less clear.
The presence of established in vitro and in vivo assays in the urinary tract infection (UTI) model system has proved to be useful in dissecting many aspects of bacterial–epithelial interactions. Escherichia coli is responsible for the vast majority of UTIs. To establish infection the microbes must be able to adhere to bladder epithelial cells (8). Type 1 pili are expressed by the majority of uropathogens and have been shown to facilitate early interactions with the bladder epithelium, including attachment to and invasion of the superficial bladder epithelial cell layer (9–11). The interaction between adherent/invasive E. coli and the bladder epithelium results in the induction of a vigorous inflammatory response. The activation of bladder epithelial cells by type 1-piliated E. coli occurs through an LPS-dependent recognition pathway that is enhanced by bacterial invasion mediated by the adhesin of the type 1 pilus FimH (4, 6, 11).
Host-cell recognition of LPS, a primary component of Gram-negative bacterial outer membranes, is mediated primarily by TLR4. However, other proteins such as CD14 and MD-2 significantly enhance LPS-mediated activation of TLR4 (12). C3H/HeJ mice have an inactivating point mutation in the cytoplasmic tail of TLR4, rendering them nonresponsive to LPS (13). These mice are classically designated Lpsd (for defective response to LPS) and can be considered TLR4 null. In addition, C3H/HeJ mice are unable to control acute infections with uropathogenic E. coli (UPEC) (4, 14, 15), defining a role for TLR4-mediated signaling in acute cystitis. However, the contribution of epithelial cells in innate defense could not be evaluated in C3H/HeJ mice, because both epithelial cells and bone marrow-derived cells were Lpsd. In this study a reciprocal bone marrow transplantation approach between LPS-responsive (Lpsn; C3H, TLR4 wild type) and Lpsd (C3H/HeJ) mice was used to investigate the separate contributions of TLR4 on hematopoietic cells and on stromal cells during an acute UTI.
Materials and Methods
Irradiation and Reconstitution.
Female C3H.SW-H2b/SnJ (H-2b, Lpsn) and C3H/HeJ (H-2k, Lpsd) mice were obtained from The Jackson Laboratory. Six-week-old mice were lethally irradiated with 9 Gy of total-body irradiation. Bone marrow was obtained from the femurs of donor mice and collected in RPMI medium 1640 (Life Technologies, Rockville, MD) + 10% FBS (Sigma) containing 0.5% gentamycin, 1% penicillin/streptomycin, 1% Glutamax (stabilized glutamine), 1% sodium pyruvate, 0.1% 2-mercaptoethanol, 1% nonessential amino acids, and 1% sodium bicarbonate (all supplements from Life Technologies). T cell-depleted bone marrow was prepared by incubating the cells with anti-Thy1 antibody (kind gift of Osami Kanagawa, Washington University) and rabbit Low-Tox-M complement (Accurate Chemicals) at 37°C for 45 min. The cells were subsequently washed twice, counted, and resuspended in sterile PBS at a concentration of 1.0 × 107 cells per ml. Irradiated recipient mice were reconstituted with 200 μl of the appropriate cell suspension via the tail vein. The reconstituted mice were maintained in a clean facility for 8 weeks to allow for complete engraftment with donor bone marrow. To assay bone marrow reconstitution, spleens were harvested from infected mice, and single cell suspensions of splenocytes were prepared in Hanks' balanced salt solution (Life Technologies) + 25 mM Hepes (Life Technologies)/1% FBS (Sigma)/1% penicillin/streptomycin. Tissue clumps were removed by filtration through a 0.22-μm filter, and the cell suspensions were pelleted by centrifugation. The splenocytes were resuspended in lysis buffer (9:1, 140 μM NH4CL/17 μM Tris) and incubated for 5 min at room temperature. After this incubation, the cells were pelleted by centrifugation, washed with the Hanks' balanced salt solution medium, and resuspended in RPMI medium 1640 + 10% FBS containing 1% Glutamax and 0.5% gentamycin at a concentration of 5 × 106 cells per ml. Cells (1 × 106) were stained with α-H2-Kk-FITC and α-H2-Kb-phycoerythrin antibodies (BD PharMingen) and analyzed by flow cytometry to determine donor/recipient chimerism of the hematopoietic compartment.
Mouse Inoculations.
A 48-h standing culture of E. coli UTI89, a cystitis isolate, was pelleted and resuspended in sterile PBS at a concentration of ≈2 × 109 colony-forming units (cfu)/ml. Mice were infected via intraurethral inoculation with 50 μl of the bacterial suspension (1 × 108 cfu). Forty-eight hours after infection, mice were killed by cervical dislocation, and the bladders, kidneys, and spleens were harvested. Bladders were bisected, and one half was either fixed in neutral buffered formalin for histological analysis or quick-frozen in OCT compound (VWR Scientific) for immunohistochemical analysis. The remaining half-bladder was homogenized in sterile 0.025% Triton X-100/PBS and titered for surviving bacteria. For histological analysis, the formalin-fixed bladders were embedded in paraffin, and sections were stained with hematoxylin/eosin.
Inflammatory Scores.
To assess the degree of inflammation, hematoxylin/eosin-stained sections from neutral buffered formalin-fixed tissue were scored in a blinded fashion by using a five-point scale as described (15): 0, no inflammatory changes; 1, focal and diffuse subepithelial inflammatory cell infiltrate; 2, edema and subepithelial inflammatory cell infiltrate; 3, neutrophils in and on the bladder epithelium, edema, and subepithelial inflammatory cell infiltrate; 4, neutrophils in and on the bladder epithelium, edema, and subepithelial inflammatory cell infiltrate extending into the muscle; 5, loss of surface epithelium (necrosis with full-thickness inflammatory cell infiltration).
Immunohistochemistry.
For immunohistochemical analysis of bladder tissue, 7-μm-thick sections were prepared from frozen tissue and fixed in acetone (−20°C) for 10 min. After rehydration in PBS for 5 min, endogenous peroxidase activity was quenched by treatment with 0.3% H2O2 for 10 min. After PBS washing (three times, 5 min each), tissue sections were blocked with 1% BSA and 0.2% milk in PBS (PBS-BB). Biotin-conjugated primary antibody was added in PBS-BB and incubated overnight at 4°C. After PBS washing (three times, 5 min each), tissue was incubated with streptavidin-conjugated horseradish peroxidase (HRP) in PBS-BB for 1 h at room temperature. After PBS washing (three times, 5 min each), Cy3-tyramide (NEN/Life Sciences) was deposited for visualization of antibody staining. Tissue was counterstained with bis-benzimide (Sigma) to reveal nuclear morphology. For dual labeling of CD11c and MHC class II, tissue sections were treated with 0.3% H2O2 and PBS-BB as described above. Tissue sections were incubated with biotin-conjugated anti-MHC II (I-A) in PBS-BB for 1 h at room temperature. After PBS washing, tissue was incubated with streptavidin-conjugated HRP in PBS-BB for 1 h at room temperature. After PBS washing, FITC-tyramide was deposited to visualize antibody staining. Tissue sections were incubated in 3% H2O2 for 10 min to quench HRP activity and then incubated with hamster anti-CD11c in PBS-BB overnight at 4°C. After PBS washing, tissue sections were incubated with HRP-conjugated anti-hamster antibody in PBS-BB for 1 h at room temperature. After PBS washing, tissue sections were treated with Cy3-tyramide followed by bis-benzimide. Biotin-conjugated anti-Gr-1, intercellular adhesion molecule (ICAM)-1, MHC II (I-A), and purified hamster anti-CD11c antibodies were purchased from BD PharMingen. HRP anti-hamster and streptavidin-conjugated HRP were purchased from Jackson ImmunoResearch.
Statistical Analysis.
Bladder and kidney titers were analyzed with the Mann–Whitney nonparametric test by using the PRISM statistics program (GraphPad, San Diego).
Results
Generation of Chimeric Mice.
To assess the donor/recipient chimerism of the hematopoietic system after bone marrow transplantation in C3H mice, C3H.SW-H2b/SnJ mice, which are congenic at the MHC I locus [H-2b instead of H-2k (C3H)], were used as the TLR4 wild-type (Lpsn) control for these experiments. The C3H.SW-H2b/SnJ mice were shown to have similar bacterial clearance and inflammatory profiles as Lpsn C3H/HeN mice after infection with UPEC (data not shown). After lethal irradiation, the C3H mouse strains were reconstituted with either bone marrow from the same strain or from the opposite strain. In this way four mouse strains were created: C3H.SW-H2b/SnJ into C3H.SW-H2b/SnJ (Lpsn→Lpsn), C3H/HeJ into C3H/HeJ (Lpsd→Lpsd), C3H/HeJ into C3H.SW-H2b/SnJ (Lpsd→Lpsn), and C3H.SW-H2b/SnJ into C3H/HeJ (Lpsn→Lpsd). Importantly, Lpsd→Lpsn mice have an Lpsn stromal compartment but an Lpsd hematopoietic compartment; and the Lpsn→Lpsd mice have an Lpsd stromal compartment and an Lpsn hematopoietic compartment. After bone marrow reconstitution occurred, the mice were infected intraurethrally with UPEC and analyzed 48 h after infection. Flow-cytometric analysis of splenocytes stained with α-H-2Kb antibodies or α-H-2Kk antibodies demonstrated that the hematopoietic compartment of all the mice consisted of at least 80%, and in most cases >90%, donor-derived cells (data not shown). Donor bone marrow-derived H-2Kb/H-2Db MHC class I molecules could be detected by immunofluorescence in the bladders of infected Lpsn→Lpsd mice but not the bladders of Lpsd→Lpsd mice (data not shown).
Bladder Colonization of Chimeric Mice After Infection with UPEC.
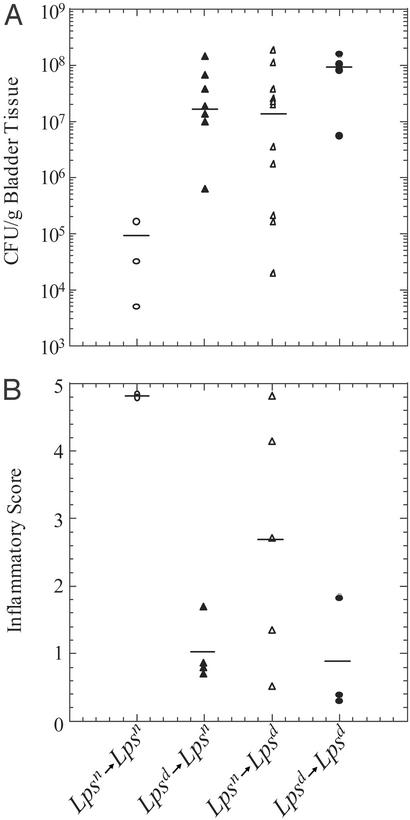
As described previously for the parental strains, the absence of a functional TLR4 rendered the Lpsd→Lpsd mice significantly more susceptible to infection in the bladder than Lpsn→Lpsn mice. At 48 h after infection, bacterial titers were 9.4 × 107 cfu per gram of bladder tissue in Lpsd→Lpsd mice and 9.6 × 104 cfu per gram of bladder tissue in Lpsn→Lpsn mice (P ≤ 0.0079; Fig. 1A). Interestingly, both the Lpsd→Lpsn and Lpsn→Lpsd mice were also impaired significantly in their ability to control infection with UPEC in the bladder as compared with Lpsn→Lpsn mice with median bacterial titers of 1.5 × 107 cfu per gram of bladder tissue (P ≤ 0.0016) and 1.3 × 107 cfu per gram of bladder tissue (P ≤ 0.0173), respectively. Although slightly lower in median bacterial titer, neither Lpsd→Lpsn nor Lpsn →Lpsd mice were significantly different from Lpsd→Lpsd mice. Thus, innate control of UPEC infection in the bladder requires Lpsn cells in both the stromal and hematopoietic compartments. Trends in bacterial titers from the kidney were the same; however, the phenotype was less pronounced (data not shown).
Figure 1.
Bladder colonization and histology scores of infected bone marrow chimeras. (A) Bladder tissue (cfu/g) from infected bone marrow chimeras 48 h postinfection in the Lpsn→Lpsn mice is identical to wild-type C3H.SW-H2b/SnJ and C3H/HeN mice (data not shown). Increased titers are seen in the Lpsd→Lpsd as well as the Lpsd→Lpsn and Lpsn→Lpsd mice. (B) Inflammatory scores for bladders from infected mice. Scores for Lpsn→Lpsn mice are identical to wild-type C3H.SW-H2b/SnJ and C3H/HeN mice (data not shown). Decreased inflammatory scores are seen in the Lpsd→Lpsd as well as the Lpsd→Lpsn and Lpsn→Lpsd mice.
Histologic Evaluation of UPEC-Infected Bladders from Chimeric Mice.
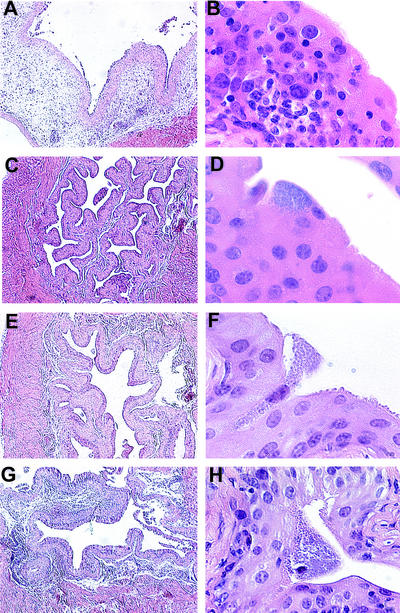
To gain more insight regarding the specific function of stromal vs. hematopoietic cells in the induction of a local inflammatory response, bladder tissue from each of the four mouse strains was analyzed by light microscopy of hematoxylin/eosin-stained tissue sections. Two independent scorers assessed the degree of inflammation in each of the samples in a blinded fashion [scoring was performed as described in Materials and Methods on a scale of 0–5, with 5 representing the highest degree of inflammation and 0 representing no inflammation (15)]. The average scores for the four mouse strains were 4.85 for Lpsn→Lpsn (n = 2), 1.0 for Lpsd→Lpsn (n = 4), 2.7 for Lpsn→Lpsd (n = 5), and 0.83 for Lpsd→Lpsd (n = 3) (Fig. 1B). The fact that Lpsn→Lpsn mice had a robust inflammatory response (Fig. 2 A and B), whereas Lpsd→Lpsd mice had only minimal inflammation (Fig. 2 C and D), confirms previous observations that TLR4 is required for the induction of an inflammatory response after UPEC challenge (4, 16). Moreover, the absence of an inflammatory response in the Lpsd→Lpsn mice (Fig. 2 E and F) indicates a critical role for Lpsn hematopoietic cell type(s) in the induction of an inflammatory response in the bladder after bacterial challenge. The reduced level of inflammation in the Lpsn→Lpsd mice (Fig. 2 G and H) argues that stromal cells are required for an efficient inflammatory response after UPEC challenge; however, the presence of inflammation in some of these mice indicates that TLR4-mediated epithelial activation is not absolutely required to induce an innate response. The variability in inflammatory scores observed in Lpsn→Lpsd mice is not related to the level of bone marrow reconstitution. This was demonstrated by showing that mice with similar degrees of reconstitution showed different levels of inflammation (data not shown). We also showed that donor MHC-expressing cells could be detected in inflamed bladders from Lpsn→Lpsd mice. The observed variability is likely the result of analyzing a dynamic inflammatory process at a single cross-sectional time point.
Figure 2.
Histology of infected bone marrow chimera bladders. Low- and high-power magnifications of infected bladder tissue from chimeric mice are shown. (Magnification: A, C, E, and G, ×5; B, D, F, and H, ×60.) Lpsn→Lpsn mice after infection have edema (A) and lymphocytic infiltrates (A and B). Infected Lpsd→Lpsd mouse bladders show minimal pathology (C) and infiltrating lymphocytes (C and D). Infected Lpsd→Lpsn mouse bladders have similar histology to Lpsd→Lpsd mouse bladders (E and F). Bladders from infected Lpsn→Lpsd mice show some edema (G) and lymphocytic infiltration (H).
Immunohistochemical Evaluation of UPEC-Infected Bladders.
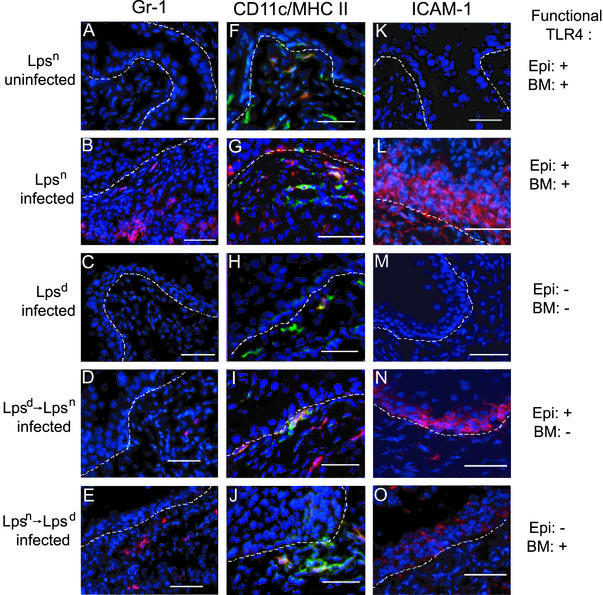
The recruitment of neutrophils to the epithelium is a hallmark of acute UTIs. To visualize the level of neutrophilic inflammation in the bladder mucosa, bladder tissue from each of the mouse strains was stained with antibody to Gr-1, a cell-surface marker of granulocytes, including neutrophils. Gr-1+ cells were absent in uninfected Lpsn mice as well as in infected Lpsd mice (Fig. 3 A and C) but were abundant in infected Lpsn mice (Fig. 3B). In Lpsd→Lpsn mice it was very difficult to find Gr-1+ cells (Fig. 3D), whereas in the Lpsn→Lpsd mice some Gr-1+ cells were present (Fig. 3E) but at levels lower than those seen in Lpsn mice (Fig. 3B). These results further demonstrate that an Lpsn hematopoietic cell type(s) is required for the recruitment of Gr-1+ cells to the bladder and that Lpsn stromal cells seem to help facilitate this process.
Figure 3.
Fluorescent immunohistochemical analysis of infected bone marrow chimera bladders. Immunofluorescent analysis of bladder tissue is shown. (A–E) Gr-1 (Ly-6G) expression by neutrophils is shown in red. (F–J) CD11c (DC marker) staining is shown in red, and MHC II (macrophage/activated DC marker) is shown in green; coexpression of CD11c and MHC II by activated DCs appears in orange. (K–O) ICAM-1 expression by activated epithelium is shown in red. Bladder tissue was analyzed from uninfected Lpsn (A, F, and K), UPEC-infected Lpsn (B, G, and L), infected Lpsd (C, H, and M), Lpsd→Lpsn (D, I, and N), and Lpsn→Lpsd mice (E, J, and O). Nuclear morphology in all fields is shown in blue. The dashed line represents the basement membrane. Expression of functional TLR4 on the epithelial/stromal compartments (Epi) or on the bone marrow-derived compartment (BM) is indicated on the right. Neutrophils are present in UPEC-infected Lpsn (B) and, to a lesser degree, in infected Lpsn→Lpsd mice (E). (D) Occasionally, neutrophils could be seen in infected Lpsd→Lpsn mice. (F–J) DCs (red), macrophages (green), and activated DCs (orange) could be seen in all mice regardless of infection status. ICAM-1 expression could be clearly seen when infected mice express functional TLR4 on the epithelium [Lpsn (L) and Lpsd→Lpsn (N) mice]. (O) Weak ICAM-1 expression could be detected in the infected Lpsn→Lpsd mice.
Macrophages and DCs are cells of the innate immune system that can respond rapidly to bacterial products and are known to populate a variety of tissues in the absence of infection, making these hematopoietic cells prime candidates for initiating an inflammatory response. To analyze the distribution of these cell types in the mouse bladder before and after infection, bladder tissue sections were stained with antibodies against CD11c and MHC class II molecules. Cells that were CD11c/MHC II double-positive were denoted as activated DCs, whereas MHC II single-positive cells were considered to be tissue-resident macrophages. In uninfected mice CD11c+, MHC II+ cells could be detected, and the majority of these cells resided at the junction of the epithelium and the lamina propria (Fig. 3F). Interestingly, it has been reported that DCs also reside in this location in the human bladder (17). In addition, a few CD11c−, MHC II+ cells as well as CD11c+, MHC II− cells, which potentially represent unactivated DCs, could be seen scattered throughout the lamina propria. After infection, CD11c+, MHC II+ double-positive cells remained at the junction between the epithelium and the underlying connective tissue (Fig. 3G) either as single cells or in clusters. Only rarely did these cells appear to enter the epithelium. There were no significant differences in the number or distribution of CD11c+, MHC II+ double-positive cells or MHC II+ cells in infected Lpsn, Lpsd, Lpsd→Lpsn or Lpsn→Lpsd mice (Fig. 3 G–J). In addition to activating naive T cells in the lymph node, DCs challenged with E. coli or E. coli LPS produce numerous acute inflammatory mediators including the neutrophil chemokines IL-8 in humans and MIP-2 in mice, suggesting that DCs could contribute to the induction of an inflammatory response (18, 19).
To assess the activation status of the epithelium in the various mouse strains at 48 h after infection we analyzed ICAM-1 expression by bladder epithelial cells by using an immunohistochemical approach. Activated epithelial cells up-regulate ICAM-1 expression on their cell surface either as a consequence of direct bacterial stimulation or after contact with cytokines such as IL-1, tumor necrosis factor, or IFN-γ (20). Moreover, the expression of ICAM-1 on bladder epithelial cells facilitates transepithelial neutrophil migration (20). In the absence of infection or functional TLR4, ICAM-1 was not detected on the epithelium (Fig. 3 K and M). However, in infected Lpsn and Lpsd→Lpsn mice, ICAM-1 expression could be readily demonstrated (Fig. 3 L and N). Some ICAM-1 staining was also present in the bladder epithelium of mice lacking functional TLR4 on epithelial cells (Lpsn→Lpsd); however, this staining was less intense than that observed for the Lpsn or Lpsd→Lpsn mice (Fig. 3O). Thus, UPEC infection induces the expression of ICAM-1 on bladder epithelial cells via a TLR4-dependent mechanism. The induction of ICAM-1 can occur either as a consequence of direct stimulation of TLR4 on epithelial cells (Lpsn or Lpsd→Lpsn) or, to a lesser extent, indirectly via TLR4-dependent activation of hematopoietic cells (Lpsn→Lpsd). These results argue that activation status of the epithelium in vivo can be modified from both the luminal (by bacteria) and basal (by hematopoietic innate immune cells) sides.
Discussion
The interaction between bacteria and epithelial cells defines the initial stage of many infectious diseases. Recently epithelial cells were proposed to be a component of the innate immune system by virtue of their ability to produce cytokines, chemokines, and antimicrobial substances after contact with bacteria (7). Although significant progress has been made regarding epithelial cell pathogen recognition, the consequences of epithelial activation in vivo are less well understood. In the UTI experimental system, it has been shown that activation of bladder epithelial cells infected with E. coli occurs through an LPS-dependent recognition event (4, 6). Furthermore, the Lpsd mouse strain, C3H/HeJ, fails to initiate an inflammatory response or clear bacteria from the bladder after an infection with UPEC (4, 14, 15). Based on these observations, we performed reciprocal bone marrow transplants between TLR4 wild-type and TLR4 mutant mouse strains to separate the contribution of epithelial/stromal cells to innate defense from those of hematopoietic cells. By using this approach, it was demonstrated that bacterial clearance from the bladder during an acute UTI requires TLR4 on cells from both stromal and hematopoietic lineages.
The observation that mice lacking either stromal or hematopoietic TLR4 are more susceptible to UPEC infection argues that these compartments must fulfill nonredundant and complementary functions that result in the efficient clearance of UPEC from the bladder during the acute phase of the infection. This result clearly demonstrates a critical role for epithelial cells in the control of acute infections. Insight into the respective roles of hematopoietic and stromal cells came from analysis of the inflammatory responses in mice lacking TLR4 in either of these compartments.
The induction of an inflammatory response required the presence of wild-type TLR4 on hematopoietic cells. Thus, it seems that hematopoietic cells must directly recognize a TLR4 ligand such as bacterial LPS to initiate the inflammatory cascade. In light of this observation, it is intriguing that CD11c+, MHC II+ DCs are present just below the epithelium where they could potentially make contact with pathogens early in the course of the infection. Another hematopoietic cell type that resides in the bladder mucosa is the mast cell (21, 22). Interestingly, TLR4 on mast cells has been implicated in neutrophil recruitment in other infection models (23, 24). Both DCs and mast cells produce cytokines and chemokines after activation and therefore can drive an immune response. Additional studies will be necessary to determine which components of the hematopoietic system are required for the initiation of the local inflammatory response.
Although some inflammation can be induced in the absence of TLR4 on the epithelium, TLR4 wild-type epithelial/stromal cells are still required to clear bacteria from the bladder during an acute infection. This result argues that rather than initiating an inflammatory response, the epithelial/stromal cells provide an additional source of cytokine production and adhesion-molecule expression, leading to an augmentation of the immune response. Future experiments may address the kinetics of the inflammatory response in the mice lacking TLR4 on the epithelium to determine whether the onset of inflammation is delayed or impaired.
During a UTI, E. coli invade into superficial umbrella cells of the bladder and replicate into large masses of bacteria. The regulation of cytokine and ICAM expression in a TLR4-dependent manner in the epithelium may be required for the efficient homing of neutrophils to the bacterial foci for decreases in bacterial number to occur. Alternatively, epithelial cells could contribute to pathogen clearance independent of influencing local inflammation, possibly by induction of inducible NOS and other enzymes. The findings presented in this article identify unique roles for both bone marrow-derived cells and epithelial cells in the recognition and control of pathogenic bacteria on uroepithelial cells as a model surface epithelium. Additional studies using other infection systems should be performed to determine the role of other mucosal epithelial surfaces in recognizing pathogens and initiating immune responses.
Acknowledgments
We thank Jacquelyn McDonough for technical expertise with bone marrow transplantation and Matt Chapman for many helpful conversations. This work was supported by National Institutes of Health Grants RO1DK51406 (to S.J.H.), RO1AI29549 (to S.J.H.), P50DK064540 (to S.J.H.), and R21DK57936 (to R.G.L.).
Abbreviations
- TLR
Toll-like receptor
- LPS
lipopolysaccharide
- DC
dendritic cell
- UTI
urinary tract infection
- Lpsd
LPS-defective (nonresponsive) or TLR4 mutant
- UPEC
uropathogenic Escherichia coli
- Lpsn
LPS-responsive
- cfu
colony-forming unit(s)
- HRP
horseradish peroxidase
- ICAM
intercellular adhesion molecule
- Lpsn→Lpsn
TLR4 wild-type bone marrow transferred into a TLR4 wild-type recipient
- Lpsd→Lpsd
TLR4 mutant bone marrow transferred into a TLR4 mutant recipient
- Lpsd→Lpsn
TLR4 mutant bone marrow transferred into a TLR4 wild-type recipient
- Lpsn→Lpsd
TLR4 wild-type bone marrow transferred into a TLR4 mutant recipient
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Medzhitov R, Janeway C., Jr N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 2.Kaisho T, Akira S. Crit Rev Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- 3.Cario E, Rosenberg I M, Brandwein S L, Beck P L, Reinecker H C, Podolsky D K. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 4.Schilling J D, Mulvey M A, Vincent C D, Lorenz R G, Hultgren S J. J Immunol. 2001;166:1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 5.Hedlund M, Frendeus B, Wachtler C, Hang L, Fischer H, Svanborg C. Mol Microbiol. 2001;39:542–552. doi: 10.1046/j.1365-2958.2001.02205.x. [DOI] [PubMed] [Google Scholar]
- 6.Backhed F, Soderhall M, Ekman P, Normark S, Richter-Dahlfors A. Cell Microbiol. 2001;3:153–158. doi: 10.1046/j.1462-5822.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- 7.Kagnoff M F, Eckmann L. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooton T M, Stamm W E. Infect Dis Clin North Am. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 9.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, Hultgren S J. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 10.Mulvey M A, Lopez-Boado Y S, Wilson C L, Roth R, Parks W C, Heuser J, Hultgren S J. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 11.Martinez J J, Mulvey M A, Schilling J D, Pinkner J S, Hultgren S J. EMBO J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beutler B. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 13.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 14.Hagberg L, Hull R, Hull S, McGhee J R, Michalek S M, Svanborg Eden C. Infect Immun. 1984;46:839–844. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins W, Gendron-Fitzpatrick A, McCarthy D O, Haine J E, Uehling D T. Infect Immun. 1996;64:1369–1372. doi: 10.1128/iai.64.4.1369-1372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins W J, Gendron-Fitzpatrick A, Balish E, Uehling D T. Infect Immun. 1998;66:2798–2802. doi: 10.1128/iai.66.6.2798-2802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christmas T J, Bottazzo G F. Clin Exp Immunol. 1992;87:450–454. doi: 10.1111/j.1365-2249.1992.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Q, Liu D, Majewski P, Schulte L C, Korn J M, Young R A, Lander E S, Hacohen N. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 19.Foti M, Granucci F, Aggujaro D, Liboi E, Luini W, Minardi S, Mantovani A, Sozzani S, Ricciardi-Castagnoli P. Int Immunol. 1999;11:979–986. doi: 10.1093/intimm/11.6.979. [DOI] [PubMed] [Google Scholar]
- 20.Agace W W, Patarroyo M, Svensson M, Carlemalm E, Svanborg C. Infect Immun. 1995;63:4054–4062. doi: 10.1128/iai.63.10.4054-4062.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christmas T J, Rode J. Br J Urol. 1991;68:473–478. doi: 10.1111/j.1464-410x.1991.tb15388.x. [DOI] [PubMed] [Google Scholar]
- 22.Bjorling D E, Jerde T J, Zine M J, Busser B W, Saban M R, Saban R. J Urol. 1999;162:231–236. doi: 10.1097/00005392-199907000-00073. [DOI] [PubMed] [Google Scholar]
- 23.Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. J Immunol. 2001;167:2250–2256. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]
- 24.Malaviya R, Ikeda T, Ross E, Abraham S N. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]